Straightforward Approach for Preparing Durable Antibacterial ZnO Nanoparticle Coatings on Flexible Substrates
Abstract
1. Introduction
2. Results and Discussion
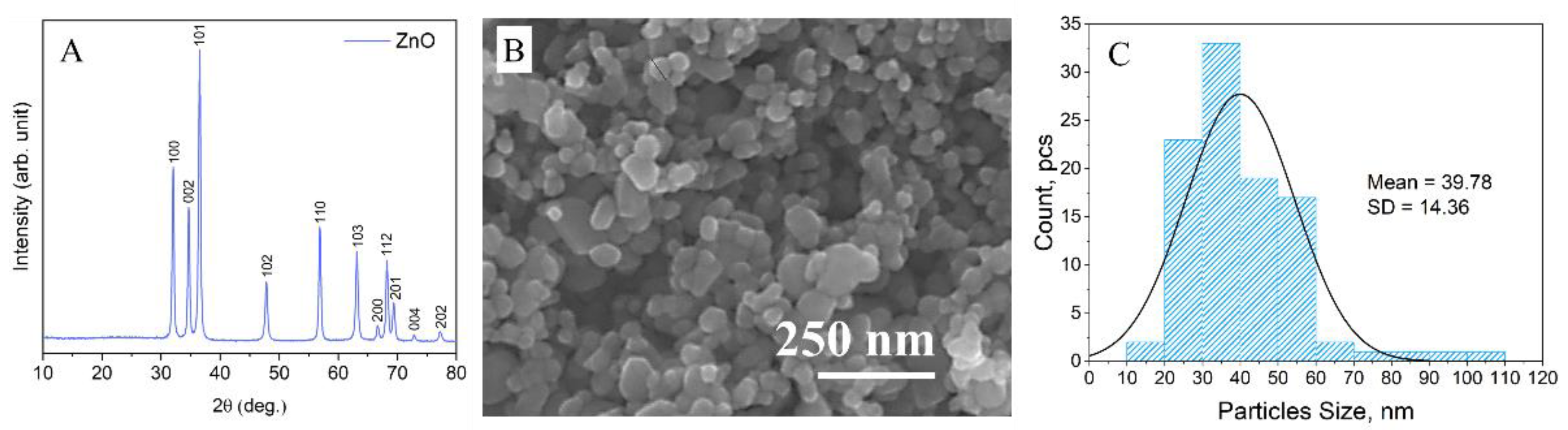
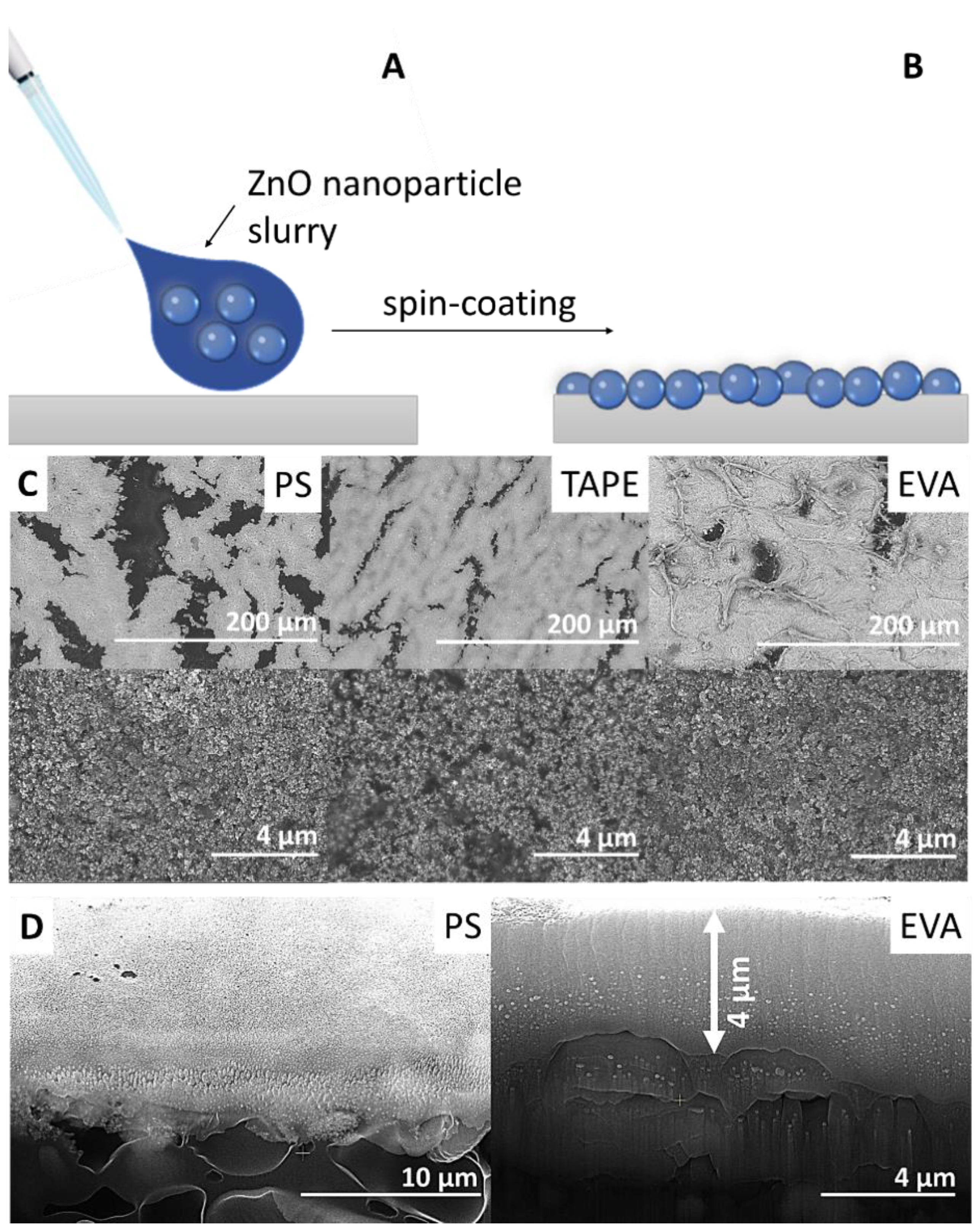
2.1. Characterization of ZnO Nanoparticles and ZnO Nanoparticle Coatings
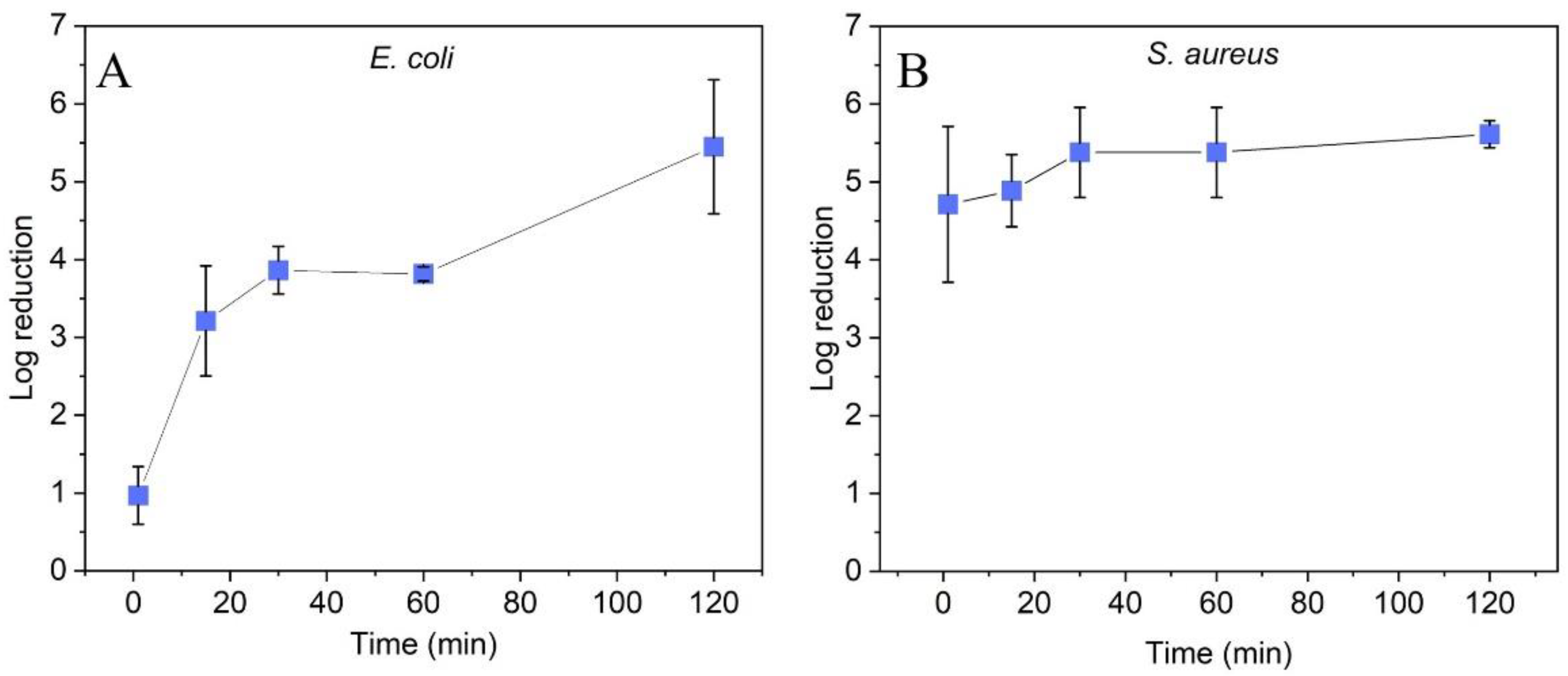
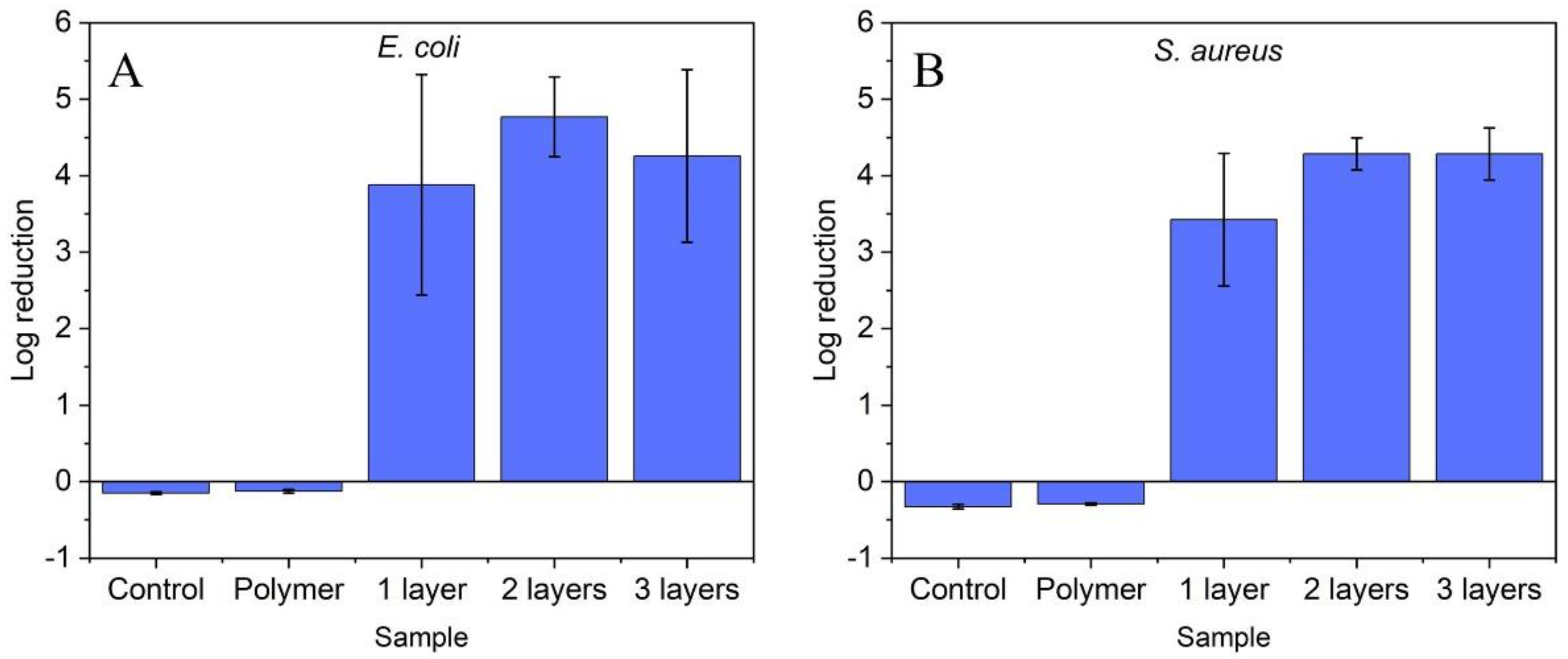
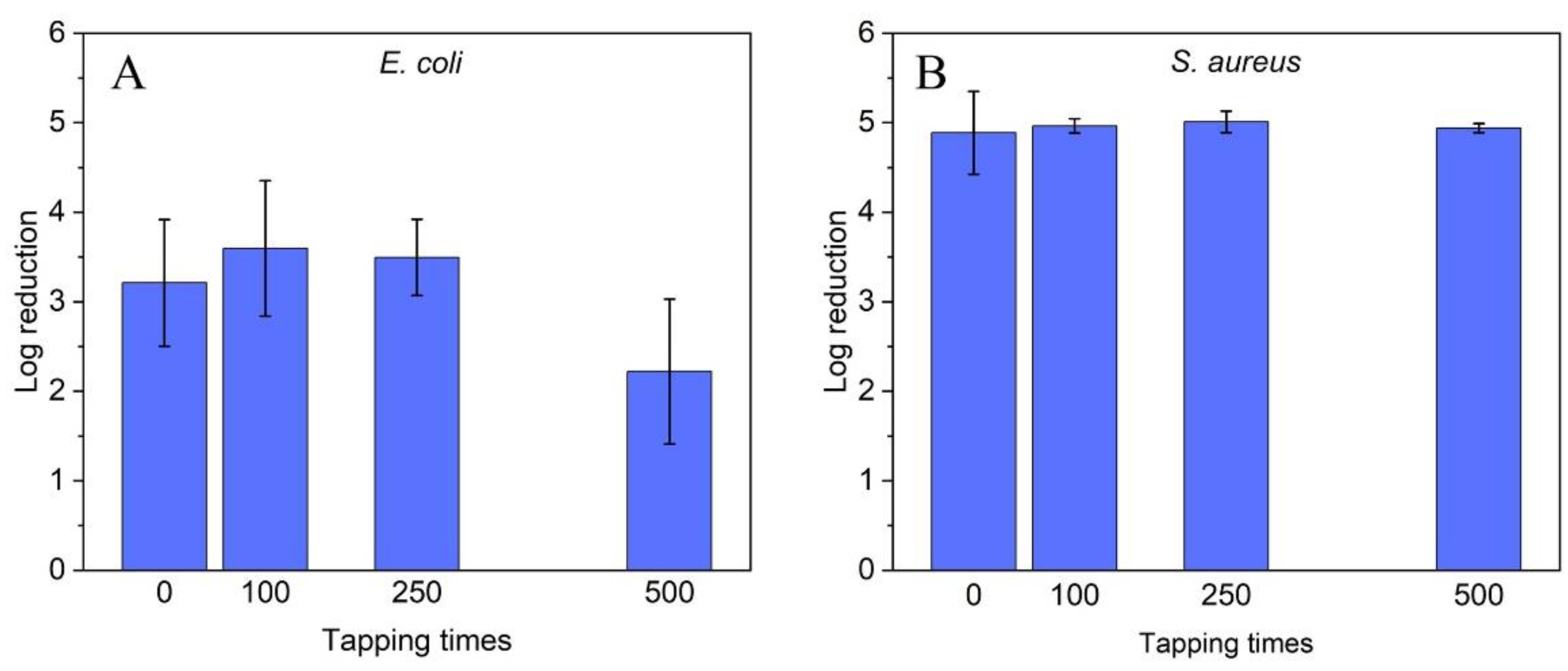
2.2. Antibacterial Efficacy of ZnO Nanoparticle Coatings
3. Materials and Methods
3.1. Materials
3.2. Samples Preparation
3.3. Characterization
3.4. Antibacterial Efficacy Assay
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasanthi, M.; Ravichandran, K.; Jabena Begum, N.; Muruganantham, G.; Snega, S.; Panneerselvam, A.; Kavitha, P. Influence of Sn doping level on antibacterial activity and certain physical properties of ZnO films deposited using a simplified spray pyrolysis technique. Superlattices Microstr. 2013, 55, 180–190. [Google Scholar] [CrossRef]
- Manoharan, C.; Pavithra, G.; Bououdina, M.; Dhanapandian, S.; Dhamodharan, P. Characterization and study of antibacterial activity of spray pyrolysed ZnO:Al thin films. Appl. Nanosci. 2016, 6, 815–825. [Google Scholar] [CrossRef]
- Khademjafai, S.; Rabiee, S.M.; Nourouzi, S.; Rami, R.S. In-vitro evaluation and antibacterial activity of ZnO nanoparticles deposited on hydroxyapatite tablets by RF magnetron sputtering. Mater. Today Commun. 2021, 28, 102520. [Google Scholar] [CrossRef]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.; Ann, L.C.; Bakhori, S.; Hasan, H.; Mohamad, D. Review on Zinc Oxide Nanoparticles: Antibacterial Activity and Toxicity Mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef]
- Luo, Q.; Cao, H.; Wang, L.; Ma, X.; Liu, X. ZnO@ZnS nanorod-array coated titanium: Good to fibroblasts but bad to bacteria. J. Colloid Interface Sci. 2020, 579, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Leśniak-Ziółkowska, K.; Kazek-Kęsik, A.; Rokosz, K.; Raaen, S.; Stolarczyk, A.; Krok-Borkowicz, M.; Pamuła, E.; Gołda-Cępa, M.; Brzychczy-Włoch, M.; Simka, W. Electrochemical modification of the Ti-15Mo alloy surface in solutions containing ZnO and Zn3(PO4)2 particles. Mater. Sci. Eng. C 2020, 115, 111098. [Google Scholar] [CrossRef] [PubMed]
- Uribe, P.A.; Ruiz, J.; Ortiz, C.C.; Blanco, S.I.; Gutierrez, J.A. Antibacterial activity of ZnO nanoparticle coatings formed by electrophoretic deposition. J. Phys. Conf. Ser. 2020, 1541, 012007. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Villani, F.; Rizzo, A.; Caputo, I.; Paolella, G.; Vigliotta, G. Antibacterial Al-doped ZnO coatings on PLA films. J. Mater. Sci. 2020, 55, 4830–4847. [Google Scholar] [CrossRef]
- Dimapilis, E.A.S.; Hsu, C.-S.; Mendoza, R.M.O.; Lu, M.-C. Zinc oxide nanoparticles for water disinfection. Sustain. Environ. Res. 2018, 28, 47–56. [Google Scholar] [CrossRef]
- Sun, J.; Cai, S.; Li, Q.; Li, Z.; Xu, G. UV-irradiation induced biological activity and antibacterial activity of ZnO coated magnesium alloy. Mater. Sci. Eng. C 2020, 114, 110997. [Google Scholar] [CrossRef]
- Visnapuu, M.; Rosenberg, M.; Truska, E.; Nõmmiste, E.; Šutka, A.; Kahru, A.; Rähn, M.; Vija, H.; Orupõld, K.; Kisand, V.; et al. UVA-induced antimicrobial activity of ZnO/Ag nanocomposite covered surfaces. Colloids Surf. B 2018, 169, 222232. [Google Scholar] [CrossRef] [PubMed]
- Dimitrakellis, P.; Kaprou, G.D.; Papavieros, G.; Mastellos, D.C.; Constantoudis, V.; Tserepi, A.; Gogolides, E. Enhanced antibacterial activity of ZnO-PMMA nanocomposites by selective plasma etching in atmospheric pressure. Micro Nano Eng. 2021, 13, 100098. [Google Scholar] [CrossRef]
- Kumari, P.; Bharti, A.; Agarwal, V.; Kumar, N.; Saxena, K. ZnO coating on silicon rubber urinary catheter to reduce the biofilm infections. Adv. Mater. Process. Technol. 2021. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Amir, B.; Riaz, S.; Naseem, S. Antibacterial, magnetic, optical and dielectric analysis of novel La2O3 doped ZnO thin films. Opt. Mater. 2020, 109, 110287. [Google Scholar] [CrossRef]
- Zhai, X.; Ju, P.; Guan, F.; Ren, Y.; Liu, X.; Wang, N.; Zhang, Y.; Duan, J.; Wang, C.; Hou, B. Electrodeposition of capsaicin-induced ZnO/Zn nanopillar films for marine antifouling and antimicrobial corrosion. Surf. Coat. Technol. 2020, 397, 125959. [Google Scholar] [CrossRef]
- Rajab, F.H.; Korshed, P.; Liu, Z.; Wang, T.; Li, L. How did the structural ZnO nanowire as antibacterial coatings control the switchable wettability. Appl. Surf. Sci. 2018, 469, 593–606. [Google Scholar] [CrossRef]
- Valerini, D.; Tammaro, L.; Vigliotta, G.; Picariello, E.; Banfi, F.; Cavaliere, E.; Ciambriello, L.; Gavioli, L. Ag Functionalization of Al-Doped ZnO Nanostructured Coatings on PLA Substrate for Antibacterial Applications. Coatings 2020, 10, 1238. [Google Scholar] [CrossRef]
- Yuvaraj, D.; Kaushik, R.; Narasimha Rao, K. Optical, Field-Emission, and Antimicrobial Properties of ZnO Nanostructured Films Deposited at Room Temperature by Activated Reactive Evaporation. ACS Appl. Mater. Interfaces 2010, 2, 1019–1024. [Google Scholar] [CrossRef]
- Popescu, M.C.; Ungureanu, C.; Buse, E.; Nastase, F.; Tucureanu, V.; Suchea, M.; Draga, S.; Popescu, M.A. Antibacterial efficiency of cellulose-based fibers covered with ZnO and Al2O3 by Atomic Layer Deposition. Appl. Surf. Sci. 2019, 481, 1287–1298. [Google Scholar] [CrossRef]
- Yemini, R.; Frank, S.; Natan, M.; Jacobi, G.; Aviv, H.; Zysler, M.; Banin, E.; Noked, M. Biofilm-Protected Catheters Nanolaminated by Multiple Atomic-Layer-Deposited Oxide Films. ACS Appl. Nano Mater. 2021, 4, 6398–6406. [Google Scholar] [CrossRef]
- Kayani, Z.N.; Bashir, Z.; Riaz, S.; Naseem, S.; Saddiqe, Z. Transparent boron-doped zinc oxide films for antibacterial and magnetic applications. J. Mater. Sci. Mater. Electron. 2020, 31, 11911–11926. [Google Scholar] [CrossRef]
- Al-Jawad, S.M.H.; Sabeeh, S.H.; Taha, A.A.; Jassim, H.A. Synthesis and Characterization of Fe–ZnO Thin Films for Antimicrobial Activity. Surf. Rev. Lett. 2018, 26, 1850197. [Google Scholar] [CrossRef]
- Ibrahim, S.; El-Naggar, M.E.; Youssef, A.M.; Abdel-Aziz, M.S. Functionalization of Polystyrene Nanocomposite with Excellent Antimicrobial Efficiency for Food Packaging Application. J. Clust. Sci. 2020, 31, 1371–1382. [Google Scholar] [CrossRef]
- Thamaraiselvan, C.; Carmiel, Y.; Eliad, G.; Sukenik, C.N.; Semiat, R.; Dosoretz, C.G. Modification of a polypropylene feed spacer with metal oxide-thin film by chemical bath deposition for biofouling control in membrane filtration. J. Membr. Sci. 2018, 573, 511–519. [Google Scholar] [CrossRef]
- Agrawal, N.; Tan, J.S.J.; Low, P.S.; Fong, E.W.M.; Lai, Y.; Chen, Z. Green Synthesis of Robust Superhydrophobic Antibacterial and UV-Blocking Cotton Fabrics by a Dual-Stage Silanization Approach. Adv. Mater. Interfaces 2019, 6, 1900032. [Google Scholar] [CrossRef]
- Uğur, S.S.; Sarıışık, M.; Aktaş, A.H.; Çiğdem Uçar, M.; Erden, E. Modifying of Cotton Fabric Surface with Nano-ZnO Multilayer Films by Layer-by-Layer Deposition Method. Nanoscale Res. Lett. 2010, 5, 1204. [Google Scholar] [CrossRef]
- Hammad, S.M.; El-Wassefy, N.A.; Shamaa, M.S.; Fathy, A. Evaluation of zinc-oxide nanocoating on the characteristics and antibacterial behaviour of nickel-titanium alloy. Dent. Press J. Orthod. 2020, 25, 51–58. [Google Scholar] [CrossRef]
- Emami-Karvani, Z.; Chehrazi, P. Antibacterial activity of ZnO nanoparticle on Gram-positive and Gram-negative bacteria. Afr. J. Microbiol. Res. 2012, 5, 1368–1373. [Google Scholar]
- Pulit-Prociak, J.; Staroń, A.; Staroń, P.; Chmielowiec-Korzeniowska, A.; Drabik, A.; Tymczyna, L.; Banach, M. Preparation and of PVA-based compositions with embedded silver, copper and zinc oxide nanoparticles and assessment of their antibacterial properties. J. Nanobiotechnol. 2020, 18, 148. [Google Scholar] [CrossRef]
- Gudkov, S.V.; Burmistrov, D.E.; Serov, D.A.; Rebezov, M.B.; Semenova, A.A.; Lisitsyn, A.B. A mini review of antibacterial properties of ZnO Nanoparticles. Front. Phys. 2021, 9, 641481. [Google Scholar] [CrossRef]
- Lakshmi Prasanna, V.; Vijayaraghavan, R. Insight into the Mechanism of Antibacterial Activity of ZnO: Surface Defects Mediated Reactive Oxygen Species Even in the Dark. Langmuir 2015, 31, 9155–9162. [Google Scholar] [CrossRef] [PubMed]
| Polymer Substrate | Solubility in Toluene | Coating Properties |
|---|---|---|
| PS | + | durable |
| EVA | + | durable |
| PMMA | − | easy to remove |
| PP | − | easy to remove |
| HDPE | − | easy to remove |
| Acrylate tape | + | durable |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šutka, A.; Mežule, L.; Denisova, V.; Meier-Haack, J.; Kulkarni, A.; Bitina, S.; Smits, K.; Vihodceva, S. Straightforward Approach for Preparing Durable Antibacterial ZnO Nanoparticle Coatings on Flexible Substrates. Molecules 2022, 27, 7672. https://doi.org/10.3390/molecules27227672
Šutka A, Mežule L, Denisova V, Meier-Haack J, Kulkarni A, Bitina S, Smits K, Vihodceva S. Straightforward Approach for Preparing Durable Antibacterial ZnO Nanoparticle Coatings on Flexible Substrates. Molecules. 2022; 27(22):7672. https://doi.org/10.3390/molecules27227672
Chicago/Turabian StyleŠutka, Andris, Linda Mežule, Viktorija Denisova, Jochen Meier-Haack, Akshay Kulkarni, Sanda Bitina, Krisjanis Smits, and Svetlana Vihodceva. 2022. "Straightforward Approach for Preparing Durable Antibacterial ZnO Nanoparticle Coatings on Flexible Substrates" Molecules 27, no. 22: 7672. https://doi.org/10.3390/molecules27227672
APA StyleŠutka, A., Mežule, L., Denisova, V., Meier-Haack, J., Kulkarni, A., Bitina, S., Smits, K., & Vihodceva, S. (2022). Straightforward Approach for Preparing Durable Antibacterial ZnO Nanoparticle Coatings on Flexible Substrates. Molecules, 27(22), 7672. https://doi.org/10.3390/molecules27227672







