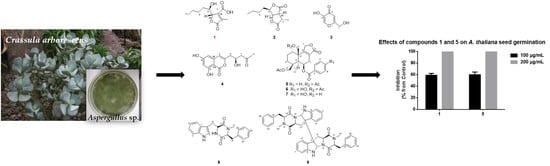
Phytotoxic Metabolites Isolated from Aspergillus sp., an Endophytic Fungus of Crassula arborescens
Abstract
1. Introduction
2. Results
2.1. Identification of Strain MJ01
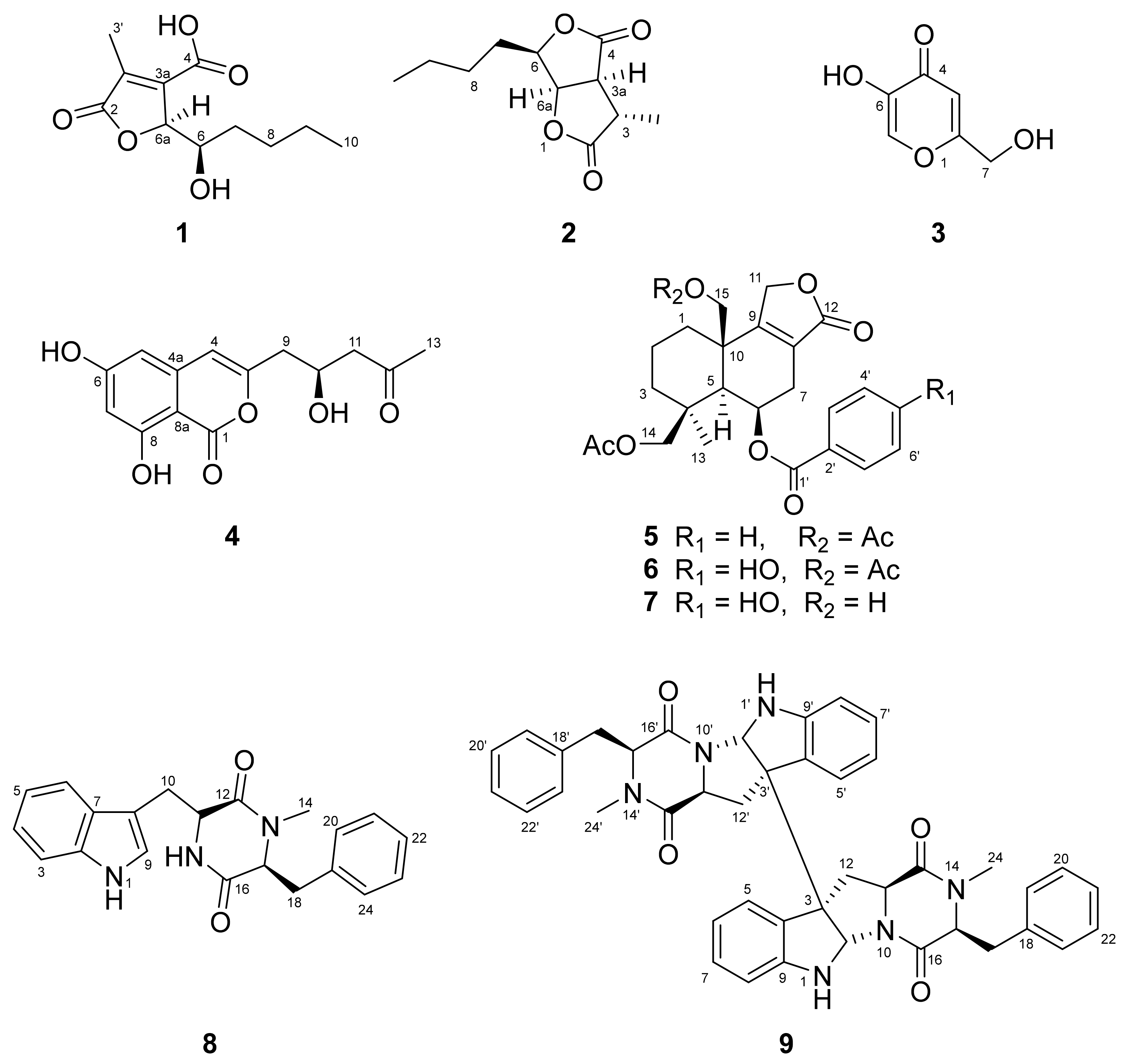
2.2. Isolation, Purification and Structure Elucidation
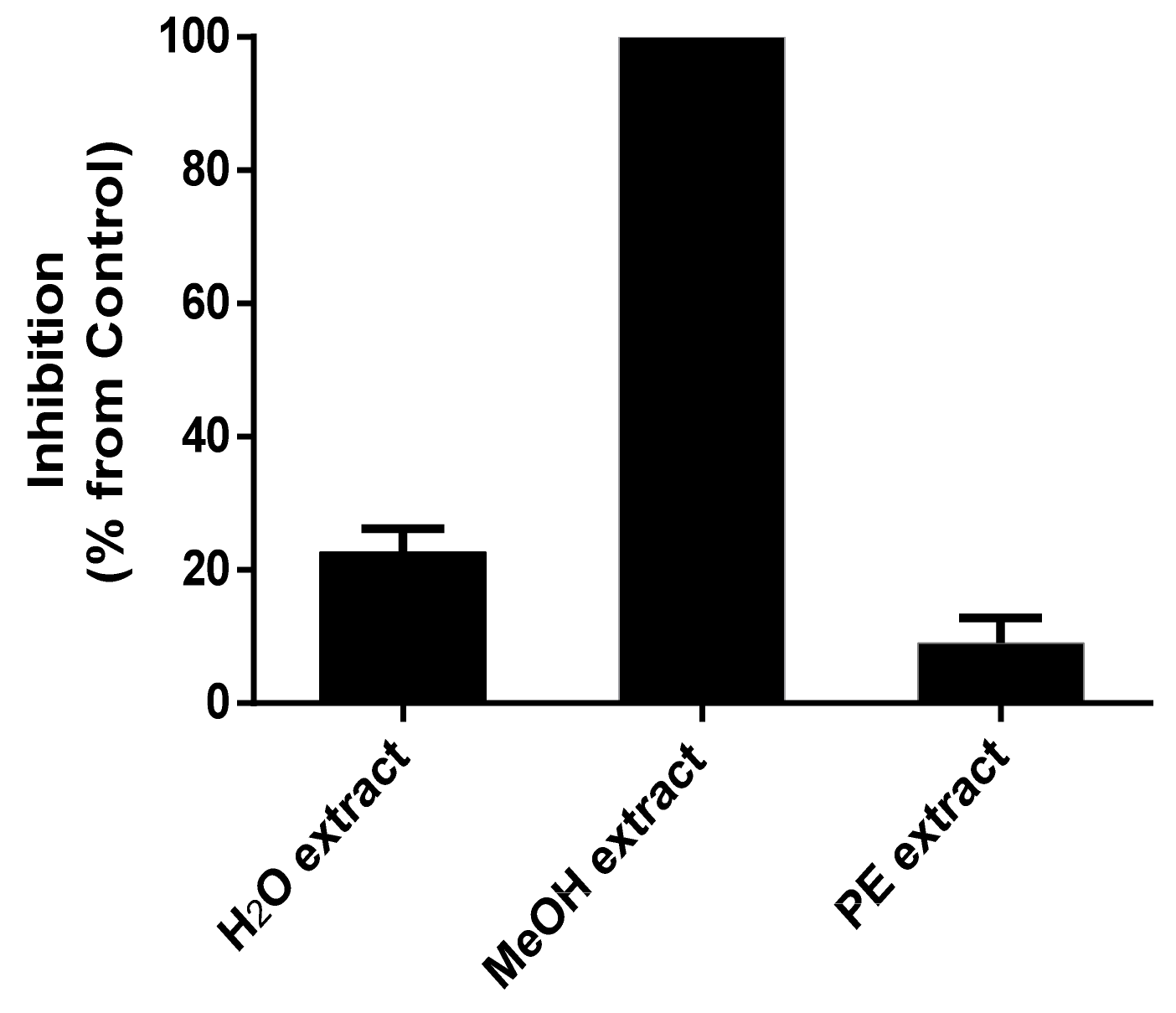
2.3. Bioactivity of Purified Compounds
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungus Isolation
4.3. Fermentation, Extraction, Bioassay-Guided Fractionation and Purification of Compounds
4.3.1. Aspertamarinoic Acid (1)
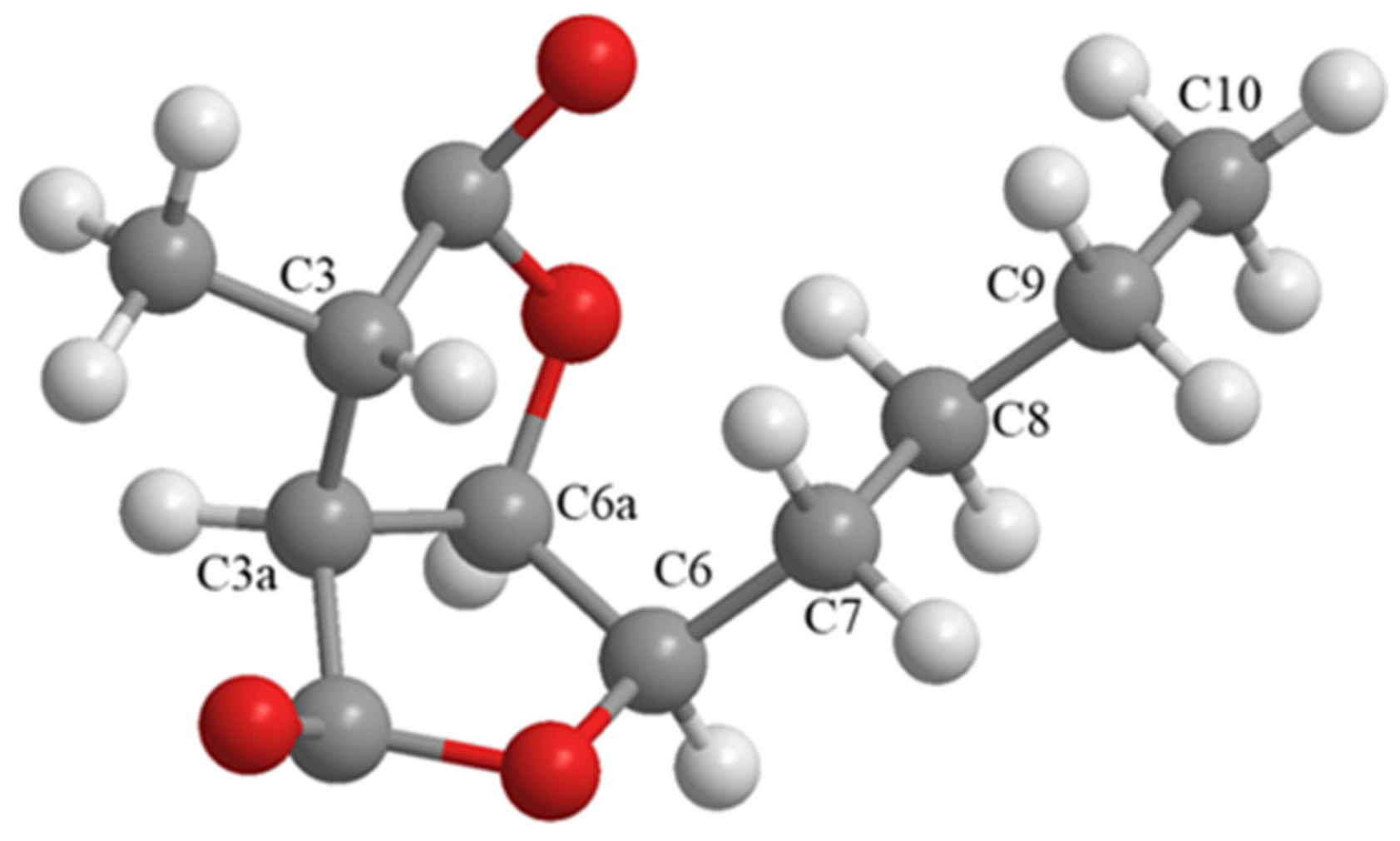
4.3.2. (−)-Dihydrocanadensolide (2)
4.3.3. Kojic Acid (3)
4.3.4. Citreoisocoumarin (4)
4.3.5. Astellolide A (5)
4.3.6. Astellolide B (6)
4.3.7. Astellolide G (7)
4.3.8. Cyclo-N-Methylphenylalanyltryptophenyl (8)
4.3.9. (−)-Ditryptophenaline (9)
4.4. X-ray Crystallographic Analysis of Compound 2
4.5. Germination-Inhibition Assay
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z. Advances in cropland weed management in China. Plant Prot. 2004, 30, 28–33. [Google Scholar]
- Schwarzlander, M.; Hinz, H.L.; Winston, R.L.; Day, M.D. Biological control of weeds: An analysis of introductions, rates of establishment and estimates of success, worldwide. BioControl 2018, 63, 319–331. [Google Scholar] [CrossRef]
- Marshall, E.J.P.; Brown, V.K.; Boatman, N.D.; Lutman, P.J.W.; Squire, G.R.; Ward, L.K. The role of weeds in supporting biological diversity within crop fields*. Weed Res. 2003, 43, 77–89. [Google Scholar] [CrossRef]
- Singh, M.; Kukal, M.S.; Irmak, S.; Jhala, A.J. Water Use Characteristics of Weeds: A Global Review, Best Practices, and Future Directions. Front. Plant Sci. 2021, 12, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for Weed Control in Agricultural Systems. Crop. Prot. 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Weir, T.L.; Park, S.W.; Vivanco, J.M. Biochemical and Physiological Mechanisms Mediated by Allelochemicals. Curr. Opin. Plant Biol. 2004, 7, 472–479. [Google Scholar] [CrossRef]
- Macias, F.A.; Molinillo, J.M.G.; Varela, R.M.; Galindo, J.C.G. Allelopathy—A Natural Alternative for Weed Control. Pest Manag. Sci. 2007, 63, 327–348. [Google Scholar] [CrossRef]
- El-Hawary, S.S.; Mohammed, R.; Abou Zid, S.; Ali, Z.Y.; El-Gendy, A.O.; Elwekeel, A. In-vitro Cyclooxygenase Inhibitory, Antioxidant and Antimicrobial Activities of Phytochemicals Isolated from Crassula arborescens (Mill.) willd. Int. J. Appl. Res. Nat. Prod. 2016, 9, 8–14. [Google Scholar]
- Wang, M.; Sun, M.; Hao, H.; Lu, C. Avertoxins A-D, Prenyl Asteltoxin Derivatives from Aspergillus versicolor Y10, an Endophytic Fungus of Huperzia serrata. J. Nat. Prod. 2015, 78, 3067–3070. [Google Scholar] [CrossRef]
- Mulzer, J.; Kattner, L. Doubly Stereodifferentiating Hiyama Addition with Mismatched Reactants: Enantio- and Diastereo-controlled Synthesis of Dihydrocanadensolide. Angew. Chem. Int. Ed. 1990, 29, 679–680. [Google Scholar] [CrossRef]
- Li, X.; Jeong, J.H.; Lee, K.T.; Rho, J.R.; Choi, H.D.; Kang, J.S.; Son, B.W. γ-Pyrone Derivatives, Kojic Acid Methyl Ethers from a Marine-derived Fungus Altenaria sp. Arch. Pharmacal Res. 2003, 26, 532–534. [Google Scholar] [CrossRef]
- Cui, H.; Liu, Y.; Nie, Y.; Liu, Z.; Chen, S.; Zhang, Z.; Lu, Y.; He, L.; Huang, X.; She, Z. Polyketides from the Mangrove-derived Endophytic Fungus Nectria sp. HN001 and Their α-Glucosidase Inhibitory Activity. Mar. Drugs 2016, 14, 86. [Google Scholar] [CrossRef]
- Yamamura, S.; Lai, S.; Shizuri, Y.; Kawai, K.; Furukawa, H. Three New Phenolic Metalolites from Penicillium Species. Heterocycles 1991, 32, 297–305. [Google Scholar] [CrossRef]
- Gould, R.O.; Simpson, T.J.; Walkinshaw, M.D. Isolation and X-ray Crystal Structures of Astellolides A and B, Sesquiterpenoid Metabolites of Aspergillus variecolor. Tetrahedron Lett. 1981, 22, 1047–1050. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Gong, Q.; Zhu, G.; Yuan, C.; Zuo, M.; Rao, Q.; Zhu, W.; Hao, X. Kojic Acid Derivatives and Sesquiterpenes from the Aspergillus flavus GZWMJZ-288, A Fungal Endophyte of Garcinia multiflora. Nat. Prod. Commun. 2018, 13, 1421–1424. [Google Scholar] [CrossRef]
- Ren, R.; Chen, C.J.; Hu, S.S.; Ge, H.M.; Zhu, W.Y.; Tan, R.X.; Jiao, R.H. Drimane Sesquiterpenoids from the Aspergillus oryzae QXPC-4. Chem. Biodivers. 2015, 12, 371–379. [Google Scholar] [CrossRef]
- Luo, S.L.; Li, G.H.; Liu, F.F.; Lei, L.P.; Xia, Z.Y.; Zhang, K.Q. A New Sesquiterpene from Endophytic Fungus Aspergillus sp. Nat. Prod. Res. 2012, 26, 1334–1338. [Google Scholar] [CrossRef]
- Saruwatari, T.; Yagishita, F.; Mino, T.; Noguchi, H.; Hotta, K.; Watanabe, K. Cytochrome P450 as Dimerization Catalyst in Diketopiperazine Alkaloid Biosynthesis. ChemBioChem 2014, 15, 656–659. [Google Scholar] [CrossRef]
- Saetang, P.; Rukachaisirikul, V.; Phongpaichit, S.; Preedanon, S.; Sakayaroj, J.; Hadsadee, S.; Jungsuttiwong, S. Aspertamarinolides A-C: γ-butenolides from the Marine-Derived Fungus Aspergillus tamarii PSU-MF90. Tetrahedron Lett. 2020, 61, 152529–152533. [Google Scholar] [CrossRef]
- Sharma, G.V.M.; Gopinath, T. Radical Cyclisation Approach for the Synthesis of (+)Dihydrocanadensolide, (+)Dihydrosporothriolide and their C-3 Epimers from D-xylose. Tetrahedron 2003, 59, 6521–6530. [Google Scholar] [CrossRef]
- Nubbemeyer, U. Diastereoselective Zwitterionic Aza-Claisen Rearrangement: The Synthesis of Bicyclic Tetrahydrofurans and a Total Synthesis of (+)-Dihydrocanadensolide. J. Org. Chem. 1996, 61, 3677–3686. [Google Scholar] [CrossRef] [PubMed]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical Ecology of Endophytic Fungi: Origins of Secondary Metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef] [PubMed]
- Strobel, G.A. Endophytes as Sources of Bioactive Products. Microbes Infect. 2003, 5, 535–544. [Google Scholar] [CrossRef]
- Qin, S.; Xing, K.; Jiang, J.H.; Xu, L.H.; Li, W.J. Biodiversity, Bioactive Natural Products and Biotechnological Potential of Plant-associated Endophytic Actinobacteria. Appl. Microbiol. Biotechnol. 2011, 89, 457–473. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The Endophytic Continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Porras-Alfaro, A.; Bayman, P. Hidden Fungi, Emergent Properties: Endophytes and Microbiomes. Annu. Rev. Phytopathol. 2011, 49, 291–315. [Google Scholar] [CrossRef]
- Aghdam, S.A.; Brown, A.M.V. Deep Learning Approaches for Natural Product Discovery from Plant Endophytic Microbiomes. Environ. Microbiome 2021, 16, 601–620. [Google Scholar] [CrossRef]
- Staniek, A.; Woerdenbag, H.J.; Kayser, O. Endophytes: Exploiting Biodiversity for the Improvement of Natural Product-based Drug Discovery. J. Plant Interact. 2008, 3, 75–93. [Google Scholar] [CrossRef]
- Pimentel, M.R.; Molina, G.; Dionisio, A.P.; Marostica Junior, M.R.; Pastore, G.M. The Use of Endophytes to Obtain Bioactive Compounds and Their Application in Biotransformation Process. Biotechnol. Res. Int. 2011, 2011, 576286–576296. [Google Scholar] [CrossRef]
- Suryanarayanan, T.S.; Thirunavukkarasu, N.; Govindarajulu, M.B.; Sasse, F.; Jansen, R.; Murali, T.S. Fungal Endophytes and Bioprospecting. Fungal Biol. Rev. 2009, 23, 9–19. [Google Scholar] [CrossRef]
- Cimmino, A.; Zonno, M.C.; Andolfi, A.; Troise, C.; Motta, A.; Vurro, M.; Evidente, A. Agropyrenol, a Phytotoxic Fungal Metabolite, and its Derivatives: A Structure-activity Relationship Study. J. Agric. Food Chem. 2013, 61, 1779–1783. [Google Scholar] [CrossRef]
- Li, H.; Xiao, J.; Gao, Y.Q.; Tang, J.J.; Zhang, A.L.; Gao, J.M. Chaetoglobosins from Chaetomium clobosum, an endophytic fungus in Ginkgo biloba, and their Phytotoxic and Cytotoxic Activities. J. Agric. Food Chem. 2014, 62, 3734–3741. [Google Scholar] [CrossRef]
- Zhang, Q.; Xiao, J.; Sun, Q.Q.; Qin, J.C.; Pescitelli, G.; Gao, J.M. Characterization of Cytochalasins from the Endophytic Xylaria sp. and their Biological Functions. J. Agric. Food Chem. 2014, 62, 10962–10969. [Google Scholar] [CrossRef]
- El-Hasan, A.; Buchenauer, H. Actions of 6-Pentyl-alpha-pyrone in Controlling Seedling Blight Incited by Fusarium moniliforme and Inducing Defence Responses in Maize. J. Phytopathol. 2009, 157, 697–707. [Google Scholar] [CrossRef]
- Li, H.; Hu, J.; Wei, H.; Solomon, P.S.; Vuong, D.; Lacey, E.; Stubbs, K.A.; Piggott, A.M.; Chooi, Y.H. Chemical Ecogenomics-Guided Discovery of Phytotoxic alpha-Pyrones from the Fungal Wheat Pathogen Parastagonospora nodorum. Org. Lett. 2018, 20, 6148–6152. [Google Scholar] [CrossRef]
- Li, F.; Ye, Z.; Huang, Z.; Chen, X.; Sun, W.; Gao, W.; Zhang, S.; Cao, F.; Wang, J.; Hu, Z.; et al. New alpha-pyrone derivatives with herbicidal activity from the endophytic fungus Alternaria brassicicola. Bioorg. Chem. 2021, 117, 105452. [Google Scholar] [CrossRef]
- Petersen, F.; Zahner, H.; Metzger, J.W.; Freund, S.; Hummel, R.P. Germicidin, an Autoregulative Germination Inhibitor of Streptomyces viridochromogenes NRRL B-1551. J. Antibiot. 1993, 46, 1126–1138. [Google Scholar] [CrossRef]
- Garcia-Mendez, M.C.; Macias-Ruvalcaba, N.A.; Lappe-Oliveras, P.; Hernandez-Ortega, S.; Macias-Rubalcava, M.L. Phytotoxic Potential of Secondary Metabolites and Semisynthetic Compounds from Endophytic Fungus Xylaria feejeensis Strain SM3e-1b Isolated from Sapium macrocarpum. J. Agric. Food Chem. 2016, 64, 4255–4263. [Google Scholar] [CrossRef]
- Ayeb-Zakhama, A.E.; Beyaoui, A.; Salem, S.B.; Sakka-Rouis, L.; Bouajila, J.; Jannet, H.B.; Harzallah-Skhiri, F. Phytochemical and phytotoxic investigation of the flowers from Citharexylum spinosum L. Ind. Crops Prod. 2015, 76, 653–659. [Google Scholar] [CrossRef]

| Compounds | 1 | 2 | 3 | 4 | 5 | 8 |
|---|---|---|---|---|---|---|
| MIC (μg/mL) | 200 | 800 | 400 | 800 | 200 | 400 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Lu, C.; Tang, Y.; Shen, Y. Phytotoxic Metabolites Isolated from Aspergillus sp., an Endophytic Fungus of Crassula arborescens. Molecules 2022, 27, 7710. https://doi.org/10.3390/molecules27227710
Ma J, Lu C, Tang Y, Shen Y. Phytotoxic Metabolites Isolated from Aspergillus sp., an Endophytic Fungus of Crassula arborescens. Molecules. 2022; 27(22):7710. https://doi.org/10.3390/molecules27227710
Chicago/Turabian StyleMa, Jingjing, Chunhua Lu, Yajie Tang, and Yuemao Shen. 2022. "Phytotoxic Metabolites Isolated from Aspergillus sp., an Endophytic Fungus of Crassula arborescens" Molecules 27, no. 22: 7710. https://doi.org/10.3390/molecules27227710
APA StyleMa, J., Lu, C., Tang, Y., & Shen, Y. (2022). Phytotoxic Metabolites Isolated from Aspergillus sp., an Endophytic Fungus of Crassula arborescens. Molecules, 27(22), 7710. https://doi.org/10.3390/molecules27227710