High-Temperature Magnesiothermic Reduction Enables HF-Free Synthesis of Porous Silicon with Enhanced Performance as Lithium-Ion Battery Anode
Abstract
1. Introduction
2. Experiment
2.1. Materials
2.2. Sample Preparation
2.3. Structure Characterization
2.4. Electrochemical Measurement
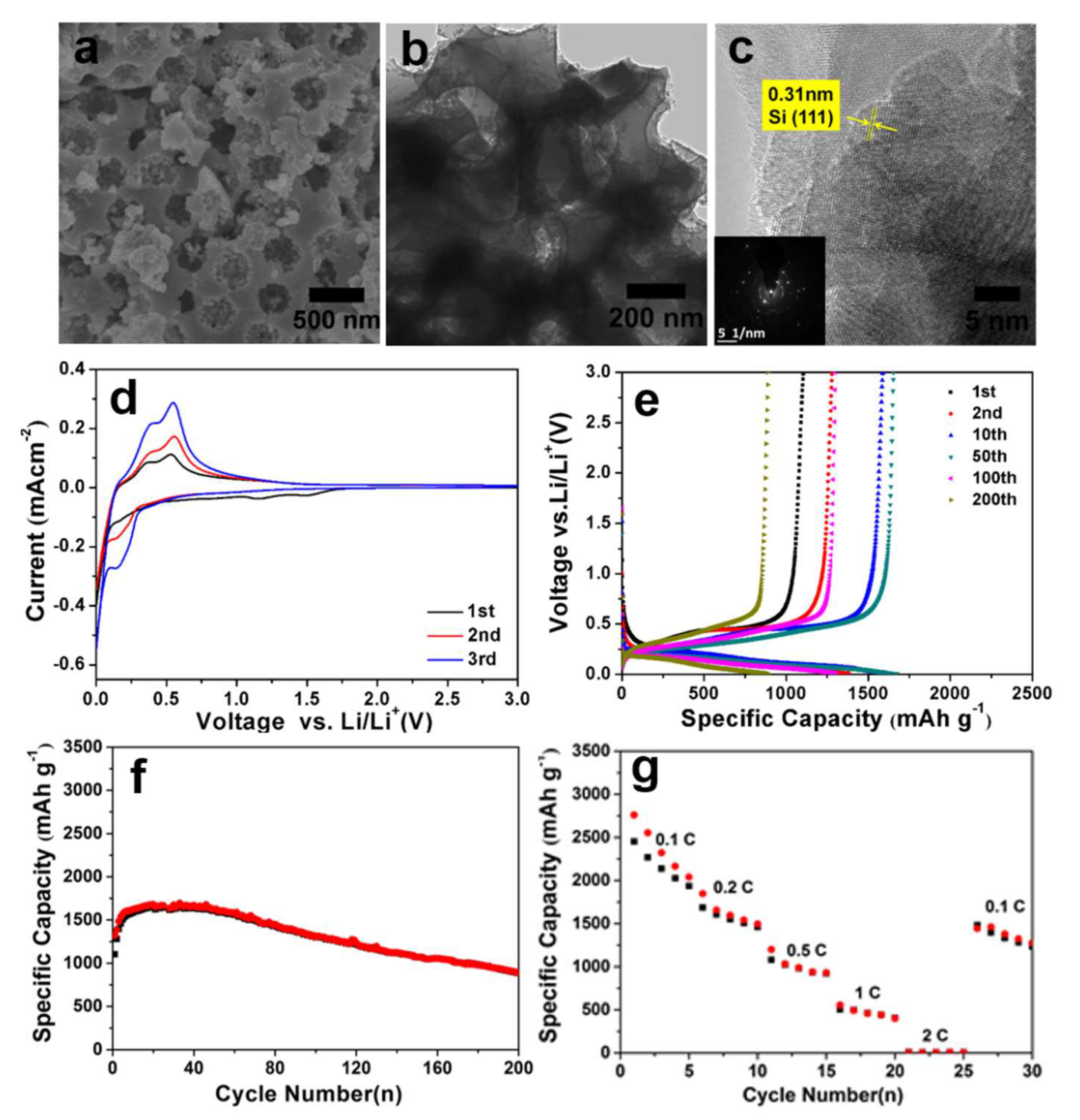
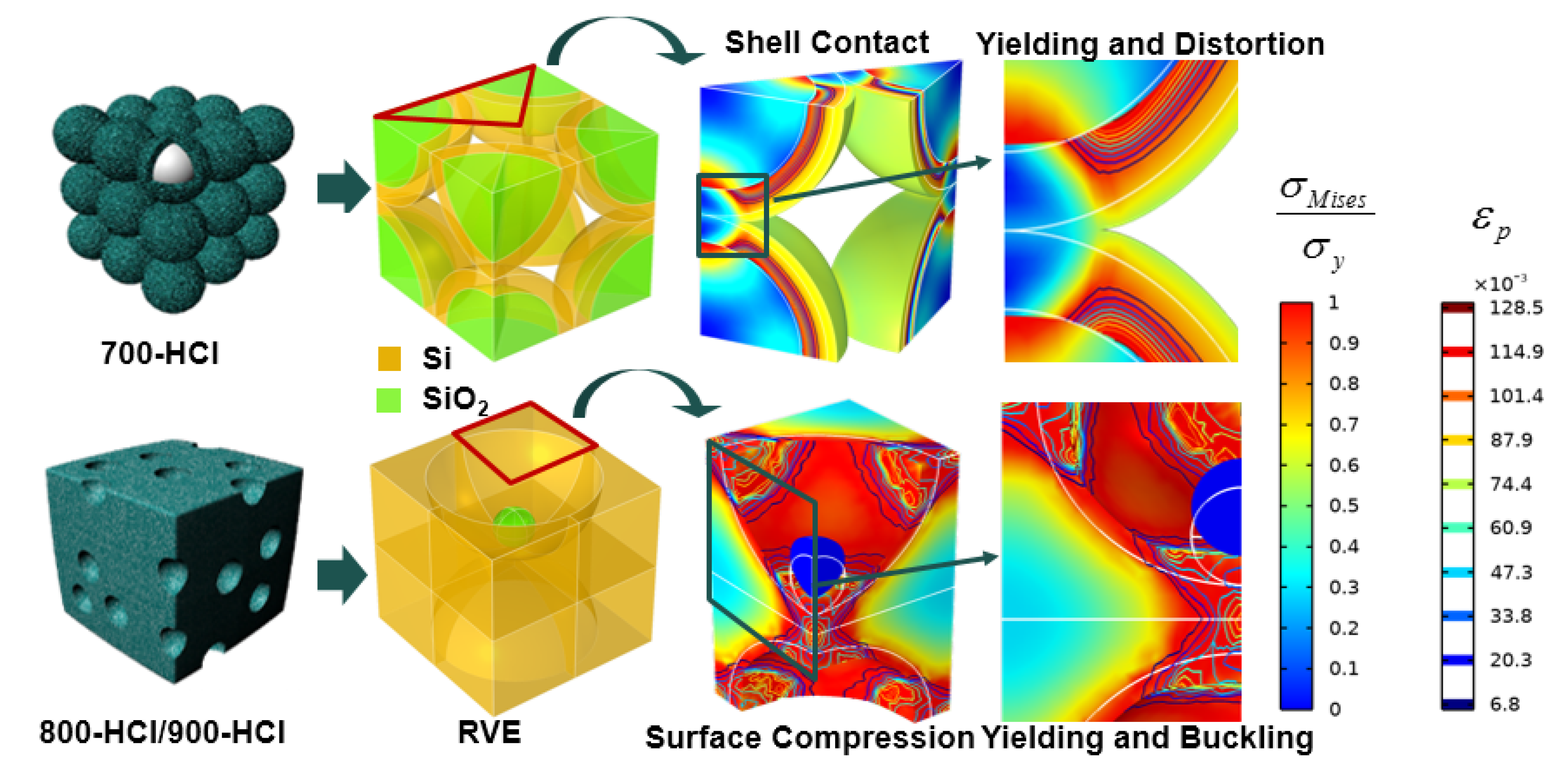
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Tarascon, J.-M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, B.; Mu, G.; Ma, C.; Mu, D.; Wu, F. Recent progress and perspectives on silicon anode: Synthesis and prelithiation for LIBs energy storage. J. Energy Chem. 2022, 64, 615–650. [Google Scholar] [CrossRef]
- Wang, H.; Yang, X.; Wu, Q.; Zhang, Q.; Chen, H.; Jing, H.; Wang, J.; Mi, S.-B.; Rogach, A.L.; Niu, C. Encapsulating Silica/Antimony into Porous Electrospun Carbon Nanofibers with Robust Structure Stability for High-Efficiency Lithium Storage. Acs Nano 2018, 12, 3406–3416. [Google Scholar] [CrossRef] [PubMed]
- Liang, B.; Liu, Y.; Xu, Y. Silicon-based materials as high capacity anodes for next generation lithium ion batteries. J. Power Sources 2014, 267, 469–490. [Google Scholar] [CrossRef]
- Ashuri, M.; He, Q.; Shaw, L.L. Silicon as a potential anode material for Li-ion batteries: Where size, geometry and structure matter. Nanoscale 2016, 8, 74–103. [Google Scholar] [CrossRef]
- Zuo, X.; Zhu, J.; Müller-Buschbaum, P.; Cheng, Y.-J. Silicon based lithium-ion battery anodes: A chronicle perspective review. Nano Energy 2017, 31, 113–143. [Google Scholar] [CrossRef]
- Shivaraju, G.; Sudakar, C.; Prakash, A. High-rate and long-cycle life performance of nano-porous nano-silicon derived from mesoporous MCM-41 as an anode for lithium-ion battery. Electrochim. Acta 2019, 294, 357–364. [Google Scholar] [CrossRef]
- Jia, H.; Li, X.; Song, J.; Zhang, X.; Luo, L.; He, Y.; Li, B.; Cai, Y.; Hu, S.; Xiao, X.; et al. Hierarchical porous silicon structures with extraordinary mechanical strength as high-performance lithium-ion battery anodes. Nat. Commun. 2020, 11, 1474. [Google Scholar] [CrossRef]
- An, W.; Gao, B.; Mei, S.; Xiang, B.; Fu, J.; Wang, L.; Zhang, Q.; Chu, P.K.; Huo, K. Scalable synthesis of ant-nest-like bulk porous silicon for high-performance lithium-ion battery anodes. Nat. Commun. 2019, 10, 1447. [Google Scholar] [CrossRef]
- Entwistle, J.; Rennie, A.; Patwardhan, S. A review of magnesiothermic reduction of silica to porous silicon for lithium-ion battery applications and beyond. J. Mater. Chem. A 2018, 6, 18344–18356. [Google Scholar] [CrossRef]
- Wang, B.; Li, W.; Wu, T.; Guo, J.; Wen, Z. Self-template construction of mesoporous silicon submicrocube anode for advanced lithium ion batteries. Energy Storage Mater. 2018, 15, 139–147. [Google Scholar] [CrossRef]
- Yoon, N.; Young, C.; Kang, D.; Park, H.; Lee, J.K. High-conversion reduction synthesis of porous silicon for advanced lithium battery anodes. Electrochim. Acta 2021, 391, 138967. [Google Scholar] [CrossRef]
- Yu, J.; Wang, K.; Song, W.; Huang, H.; Liang, C.; Xia, Y.; Zhang, J.; Gan, Y.; Wang, F.; Zhang, W. A low temperature MgH2-AlCl3-SiO2 system to synthesize nano-silicon for high-performance Li-ion batteries. Chem. Eng. J. 2021, 406, 126805. [Google Scholar] [CrossRef]
- Du, F.-H.; Ni, Y.; Wang, Y.; Wang, D.; Ge, Q.; Chen, S.; Yang, H.Y. Green Fabrication of Silkworm Cocoon-like Silicon-Based Composite for High-Performance Li-Ion Batteries. Acs Nano 2017, 11, 8628–8635. [Google Scholar] [CrossRef]
- Zuo, X.; Xia, Y.; Ji, Q.; Gao, X.; Yin, S.; Wang, M.; Wang, X.; Qiu, B.; Wei, A.; Sun, Z.; et al. Self-Templating Construction of 3D Hierarchical Macro-/Mesoporous Silicon from 0D Silica Nanoparticles. ACS Nano 2016, 11, 889–899. [Google Scholar] [CrossRef] [PubMed]
- Zuo, X.; Wen, Y.; Qiu, Y.; Cheng, Y.-J.; Yin, S.; Ji, Q.; You, Z.; Zhu, J.; Müller-Buschbaum, P.; Ma, L.; et al. Rational design and mechanical understanding of three-dimensional macro-/mesoporous silicon lithium-ion battery anodes with a tunable pore size and wall thickness. ACS Appl. Mater. Inter. 2020, 12, 43785–43797. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled growth of monodisperse silica spheres in the micron size range. J. Colloid Interf. Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Wang, W.; Favors, Z.; Ionescu, R.; Ye, R.; Bay, H.H.; Ozkan, M.; Ozkan, C.S. Monodisperse Porous Silicon Spheres as Anode Materials for Lithium Ion Batteries. Sci. Rep. 2015, 5, 6. [Google Scholar] [CrossRef]
- Entwistle, J.E.; Beaucage, G.; Patwardhan, S.V. Mechanistic understanding of pore evolution enables high performance mesoporous silicon production for lithium-ion batteries. J. Mater. Chem. A 2020, 8, 4938–4949. [Google Scholar] [CrossRef]
- Ren, W.; Wang, Y.; Tan, Q.; Yu, J.; Etim, U.J.; Zhong, Z.; Su, F. Nanosized Si particles with rich surface organic functional groups as high-performance Li-battery anodes. Electrochim. Acta 2019, 320, 134625. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, C.; Li, S.; Qiu, L.; Yang, Z.; Zhong, Y.; Zhong, B.; Song, Y.; Wang, G.; Liu, Y.; et al. A Unique Structure of Highly Stable Interphase and Self-Consistent Stress Distribution Radial-Gradient Porous for Silicon Anode. Adv. Funct. Mater. 2021, 32, 2107897. [Google Scholar] [CrossRef]
- Cui, M.; Wang, L.; Guo, X.; Wang, E.; Yang, Y.; Wu, T.; He, D.; Liu, S.; Yu, H. Designing of hierarchical mesoporous/macroporous silicon-based composite anode material for low-cost high-performance lithium-ion batteries. J. Mater. Chem. A 2019, 7, 3874–3881. [Google Scholar] [CrossRef]
- Shen, T.; Xia, X.-H.; Xie, D.; Yao, Z.-J.; Zhong, Y.; Zhan, J.-Y.; Wang, D.-H.; Wu, J.-B.; Wang, X.-L.; Tu, J.-P. Encapsulating silicon nanoparticles into mesoporous carbon forming pomegranate-structured microspheres as a high-performance anode for lithium ion batteries. J. Mater. Chem. A 2017, 5, 11197–11203. [Google Scholar] [CrossRef]
- Shi, L.; Wang, W.; Wang, A.; Yuan, K.; Yang, Y. Understanding the impact mechanism of the thermal effect on the porous silicon anode material preparation via magnesiothermic reduction. J. Alloys Compd. 2016, 661, 27–37. [Google Scholar] [CrossRef]
- Liu, T.; Qu, Y.; Liu, J.; Zhang, L.; Cheng, B.; Yu, J. Core-Shell Structured C@SiO2 Hollow Spheres Decorated with Nickel Nanoparticles as Anode Materials for Lithium-Ion Batteries. Small 2021, 17, 2103673. [Google Scholar] [CrossRef]
- Liu, F.; Liu, Y.; Wang, E.; Ruan, J.; Chen, S. Double-buffer silicon-carbon anode material by a dynamic self-assembly process for lithium-ion batteries. Electrochim. Acta 2021, 393, 139041. [Google Scholar] [CrossRef]
- Liu, N.; Lu, Z.; Zhao, J.; McDowell, M.T.; Lee, H.-W.; Zhao, W.; Cui, Y. A pomegranate-inspired nanoscale design for large-volume-change lithium battery anodes. Nat. Nanotechnol. 2014, 9, 187–192. [Google Scholar] [CrossRef]
- Jiao, L.-S.; Liu, J.-Y.; Li, H.-Y.; Wu, T.-S.; Li, F.; Wang, H.-Y.; Niu, L. Facile synthesis of reduced graphene oxide-porous silicon composite as superior anode material for lithium-ion battery anodes. J. Power Sources 2016, 315, 9–15. [Google Scholar] [CrossRef]
- Wang, X.; Li, G.; Seo, M.H.; Lui, G.; Hassan, F.; Feng, K.; Xiao, X.; Chen, Z. Carbon-Coated Silicon Nanowires on Carbon Fabric as Self-Supported Electrodes for Flexible Lithium-Ion Batteries. ACS Appl. Mater. Inter. 2017, 9, 9551–9558. [Google Scholar] [CrossRef]
- Sun, Z.; Wang, X.; Ying, H.; Wang, G.; Han, W.-Q. Facial Synthesis of Three-Dimensional Cross-Linked Cage for High-Performance Lithium Storage. ACS Appl. Mater. Inter. 2016, 8, 15279–15287. [Google Scholar] [CrossRef]
- Morita, T.; Takami, N. Nano Si Cluster- SiO x -C Composite Material as High-Capacity Anode Material for Rechargeable Lithium Batteries. J. Electrochem. Soc. 2006, 153, A425–A430. [Google Scholar] [CrossRef]
- Cook, J.B.; Kim, H.-S.; Lin, T.C.; Robbennolt, S.; Detsi, E.; Dunn, B.S.; Tolbert, S.H. Tuning Porosity and Surface Area in Mesoporous Silicon for Application in Li-Ion Battery Electrodes. ACS Appl. Mater. Inter. 2017, 9, 19063–19073. [Google Scholar] [CrossRef]
- Zhao, Y.; Stein, P.; Bai, Y.; Al-Siraj, M.; Yang, Y.; Xu, B.-X. A review on modeling of electro-chemo-mechanics in lithium-ion batteries. J. Power Sources 2019, 413, 259–283. [Google Scholar] [CrossRef]
- Wen, J.; Wei, Y.; Cheng, Y.-T. Examining the validity of Stoney-equation for in-situ stress measurements in thin film electrodes using a large-deformation finite-element procedure. J. Power Sources 2018, 387, 126–134. [Google Scholar] [CrossRef]
- Zhang, X.-Y.; Hao, F.; Chen, H.-S.; Fang, D.-N. Diffusion-induced stress and delamination of layered electrode plates with composition-gradient. Mech. Mater. 2015, 91, 351–362. [Google Scholar] [CrossRef]
- Korte, S.; Barnard, J.; Stearn, R.; Clegg, W. Deformation of silicon—Insights from microcompression testing at 25–500 °C. Int. J. Plast. 2011, 27, 1853–1866. [Google Scholar] [CrossRef]
- He, Y.-L.; Hu, H.; Song, Y.-C.; Guo, Z.-S.; Liu, C.; Zhang, J.-Q. Effects of concentration-dependent elastic modulus on the diffusion of lithium ions and diffusion induced stress in layered battery electrodes. J. Power Sources 2014, 248, 517–523. [Google Scholar] [CrossRef]
- Spearing, S.M. Materials issues in microelectromechanical systems (MEMS). Acta Mater. 2000, 48, 179–196. [Google Scholar] [CrossRef]
- Song, Y.; Shao, X.; Guo, Z.; Zhang, J. Role of material properties and mechanical constraint on stress-assisted diffusion in plate electrodes of lithium ion batteries. J. Phys. D Appl. Phys. 2013, 46, 105307. [Google Scholar] [CrossRef]
- Xu, Q.; Li, J.-Y.; Sun, J.-K.; Yin, Y.-X.; Wan, L.-J.; Guo, Y.-G. Watermelon-Inspired Si/C Microspheres with Hierarchical Buffer Structures for Densely Compacted Lithium-Ion Battery Anodes. Adv. Energy Mater. 2016, 1601481. [Google Scholar] [CrossRef]
- Yan, L.J.; Liu, J.; Wang, Q.Q.; Sun, M.H.; Jiang, Z.G.; Liang, C.D.; Pan, F.; Lin, Z. In Situ Wrapping Si Nanoparticles with 2D Carbon Nanosheets as High-Areal-Capacity Anode for Lithium-Ion Batteries. ACS Appl. Mater. Inter. 2017, 9, 38159–38164. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Wang, Y.H.; Ren, W.F.; Tan, Q.Q.; Chen, Y.F.; Li, H.; Zhong, Z.Y.; Su, F.B. Scalable Synthesis of Interconnected Porous Silicon/Carbon Composites by the Rochow Reaction as High-Performance Anodes of Lithium Ion Batteries. Angew. Chem. Int. Ed. 2014, 53, 5165–5169. [Google Scholar] [CrossRef]
- Lu, Z.; Liu, N.; Lee, H.-W.; Zhao, J.; Li, W.; Li, Y.; Cui, Y. Nonfilling Carbon Coating of Porous Silicon Micrometer-Sized Particles for High-Performance Lithium Battery Anodes. ACS Nano 2015, 9, 2540–2547. [Google Scholar] [CrossRef]
- Lin, D.C.; Lu, Z.D.; Hsu, P.C.; Lee, H.R.; Liu, N.; Zhao, J.; Wang, H.T.; Liu, C.; Cui, Y. A high tap density secondary silicon particle anode fabricated by scalable mechanical pressing for lithium-ion batteries. Environ. Sci. Technol. 2015, 8, 2371–2376. [Google Scholar] [CrossRef]
- Wasalathilake, K.C.; Hapuarachchi, S.N.S.; Zhao, Y.B.; Fernando, J.F.S.; Chen, H.; Nerkar, J.Y.; Golberg, D.; Zhang, S.Q.; Yan, C. Unveiling the Working Mechanism of Graphene Bubble Film/Silicon Composite Anodes in Li-Ion Batteries: From Experiment to Modeling. ACS Appl. Mater. Inter. 2020, 3, 521–531. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuo, X.; Yang, Q.; He, Y.; Cheng, Y.-J.; Yin, S.; Zhu, J.; Müller-Buschbaum, P.; Xia, Y. High-Temperature Magnesiothermic Reduction Enables HF-Free Synthesis of Porous Silicon with Enhanced Performance as Lithium-Ion Battery Anode. Molecules 2022, 27, 7486. https://doi.org/10.3390/molecules27217486
Zuo X, Yang Q, He Y, Cheng Y-J, Yin S, Zhu J, Müller-Buschbaum P, Xia Y. High-Temperature Magnesiothermic Reduction Enables HF-Free Synthesis of Porous Silicon with Enhanced Performance as Lithium-Ion Battery Anode. Molecules. 2022; 27(21):7486. https://doi.org/10.3390/molecules27217486
Chicago/Turabian StyleZuo, Xiuxia, Qinghua Yang, Yaolong He, Ya-Jun Cheng, Shanshan Yin, Jin Zhu, Peter Müller-Buschbaum, and Yonggao Xia. 2022. "High-Temperature Magnesiothermic Reduction Enables HF-Free Synthesis of Porous Silicon with Enhanced Performance as Lithium-Ion Battery Anode" Molecules 27, no. 21: 7486. https://doi.org/10.3390/molecules27217486
APA StyleZuo, X., Yang, Q., He, Y., Cheng, Y.-J., Yin, S., Zhu, J., Müller-Buschbaum, P., & Xia, Y. (2022). High-Temperature Magnesiothermic Reduction Enables HF-Free Synthesis of Porous Silicon with Enhanced Performance as Lithium-Ion Battery Anode. Molecules, 27(21), 7486. https://doi.org/10.3390/molecules27217486








