Neurite Outgrowth-Promoting Compounds from the Petals of Paeonia lactiflora in PC12 Cells
Abstract
1. Introduction
2. Results and Discussion
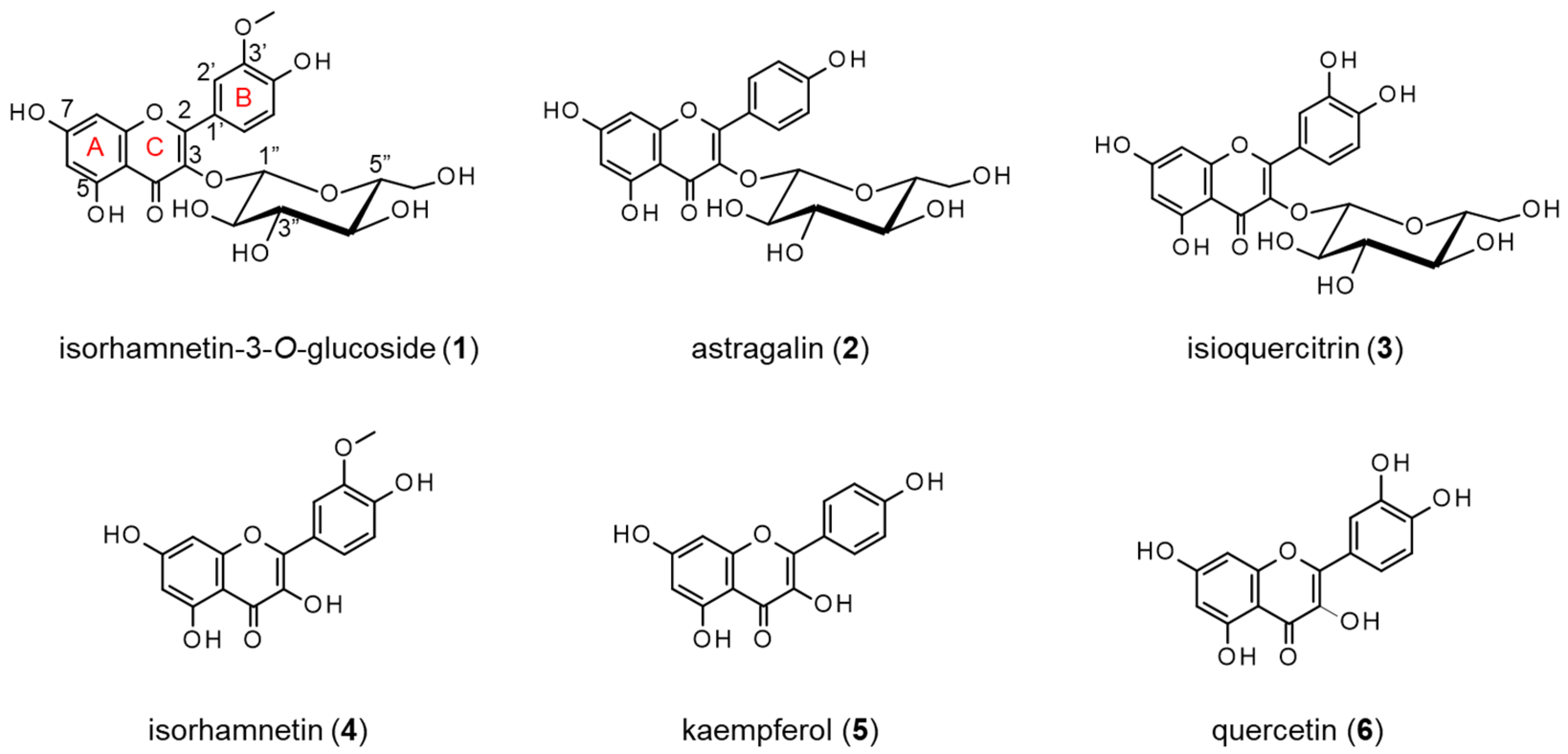
2.1. Isolation of Compounds 1 and 2 from the Extract of the Petals of P. lactiflora
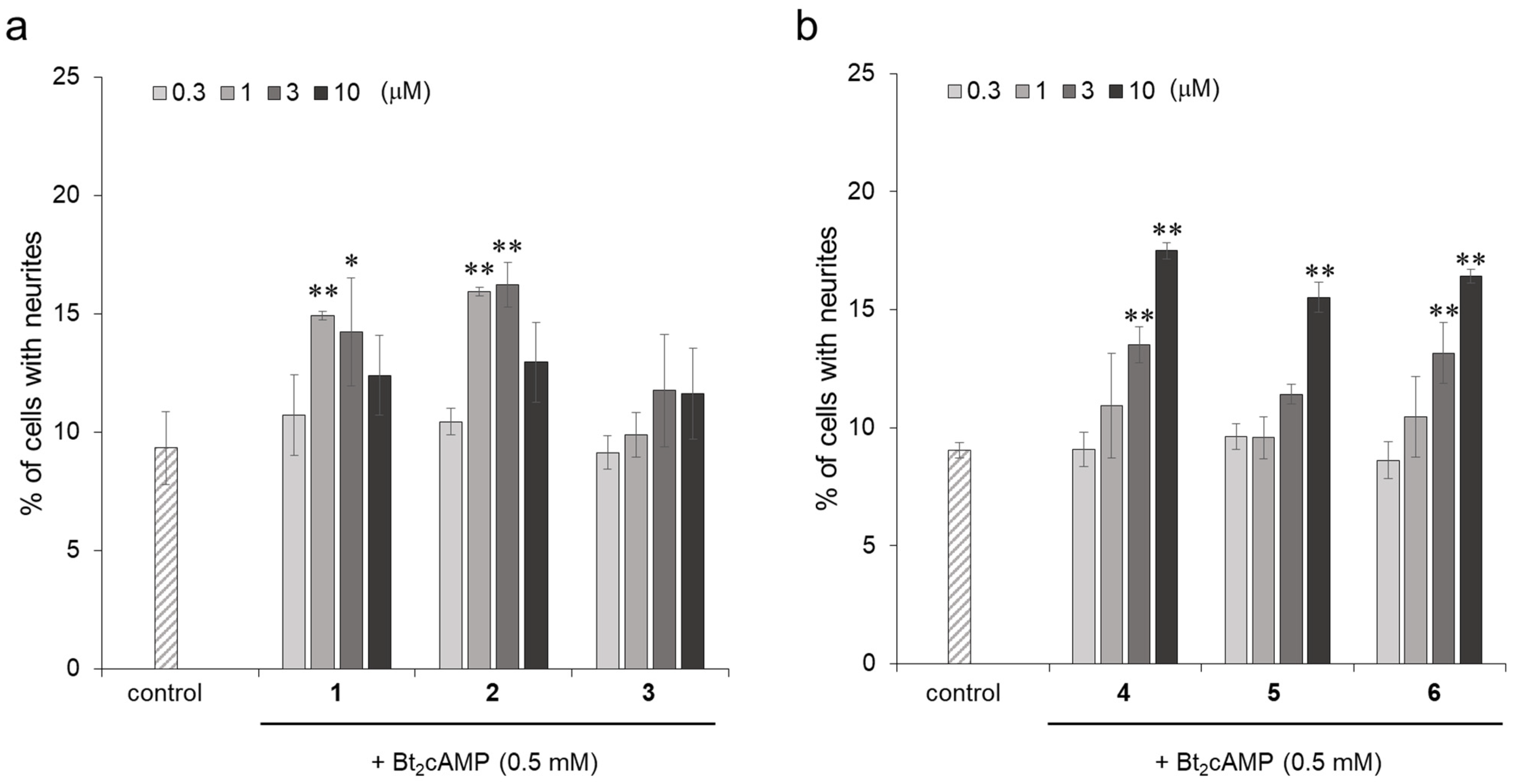
2.2. Structure-Activity Relationships of Compounds 1 and 2 and Their Analogs
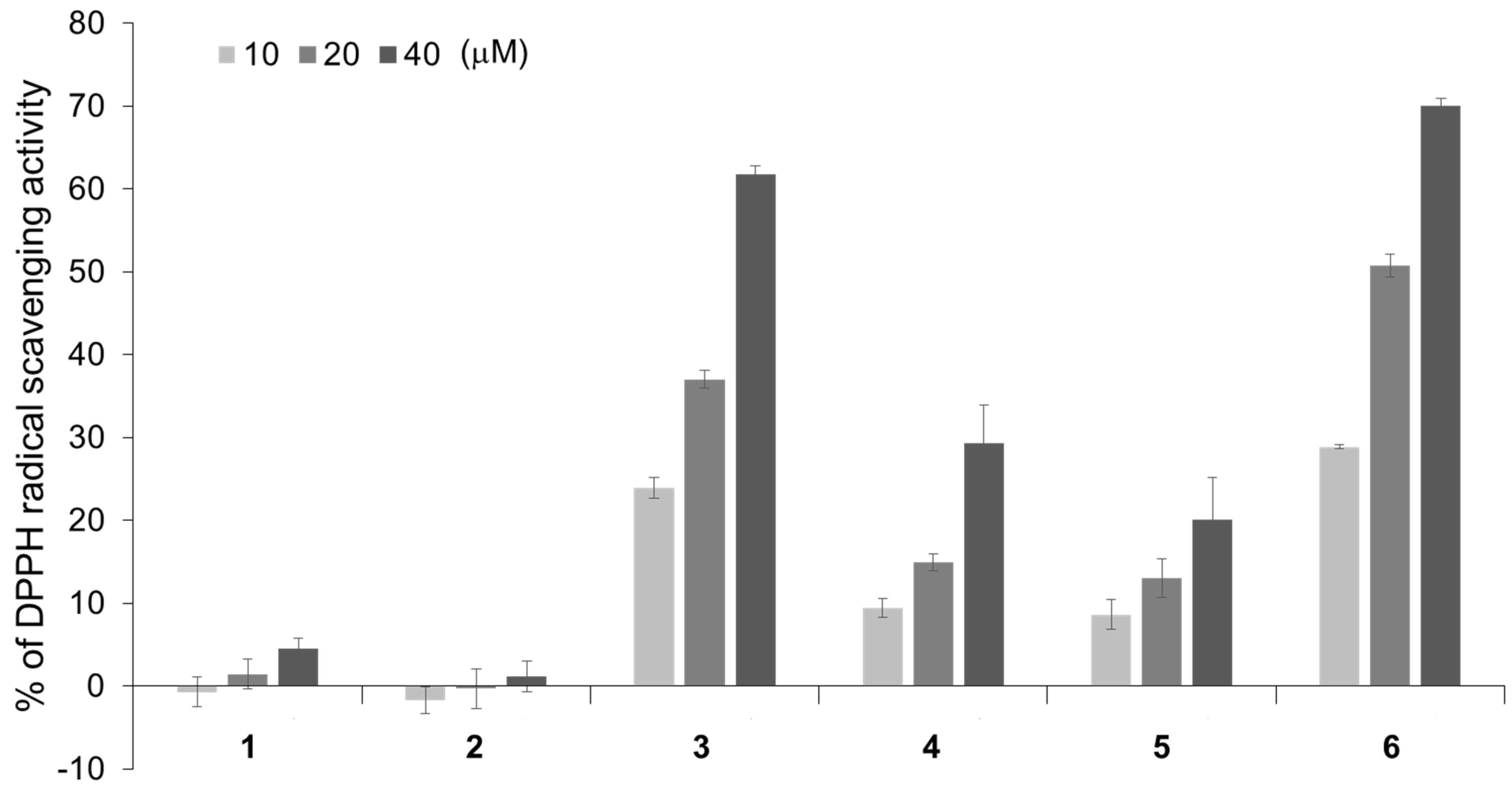
2.3. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Activities of Isorhamnetin-3-O-glucoside (1), Astragalin (2), and Their Analogs
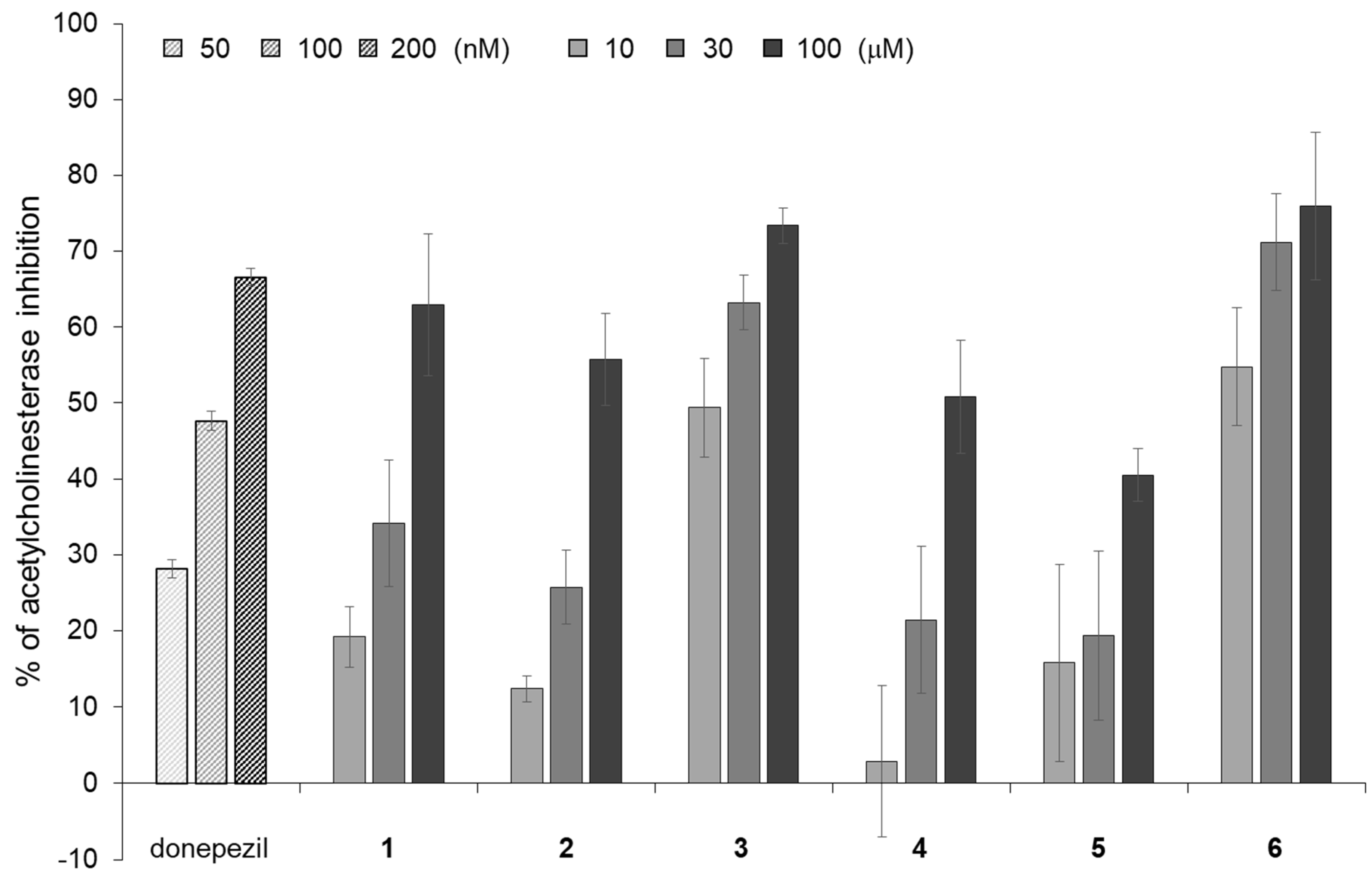
2.4. Acetylcholinesterase (AChE) Inhibitory Activities of Isorhamnetin-3-O-glucoside (1), Astragalin (2) and Their Analogs
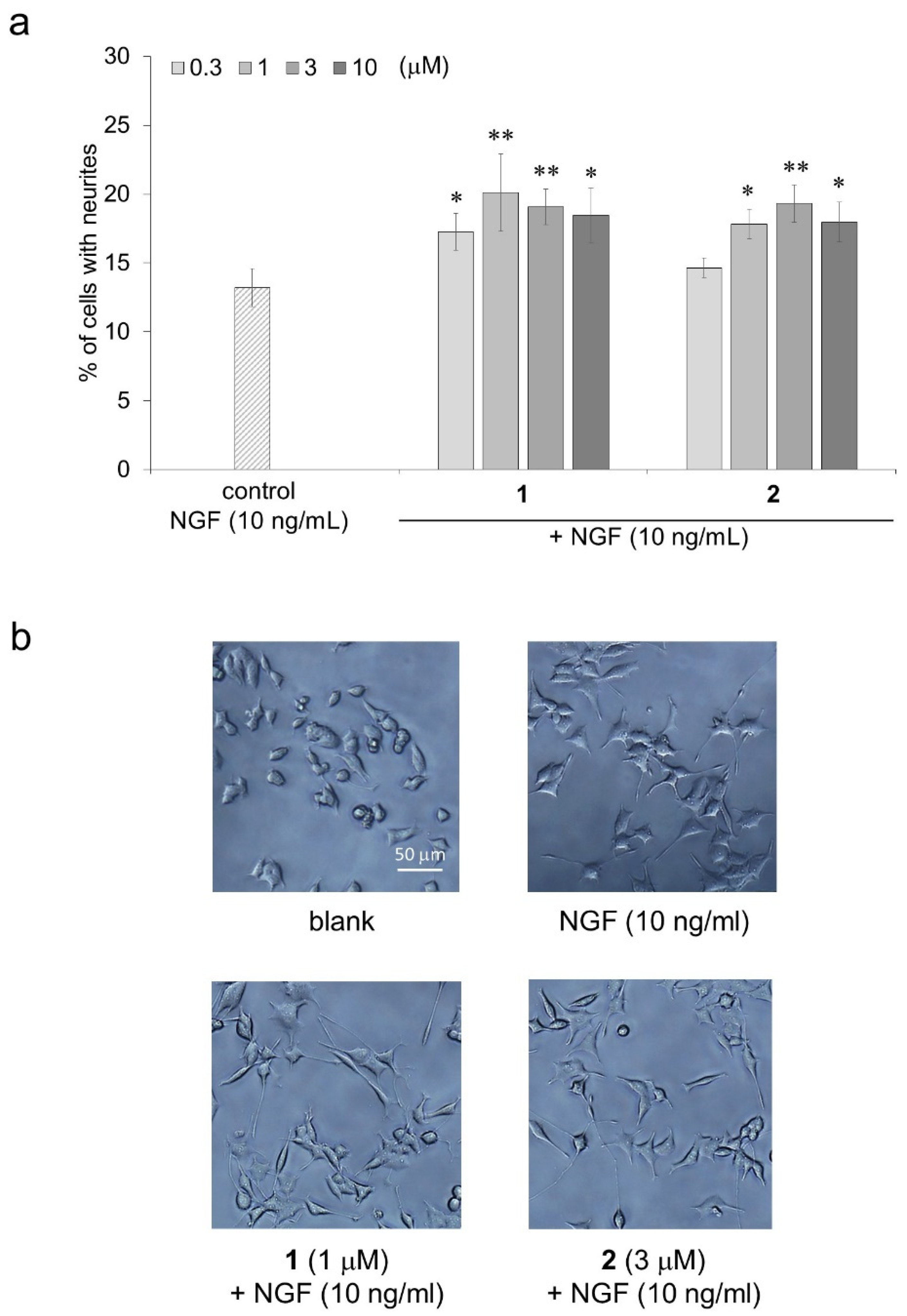
2.5. Promoting the Activity of Isorhamnetin-3-O-glucosides (1) and Astragalin (2) for Neurite Formation Induced by NGF in PC12 Cells
3. Materials and Methods
3.1. Chemicals
3.2. Instruments
3.3. Extraction and Isolation
3.4. Spectroscopic Data of Compounds
3.5. Cell Culture
3.6. Neurite Outgrowth-Promoting Activity
3.7. 2,2-Diphenyl-1-Picrylhydrazyl (DPPH) Radical Scavenging Assay
3.8. Acetylcholinesterase (AChE) Inhibitory Activity
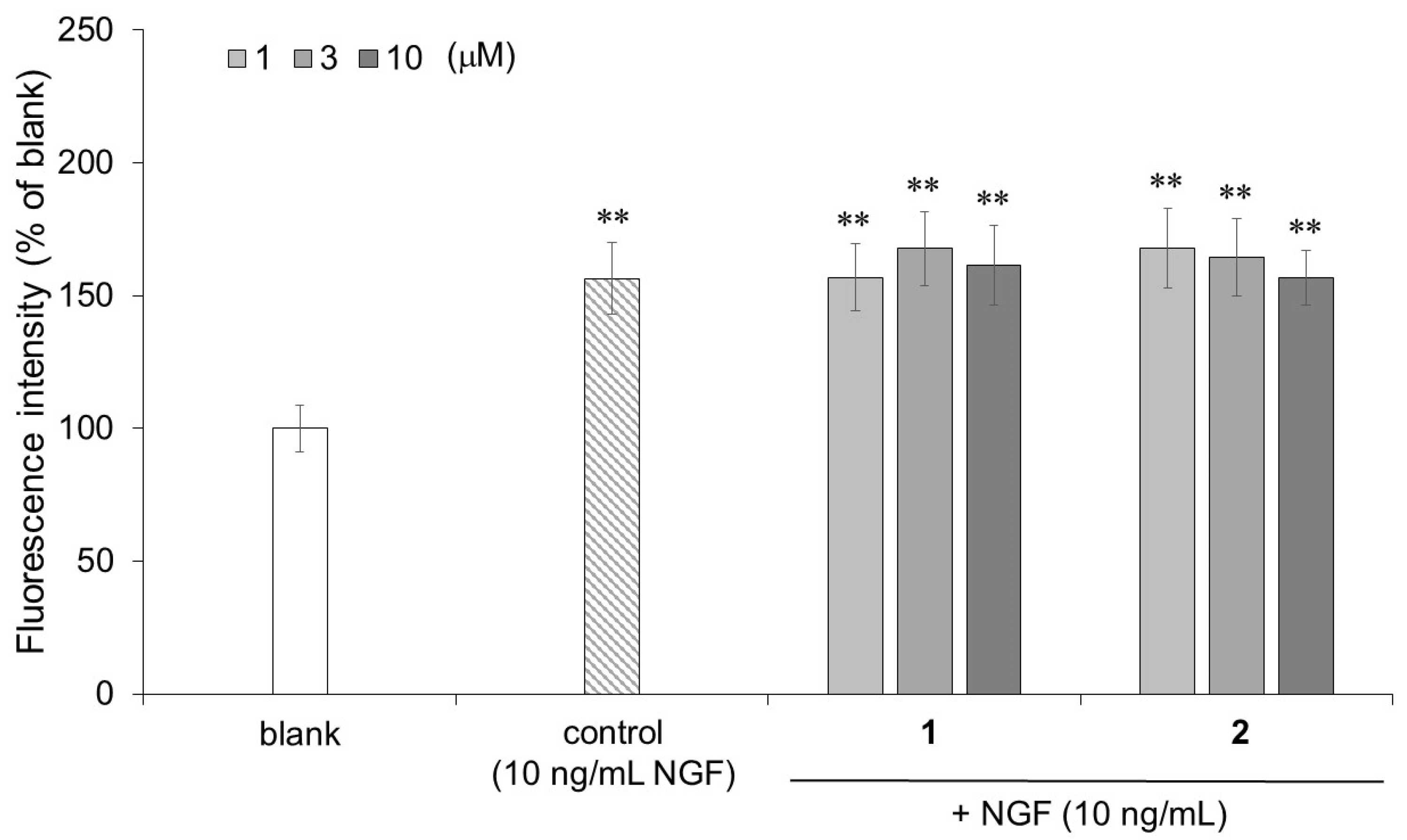
3.9. Detection of Neurofilaments in PC12 Cells with Cell-ELISA
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ukiya, M.; Akihisa, T.; Yasukawa, K.; Kasahara, Y.; Kimura, Y.; Koike, K.; Nikaido, T.; Takido, M. Constituents of compositae plants. 2. Triterpene diols, triols, and their 3-O-fatty acid esters from edible chrysanthemum flower extract and their anti-inflammatory effects. J. Agric. Food Chem. 2001, 49, 3187–3197. [Google Scholar] [CrossRef]
- Yang, R.; Wang, W.X.; Chen, H.J.; He, Z.C.; Jia, A.Q. The inhibition of advanced glycation end-products by five fractions and three main flavonoids from Camellia nitidissima Chi flowers. J. Food Drug Anal. 2018, 26, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Shimoda, H.; Nakamura, S.; Morioka, M.; Tanaka, J.; Matsuda, H.; Yoshikawa, M. Effect of cinnamoyl and flavonol glucosides derived from cherry blossom flowers on the production of advanced glycation end products (AGEs) and AGE-induced fibroblast apoptosis. Phytother. Res. 2011, 25, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Kim, J.Y.; Qi, Y.; Park, S.; Lee, H.L.; Yamabe, N.; Kim, H.; Jang, D.S.; Kang, K.S. Phytochemicals from the flowers of Prunus persica (L.) Batsch: Anti-adipogenic effect of mandelamide on 3T3-L1 preadipocytes. Bioorg. Med. Chem. Lett. 2021, 49, 128326. [Google Scholar] [CrossRef] [PubMed]
- Sang, T.; Crawford, D.; Stuessy, T. Chloroplast DNA phylogeny, reticulate evolution, and biogeography of Paeonia (Paeoniaceae). Am. J. Bot. 1997, 84, 1120. [Google Scholar] [CrossRef]
- He, D.Y.; Dai, S.M. Anti-inflammatory and immunomodulatory effects of Paeonia lactiflora pall., a traditional Chinese herbal medicine. Front. Pharm. 2011, 2, 10. [Google Scholar] [CrossRef]
- Hsu, F.L.; Lai, C.W.; Cheng, J.T. Antihyperglycemic effects of paeoniflorin and 8-debenzoylpaeoniflorin, glucosides from the root of Paeonia lactiflora. Planta Med. 1997, 63, 323–325. [Google Scholar] [CrossRef]
- Zhu, X.; Fang, Z.H. New monoterpene glycosides from the root cortex of Paeonia suffruticosa and their potential anti-inflammatory activity. Nat. Prod. Res. 2014, 28, 301–305. [Google Scholar] [CrossRef]
- Zhong, S.Z.; Ge, Q.H.; Li, Q.; Qu, R.; Ma, S.P. Peoniflorin attentuates Aβ(1-42)-mediated neurotoxicity by regulating calcium homeostasis and ameliorating oxidative stress in hippocampus of rats. J. Neurol. Sci. 2009, 280, 71–78. [Google Scholar] [CrossRef]
- Seow, S.L.S.; Hong, S.L.; Lee, G.S.; Malek, S.N.A.; Sabaratnam, V. 6-Shogaol, a neuroactive compound of ginger (jahe gajah) induced neuritogenic activity via NGF responsive pathways in PC-12 cells. BMC Complement. Altern. Med. 2017, 17, 334. [Google Scholar] [CrossRef]
- Mustafa, A.M.; Maggi, F.; Papa, F.; Kaya, E.; Dikmen, M.; Öztürk, Y. Isofuranodiene: A neuritogenic compound isolated from wild celery (Smyrnium olusatrum L., Apiaceae). Food Chem. 2016, 192, 782–787. [Google Scholar] [CrossRef] [PubMed]
- Duan, L.; Hope, J.M.; Guo, S.; Ong, Q.; François, A.; Kaplan, L.; Scherrer, G.; Cui, B. Optical activation of trkA signaling. Acs Synth. Biol. 2018, 7, 1685–1693. [Google Scholar] [CrossRef] [PubMed]
- Vaudry, D.; Stork, P.J.; Lazarovici, P.; Eiden, L.E. Signaling pathways for PC12 cell differentiation: Making the right connections. Science 2002, 296, 1648–1649. [Google Scholar] [CrossRef] [PubMed]
- Ravni, A.; Vaudry, D.; Gerdin, M.J.; Eiden, M.V.; Falluel-Morel, A.; Gonzalez, B.J.; Vaudry, H.; Eiden, L.E. A cAMP-dependent, protein kinase A-independent signaling pathway mediating neuritogenesis through Egr1 in PC12 cells. Mol. Pharm. 2008, 73, 1688–1708. [Google Scholar] [CrossRef]
- Shu, X.; Duan, W.; Liu, F.; Shi, X.; Geng, Y.; Wang, X.; Yang, B. Preparative separation of polyphenols from the flowers of Paeonia lactiflora Pall. by high-speed counter-current chromatography. J. Chromatogr. B 2014, 947–948, 62–67. [Google Scholar] [CrossRef]
- Muhammad, A.; Guerrero-Analco, J.A.; Martineau, L.C.; Musallam, L.; Madiraju, P.; Nachar, A.; Saleem, A.; Haddad, P.S.; Arnason, J.T. Antidiabetic compounds from Sarracenia purpurea used traditionally by the Eeyou Istchee Cree First Nation. J. Nat. Prod. 2012, 75, 1284–1288. [Google Scholar] [CrossRef]
- Kong, C.S.; Seo, Y. Antiadipogenic activity of isorhamnetin 3-O-β-D-glucopyranoside from Salicornia herbacea. Immunopharmacol. Immunotoxicol. 2012, 34, 907–911. [Google Scholar] [CrossRef]
- Liu, H.; Mou, Y.; Zhao, J.; Wang, J.; Zhou, L.; Wang, M.; Wang, D.; Han, J.; Yu, Z.; Yang, F. Flavonoids from Halostachys caspica and their antimicrobial and antioxidant activities. Molecules 2010, 15, 7933–7945. [Google Scholar] [CrossRef]
- Je Ma, C.; Jung, W.J.; Lee, K.Y.; Kim, Y.C.; Sung, S.H. Calpain inhibitory flavonoids isolated from Orostachys japonicus. J. Enzym. Inhib. Med. Chem. 2009, 24, 676–679. [Google Scholar] [CrossRef]
- Kameda, K.; Takaku, T.; Okuda, H.; Kimura, Y.; Okuda, T.; Hatano, T.; Agata, I.; Arichi, S. Inhibitory effects of various flavonoids isolated from leaves of persimmon on angiotensin-converting enzyme activity. J. Nat. Prod. 1987, 50, 680–683. [Google Scholar] [CrossRef]
- Kim, H.Y.; Moon, B.H.; Lee, H.J.; Choi, D.H. Flavonol glycosides from the leaves of Eucommia ulmoides O. with glycation inhibitory activity. J. Ethnopharmacol. 2004, 93, 227–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.L.; Choi, R.C.; Zhu, K.Y.; Leung, K.W.; Guo, A.J.; Bi, D.; Xu, H.; Lau, D.T.; Dong, T.T.; Tsim, K.W. Isorhamnetin, a flavonol aglycone from Ginkgo biloba L., induces neuronal differentiation of cultured PC12 cells: Potentiating the effect of nerve growth factor. Evid. Based Complement. Altern. Med. 2012, 2012, 278273. [Google Scholar] [CrossRef]
- Chan, G.K.L.; Hu, W.W.H.; Zheng, Z.X.; Huang, M.; Lin, Y.X.Y.; Wang, C.Y.; Gong, A.G.W.; Yang, X.Y.; Tsim, K.W.K.; Dong, T.T.X. Quercetin potentiates the NGF-induced effects in cultured PC 12 cells: Identification by HerboChips showing a binding with NGF. Evid. Based Complement. Altern. Med. 2018, 2018, 1502457. [Google Scholar] [CrossRef] [PubMed]
- Eik, L.F.; Naidu, M.; David, P.; Wong, K.H.; Tan, Y.S.; Sabaratnam, V. Lignosus rhinocerus (Cooke) ryvarden: A medicinal mushroom that stimulates neurite outgrowth in PC-12 cells. Evid. Based Complement. Altern. Med. 2012, 2012, 320308. [Google Scholar] [CrossRef] [PubMed]
- Tai, A.; Aburada, M.; Ito, H. A simple efficient synthesis and biological evaluation of 3-O-methylascorbic acid. Biosci. Biotechnol. Biochem. 2014, 78, 1984–1987. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Tai, A.; Yamamoto, I. Enhancement of neurite outgrowth in PC12 cells stimulated with cyclic AMP and NGF by 6-acylated ascorbic acid 2-O-α-glucosides (6-Acyl-AA-2G), novel lipophilic ascorbate derivatives. Biol. Pharm. Bull. 2003, 26, 341–346. [Google Scholar] [CrossRef]
- Suzukawa, K.; Miura, K.; Mitsushita, J.; Resau, J.; Hirose, K.; Crystal, R.; Kamata, T. Nerve growth factor-induced neuronal differentiation requires generation of Rac1-regulated reactive oxygen species. J. Biol. Chem. 2000, 275, 13175–13178. [Google Scholar] [CrossRef]
- Haraguchi, H.; Ishikawa, H.; Sanchez, Y.; Ogura, T.; Kubo, Y.; Kubo, I. Antioxidative constituents in Heterotheca inuloides. Bioorg. Med. Chem. 1997, 5, 865–871. [Google Scholar] [CrossRef]
- Arya, A.; Chahal, R.; Rao, R.; Rahman, M.H.; Kaushik, D.; Akhtar, M.F.; Saleem, A.; Khalifa, S.M.A.; El-Seedi, H.R.; Kamel, M.; et al. Acetylcholinesterase inhibitory potential of various sesquiterpene analogues for Alzheimer’s disease therapy. Biomolecules 2021, 11, 350. [Google Scholar] [CrossRef]
- Fukuyama, Y.; Kubo, M.; Harada, K. The search for, and chemistry and mechanism of, neurotrophic natural products. J. Nat. Med. 2020, 74, 648–671. [Google Scholar] [CrossRef]
- Du, L.Y.; Zhao, M.; Xu, J.; Qian, D.W.; Jiang, S.; Shang, E.X.; Guo, J.M.; Duan, J.A. Analysis of the metabolites of isorhamnetin 3-O-glucoside produced by human intestinal flora in vitro by applying ultraperformance liquid chromatography/quadrupole time-of-flight mass spectrometry. J. Agric. Food Chem. 2014, 62, 2489–2495. [Google Scholar] [CrossRef] [PubMed]
- Aloe, L.; Tuveri, M.A.; Carcassi, U.; Levi-Montalcini, R. Nerve growth factor in the synovial fluid of patients with chronic arthritis. Arthritis Rheum. 1992, 35, 351–355. [Google Scholar] [CrossRef] [PubMed]
- Lowe, E.M.; Anand, P.; Terenghi, G.; Williams-Chestnut, R.E.; Sinicropi, D.V.; Osborne, J.L. Increased nerve growth factor levels in the urinary bladder of women with idiopathic sensory urgency and interstitial cystitis. Br. J. Urol. 1997, 79, 572–577. [Google Scholar] [CrossRef]
- Miller, L.J.; Fischer, K.A.; Goralnick, S.J.; Litt, M.; Burleson, J.A.; Albertsen, P.; Kreutzer, D.L. Nerve growth factor and chronic prostatitis/chronic pelvic pain syndrome. Urology 2002, 59, 603–608. [Google Scholar] [CrossRef]
- Wu, C.; Boustany, L.; Liang, H.; Brennan, T.J. Nerve growth factor expression after plantar incision in the rat. Anesthesiology 2007, 107, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Rangel-Ordonez, L.; Noldner, M.; Schubert-Zsilavecz, M.; Wurglics, M. Plasma levels and distribution of flavonoids in rat brain after single and repeated doses of standardized Ginkgo biloba Extract EGb 761. Planta Med. 2010, 76, 1683–1690. [Google Scholar] [CrossRef]
- Paula, P.C.; Angelica Maria, S.G.; Luis, C.H.; Gloria Patricia, C.G. Preventive effect of quercetin in a triple transgenic Alzheimer’s disease mice model. Molecules 2019, 24, 2287. [Google Scholar] [CrossRef]
- Kim, A.R.; Jin, Q.; Jin, H.G.; Ko, H.J.; Woo, E.R. Phenolic compounds with IL-6 inhibitory activity from Aster yomena. Arch. Pharm. Res. 2014, 37, 845–851. [Google Scholar] [CrossRef]
- Han, S.; Hanh Nguyen, T.T.; Hur, J.; Kim, N.M.; Kim, S.B.; Hwang, K.H.; Moon, Y.H.; Kang, C.; Chung, B.; Kim, Y.M.; et al. Synthesis and characterization of novel astragalin galactosides using β-galactosidase from Bacillus circulans. Enzym. Microb. Technol. 2017, 103, 59–67. [Google Scholar] [CrossRef]
- Doi, N.; Togari, H.; Minagi, K.; Iwaoka, Y.; Tai, A.; Nakaoji, K.; Hamada, K.; Tatsuka, M. 2-O-Octadecylascorbic acid represses RhoGDIβ expression and ameliorates DNA damage-induced abnormal spindle orientations. J. Cell. Biochem. 2021, 122, 739–751. [Google Scholar] [CrossRef]
- Olennikov, D.N.; Kashchenko, N.I.; Chirikova, N.K.; Akobirshoeva, A.; Zilfikarov, I.N.; Vennos, C. Isorhamnetin and quercetin derivatives as anti-acetylcholinesterase principles of marigold (Calendula officinalis) flowers and preparations. Int. J. Mol. Sci. 2017, 18, 1685. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koga, T.; Ito, H.; Iwaoka, Y.; Noshita, T.; Tai, A. Neurite Outgrowth-Promoting Compounds from the Petals of Paeonia lactiflora in PC12 Cells. Molecules 2022, 27, 7670. https://doi.org/10.3390/molecules27227670
Koga T, Ito H, Iwaoka Y, Noshita T, Tai A. Neurite Outgrowth-Promoting Compounds from the Petals of Paeonia lactiflora in PC12 Cells. Molecules. 2022; 27(22):7670. https://doi.org/10.3390/molecules27227670
Chicago/Turabian StyleKoga, Takeru, Hideyuki Ito, Yuji Iwaoka, Toshiro Noshita, and Akihiro Tai. 2022. "Neurite Outgrowth-Promoting Compounds from the Petals of Paeonia lactiflora in PC12 Cells" Molecules 27, no. 22: 7670. https://doi.org/10.3390/molecules27227670
APA StyleKoga, T., Ito, H., Iwaoka, Y., Noshita, T., & Tai, A. (2022). Neurite Outgrowth-Promoting Compounds from the Petals of Paeonia lactiflora in PC12 Cells. Molecules, 27(22), 7670. https://doi.org/10.3390/molecules27227670






