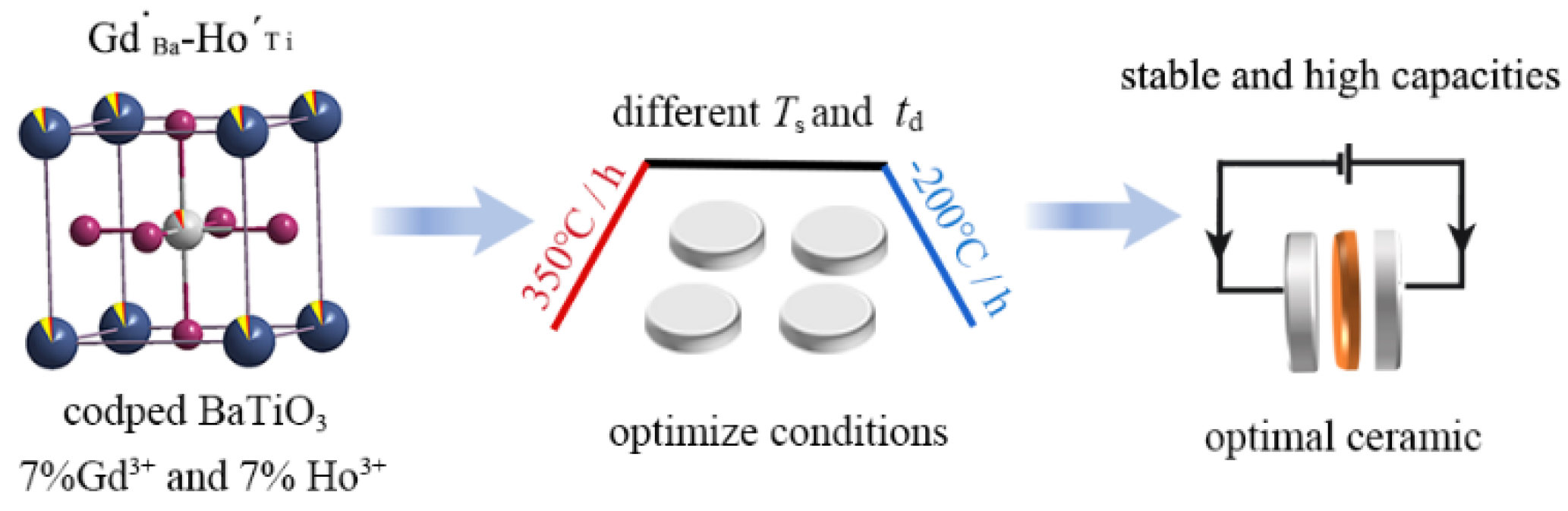
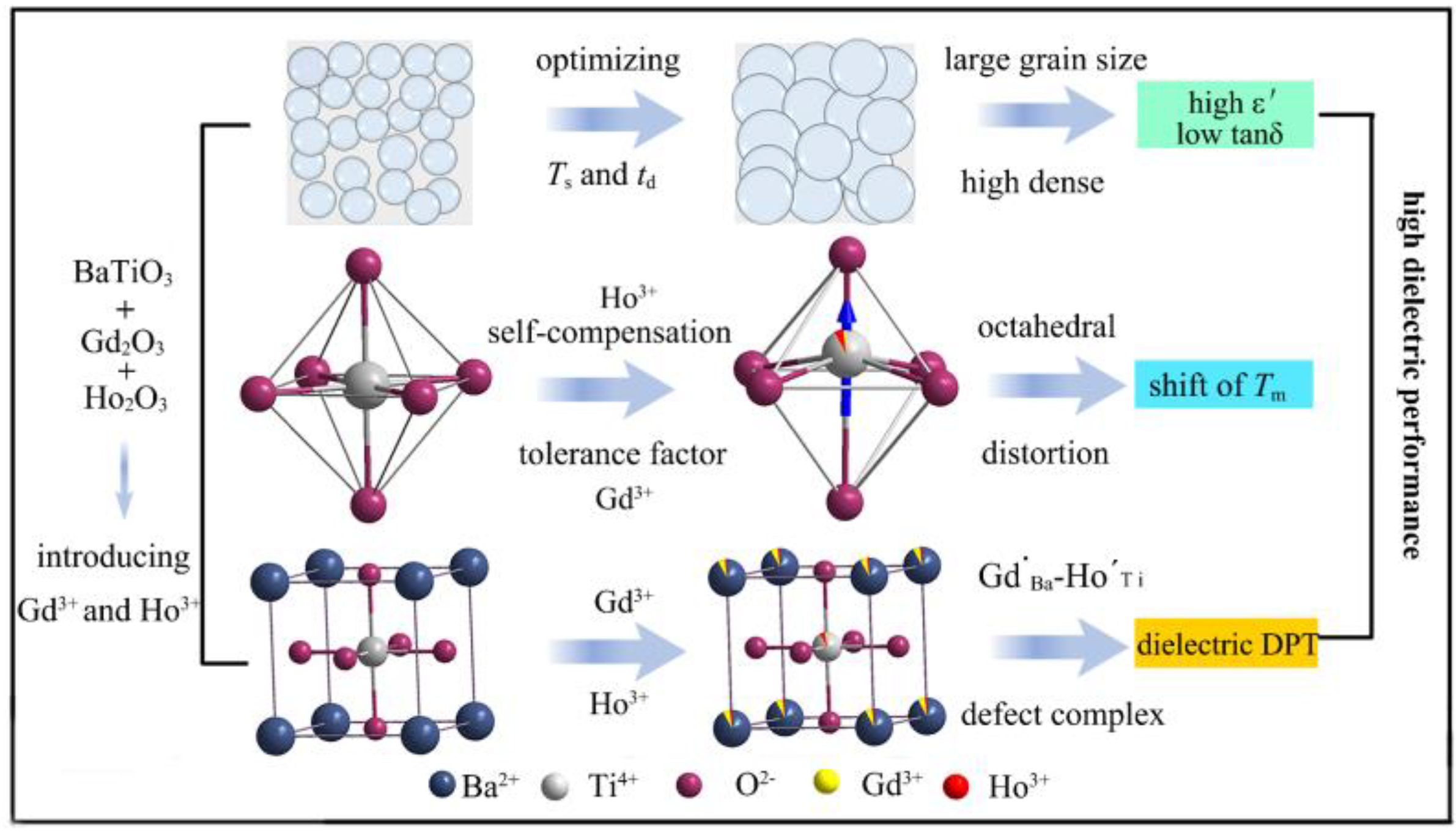
Optimization of Sintering Conditions to Enhance the Dielectric Performance of Gd3+ and Ho3+ Codoped BaTiO3 Ceramics
Abstract
1. Introduction
2. Results and Discussion
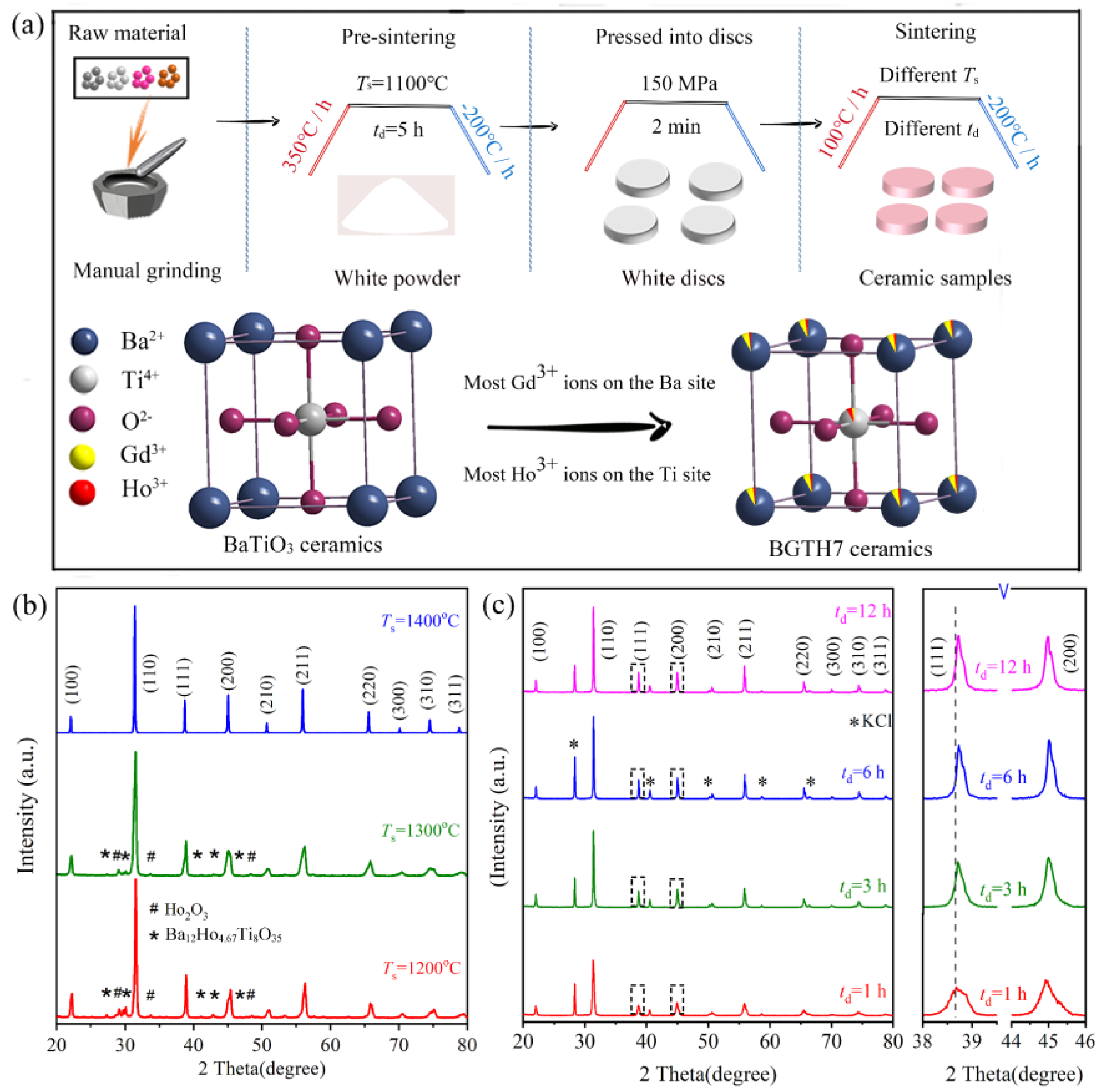
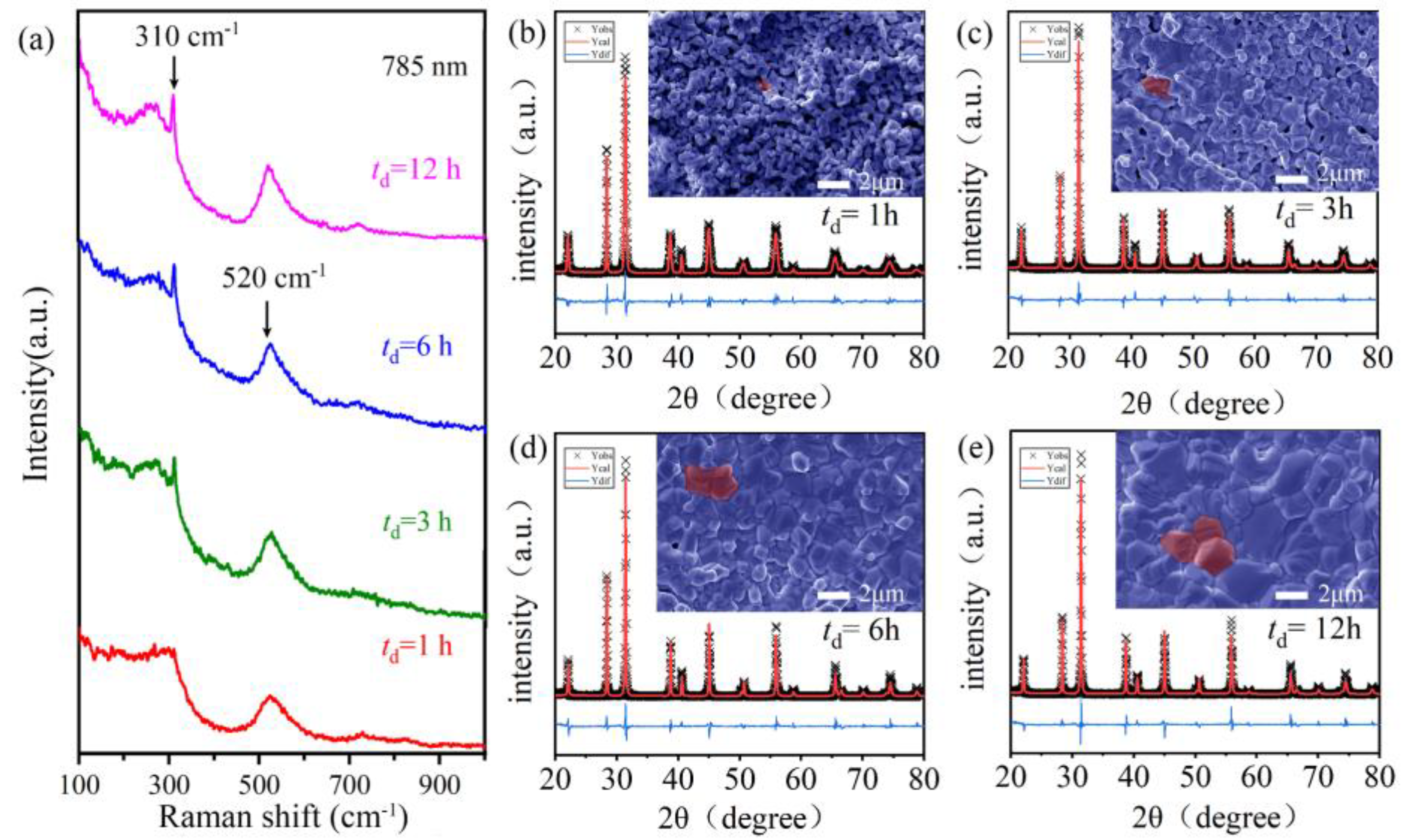
2.1. Effect of Sintering Temperature and Dwell Time on Phase Purity and Morphology
2.2. Effect of Dwell Time on the Site Occupation of BGTH7 Ceramics Sintered at 1400 °C
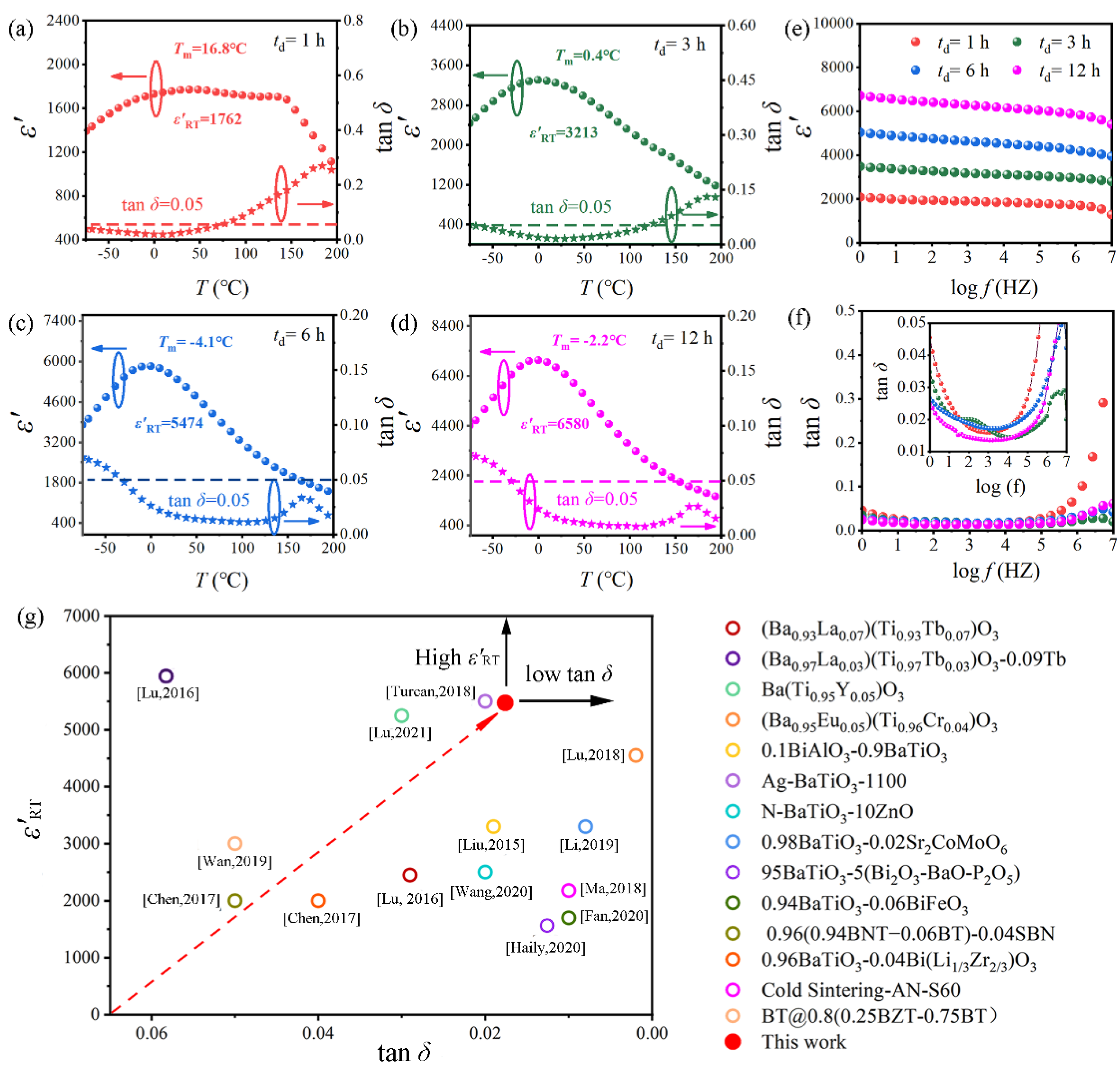
2.3. Dielectric Properties of BGTH7 Ceramics Sintered at 1400 °C
2.4. Understanding the Excellent Dielectric Properties of BGTH7 Ceramics Sintered at 1400 °C
2.4.1. High Dielectric Constant at Room Temperature
2.4.2. Shift of the Dielectric Peak
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, M.; Liang, R.; Zhou, Z.; Dong, X. Novel BaTiO3-based lead-free ceramic capacitors featuring high energy storage density, high power density, and excellent stability. J. Mater. Chem. C 2018, 6, 8528–8537. [Google Scholar] [CrossRef]
- Wang, X.; Ren, P.; Wang, J.; Xu, J.; Xi, Y. Multi-phase coexistence and temperature-stable dielectric properties in BaTiO3/ZnO composite ceramics. J. Eur. Ceram. Soc. 2020, 40, 1896–1901. [Google Scholar] [CrossRef]
- Tawade, B.; Apata, I.; Pradhan, N.; Karim, A.; Raghavan, D. Recent Advances in the Synthesis of Polymer-Grafted Low-K and High-K Nanoparticles for Dielectric and Electronic Applications. Molecules 2021, 26, 2942. [Google Scholar] [CrossRef] [PubMed]
- Boonlakhorn, J.; Manyam, J.; Srepusharawoot, P.; Krongsuk, S.; Thongbai, P. Effects of charge compensation on colossal permittivity and electrical properties of grain boundary of CaCu3Ti4O12 ceramics Substituted by Al3+ and Ta5+/Nb5+. Molecules 2021, 26, 3294. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Li, F.; Ma, L.P. Advanced materials for energy storage. Adv. Mater. 2010, 22, E28–E62. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Zhu, X.N.; Ren, G.R.; Chen, X.M. Enhanced energy storage density and its variation tendency in CaZrxTi1-xO3 ceramics. J. Alloy Compd. 2016, 688, 687–691. [Google Scholar] [CrossRef]
- Bokov, A.; Ye, Z.-G. Recent progress in relaxor ferroelectrics with perovskite structure. J. Mater. Sci. 2006, 41, 31–52. [Google Scholar] [CrossRef]
- Hong, K.; Lee, T.H.; Suh, J.M.; Yoon, S.-H.; Jang, H.W. Perspectives and challenges in multilayer ceramic capacitors for next generation electronics. J. Mater. Chem. C 2019, 7, 9782–9802. [Google Scholar] [CrossRef]
- Jiang, B.; Iocozzia, J.; Zhao, L.; Zhang, H.; Harn, Y.-W.; Chen, Y.; Lin, Z. Barium titanate at the nanoscale: Controlled synthesis and dielectric and ferroelectric properties. Chem. Soc. Rev. 2019, 48, 1194–1228. [Google Scholar] [CrossRef]
- Saini, P.; Arora, M.; Gupta, G.; Gupta, B.K.; Singh, V.N.; Choudhary, V. High permittivity polyaniline-barium titanate nanocomposites with excellent electromagnetic interference shielding response. Nanoscale 2013, 5, 4330–4336. [Google Scholar] [CrossRef]
- Jia, H.; Chen, J. Tailoring the tetragonal distortion to obtain high Curie temperature and large piezoelectric properties in BiFeO3-PbTiO3-BaTiO3 solid solutions. J. Eur. Ceram. Soc. 2020, 41, 2443–2449. [Google Scholar] [CrossRef]
- Xie, Y.; Yin, S.; Hashimoto, T.; Tokano, Y.; Sasaki, A.; Sato, T. Low temperature synthesis of tetragonal BaTiO3 by a novel composite-hydroxide-mediated approach and its dielectric properties. J. Eur. Ceram. Soc. 2010, 30, 699–704. [Google Scholar] [CrossRef]
- Ma, R.; Cui, B.; Shangguan, M.; Wang, S.; Wang, Y.; Chang, Z.; Wang, Y. A novel double-coating approach to prepare fine-grained BaTiO3@La2O3@SiO2 dielectric ceramics for energy storage application. J. Alloy. Compd. 2017, 690, 438–445. [Google Scholar] [CrossRef]
- Jin, S.H.; Lee, H.W.; Kim, N.W.; Lee, B.-W.; Lee, G.-G.; Hong, Y.-W.; Nam, W.H.; Lim, Y.S. Sonochemically activated solid-state synthesis of BaTiO3 powders. J. Eur. Ceram. Soc. 2021, 41, 4826–4834. [Google Scholar] [CrossRef]
- Ben, L.; Sinclair, D.C. Anomalous Curie temperature behavior of A-site Gd-doped BaTiO3 ceramics: The influence of strain. Appl. Phys. Lett. 2011, 98, 092907. [Google Scholar] [CrossRef]
- Han, F.; Bai, Y.; Qiao, L.-J.; Guo, D. A systematic modification of the large electrocaloric effect within a broad temperature range in rare-earth doped BaTiO3 ceramics. J. Mater. Chem. C 2016, 4, 1842–1849. [Google Scholar] [CrossRef]
- Huang, Q.; Si, F.; Tang, B. The effect of rare-earth oxides on the energy storage performances in BaTiO3 based ceramics. Ceram. Int. 2022, 48, 17359–17368. [Google Scholar] [CrossRef]
- Jeon, S.-C.; Kang, S.-J.L. Coherency strain enhanced dielectric-temperature property of rare-earth doped BaTiO3. Appl. Phys. Lett. 2013, 102, 112915. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Z.; Lu, Z.; Wang, X.; Fu, X. Effect of Bi2O3 and Ho2O3 co-doping on the dielectric properties and temperature reliability of X8R BaTiO3-based ceramics. Ceram. Int. 2021, 47, 24982–24987. [Google Scholar] [CrossRef]
- Lu, D.Y.; Guan, D.X.; Li, H.B. Multiplicity of photoluminescence in Raman spectroscopy and defect chemistry of (Ba1−xRx)(Ti1−xHox)O3 (R = La, Pr, Nd, Sm) dielectric ceramics. Ceram. Int. 2018, 44, 1483–1492. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Gao, X.-L.; Wang, S. Abnormal Curie-temperature shift in Ho-doped BaTiO3 ceramics with the self-compensation mode. Results Phys. 2018, 12, 585–591. [Google Scholar] [CrossRef]
- Yuan, L.; Hu, W.; Fang, S.; Li, G.; Wang, X.; Wu, X.; Li, L. CdO-CuO-TiO2 ternary dielectric systems: Subsolidus phase diagram and the effects of Cu segregation. J. Eur. Ceram. Soc. 2018, 38, 4978–4985. [Google Scholar] [CrossRef]
- Tsuji, K.; Ndayishimiye, A.; Lowum, S.; Floyd, R.; Wang, K.; Wetherington, M.; Maria, J.-P.; Randall, C.A. Single step densification of high permittivity BaTiO3 ceramics at 300 °C. J. Eur. Ceram. Soc. 2019, 40, 1280–1284. [Google Scholar] [CrossRef]
- Wang, H.; Li, G.; Li, L. Molten-salt-mediated synthesis and low-temperature electrical conduction of LnCoO3 (Ln=Pr, Nd, Sm, and Gd). J. Alloy Compd. 2014, 612, 227–232. [Google Scholar] [CrossRef]
- Wang, X.; Deng, X.; Wen, H.; Li, L. Phase transition and high dielectric constant of bulk dense nanograin barium titanate ceramics. Appl. Phys. Lett. 2006, 89, 162902. [Google Scholar] [CrossRef]
- Manika, G.C.; Andrikopoulos, K.S.; Psarras, G.C. On the ferroelectric to paraelectric structural transition of BaTiO3 micro-/nanoparticles and their epoxy nanocomposites. Molecules 2020, 25, 2686. [Google Scholar] [CrossRef] [PubMed]
- Naik, R.; Nazarko, J.J.; Flattery, C.S.; Venkateswaran, U.D.; Naik, V.M. Temperature dependence of the Raman spectra of polycrystalline Ba1-xSixTiO3. Phys. Rev. B 2000, 61, 11367–11372. [Google Scholar] [CrossRef]
- Lin, M.-F.; Thakur, V.K.; Tan, E.J.; Lee, P.S. Dopant induced hollow BaTiO3 nanostructures for application in high performance capacitors. J. Mater. Chem. 2011, 21, 16500–16504. [Google Scholar] [CrossRef]
- Petrovsky, V.; Petrovsky, T.; Kamlapurkar, S.; Dogan, F. Dielectric Constant of Barium Titanate Powders Near Curie Temperature. J. Am. Ceram. Soc. 2008, 91, 3590–3592. [Google Scholar] [CrossRef]
- Ma, N.; Zhang, B.P.; Yang, W.G.; Guo, D. Phase structure and nano-domain in high performance of BaTiO3 piezoelectric ceramics. J. Eur. Ceram. Soc. 2012, 32, 1059–1066. [Google Scholar] [CrossRef]
- Alkathy, M.S.; Hezam, A.; Manoja, K.; Wang, J.; Cheng, C.; Byrappa, K.; Raju, K.J. Effect of sintering temperature on structural, electrical, and ferroelectric properties of lanthanum and sodium co-substituted barium titanate ceramics. J. Alloy Compd. 2018, 762, 49–61. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Zhang, X.; Sui, Y.; Zhang, Y.; Liu, Z.; Lv, Z.; Wang, Y.; Xu, P.; Song, B. The contribution of doped-Al to the colossal permittivity properties of AlxNb0.03Ti0.97-xO2 rutile ceramics. J. Mater. Chem. C 2016, 4, 6798–6805. [Google Scholar] [CrossRef]
- Gao, B.; Xu, S.; Xu, Q. CO2 Induced Exposure of the Intrinsic Magnetic Surface of BaTiO3 to Give Room-Temperature Ferromagnetism. Angew. Chem. Int. Ed. Engl. 2022, 61, e202117084. [Google Scholar] [CrossRef]
- Lu, D.Y.; Liu, T.T. Dielectric properties and defect chemistry of (Ba1−xLax)(Ti1−xLux)O3 ceramics. J. Alloys Compd. 2017, 698, 967–976. [Google Scholar] [CrossRef]
- Kolodiazhnyi, T.; Petric, A. Analysis of point defects in polycrystalline BaTiO3 by electron paramagnetic resonance. J. Phys. Chem. Solids 2003, 64, 953–960. [Google Scholar] [CrossRef]
- Munteanu, A.-C.; Badea, M.; Olar, R.; Silvestro, L.; Dulea, C.; Negut, C.-D.; Uivarosi, V. Synthesis and Structural Investigation of New Bio-Relevant Complexes of Lanthanides with 5-Hydroxyflavone: DNA Binding and Protein Interaction Studies. Molecules 2016, 21, 1737. [Google Scholar] [CrossRef]
- Singh, V.; Singh, N.; Watanabe, S.; Rao, T.K.G.; Pathak, M.S.; Srivastava, A.K.; Singh, P.K.; Dhoble, S.J. Gadolinium-Activated CaZr4O9 Ultraviolet-B-Emitting Phosphor: A Luminescence and EPR Study. J. Electron. Mater. 2017, 46, 1943–1947. [Google Scholar] [CrossRef]
- Aminov, L.K.; Kurkin, I.N.; Malkin, B.Z. Superhyperfine structure in the EPR spectra and optical spectra of impurity f ions in dielectric crystals: A review. Phys. Solid State 2013, 55, 1343–1363. [Google Scholar] [CrossRef]
- Lu, D.-Y.; Guan, D.-X. Photoluminescence associated with the site occupations of Ho3+ ions in BaTiO3. Sci. Rep. 2017, 7, 6125. [Google Scholar] [CrossRef]
- Pan, M.J.; Randall, C.A. A brief introduction to ceramic capacitors. Electr. Insul. M. 2010, 26, 44. [Google Scholar] [CrossRef]
- Tavernor, A.W.; Lia, H.-P.S.; Stevens, R. Production and characterisation of composite relaxor ferroelectric multi-layer structures. J. Eur. Ceram. Soc. 1999, 19, 1859. [Google Scholar] [CrossRef]
- Furukawa, O.; Harata, M.; Imai, M.; Yamashita, Y.; Mukaeda, S. Low firing and high dielectric constant X7R ceramic dielectric for multilayer capacitors based on relaxor and barium titanate composite. J. Mater. Sci. 1991, 26, 5838. [Google Scholar] [CrossRef]
- Hino, T.; Matsumoto, N.; Nishida, M.; Araki, T. PLD of X7R for thin film capacitors. Appl. Surf. Sci. 2008, 254, 2638. [Google Scholar] [CrossRef]
- Lu, D.Y.; Peng, Y.Y.; Yu, X.Y.; Sun, X.Y. Dielectric properties and defect chemistry of La and Tb co-doped BaTiO3 ceramics. J. Alloys Compd. 2016, 681, 128–138. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Q.; Lv, J.-H.; Zhao, X.; Wang, C.-M. Dielectric properties and electrocaloric effect of yttrium-modified BaTiO3 ceramics. Ceram. Int. 2021, 47, 18610–18618. [Google Scholar] [CrossRef]
- Lu, D.Y.; Liang, Y. Valence states and dielectric properties of fine-grained BaTiO3 ceramics co-doped with double valence-variable europium and chromium. Ceram. Int. 2018, 44, 14717–14727. [Google Scholar] [CrossRef]
- Liu, M.; Hao, H.; Zhen, Y.; Wang, T.; Zhou, D.; Liu, H.; Cao, M.; Yao, Z. Temperature stability of dielectric properties for xBiAlO3-(1−x)BaTiO3 ceramics. J. Eur. Ceram. Soc. 2015, 35, 2303–2311. [Google Scholar] [CrossRef]
- Turcan, I.; Lukacs, V.A.; Curecheriu, L.; Padurariu, L.; Ciomaga, C.E.; Airimioaei, M.; Stoian, G.; Lupu, N.; Mitoseriu, L. Microstructure and dielectric properties of Ag-BaTiO3 composite ceramics. J. Eur. Ceram. Soc. 2018, 38, 5420–5429. [Google Scholar] [CrossRef]
- Li, J.; Pu, Y.; Shi, Y.; Shi, R.; Wang, X.; Yang, M.; Wang, W.; Guo, X.; Peng, X. Dielectric, multiferroic and magnetodielectric properties of (1-x)BaTiO3-xSr2CoMoO6 solid solution. Ceram. Int. 2019, 45, 16353–16360. [Google Scholar] [CrossRef]
- Haily, E.; Bih, L.; El Bouari, A.; Lahmar, A.; Elmarssi, M.; Manoun, B. Effect of BaO-Bi2O3-P2O5 glass additive on structural, dielectric and energy storage properties of BaTiO3 ceramics. Mater. Chem. Phys. 2020, 241, 123034. [Google Scholar] [CrossRef]
- Fan, T.; Ji, C.; Chen, G.; Cai, W.; Gao, R.; Deng, X.; Wang, Z.; Fu, C. Enhanced the dielectric relaxation characteristics of BaTiO3 ceramic doped by BiFeO3 and synthesized by the microwave sintering method. Mater. Chem. Phys. 2020, 250, 123034. [Google Scholar] [CrossRef]
- Wan, Y.; Tang, L.; Dang, X.; Ren, P.; Ma, M.; Song, K.; Zhao, G. High temperature dielectrics based on Bi1/2Na1/2TiO3-BaTiO3-Sr0.53Ba0.47Nb2O6 ceramics with high dielectric permittivity and wide operational temperature range. Ceram. Int. 2019, 45, 2596–2601. [Google Scholar] [CrossRef]
- Chen, X.; Huang, G.; Ma, D.; Liu, G.; Zhou, H. High thermal stability and low dielectric loss of BaTiO3-Bi(Li1/3Zr2/3)O3 solid solution. Ceram. Int. 2017, 43, 926–929. [Google Scholar] [CrossRef]
- Ma, J.P.; Chen, X.M.; Ouyang, W.Q.; Wang, J.; Li, H.; Fang, J.-L. Microstructure, dielectric, and energy storage properties of BaTiO3 ceramics prepared via cold sintering. Ceram. Int. 2018, 44, 4436–4441. [Google Scholar] [CrossRef]
- Chen, W.; Hao, H.; Yang, Y.; Chen, C.; Appiah, M.; Yao, Z.; Cao, M.; Yu, Z.; Liu, H. Dielectric properties and impedance analysis of BaTiO3 -based ceramics with core-shell structure. Ceram. Int. 2017, 43, 8449–8458. [Google Scholar] [CrossRef]
- Meng, K.; Li, W.; Tang, X.G.; Liu, Q.X.; Jiang, Y.P. A review of a good binary ferroelectric ceramic: BaTiO3–BiFeO3. ACS Appl. Electron. Mater. 2021, 4, 2109–2145. [Google Scholar] [CrossRef]
- Ghayour, H.; Abdellahi, M. A brief review of the effect of grain size variation on the electrical properties of BaTiO3-based ceramics. Powder Technol. 2016, 292, 84–93. [Google Scholar] [CrossRef]
- Buscaglia, V.; Randall, C.A. Size and scaling effects in barium titanate. An overview. J. Eur. Ceram. Soc. 2020, 40, 3744–3758. [Google Scholar] [CrossRef]
- Freeman, C.L.; Dawson, J.A.; Harding, J.H.; Ben, L.B.; Sinclair, D.C. The Influence of A-Site Rare Earth Ion Size in Controlling the Curie Temperature of Ba1−xRExTi1−x/4O3. Adv. Funct. Mater. 2013, 23, 491–495. [Google Scholar] [CrossRef]
- Khedhri, M.H.; Abdelmoula, N.; Khemakhem, H.; Douali, R.; Dubois, F. Structural, spectroscopic and dielectric properties of Ca-doped BaTiO3. Appl. Phys. A 2019, 125, 1–13. [Google Scholar] [CrossRef]
- Martirenat, H.T.; Burfoot, J.C. Grain-size effects on properties of some ferroelectric ceramics. J. Phys. C Solid State Phys. 1974, 7, 3182–3192. [Google Scholar] [CrossRef]
- Zhang, Q.W.; Zhai, J.W.; Shen, B.; Zhang, H.J.; Yao, X. Grain size effects on dielectric properties of barium strontium titanate composite ceramics. Mater. Res. Bull. 2013, 48, 973–977. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bai, J.; Liu, Q.; Li, X.; Wei, X.; Li, L. Optimization of Sintering Conditions to Enhance the Dielectric Performance of Gd3+ and Ho3+ Codoped BaTiO3 Ceramics. Molecules 2022, 27, 7464. https://doi.org/10.3390/molecules27217464
Bai J, Liu Q, Li X, Wei X, Li L. Optimization of Sintering Conditions to Enhance the Dielectric Performance of Gd3+ and Ho3+ Codoped BaTiO3 Ceramics. Molecules. 2022; 27(21):7464. https://doi.org/10.3390/molecules27217464
Chicago/Turabian StyleBai, Jianghui, Qiaoli Liu, Xia Li, Xin Wei, and Liping Li. 2022. "Optimization of Sintering Conditions to Enhance the Dielectric Performance of Gd3+ and Ho3+ Codoped BaTiO3 Ceramics" Molecules 27, no. 21: 7464. https://doi.org/10.3390/molecules27217464
APA StyleBai, J., Liu, Q., Li, X., Wei, X., & Li, L. (2022). Optimization of Sintering Conditions to Enhance the Dielectric Performance of Gd3+ and Ho3+ Codoped BaTiO3 Ceramics. Molecules, 27(21), 7464. https://doi.org/10.3390/molecules27217464







