Abstract
L-Dopa (LD), a substance used medically in the treatment of Parkinson’s disease, is found in several natural products, such as Vicia faba L., also known as broad beans. Due to its low chemical stability, LD analysis in plant matrices requires an appropriate optimization of the chosen analytical method to obtain reliable results. This work proposes an HPLC-UV method, validated according to EURACHEM guidelines as regards linearity, limits of detection and quantification, precision, accuracy, and matrix effect. The LD extraction was studied by evaluating its aqueous stability over 3 months. The best chromatographic conditions were found by systematically testing several C18 stationary phases and acidic mobile phases. In addition, the assessment of the best storage treatment of Vicia faba L. broad beans able to preserve a high LD content was performed. The best LD determination conditions include sun-drying storage, extraction in HCl 0.1 M, chromatographic separation with a Discovery C18 column, 250 × 4.6 mm, 5 µm particle size, and 99% formic acid 0.2% v/v and 1% methanol as the mobile phase. The optimized method proposed here overcomes the problems linked to LD stability and separation, thus contributing to the improvement of its analytical determination.
1. Introduction
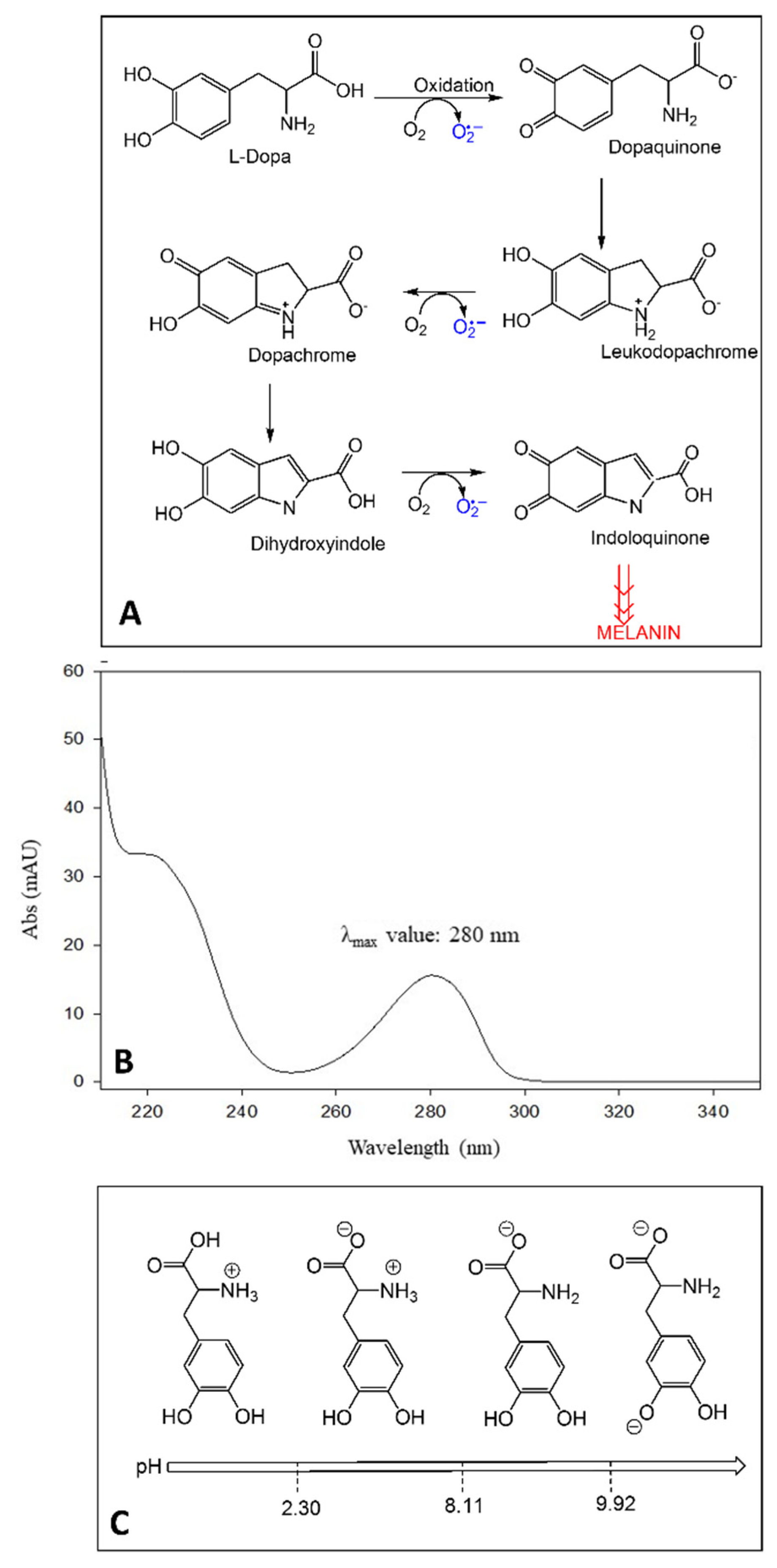
Broad bean (Vicia faba L.) has been identified as a rich source of L-Dopa or levodopa (LD), a dopamine precursor and first-line treatment for Parkinson’s disease (PD) symptoms, usually characterized by slowness of movement (bradykinesia), tremor at rest, muscle rigidity, and postural fragility [1,2,3,4,5,6]. PD affects nerve cells and their function in producing the neurotransmitter dopamine. In contrast to dopamine, LD can cross the blood-brain barrier and enter into the nerve cells, where it is decarboxylated to dopamine. In cells, LD can also be oxidized toward melanin, producing leucodopachrome and dopachrome by auto-oxidation or with the aid of tyrosinases, also known as polyphenol oxidases (PPO) (Figure 1A). During these reactions, reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), superoxide anion (O2•−), and hydroxyl radical (HO•) can be produced. Some studies report that the beneficial effect of LD drugs in PD is counterbalanced by the strong oxidative damage generated over a long period of drug treatment [7]. Furthermore, patients with advanced PD generally experience an unbalanced response pattern to L-Dopa because of fluctuating drug delivery to the brain. In the most severe form, motor fluctuations produce the typical “on-off” syndrome. Thus, the consumption of vegetables containing LD, such as broad beans or botanicals food supplements, could be recommended as adjuvants for patients with PD [7,8]. In fact, as reported by Apaydin et al. [9]., the “on” period was prolonged in patients consuming a Vicia faba broad bean meal.
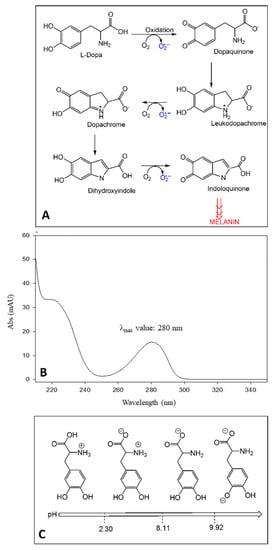
Figure 1.
Auto-oxidation reactions of LD and ROS production (A); UV absorption spectrum of LD (B); LD structures at different pH and corresponding pKa values (C).
The presence of the catechol moiety in the chemical structure characterizes LD as a chromophore with a λmax value of 280 nm and allows us to obtain UV detection results sufficiently sensitive for LD determination in vegetable matrices [10]. Since the absorption spectrum of LD is highly characteristic (Figure 1B), its determination by spectrum acquisition (LC-UV analysis) is also selective. Although several scientific works reported the separation and quantification of LD in Vicia faba L. by using liquid chromatography coupled to a UV–Vis detector (LC/UV–Vis) [8,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25], there are still some limitations regarding LD extraction/stability, chromatographic conditions, and method validation.
Firstly, LD is unstable in aqueous solutions and naturally degrades over time [26,27,28,29,30,31,32,33,34,35,36]: for this reason, it is crucial to identify the best conditions to avoid LD degradation over all the analytical steps. LD instability will also influence the choice of the extraction technique. Typical extraction techniques include liquid–solid extraction, Soxhlet extraction, microwave-assisted extraction, and ultrasound-assisted extraction [10]. However, the latter two techniques, although significantly improving the extraction procedure in terms of automation and solvent consumption, are less used because thermal effects can reduce the final concentration of L-Dopa [11].
Secondly, low-molecular-weight polar compounds, such as LD, are generally difficult to separate by using reversed-phase liquid chromatography (RP-LC) [10]. Generally, it is required to work below the pKa of the compound where it will be completely protonated, or to use an ion pair reagent to increase retention time. Chromatographic conditions as regards LC column and mobile-phase composition are also fundamental to obtain reliable results. Mostly, C18 stationary phases have been used due to the highly polar structure of the analyte [10,11,13,23]. Acetate/phosphate buffers at acidic pH values (<3) have also been used, although some papers report the use of acetate/phosphate buffers at pH of 4.00, 4.55, 4.66, or 7.00 [10,19,20,23,24,37]. It should be pointed out that, under highly acidic conditions, both analyte and residual silanol groups on the silica column packing support are fully protonated, so they cannot interact electrostatically, avoiding tailing, peaks broadening, and poor retention reproducibility [37]. However, the use of a mobile phase with a certain percentage of organic phase should be considered to prevent the dewetting problem of the C18 stationary phase, and, as a consequence, to limit the lowering of its retention capacity [38,39]. For these reasons, an optimization of chromatographic conditions is necessary.
As no systematic LC-UV method validation has been reported in the literature to analyze LD occurring in Vicia faba L. broad beans, with just a few quality parameters of the chromatographic method being ascertained [16], it is crucial to validate a reliable LC-UV method for LD quantification in this matrix.
In this work, a validated LC-UV method for LD analysis according to EURACHEM guidelines has been proposed, alongside the study of its stability in different acidic solutions and the systematic optimization of chromatographic conditions as regards both chromatographic column and mobile phase. Then, the validated method was tested to evaluate the LD contents in seven Vicia faba L. broad bean samples, differently stored (fresh, sun-dried for 10 and 30 days, freeze-dried, frozen for 10 and 30 days, and commercial long-life frozen), to find the best storage conditions to limit LD degradation.
2. Results and Discussion
2.1. Aqueous Stability Study of LD
Several studies highlighted LD instability in solution and plant matrices [14,26,27,28,29,30,31,32]. Based on its chemical structure, LD has three ionizable groups (Figure 1C). When the pH is between 2.3 and 8.11, LD takes on a zwitterionic structure, which is involved in the intermolecular bond between the protonated amino group and the deprotonated carboxylic group, thus leading to aggregated structures, responsible for the lower LD solubility and stability in neutral environments [10,11,32]. Recent studies have also shown that even oxygen tension affects LD stability. Under normoxic conditions, the analyte is prone to auto-oxidation by releasing protons and reactive oxygen species (ROS) as intermediates [33,34]. In acidic conditions, protons supplied by solvents would shift the auto-oxidation reaction equilibrium towards the reagents and avoid the formation of intermolecular bonding networks [30,32]. Instead, alkaline pH values in plants were shown to increase the enzymatic activity of phenol oxidase (PO). By going from pH 3.5 to pH 4.5, PO activity rises from 60% to 100% [35], thus encouraging the employment of acidic environments when performing the determination of LD. Therefore, LD acidic structure requires working at a suitable mobile-phase pH value to avoid a significant conversion in the corresponding ionized structures [36].
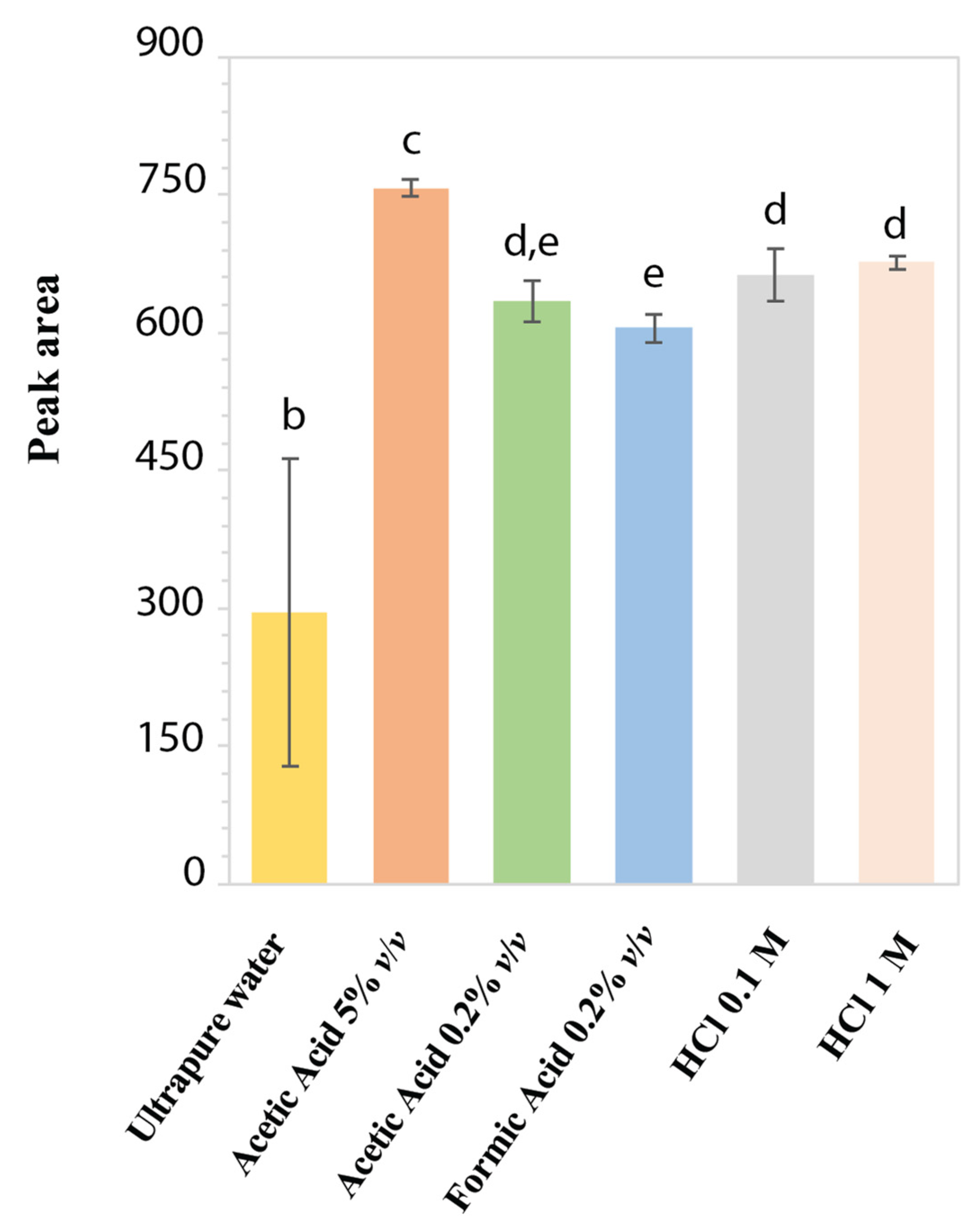
To assess the aqueous stability and to identify the best extraction solvent, the performance of different standard acidic solutions of LD at 50 mg/L was evaluated in terms of reproducibility of chromatographic peak area. In Figure 2, the stability histogram of LD peak area is reported as mean values over 3 months with the corresponding standard deviations. Since the LD solution in ultrapure H2O presented the lowest mean peak area and the highest standard deviation because of the area decreasing over time, it was ascertained as the least stable compared to the other acidic solutions. Furthermore, after two weeks, the solution acquired a dark color due to the formation of melanin [29]: this result confirmed the LD degradation and its chemical instability in H2O, as already reported for LD solutions with alkaline pH or close to neutrality [10,11,29,32]. Instead, for standard LD solutions at acidic pH, the chromatographic peak area values remained stable over 3 months, with %RSD ranging from 0.99% to 4.30%. The best reproducibility in chromatographic peak areas was obtained with standard solutions in HCl 1 M (0.99% RSD) and acetic acid 5% v/v (1.17% RSD). However, in HCl 1 M, an additional chromatographic peak was observed rising over time, probably due to hydrolysis of LD. On the other hand, LD in acetic acid at 5% v/v showed a UV-absorption spectrum completely different from the characteristic profile of LD, reported in Figure 1A. This could be related to a possible analyte acetylation due to the presence of acetic acid at a high concentration. Based on these results, HCl 0.1 M was selected to prepare LD standard solutions as an appreciable signal intensity, with a good reproducibility (4.30% RSD) of the chromatographic peak areas was obtained [9].
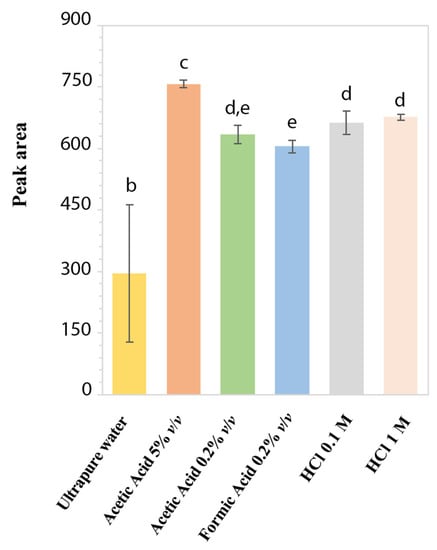
Figure 2.
Stability histogram obtained by monitoring peak areas of LD standard solutions at 50 mg/L in different acidic media. Each bar represents the means ± standard deviation of the results obtained weekly for 3 consecutive months. Values marked by the same letter are not significantly different (p < 0.05).
2.2. Chromatographic Performances
In the present work, a careful investigation of the LD separative chromatographic conditions has been carried out to develop a suitable method for analyzing Vicia faba L. extracted samples.
2.2.1. Choice of the Chromatographic Column
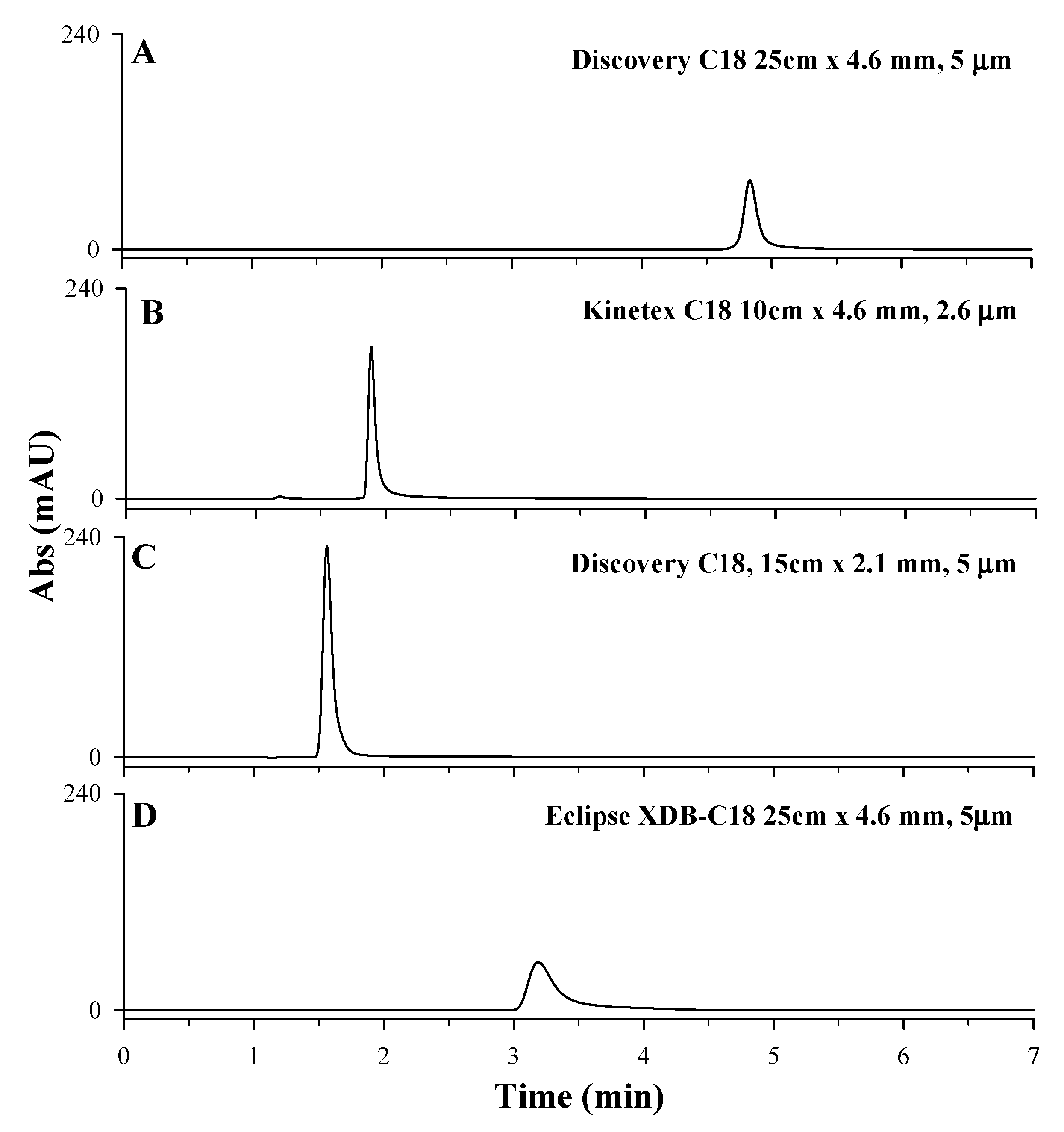
Starting from the chromatographic method proposed by Polanowska et al. [11], the performances of four different reverse-phase chromatographic columns (Agilent ZORBAX Eclipse XDB, Kinetex C18 column, 100 × 4.6 mm, 2.6 µm, Discovery C18 column, 150 × 2.1 mm, 5 µm, Discovery C18 column, 250 × 4.6 mm, 5 µm) have been tested (for columns characteristics see Section 3). Owing to its high and strong retention towards polar compounds, the porous graphitic carbon (PGC) analytical column is not the best choice for trapping very polar compounds at highly aqueous conditions and, for this reason, it was not considered in this study [40]. As reported in Figure 3, LD retention times and peak shapes changed according to the analytical chromatographic column used. The Agilent ZORBAX Eclipse XDB column was successfully used by Long et al. [36] for the separation of acids, bases, and other highly polar analytes in reversed-phase liquid chromatography. However, when used for LD separation, the HPLC-UV chromatographic profile showed a broad and asymmetric chromatographic peak; hence, the column is not suitable for the analysis (Figure 3D). Kinetex C18 column, 100 × 4.6 mm, 2.6 µm particle size, and Discovery C18 column, 150 × 2.1 mm, 5 µm particle size showed the highest intensity of chromatographic peaks but poor retention of LD analytes, with retention times lower than 2 min, too close to the solvent front (Figure 3B,C). Discovery C18 column, 250 × 4.6 mm, 5 µm particle size, instead showed an LD symmetrical peak eluted at 4.8 min. (Figure 3A) [10]. Although chromatograms in plots A and D were obtain with two columns with the same length, internal diameter (ID), and particle sizes, peaks show different retention time. This could be related to the different chemical and physical properties of the stationary phase, such as the type and surface area. In fact, the surface area accessible in a column can be considered as a key factor in influencing the retention of the analyte on different columns with the same type of stationary phase. The higher surface area of the Discovery C18 provides a greater number of binding sites compared to the Eclipse XDB (see Section 3), increasing the retention of the analyte. Based on these results, Discovery C18 column, 250 × 4.6 mm, 5 µm particle size was considered the most suitable for LD separation.
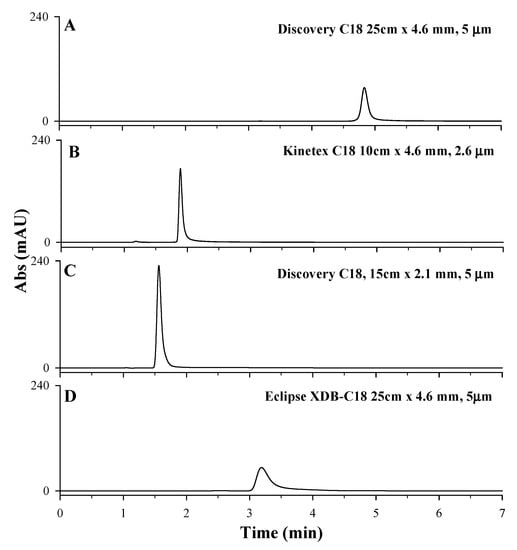
Figure 3.
HPLC-UV chromatographic profiles obtained during the optimization of stationary phase: (A) Discovery C18 column, 250 × 4.6 mm, 5 µm; (B) Kinetex C18 column, 100 × 4.6 mm, 2.6 µm particle size; (C) Discovery C18 column, 150 × 2.1 mm, 5 µm; (D) Agilent ZORBAX Eclipse XDB C18 column, 250 × 4.6 mm, 5 µm. An LD standard solution at 50 mg/L solubilized in HCl 0.1 M, 97% acetic acid 0.2% v/v, and 3% methanol as mobile phase, under isocratic conditions depending on the tested C18 column (1 mL/min for Discovery C18 column, 0.8 mL/min for Kinetex C18 column and Agilent ZORBAX Eclipse XDB, 0.5 mL/min for Discovery Supelco C18 column.), injection volume of 20 µL and λmax set of 280 nm, were used.
2.2.2. Optimization of the Mobile-Phase Composition
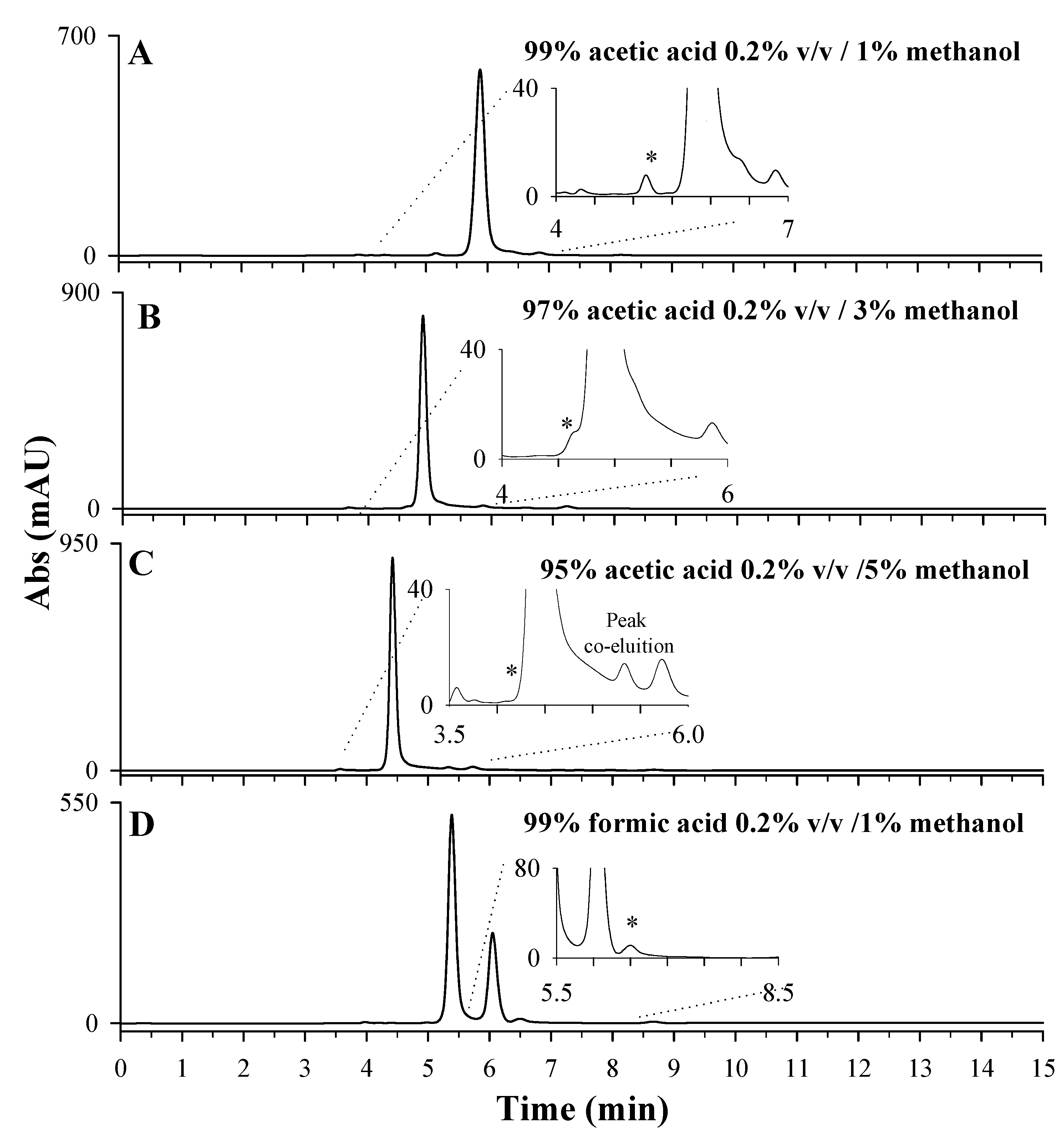
After choosing the most suitable stationary phase for LD separation, the mobile phase’s optimization tests were carried out on the sample extracts of Vicia faba L. broad beans to evaluate potential interferences. Four different mobile phases were tested, as reported in Figure 4: 99% of acetic acid 0.2% v/v containing 1% of methanol (Figure 4A); 97% of acetic acid 0.2% v/v containing 3% of methanol (Figure 4B); 95% of acetic acid 0.2% v/v containing 5% of methanol (Figure 4C); 99% of formic acid 0.2% v/v containing 1% of methanol (Figure 4D). A percentage of methanolic organic phase not higher than 5% was used to limit the collapse of the stationary phase. By comparing plots A, B, and C, it is possible to observe a clear improvement in terms of resolution of the LD chromatographic peak, due to an increase in percentage of the aqueous phase, containing 0.2% acetic acid, from 95% (Figure 4C) to 99% (Figure 4A). A similar trend was observed for 0.2% v/v formic acid as the aqueous phase. Having set the aqueous-phase percentage of 99%, the performances of the two acids, formic and acetic, were compared (Figure 4A,D). In both cases, a well resolved peak was observed for LD (see insets) even if a chromatographic separation of the most abundant interference compounds is achieved only using formic acid (Figure 4D). Furthermore, the employment of formic acid ensured a longer retention time for LD (6.5 min) compared to acetic acid (5.2 min). Formic acid is known to be an ion-pairing reagent, whereas acetic acid is not. The authors of [36] state that ionic interactions between the formate group (-COO−) and basic group (-NH3+) of LD occur and are responsible for the increase in retention time, improving the selectivity of chromatographic separation [37].
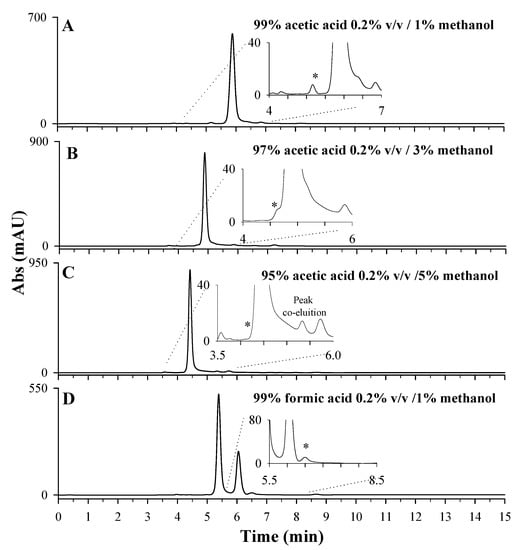
Figure 4.
HPLC-UV chromatographic profiles obtained during the optimization of mobile-phase composition: 99% acetic acid 0.2% v/v containing 1% methanol (pH 3.04) (A); 97% acetic acid 0.2% v/v containing 3% methanol (pH 3.13) (B); 95% acetic acid 0.2% v/v containing 5% methanol (pH 3.13) (C); 99% formic acid 0.2% v/v containing 1% methanol (pH 2.61) (D). The LD peak is indicated with *. A 10-day dried extract of Vicia faba L. sample, diluted 1:10 in HCl 0.1 M, Discovery C18 column, 250 × 4.6 mm, 5 µm, under isocratic conditions (flow rate 0.1 mL/min), injection volume of 20 µL and λmax set of 280 nm, were used.
After choosing 99% formic acid 0.2% v/v/1% methanol as the mobile phase, a new set of chromatographic runs of Vicia faba L. extracts were carried out on the Discovery C18 column, in order to compare the selected mobile phase with the 100% phosphate buffer 125 mM (pH 2.5) [41]. In fact, a buffered mobile phase is recommended in the literature to guarantee a stable pH system value for good peak shape and retention time reproducibility.
The use of phosphate buffer as the mobile phase improved the chromatographic separation efficiency but, over time, caused blockages to the pump and pressure drops due to the precipitation of phosphate salts at the LC valves, requiring additional cleaning of the system to ensure reproducibility and repeatability. Furthermore, considering the higher compatibility of formic acid with most powerful detection techniques such as mass spectrometry, the use of 99% of formic acid 0.2% v/v containing 1% of methanol is the most suitable choice as the mobile phase.
In conclusion, the optimal chromatographic conditions for LD separation in plant matrices of Vicia faba L. can be achieved by using the Discovery C18 column, 250 × 4.6 mm, 5 µm particle size as a stationary phase and a mobile phase composed of 99% formic acid 0.2% v/v containing 1% methanol, under isocratic conditions with a flow of 1 mL/min.
2.3. Method Validation
The performances of the analytic method were evaluated in terms of the estimation of linearity, LOD (limit of detection), LOQ (limit of quantification), accuracy, precision, matrix effect, and measurement uncertainties, according to European Action in Chemistry guidelines [42,43]. All these parameters are reported in Table 1.

Table 1.
Calibration curves, limit of detection and limit of quantification, precision, recovery, uncertainties, resolution, and tailing factor for LD determination by using LC-UV method.
The proposed method showed significant accuracy, precision, and linearity in the concentration range 0.5–50 mg/L. This range is much lower in concentration than that reported in the literature for Vicia faba L. matrix by Vora et al. [16], which validated a linear range 100–700 mg/L by using the same detector.
The calculated LOD and LOQ values of 0.0414 mg/L and 0.0452 mg/L, respectively, were lower than those of the only validated method by LC-UV reported in the literature for Vicia faba L. [16], highlighting the improved suitability of the proposed method for determination in samples with low LD content.
As regards the matrix effect (ME), a value of 100% means that there is not any kind of ME, as obtained in the proposed work. Therefore, it is possible to assess that the presence of matrix components does not interfere with the analysis of LD over the validated range.
The uncertainty measurements were expressed as expanded uncertainty (U, mg/L) for a normal distribution at a 95% confidence level [43,44], and the obtained results are reported in Table 1.
Finally, the results of tailing factor (T) and resolution (Rs) of the method at the three concentration levels, reported in Table 1, met the requirements for a good chromatographic method, showing well resolved peaks (R > 1.5) and no back tailing [45]
2.4. Quantitative Analysis of LD in Differently Stored Samples of Vicia faba L.
The proposed LC-UV method was successfully applied for the quantitative determination of LD occurring in local Vicia faba L. broad beans. Extracts from fresh broad bean sample were firstly analyzed. Then, the same sample was subjected to different storage treatments: sun-drying, freezing, and freeze-drying. Drying and freezing times were also varied (10 and 30 days). The aim was to suggest the best conditions able to preserve the LD content found in fresh samples. Table 2 shows the LD content in mg/g dry weight (dw) occurring in fresh sample, 10-day sun-dried sample, 30-day sun-dried sample, freeze-dried sample, 10-day frozen sample, 30-day frozen sample, and commercial long-life frozen sample.

Table 2.
LD quantification in seven Vicia faba L. broad beans, stored by different processes: fresh sample, 10-day sun-dried sample, 30-day sun-dried sample, freeze-dried sample, 10-day frozen sample, 30-day frozen sample, and commercial long-life frozen sample. LD content is expressed as (mg/g dw) ± U(mg/g). Values marked by the same letter are not significantly different (p < 0.05).
The most abundant LD concentration was detected for 10-day sun-dried sample (1.26 ± 0.15 mg/g dw), which was not significantly different from the content occurring in the fresh sample (1.21 ± 0.17 mg/g dw). Interestingly, the LD content decreased by prolonging sun-drying up to 30 days. Freezing proved to be a less effective conservation method since after 10 days, LD content was already reduced to 1.03 ± 0.14 mg/g dw and after 30 days, it further decreased. For the extreme situation of indefinitely prolonged freezing time, as the commercial long-life frozen sample, LD content decreases so much as to become undetectable using the proposed HPLC-UV method. The freeze-drying storage preserved the LD content better than the 30-day freezing treatment. Furthermore, for samples with a short storage time (10 days), a difference between the freezing and drying treatments was observed. Compared to freezing storage, it seems that the conservation of broad beans by sun-drying allows for better preservation of the analyte, in agreement with the literature data [46,47]. Some papers highlight that sudden changes in temperature cause inhibition of the plant enzymatic activity of cytochrome P450, responsible for the synthesis of LD. Additionally, LD instability under strong thermal processes was largely demonstrated (e.g., freeze-drying, freezing, cooking, and high-temperature heating) [11,23,31,48].
Therefore, this study confirmed the convenience of using short-time sun-dried samples, which guarantee high and reproducible LD contents.
3. Materials and Methods
3.1. Chemicals
Methanol (≥99.8%) was purchased from Honeywell (Seelze, Germany). Analytical standard (≥98%) of 3,4-Dihydroxy-L-phenylalanine (LD) was purchased from Sigma-Aldrich (Milano, Italy). Formic acid was purchased from Fluka (Buchs, Switzerland). Potassium dihydrogen phosphate (≥98%), acetic acid (≥96%) were acquired from Carlo Erba (Rodano, Italy). Hydrochloric acid 37% (GR for analysis) was purchased from Merck KGaA (Darmastadt, Germany). Ultrapure water was produced using a Milli-Q RG system from Millipore (Bedford, MA, USA).
3.2. LD Stability Study
Stock solutions of LD were prepared at 1000 mg/L, in different acidic solutions: acetic acid 0.2% v/v (pH 3.29), formic acid 0.2% v/v (pH 2.62), acetic acid 5% v/v (pH 2.40), HCl 1 mol/L (pH 0), HCl 0.1 mol/L (pH 1), and ultrapure H2O (pH 5.37). LD working standard solutions at 50 mg/L were prepared by dilution from stock solutions and injected weekly by using Discovery C18 column, 250 × 4.6 mm, 5 µm particle size and the chromatographic conditions proposed by Polanowska et al. [11]. This study was performed for 3 months to monitor the analyte stability in the aqueous solutions over a long time. All solutions were stored at 4 °C in the dark to minimize thermal and photolytic degradation [32]. Student’s t-test (SPSS 19.0 for Windows; IBM SPSS Statistics, Armonk, NY, USA) was used to assess the presence of significant differences (p < 0.05) among the standard solution.
3.3. Analytical Method Validation
To optimize the method for the determination of LD by LC-UV system, the following parameters were assessed: LC performance, i.e., C18 stationary phases and mobile phases, and validation parameters, i.e., linearity, limit of detection (LOD), limit of quantification (LOQ), precision, accuracy, matrix effects, and uncertainties, according to European Action in Chemistry (EURACHEM) guidelines [35].
3.3.1. LC Performance
Experiments were performed by using an Agilent 1200 Series Gradient HPLC System (Agilent Technologies, Santa Clara, CA, USA) equipped with a quaternary gradient pump unit, a diode array detector (DAD, 190–950 nm), and a standard autosampler (0.1 μL–100 μL) set to inject 20 μL. For all samples, to improve method selectivity, chromatograms at λ = 280 nm, combined with the absorbance spectrum (190–400 nm) were acquired and purity of LD peaks was checked. All the experiments were carried out at room temperature (25 °C).
Four different C18 analytical columns (Table 3) were used to optimize the best chromatographic separation: Discovery C18 column, 250 × 4.6 mm, 5 µm particle size; Kinetex C18 column, 100 × 4.6 mm, 2.6 µm particle size; Discovery Supelco C18 column, 150 × 2.1 mm, 5 µm particle size; ZORBAX Eclipse XDB-C18, 150 × 4.6 mm, 5 µm particle size. For the stationary-phase optimization study, a standard LD solution at 50 mg/L in 0.1 M HCl was injected, and chromatographic runs were carried out by using the mobile phase proposed by Polanowska et al. [11]: 97% of acetic acid at 0.2% v/v as aqueous phase containing 3% of methanol as organic phase, under isocratic conditions at 1 mL/min for Discovery C18 column, 0.8 mL/min for Kinetex C18 column and Agilent ZORBAX Eclipse XDB, 0.5 mL/min for Discovery Supelco C18 column.

Table 3.
Characteristics of columns tested in this work.
Four different mobile phases were tested on the chosen column: 99% acetic acid 0.2% v/v (aqueous phase) containing 1% methanol (organic phase); 97% acetic acid 0.2% v/v (aqueous phase) containing 3% methanol (organic phase); 95% acetic acid 0.2% v/v (aqueous phase) containing 5% methanol (organic phase); 99% formic acid 0.2% v/v (aqueous phase) containing 1% methanol (organic phase). A 100% aqueous phase A consisting of a KH2PO4 125 mM buffer solution (adjusted to pH 2.5 with concentrated H3PO4) was also tried. For mobile-phase optimization, 10-day sun-dried extract of Vicia faba L. sample, diluted 1:10 in HCl 0.1 M, Discovery C18 column, 250 × 4.6 mm, 5 µm, under isocratic conditions (flow rate 1 mL/min) was used.
All tests were performed in triplicate. Data acquisition and analyses were accomplished using the HPLC 1200 offline (Agilent Technologies, Santa Clara, CA, USA). The chromatographic raw data were imported, elaborated, and plotted by SigmaPlot 11.0 (Systat Software, Inc., London, UK).
3.3.2. Validation Parameters
The linearity was assessed by the least-squares method in a concentration range between 0.5–50 mg/L of LD working solutions in 0.1M HCl. The linearity parameter was estimated at six concentration levels (k = 6) and the analyses were performed in three independent replicates (n = 3). Additionally, a statistic t-test was performed in order to assess the significativity of the correlation coefficient R2 [43,44,49,50].
LOD and LOQ were evaluated by analyzing ten independent blank samples, calculating the mean blank response (xb) and its standard deviation (sb) as follows: yLoD = xb + 3sb and yLoQ = xb + 10sb.
The precision of the proposed method was studied as “repeatability” and “intermediate precision”, expressed as percentage relative standard deviation (%RSD). The first parameter is the precision under the same operating conditions over a short time interval, i.e., the %RSD for six replicates (n = 6) of three levels (k = 3) over the linear range in the same day (p = 1). The second is the within-laboratories variations (different days, different analysts, different equipment, etc.) [51], i.e., the %RSD within several days (p = 3) for the ten replicates (n = 10) of three levels (k = 3) over the linear range. Furthermore, according to the EURACHEM guidelines, the repeatability limit and the intermediate repeatability limit were also calculated as follows: r = √2 × tcrit × s, where s is the standard deviation obtained under repeatability (n = 6) and intermediate precision (n = 10), the factor √2 reflects the difference between the two measurements, and the tcrit value is chosen at a 95% confidence level using a two-tailed distribution and a number of degrees of freedom equal to (n−1).
Since a certified reference material is not commercially available for the plant matrix under study, the accuracy, expressed as recovery, was evaluated by fortifying the real samples at least at three concentration levels (k = 3) over the linear range for three replicates (n = 3) at each level, over three days (p = 3). Since the long-term dried commercial broad bean sample had an LD quantity significantly lower than the LOD and LOQ values of the proposed method, it was used for recovery evaluation. The fortified LD concentration levels were: 0.5 mg/L (5 mg/Kg), 15 mg/L (150 mg/kg), and 50 mg/L (500 mg/kg). The fortified broad bean samples and the corresponding blank samples without fortification were analyzed to calculate the concentration of the analyte from the calibration curve. The difference between the analyte amount in spiked (Csample_spk) and unspiked (Csample_unspk) samples was divided by the amount of spike (Cspk), to estimate the recovery as follows [42,43,49]: Rm = (Csample_spk − Csample_unspk)/(Cspk). The recovery standard deviation (U(Rm)) was calculated by following this equation [44,52]:
where ssample_spk and ssample_unspk are the standard deviations of LD occurring in spiked and unspiked samples, and sspk is the spike uncertainty.
Matrix effects (ME) were assessed using the post-extraction additions, which involve the calibration curve preparation with real extracts. Therefore, the calibration curve of the real samples was compared to the one achieved for the same standards in HCl 0.1 mol/L solvent. If both curves were parallel and overlapped, compounds are not subjected to any matrix effects. The ME was estimated by dividing the slopes of the matrix-matched calibration curves prepared with real extracts (slope matrix) and the slopes of the calibration curves prepared with solvent (slope std): ME(%) = 100 × (slopematrix/slopestd) [43].
Finally, the expanded uncertainty was estimated as a combination of different contributions by using the bottom-up approach [43,44,52], as follows:
where uc is the combined standard uncertainty (mg/L); C0 is the concentration level (mg/L); Rm is the recovery (%); LOD is the limit of detection (mg/L); uprep is the uncertainty related to concentration levels preparation (mg/L); ucal is the uncertainty of calibration curves (mg/L); uRm is the recovery uncertainty; uLoD is the LoD uncertainty. Finally, extended uncertainties U, for n = 3 replicates, was estimated by multiplying the compound uncertainty by a coverage factor corresponding to k = 1.98, for a confidence level of 95%.
3.4. Vicia faba L. Broad Beans and LD Extraction
Vicia faba L. broad beans were purchased from a local producer in Potenza (Basilicata, Italy) as fresh sample. Vicia faba L. broad beans were then divided in different aliquots for different storage treatments: sun-drying for 10 and 30 days, freezing for 10 and 30 days, freeze-drying. Extraction conditions proposed by Polanowska et al. [11] were optimized to allow LD extraction from the fresh sample, sun-dried samples (10 and 30 days), freeze-dried sample, frozen samples (10 and 30 days), and commercial long-life frozen sample. Briefly, ultrasonic assisted extraction (UAE) was applied by using an extraction ratio of 1:10 weight/dry volume and HCl 0.1 mol/L as extracting solution; a sonication time of 20 min in an ice bath (4 °C) and a centrifugation for 10 min at 6000× g was applied. This procedure was performed twice, and then supernatants were collected, filtered on PTFE 0.2 µm filters, and stored at 4 °C in the dark until the LC-UV analyses. The LD quantification on the various broad bean extracts was performed by the external standard method. A botanical sample is kept in the Science Department of the University of Basilicata. The genus and species of the plant have been unambiguously identified.
4. Conclusions
An LC-UV method has been successfully optimized and validated for L-Dopa separation and quantification in Vicia faba L. broad bean samples. A strongly acidic aqueous solution, consisting of HCl 0.1 M, proved to be the best extraction solvent to assure the stability of LD over 3 months. After testing different stationary and mobile phases, a Discovery C18 column, 250 × 4.6 mm, 5 µm particle size as a stationary phase and a mobile phase consisting of 99% formic acid 0.2% v/v and 1% methanol (pH 2.61), under isocratic flow of 1 mL/min, were chosen for a reliable chromatographic separation. A rigorous LC-UV method validation, according to EURACHEM guidelines, reached LOD and LOQ values of 0.0414 and 0.0452 mg/L, respectively. High precision (less than 6.87% RSD) and accuracy (ranging between 1.01 and 1.03 of recovery) were obtained. No matrix effect was detected for the samples under study.
After validation, the proposed method was used for the LD quantitative analysis of differently stored Vicia faba L. broad bean samples, thus defining 10-day sun-drying as the best storage treatment able to preserve a high LD content in broad beans (1.26 ± 0.15 mg/g dw). The method described in this work was demonstrated to be robust and reliable for routine LD analyses in vegetable matrices, such as Vicia faba L. broad beans, which could be a potential functional food or an ingredient for food supplement preparation for PD patients.
Author Contributions
Conceptualization, F.L., R.C., G.B. and M.D.; methodology, C.T. and F.L.; validation, C.T., R.P. and G.B.; formal analysis, C.T., R.P., F.L. and R.C.; investigation, C.T., F.L., M.A.A. and A.D.C.; resources, L.S. and S.A.B.; data curation C.T., R.P. and M.A.A.; writing—original draft preparation C.T., A.D.C. and M.A.A.; writing—review and editing, F.L., R.C., G.B., A.G., M.D. and S.P.; supervision F.L., R.C. and A.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Ribeiro, R.P.; Gasparetto, J.C.; De Oliveira Vilhena, R.; De Francisco, T.M.G.; Martins, C.A.F.; Cardoso, M.A.; Pontarolo, R. Simultaneous determination of levodopa, carbidopa, entacapone, tolcapone, 3-O-methyldopa and dopamine in human plasma by an HPLC-MS/MS method. Bioanalysis 2015, 7, 207–220. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Wang, Y.; Wu, H.; Cheng, C.; Le, W. Research advances on L-DOPA-induced dyskinesia: From animal models to human disease. Neurol. Sci. 2020, 41, 2055–2065. [Google Scholar] [CrossRef] [PubMed]
- Mortezazadeh, T.; Seyedarabi, H.; Mahmoudian, B.; Islamian, J.P. Imaging modalities in differential diagnosis of Parkinson’s disease: Opportunities and challenges. Egypt. J. Radiol. Nucl. Med. 2021, 52, 79. [Google Scholar] [CrossRef]
- Balestrino, R.; Schapira, A.H.V. Parkinson disease. Eur. J. Neurol. 2020, 27, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.F.E.; Church, F.C. Integrative Medicine and Health Therapy for Parkinson Disease. Top. Geriatr. Rehabil. 2020, 36, 176–186. [Google Scholar] [CrossRef]
- Min, K.; Park, K.; Park, D.H.; Yoo, Y.J. Overview on the biotechnological production of l-DOPA. Appl. Microbiol. Biotechnol. 2015, 99, 575–584. [Google Scholar] [CrossRef] [PubMed]
- Dhull, S.B.; Kidwai, M.K.; Noor, R.; Chawla, P.; Rose, P.K. A review of nutritional profile and processing of faba bean (Vicia faba L.). Legum. Sci. 2021, 4, e129. [Google Scholar] [CrossRef]
- Benfica, J.; Morais, E.S.; Miranda, J.S.; Freire, M.G.; de Cássia Superbi de Sousa, R.; Coutinho, J.A.P. Aqueous solutions of organic acids as effective solvents for levodopa extraction from Mucuna pruriens seeds. Sep. Purif. Technol. 2021, 274, 119084. [Google Scholar] [CrossRef]
- Apaydin, H.; Ertan, S.; Ozekmekçi, S. Broad bean (Vicia faba)—A natural source of L-dopa—Prolongs “on” periods in patients with Parkinson’s disease who have “on–off” fluctuations. Mov. Disord. Off. J. Mov. Disord. Soc. 2000, 15, 164–166. [Google Scholar] [CrossRef]
- Tesoro, C.; Lelario, F.; Ciriello, R.; Bianco, G.; Di Capua, A.; Acquavia, M.A. An Overview of Methods for L-Dopa Extraction and Analytical Determination in Plant Matrices. Separations 2022, 9, 224. [Google Scholar] [CrossRef]
- Polanowska, K.; Łukasik, R.M.; Kuligowski, M. Development of a Sustainable, Simple, and Robust Method for Efficient l-DOPA Extraction. Molecules 2019, 24, 2325. [Google Scholar] [CrossRef] [PubMed]
- Rathod, B.G.; Patel, N.M. Development of validated RP-HPLC method for the estimation of L-Dopa from Mucuna pruriens, its extracts and in Aphrodisiac formulation. Int. J. Pharma Sci. Res. 2014, 5, 508–513. [Google Scholar]
- Renna, M.; De Cillis, F.; Leoni, B.; Acciardi, E.; Santamaria, P. From by-product to unconventional vegetable: Preliminary evaluation of fresh fava hulls highlights richness in L-DOPa and low content of anti-nutritional factor. Foods 2020, 9, 159. [Google Scholar] [CrossRef]
- Siddhuraju, P.; Becker, K. Rapid reversed-phase high performance liquid chromatographic method for the quantification of L-Dopa (L-3,4-dihydroxyphenylalanine), non-methylated and methylated tetrahydroisoquinoline compounds from Mucuna beans. Food Chem. 2001, 72, 389–394. [Google Scholar] [CrossRef]
- Singh, R.; Saini, P.; Mathur, S.; Singh, G.; Kumar, S. Application of high performance liquid chromatography to the determination and validation of levodopa in methanolic extract of Mucuna utilis. Int. J. Green Pharm. 2010, 4, 156–158. [Google Scholar] [CrossRef]
- Vora, R.N.; Joshi, A.N.; Joshi, N.C. Comparison of L-Dopa Content in Two Varieties of Broad Beans (Vicia faba) By Different Extraction Techniques. World J. Pharm. Med. Res. 2017, 3, 271–274. [Google Scholar]
- Yang, X.; Zhang, X.; Zhou, R. Determination of L-Dopa content and other significant nitrogenous compounds in the seeds of seven Mucuna and Stizolobium species in China. Pharm. Biol. 2001, 39, 312–316. [Google Scholar] [CrossRef]
- Aware, C.; Patil, R.; Gaikwad, S.; Yadav, S.; Bapat, V.; Jadhav, J. Evaluation of L-dopa, proximate composition with in vitro anti-inflammatory and antioxidant activity of Mucuna macrocarpa beans: A future drug for Parkinson treatment. Asian Pac. J. Trop. Biomed. 2017, 7, 1097–1106. [Google Scholar] [CrossRef]
- Baranowska, I.; Płonka, J. Simultaneous Determination of Biogenic Amines and Methylxanthines in Foodstuff—Sample Preparation with HPLC-DAD-FL Analysis. Food Anal. Methods 2015, 8, 963–972. [Google Scholar] [CrossRef]
- Bulduk, İ.; Topal, N. Development and Validation of a Quantification Method for L-DOPA in Plants and Pharmaceutical Materials. Hacettepe J. Biol. Chem. 2020, 49, 1–10. [Google Scholar] [CrossRef]
- Dhanani, T.; Singh, R.; Shah, S.; Kumari, P.; Kumar, S. Comparison of green extraction methods with conventional extraction method for extract yield, L-DOPA concentration and antioxidant activity of Mucuna pruriens seed. Green Chem. Lett. Rev. 2015, 8, 43–48. [Google Scholar] [CrossRef]
- Duan, S.; Kwon, S.J.; Lim, Y.J.; Gil, C.S.; Jin, C.; Eom, S.H. L-3,4-dihydroxyphenylalanine accumulation in faba bean (Vicia faba L.) tissues during different growth stages. Agronomy 2021, 11, 502. [Google Scholar] [CrossRef]
- Etemadi, F.; Hashemi, M.; Randhir, R.; Zand Vakili, O.; Ebadi, A. Accumulation of L-DOPA in various organs of faba bean and influence of drought, nitrogen stress, and processing methods on L-DOPA yield. Crop J. 2018, 6, 426–434. [Google Scholar] [CrossRef]
- Goyoaga, C.; Burbano, C.; Cuadrado, C.; Varela, A.; Guillamón, E.; Pedrosa, M.M.; Muzquiz, M. Content and distribution of vicine, convicine and l-DOPA during germination and seedling growth of two Vicia faba L. varieties. Eur. Food Res. Technol. 2008, 227, 1537–1542. [Google Scholar] [CrossRef]
- Pavón-Pérez, J.; Oviedo, C.A.; Elso-Freudenberg, M.; Henríquez-Aedo, K.; Aranda, M. LC-MS/MS Method For L-Dopa Quantification in Different Tissues of Vicia faba. J. Chil. Chem. Soc. 2019, 64, 4–6. [Google Scholar] [CrossRef]
- Shetty, P.; Atallah, M.T.; Shetty, K. Effects of UV treatment on the proline-linked pentose phosphate pathway for phenolics and L-DOPA synthesis in dark germinated Vicia faba. Process Biochem. 2002, 37, 1285–1295. [Google Scholar] [CrossRef]
- Neyra Recky, J.R.; Serrano, M.P.; Dántola, M.L.; Lorente, C. Oxidation of tyrosine: Antioxidant mechanism of L-DOPA disclosed. Free Radic. Biol. Med. 2021, 165, 360–367. [Google Scholar] [CrossRef]
- Pulikkalpura, H.; Kurup, R.; Mathew, P.J.; Baby, S. Levodopa in Mucuna pruriens and its degradation. Sci. Rep. 2015, 5, 2–10. [Google Scholar] [CrossRef]
- Omotani, H.; Yasuda, M.; Ishii, R.; Ikarashi, T.; Fukuuchi, T.; Yamaoka, N.; Mawatari, K.I.; Kaneko, K.; Nakagomi, K. Analysis of l-DOPA-derived melanin and a novel degradation product formed under alkaline conditions. J. Pharm. Biomed. Anal. 2016, 125, 22–26. [Google Scholar] [CrossRef]
- Andrade, Â.L.; Cardoso, T.D.; Thomasi, S.S.; Alvarenga, M.E.; da Silva, M.A.N.; Magalhães, E.J.; Duarte, H.A.; de Almeida, K.J. A simple and efficient method for simultaneous quantification of levodopa and carbidopa based on controlled oxidation process. Chem. Pap. 2021, 75, 3091–3102. [Google Scholar] [CrossRef]
- Gurumoorthi, P.; Janardhanan, K.; Myhrman, R.V. Effect of differential processing methods on L-Dopa and protein quality in velvet bean, an underutilized pulse. LWT 2008, 41, 588–596. [Google Scholar] [CrossRef]
- Zhou, Y.Z.; Alany, R.G.; Chuang, V.; Wen, J. Studies of the rate constant of L-DOPA oxidation and decarboxylation by HPLC. Chromatographia 2012, 75, 597–606. [Google Scholar] [CrossRef]
- Kostrzewa, R.M.; Kostrzewa, J.P.; Brus, R. Neuroprotective and neurotoxic roles of levodopa (L-DOPA) in neurodegenerative disorders relating to Parkinson’s disease. Amino Acids 2002, 23, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Hörmann, P.; Delcambre, S.; Hanke, J.; Geffers, R.; Leist, M.; Hiller, K. Impairment of neuronal mitochondrial function by l-DOPA in the absence of oxygen-dependent auto-oxidation and oxidative cell damage. Cell Death Discov. 2021, 7, 151. [Google Scholar] [CrossRef] [PubMed]
- Tahvanainen, T.; Haraguchi, A. Effect of pH on phenol oxidase activity on decaying Sphagnum mosses. Eur. J. Soil Biol. 2013, 54, 41–47. [Google Scholar] [CrossRef]
- Long, W.J.; Brooks, A.E.; Biazzo, W. Analysis of Polar Compounds Using 100% Aqueous Mobile Phases with Agilent ZORBAX Eclipse Plus Phenyl-Hexyl and Other ZORBAX Phenyl Columns. Agil. Technol. Publ. 2009, 1–8. [Google Scholar]
- Cai, B.; Li, J. Evaluation of trifluoroacetic acid as an ion-pair Analysis of Polar Compounds Using 100% Aqueous Mobile Phases with Agilent ZORBAX Eclipse Plus Phenyl-Hexyl and Other ZORBAX Phenyl Columnsreagent in the separation of small ionizable molecules by reversed-phase liquid chromatography. Anal. Chim. Acta 1999, 399, 249–258. [Google Scholar] [CrossRef]
- Bidlingmeyer, B.A.; Broske, A.D. The Role of Pore Size and Stationary Phase Composition in Preventing Aqueous-Induced Retention Time Loss in Reversed-Phase HPLC. J. Chromatogr. Sci. 2004, 42, 100–106. [Google Scholar] [CrossRef]
- Buszewski, B.; Noga, S. Hydrophilic interaction liquid chromatography (HILIC)-a powerful separation technique. Anal. Bioanal. Chem. 2012, 402, 231–247. [Google Scholar] [CrossRef]
- Thiébaut, D.; Vial, J.; Michel, M.; Hennion, M.C.; Greibrokk, T. Evaluation of reversed phase columns designed for polar compounds and porous graphitic carbon in “trapping” and separating neurotransmitters. J. Chromatogr. A 2006, 1122, 97–104. [Google Scholar] [CrossRef]
- Arnetoli, M.; Montegrossi, G.; Buccianti, A.; Gonnelli, C. Determination of organic acids in plants of Silene paradoxa L. by HPLC. J. Agric. Food Chem. 2008, 56, 789–795. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, B.; Örnemark, U. Eurachem Guide: The Fitness for Purpose of Analytical Methods—A Laboratory Guide to Method Validation and Related Topics, 2nd ed.; Örnemark, U., Ed.; Eurachem: Gembloux, Belgium, 2014; ISBN 0-94948926-12-0. [Google Scholar]
- Pascale, R.; Bianco, G.; Coviello, D.; Cristina Lafiosca, M.; Masi, S.; Mancini, I.M.; Bufo, S.A.; Scrano, L.; Caniani, D. Validation of a liquid chromatography coupled with tandem mass spectrometry method for the determination of drugs in wastewater using a three-phase solvent system. J. Sep. Sci. 2020, 43, 886–895. [Google Scholar] [CrossRef] [PubMed]
- Pascale, R.; Bianco, G.; Calace, S.; Masi, S.; Mancini, I.M.; Mazzone, G.; Caniani, D. Method development and optimization for the determination of benzene, toluene, ethylbenzene and xylenes in water at trace levels by static headspace extraction coupled to gas chromatography–barrier ionization discharge detection. J. Chromatogr. A 2018, 1548, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.R.; Kirkland, J.J.; Glajchand, J.L. Practical HPLC Method Development, 2nd ed.; John Wiley & Sons: New York, NY, USA, 1997. [Google Scholar]
- Saranya, G.; Jiby, M.V.; Jayakumar, K.S.; Pillai, P.P.; Jayabaskaran, C. L-DOPA synthesis in Mucuna pruriens (L.) DC. is regulated by polyphenol oxidase and not CYP 450/tyrosine hydroxylase: An analysis of metabolic pathway using biochemical and molecular markers. Phytochemistry 2020, 178, 112467. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, H.L.; Beyer, N.; Janssen, D.B.; Fraaije, M.W. Lyophilization conditions for the storage of monooxygenases. J. Biotechnol. 2015, 203, 41–44. [Google Scholar] [CrossRef]
- Sharma, A.; Khamar, D.; Cullen, S.; Hayden, A.; Hughes, H. Innovative Drying Technologies for Biopharmaceuticals. Int. J. Pharm. 2021, 609, 121115. [Google Scholar] [CrossRef]
- Coviello, D.; Pascale, R.; Ciriello, R.; Salvi, A.M.; Guerrieri, A.; Contursi, M.; Scrano, L.; Bufo, S.A.; Cataldi, T.R.I.; Bianco, G. Validation of an analytical method for nitrite and nitrate determination in meat foods for infants by ion chromatography with conductivity detection. Foods 2020, 9, 1238. [Google Scholar] [CrossRef]
- Araujo, P. Key aspects of analytical method validation and linearity evaluation. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2009, 877, 2224–2234. [Google Scholar] [CrossRef]
- Guideline, ICH Harmonised tripartite. Validation of analytical procedures: Text and methodology Q2(R1). Guidance 2005, 1, 5. [Google Scholar]
- Ellison, S.L.R.; Williams, A. (Eds.) Eurachem/CITAC Guide: Quantifying Uncertainty in Analytical Measurement, 3rd ed.; Eurachem/CITAC: Teddington, UK, 2012. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).