Parkia platycephala Lectin (PPL) Inhibits Orofacial Nociception Responses via TRPV1 Modulation
Abstract
1. Introduction
2. Results
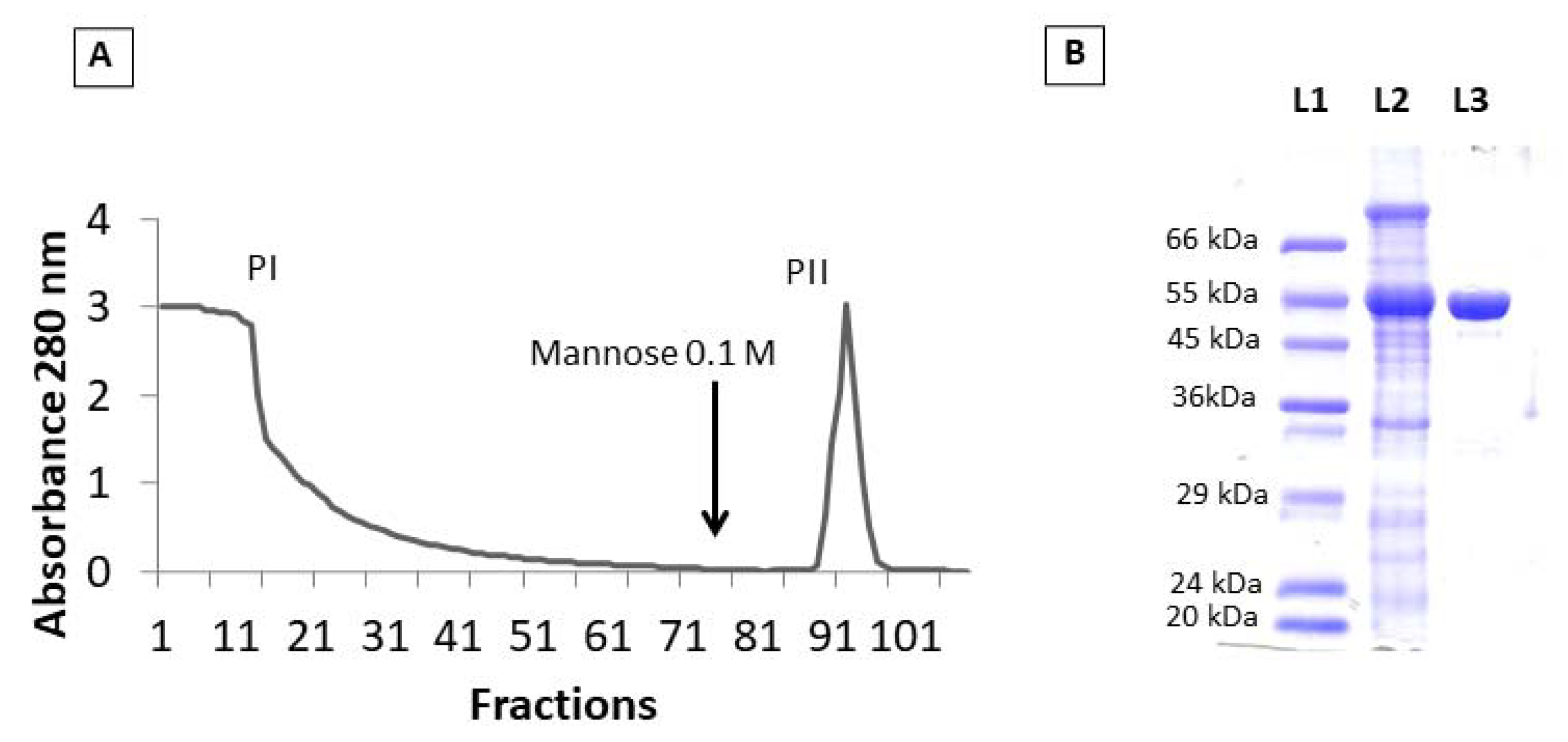
2.1. Lectin Purification
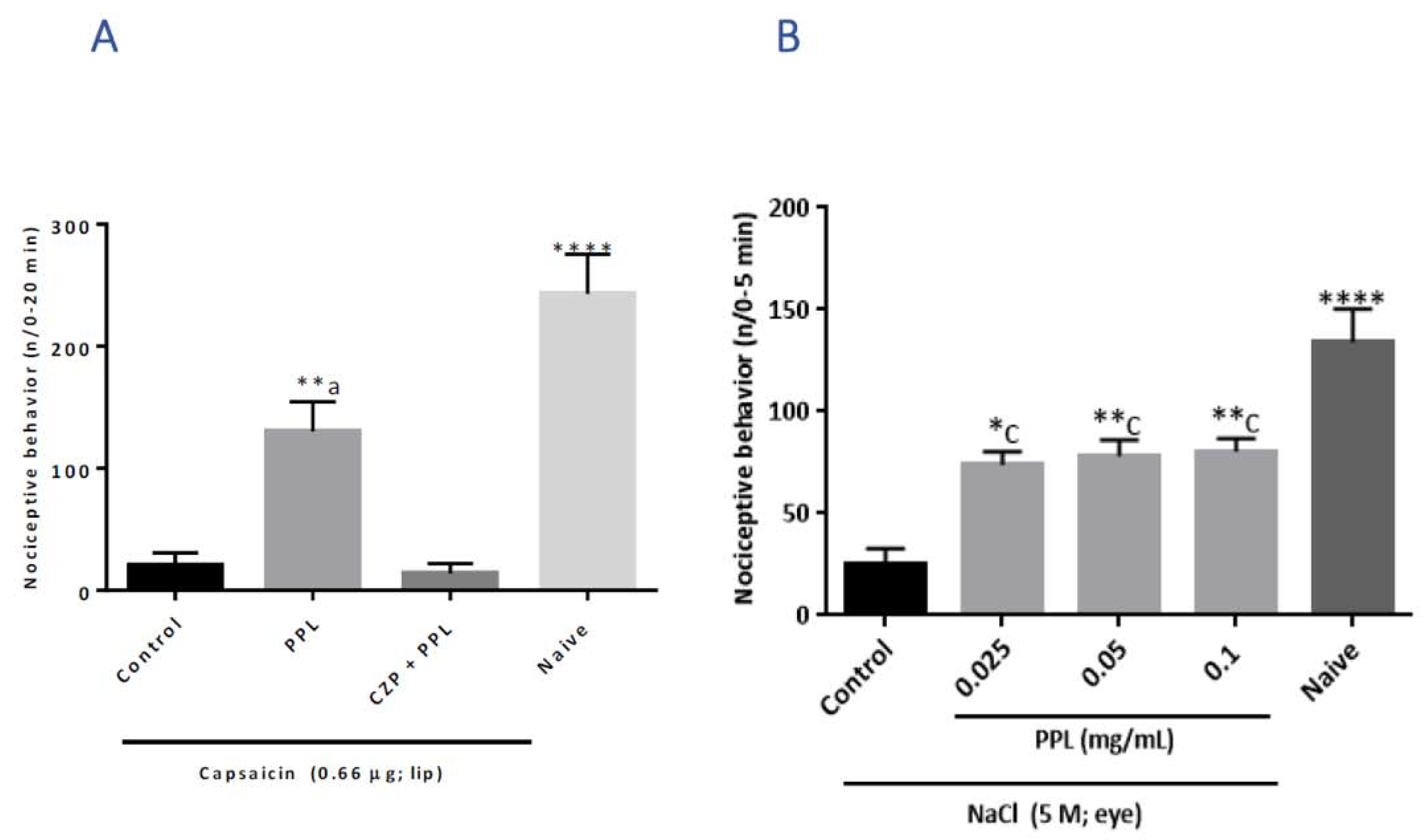
2.2. Orofacial and Corneal Antinociceptive Activity in Adult Zebrafish
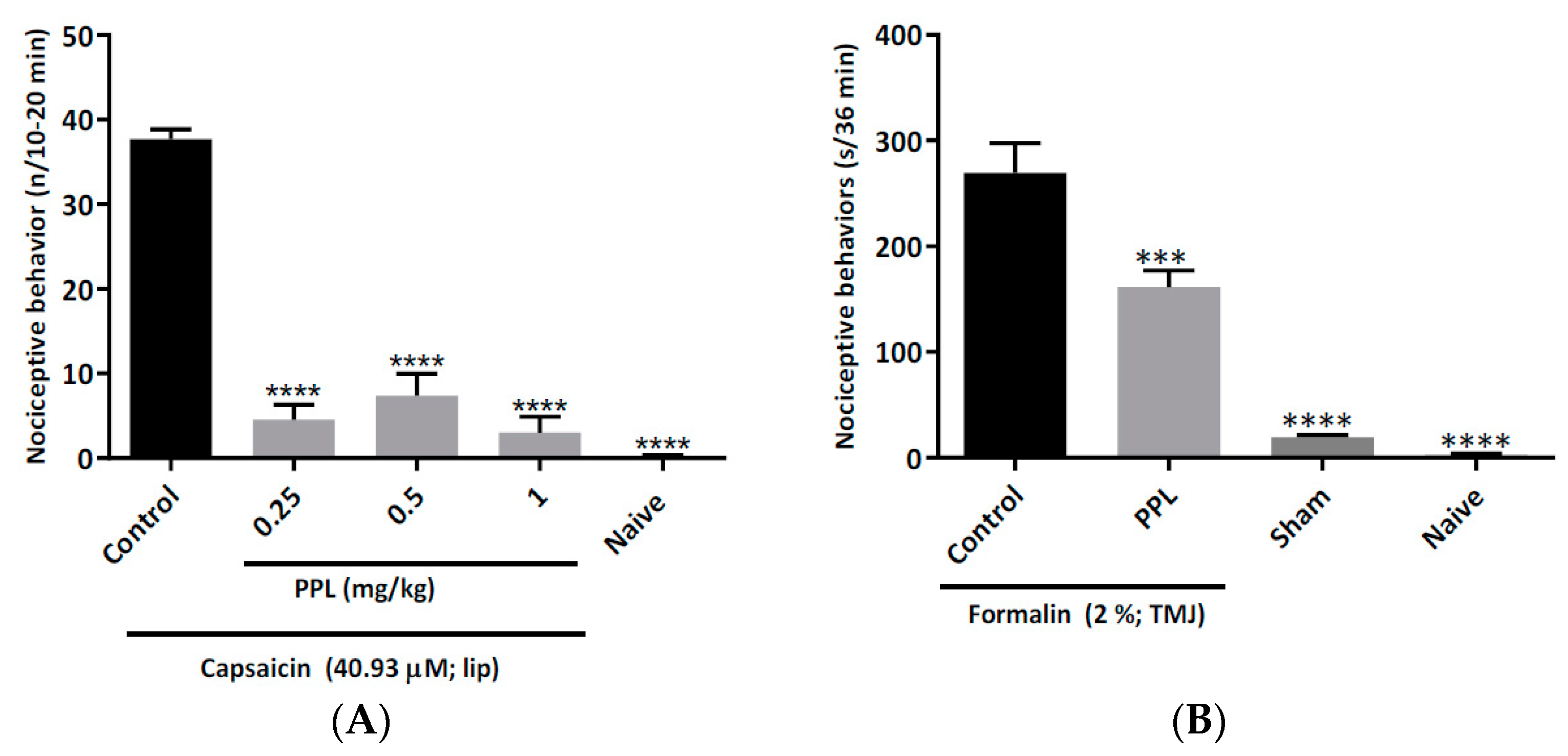
2.3. Capsaicin-Induced Orofacial Nociceptive Behaviour in Mice
2.4. Formalin-Induced Temporomandibular Nociception in Rats
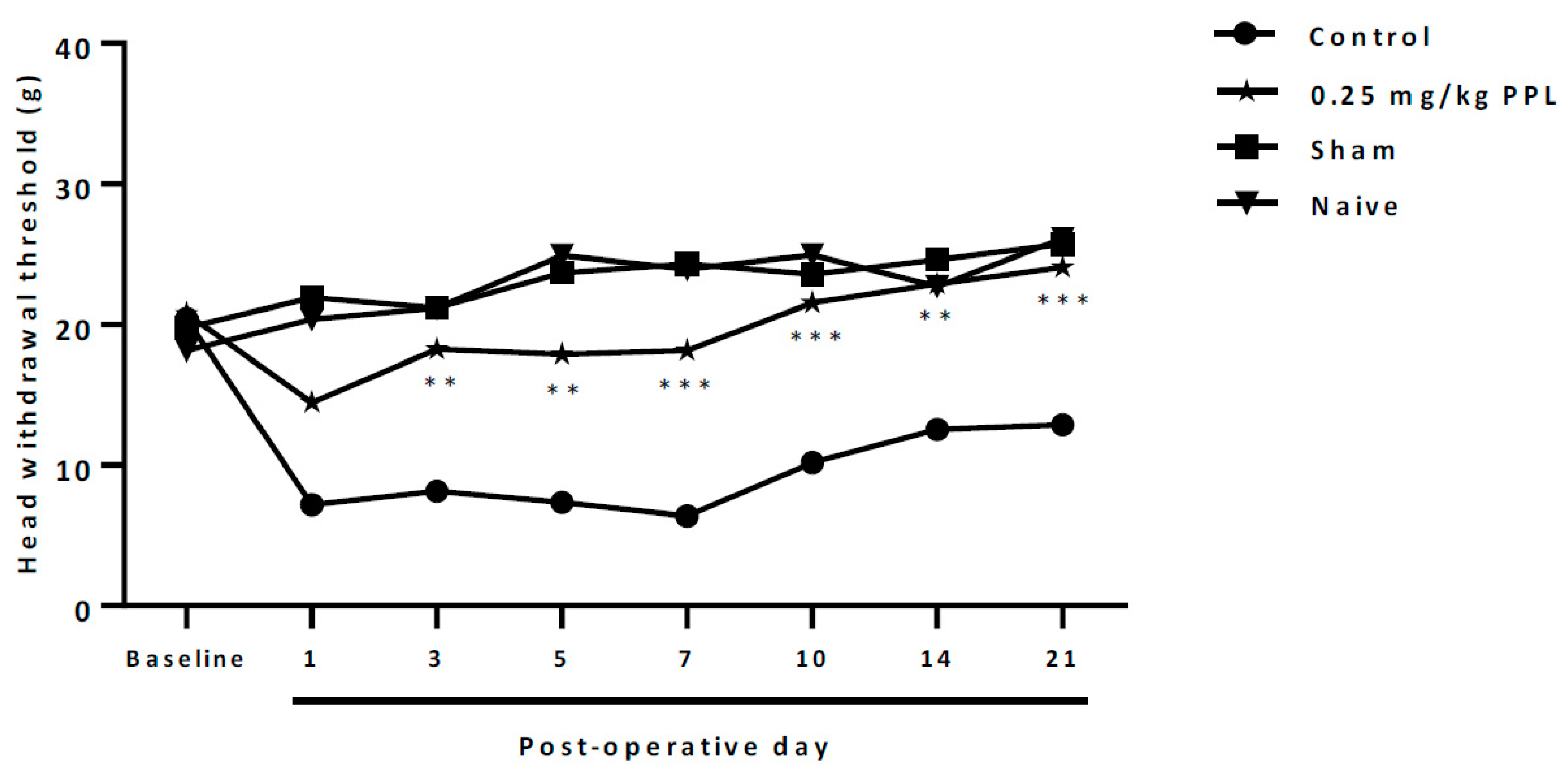
2.5. Neuropathic Orofacial Nociception Induced by Infraorbital Nerve Transection
3. Material and Methods
3.1. Animals
3.1.1. Zebrafish
3.1.2. Rodents
3.2. Lectin Purification
3.3. General Protocol
3.4. Orofacial Antinociceptive Activity in Adult Zebrafish
3.4.1. Capsaicin-Induced Orofacial Nociceptive Behaviour
3.4.2. Evaluation of the Involvement of Central Afferent Fibres to Capsaicin
3.4.3. Corneal Nociception Model in Adult Zebrafish
3.5. Capsaicin-Induced Orofacial Nociceptive Behaviour in Mice
3.6. Formalin-Induced Temporomandibular Nociception in Rats
3.7. Neuropathic Orofacial Nociception Induced by Infraorbital Nerve Transection
3.8. Statistical Analyses
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Nomenclature and Abbreviations
| ANOVA | analysis of variance |
| Capz | capsazepine |
| DC | Desensitized Control |
| ION | infraorbital nerve |
| IONX | infraorbital nerve transection |
| NaCl | sodium chloride |
| PBS | phosphate-buffered saline |
| PPL | Parkia platycephala |
| TMJ | temporomandibular joint |
| TRPV1 | transient receptor potential vanilloid type 1 |
References
- Verri, W.A., Jr.; Cunha, T.M.; Parada, C.A.; Poole, S.; Cunha, F.Q.; Ferreira, S.H. Hypernociceptive role of cytokines and chemokines: Targets for anal, gesic drug development? Pharmacol. Ther. 2006, 112, 116–138. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Mohan, V.; Mahay, P.; Yadav, P.K. Orofacial Pain: A Review. Dentistry 2016, 6, 367. [Google Scholar] [CrossRef]
- Rivanor, R.L.D.C.; Do Val, D.R.; Ribeiro, N.A.; Silveira, F.D.; de Assis, E.L.; Franco, Á.X.; Vieira, L.V.; de Queiroz, I.N.L.; Chaves, H.V.; Bezerra, M.M.; et al. A lectin fraction from green seaweed Caulerpa cupressoides inhibits inflammatory nociception in the temporomandibular joint of rats dependent from peripheral mechanisms. Int. J. Biol. Macromol. 2018, 115, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.L.E.; Leite, G.O.; Carneiro, R.F.; Roma, R.R.; Santos, V.F.; Santos, M.H.C.; Pereira, R.O.; Silva, R.C.; Nagano, C.S.; Sampaio, A.H.; et al. Purification and biophysical characterization of a mannose/N-acetyl-d-glucosamine-specific lectin from Machaerium acutifolium and its effect on inhibition of orofacial pain via TRPV1 receptor. Arch. Biochem. Biophys. 2019, 664, 149–156. [Google Scholar] [CrossRef]
- de Oliveira Leite, G.; Santos, S.A.A.R.; Bezerra, F.M.D.H.; Sena Silva, F.E.; de Castro Ribeiro, A.D.; Roma, R.R.; Silva, R.R.S.; Santos, M.H.C.; Santos, A.L.E.; Teixeira, C.S.; et al. Is the orofacial antinociceptive effect of lectins intrinsically related to their specificity to monosaccharides? Int. J. Biol. Macromol. 2020, 161, 1079–1085. [Google Scholar] [CrossRef]
- Van Damme, E.J.M.; Peumans, W.J.; Barre, A.; Rouge, P. Plant Lectins: A composite of several distinct families of structurally and evolutionary related proteins with diverse biological roles. Crit. Rev. Plant. Sci. 1998, 17, 575–692. [Google Scholar] [CrossRef]
- Fu, L.L.; Zhou, C.C.; Yao, S.; Yu, J.Y.; Liu, B.; Bao, J.K. Plant lectins: Targeting programmed cell death pathways as antitumor agents. Int. J. Biochem. Cell Biol. 2011, 43, 1442–1449. [Google Scholar] [CrossRef]
- Abreu, T.M.; Monteiro, V.S.; Martins, A.B.S.; Teles, F.B.; Rivanor, R.L.C.; Mota, E.F.; Macedo, D.S.; Vasconcelos, S.M.M.; Honório Junior, J.E.R.; Benevides, N.M.B. Involvement of the dopaminergic system in the antidepressant-like effect of the lectin isolated from the red marine alga Solieria filiformis in mice. Int. J. Biol. Macromol. 2018, 111, 534–541. [Google Scholar] [CrossRef]
- Bezerra, G.A.; Viertlmayr, R.; Moura, T.R.; Delatorre, P.; Rocha, B.A.; do Nascimento, K.S.; Figueiredo, J.G.; Bezerra, I.G.; Teixeira, C.S.; Simões, R.C.; et al. Structural studies of an anti-inflammatory lectin from Canavalia boliviana seeds in complex with dimannosides. PLoS ONE 2014, 9, e97015. [Google Scholar] [CrossRef]
- Kumar, K.K.; Chandra, K.L.; Sumanthi, J.; Reddy, G.S.; Shekar, P.C.; Reddy, B. Biological role of lectins: A review. J. Orofac. Sci. 2012, 4, 20–25. [Google Scholar] [CrossRef]
- MacRae, C.A.; Peterson, R.T. Zebrafish as tools for drug discovery. Nat. Rev. Drug Discov. 2015, 14, 721–731. [Google Scholar] [CrossRef] [PubMed]
- Rinkwitz, S.; Mourrain, P.; Becker, T.S. Zebrafish: An integrative system for neurogenomics and neurosciences. Prog. Neurobiol. 2011, 93, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Miller, Y.I. Emerging applications for zebrafish as a model organism to study oxidative mechanisms and their roles in inflammation and vascular accumulation of oxidized lipids. Free Radic. Biol. Med. 2012, 53, 1411–1420. [Google Scholar] [CrossRef] [PubMed]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef]
- Kouadio, F.; Kanko, C.; Juge, M.; Grimaud, N.; Jean, A.; N’Guessan, Y.T.; Petit, J.Y. Analgesic and antiinflammatory activities of an extract from Parkia biglobosa used in traditional medicine in the Ivory Coast. Phytother. Res. 2000, 14, 635–637. [Google Scholar] [CrossRef]
- Santos, L.M.; Farias, S.G.G.; Silva, R.B.; Dias, B.A.S.; Silva, L.S. Ecophysiology of Germination of Parkia platycephala Benth. Seeds. Floresta Ambiente 2019, 26, 1–7. [Google Scholar] [CrossRef]
- Cavada, B.S.; Santos, C.F.; Grangeiro, T.B.; Moreira Da Silva, L.I.M.; Campos, M.J.O.; De Sousa, F.A.M.; Calvete, J.J. Isolation and partial characterization of a lectin from Parkia platycephala Benth seeds. Physiol. Mol. Biol. Plants 1997, 3, 109–115. [Google Scholar]
- Cavada, B.S.; Madeira, S.V.F.; Calvete, J.J.; Souza, L.A.; Bomfim, L.R.; Dantas, A.R.; Lopes, M.C.; Grangeiro, T.B.; Freitas, B.T.; Pinto, V.P.; et al. Purification, chemical, and immunochemical properties of a new lectin from Mimosoideae (Parkia discolor). Prep. Biochem. Biotechnol. 2000, 30, 271–280. [Google Scholar] [CrossRef]
- Silva, R.R.S.; Silva, C.R.; Santos, V.F.; Barbosa, C.R.S.; Muniz, D.F.; Santos, A.L.E.; Santos, M.H.C.; Rocha, B.A.M.; Batista, K.L.R.; Costa-Júnior, L.M.; et al. Parkia platycephala lectin enhances the antibiotic activity against multi-resistant bacterial strains and inhibits the development of Haemonchus contortus. Microb. Pathog. 2019, 135, 103629. [Google Scholar] [CrossRef]
- Bari, A.U.; Santiago, M.Q.; Osterne, V.J.; Pinto-Junior, V.R.; Pereira, L.P.; Silva-Filho, J.C.; Debray, H.; Rocha, B.A.; Delatorre, P.; Teixeira, C.S.; et al. Lectins from Parkia biglobosa and Parkia platycephala: A comparative study of structure and biological effects. Int. J. Biol. Macromol. 2016, 92, 194–201. [Google Scholar] [CrossRef]
- Nomura, E.C.; Rodrigues, M.R.; da Silva, C.F.; Hamm, L.A.; Nascimento, A.M.; de Souza, L.M.; Cipriani, T.R.; Baggio, C.H.; Werner, M.F. Antinociceptive effects of ethanolic extract from the flowers of Acmella oleracea (L.) R.K. Jansen in mice. J. Ethnopharmacol. 2013, 150, 583–589. [Google Scholar] [CrossRef]
- Baggio, C.H.; Freitas, C.S.; Marcon, R.; Werner, M.F.; Rae, G.A.; Smiderle, F.R.; Sassaki, G.L.; Iacomini, M.; Marques, M.C.; Santos, A.R. Antinociception of β-D-glucan from Pleurotus pulmonarius is possibly related to protein kinase C inhibition. Int. J. Biol. Macromol. 2012, 50, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Quintans-Júnior, L.J.; Melo, M.S.; De Sousa, D.P.; Araujo, A.A.; Onofre, A.C.; Gelain, D.P.; Gonçalves, J.C.; Araújo, D.A.; Almeida, J.R.; Bonjardim, L.R. Antinociceptive effects of citronellal in formalin-, capsaicin-, and glutamate-induced orofacial nociception in rodents and its action on nerve excitability. J. Orofac. Pain. 2010, 24, 305–312. [Google Scholar] [PubMed]
- Sakurada, T.; Matsumura, T.; Moriyama, T.; Sakurada, C.; Ueno, S.; Sakurada, S. Differential effects of intraplantar capsazepine and ruthenium red on capsaicin-induced desensitization in mice. Pharmacol. Biochem. Behav. 2003, 75, 115–121. [Google Scholar] [CrossRef]
- Soares, I.C.R.; Santos, S.A.A.R.; Coelho, R.F.; Alves, Y.A.; Vieira-Neto, A.E.; Tavares, K.C.S.; Magalhaes, F.E.A.; Campos, A.R. Oleanolic acid promotes orofacial antinociception in adult zebrafish (Danio rerio) through TRPV1 receptors. Chem. Biol. Interact. 2019, 299, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Pelisser, T.; Pajot, J.; Dallel, R. The orofacial capsaicin test in rats: Effects of different capsaicin concentrations and morphine. Pain 2002, 96, 81–87. [Google Scholar] [CrossRef]
- Santos, S.A.A.R.; Damasceno, M.B.M.V.; Magalhães, F.E.A.; Sessle, B.J.; Oliveira, B.A.; Batista, F.L.A.; Vieira-Neto, A.E.; Campos, A.R. Transient receptor potential channel involvement in antinociceptive effect of citral in orofacial acute and chronic pain models. Excli. J. 2022, 21, 869–887. [Google Scholar]
- Roveroni, R.C.; Parada, C.A.; Cecília, M.; Veiga, F.A.; Tambeli, C.H. Development of a behavioral model of TMJ pain in rats: The TMJ formalin test. Pain 2001, 94, 185–191. [Google Scholar] [CrossRef]
- Pan, Z.; Wang, Z.; Yang, H.; Zhang, F.; Reinach, P.S. TRPV1 activation is required for hypertonicity-stimulated inflammatory cytokine release in human corneal epithelial cells. Investig. Ophthalmol. Vis Sci. 2011, 52, 485–493. [Google Scholar] [CrossRef]
- Veldhuis, N.A.; Lew, M.J.; Abogadie, F.C.; Poole, D.P.; Jennings, E.A.; Ivanusic, J.J.; Eilers, H.; Bunnett, N.W.; McIntyre, P. N-glycosylation determines ionic permeability and desensitization of the TRPV1 capsaicin receptor. J. Biol. Chem. 2012, 287, 21765–21772. [Google Scholar] [CrossRef]
- Barre, A.; Van Damme, E.J.M.; Klonjkowski, B.; Simplicien, M.; Sudor, J.; Benoist, H.; Rougé, P. Legume Lectins with Different Specificities as Potential Glycan Probes for Pathogenic Enveloped Viruses. Cells 2022, 11, 339. [Google Scholar] [CrossRef] [PubMed]
- Yau, T.; Dan, X.; Ng, C.C.; Ng, T.B. Lectins with potential for anti-cancer therapy. Molecules 2015, 20, 3791–3810. [Google Scholar] [CrossRef]
- Souza, M.A.; Carvalho, F.C.; Ruas, L.P.; Ricci-Azevedo, R.; Roque-Barreira, M.C. The immunomodulatory effect of plant lectins: A review with emphasis on ArtinM properties. Glycoconj. J. 2013, 30, 641–657. [Google Scholar] [CrossRef] [PubMed]
- Prasad, S.R.; Kumar, N.R.; Shruthi, H.R.; Kalavathi, S.D. Temporomandibular pain. J. Oral. Maxillofac. Pathol. 2016, 20, 272–275. [Google Scholar] [CrossRef]
- Urano, H.; Ara, T.; Fujinami, Y.; Hiraoka, B.Y. Aberrant TRPV1 expression in heat hyperalgesia associated with trigeminal neuropathic pain. Int. J. Med. Sci. 2012, 9, 690–697. [Google Scholar] [CrossRef] [PubMed]
- Cruz, L.S.; Kopruszinski, C.M.; Chichorro, J.G. Intraganglionar resiniferatoxin prevents orofacial inflammatory and neuropathic hyperalgesia. Behav. Pharmacol. 2014, 25, 112–118. [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Oliveira Leite, G.; Santos, S.A.A.R.; dos Santos Silva, R.R.; Teixeira, C.S.; Campos, A.R. Parkia platycephala Lectin (PPL) Inhibits Orofacial Nociception Responses via TRPV1 Modulation. Molecules 2022, 27, 7506. https://doi.org/10.3390/molecules27217506
de Oliveira Leite G, Santos SAAR, dos Santos Silva RR, Teixeira CS, Campos AR. Parkia platycephala Lectin (PPL) Inhibits Orofacial Nociception Responses via TRPV1 Modulation. Molecules. 2022; 27(21):7506. https://doi.org/10.3390/molecules27217506
Chicago/Turabian Stylede Oliveira Leite, Gerlânia, Sacha Aubrey Alves Rodrigues Santos, Romério Rodrigues dos Santos Silva, Claudener Souza Teixeira, and Adriana Rolim Campos. 2022. "Parkia platycephala Lectin (PPL) Inhibits Orofacial Nociception Responses via TRPV1 Modulation" Molecules 27, no. 21: 7506. https://doi.org/10.3390/molecules27217506
APA Stylede Oliveira Leite, G., Santos, S. A. A. R., dos Santos Silva, R. R., Teixeira, C. S., & Campos, A. R. (2022). Parkia platycephala Lectin (PPL) Inhibits Orofacial Nociception Responses via TRPV1 Modulation. Molecules, 27(21), 7506. https://doi.org/10.3390/molecules27217506






