A Ferrofluid with Surface Modified Nanoparticles for Magnetic Hyperthermia and High ROS Production
Abstract
:1. Introduction
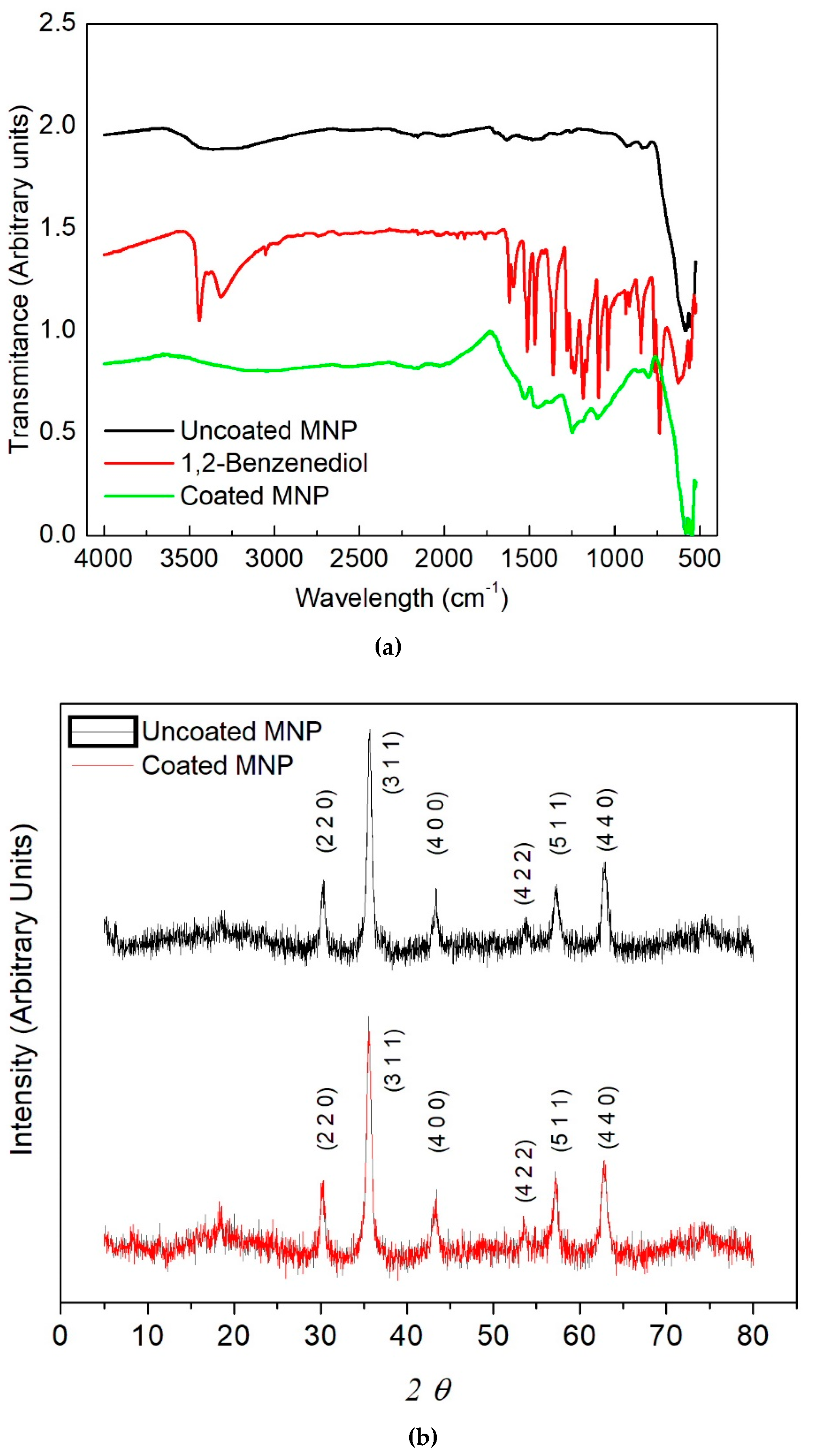
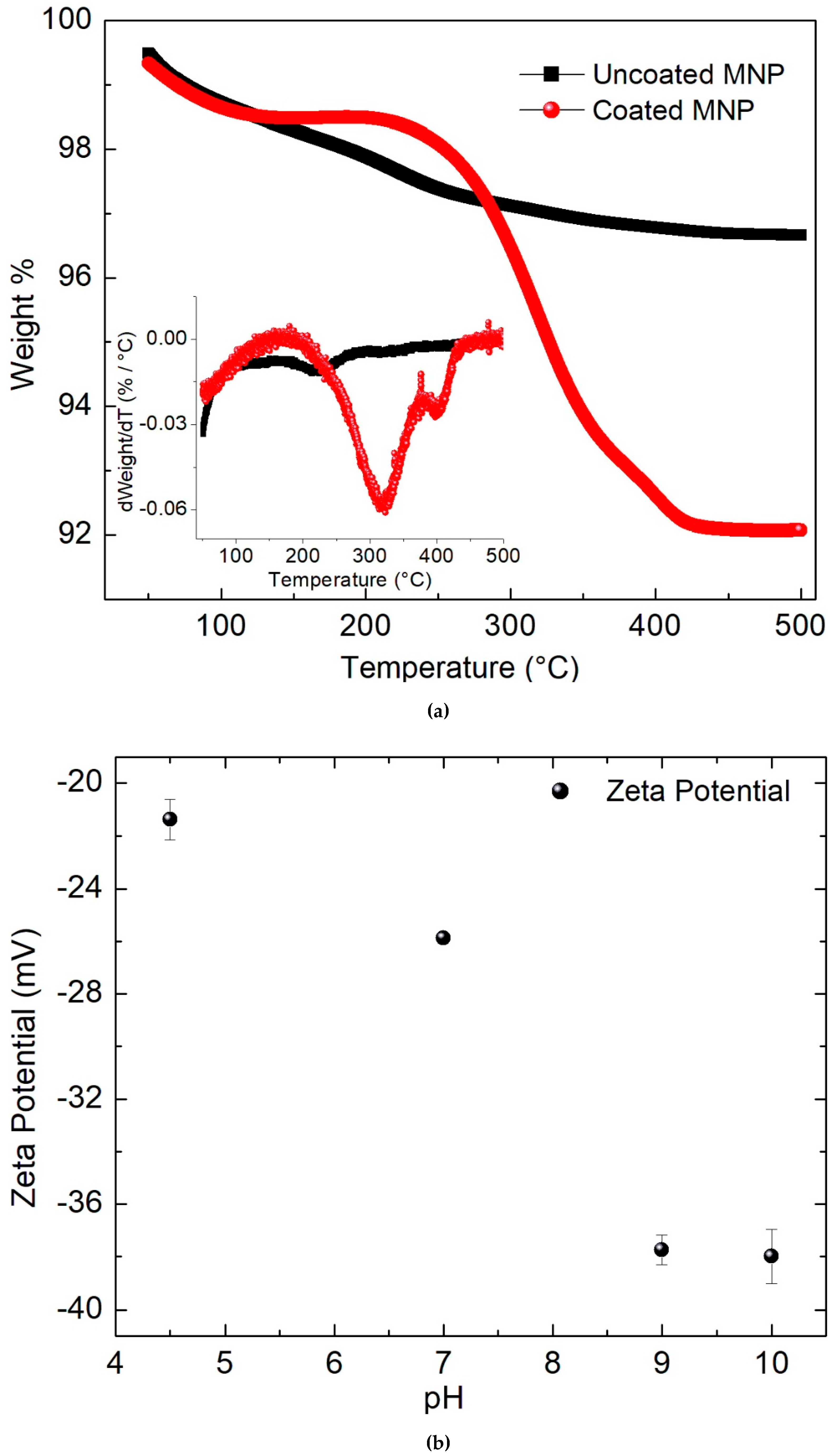
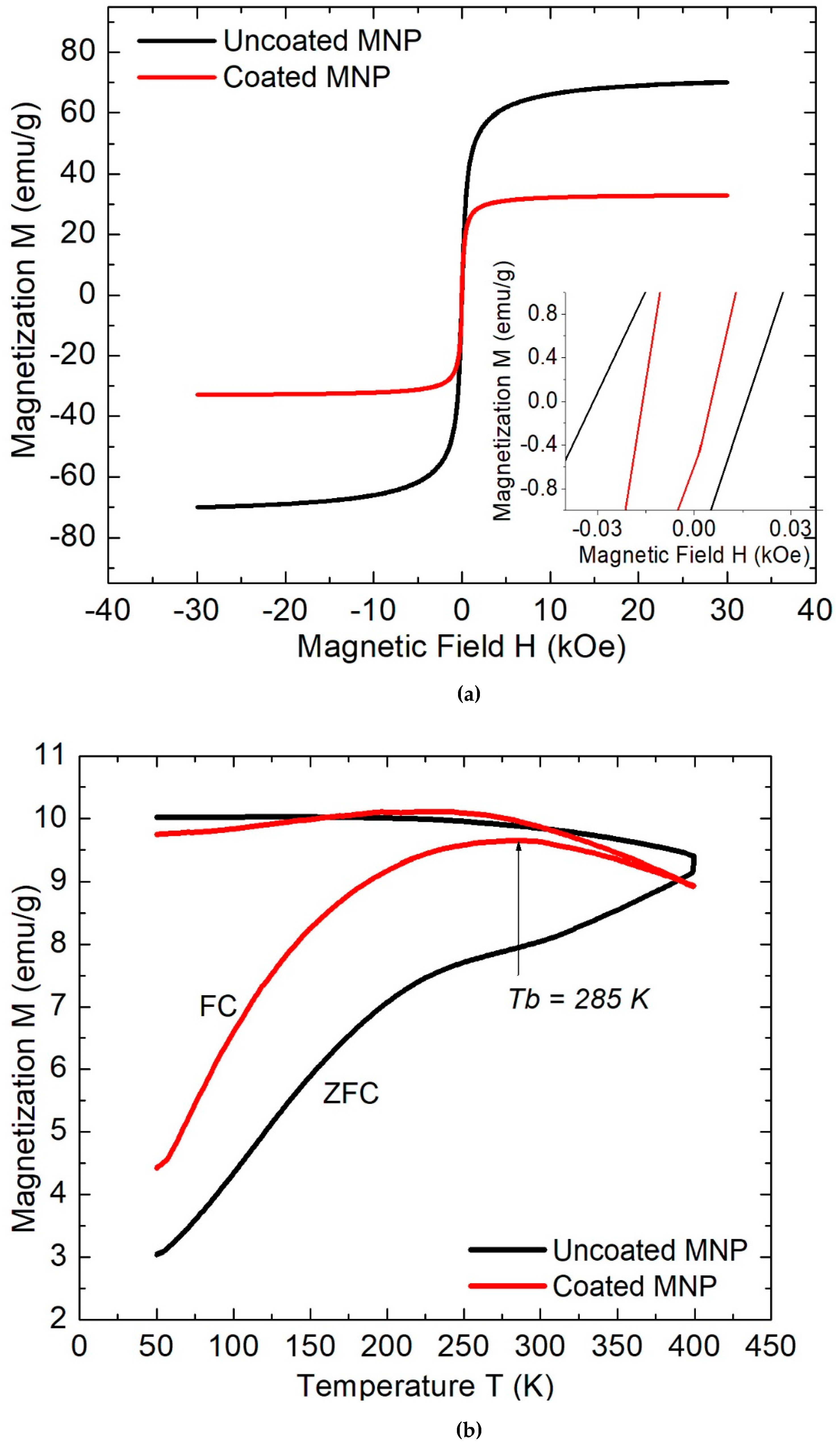
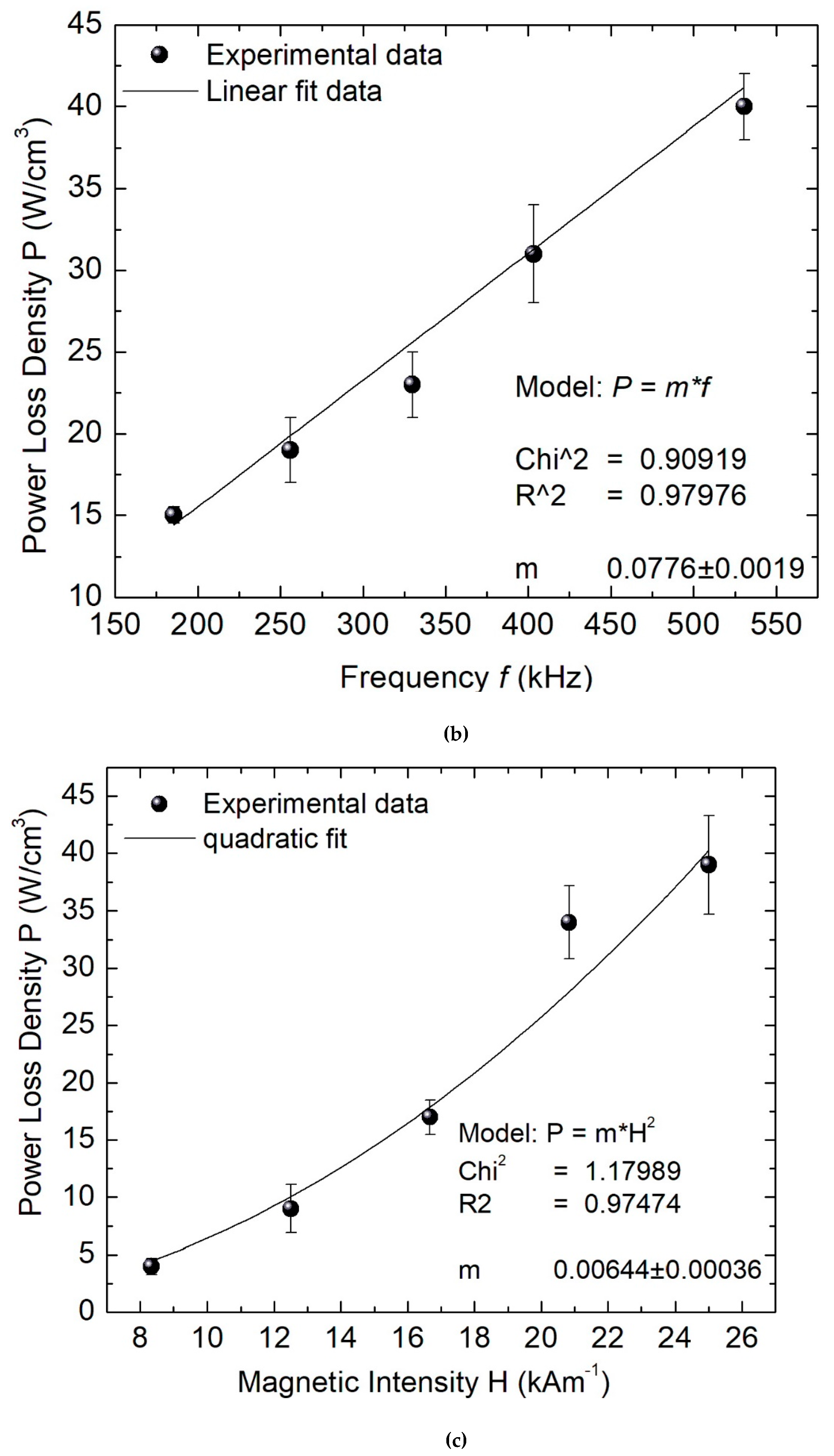
2. Results and Discussion
3. Materials and Methods
3.1. Synthesis of MNPs and Their Coating Procedure
3.2. Physicochemical Characterization
3.3. Oxidative Capability Analysis of the Catechol-Coated MNPs Ferrofluid
3.4. Cytotoxicity Analysis and Hyperthermia Assays of the Ferrofluid with CMNP
3.5. Qualitative Measurement of ROS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Pozo-Torres, E.; Caro, C.; Avasthi, A.; Páez-Muñoz, J.M.; García-Martín, M.L.; Fernández, I.; Leal, M.P. Clickable iron oxide NPs based on catechol derived ligands: Synthesis and characterization. Soft Matter 2020, 16, 3257–3266. [Google Scholar] [CrossRef]
- Woo, S.; Kim, S.; Kim, H.; Cheon, Y.W.; Yoon, S.; Oh, J.H.; Park, J. Charge-Modulated Synthesis of Highly Stable Iron Oxide Nanoparticles for In Vitro and In Vivo Toxicity Evaluation. Nanomaterials 2021, 11, 3068. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.; Zheng, X.; Cheng, H.; Rao, M.; Chen, K.; Xia, J.; Lin, L.; Zhu, H. Mn-Fe3O4 nanoparticles anchored on the urushiol functionalized 3D-graphene for the electrochemical detection of 4-nitrophenol. J. Hazard. Mater. 2021, 409, 124926. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.; Yu, H.; Wang, L.; Wang, N.; Amin, B.U. NIR Light-Triggered Shape Memory Polymers Based on Mussel-Inspired Iron–Catechol Complexes. Adv. Funct. Mater. 2021, 31, 2102621. [Google Scholar] [CrossRef]
- Wu, Y.; Lu, Z.; Li, Y.; Yang, J.; Zhang, X. Surface modification of iron oxide-based magnetic nanoparticles for cerebral theranostics: Application and prospection. Nanomaterials 2020, 10, 1441. [Google Scholar] [CrossRef] [PubMed]
- Negm, N.A.; Abubshait, H.A.; Abubshait, S.A.; Abou Kana, M.T.; Mohamed, E.A.; Betiha, M.M. Performance of chitosan polymer as platform during sensors fabrication and sensing applications. Int. J. Biol. Macromol. 2020, 165, 402–435. [Google Scholar] [CrossRef]
- Kim, J.; Lee, C.; Ryu, J.H. Adhesive Catechol-Conjugated Hyaluronic Acid for Biomedical Applications: A Mini Review. Appl. Sci. 2021, 11, 21. [Google Scholar] [CrossRef]
- Park, K.; Kim, T.; Park, J.; Yan, X.; Kim, H. Development of a carbamate-conjugated catechol ligand and its application to Cs extraction from contaminated soil by using supercritical CO2. Chemosphere 2020, 242, 125210. [Google Scholar] [CrossRef] [PubMed]
- Smolyaninov, I.V.; Pitikova, O.V.; Korchagina, E.O.; Poddel’sky, A.I.; Fukin, G.K.; Luzhnova, S.A.; Tichkomirov, A.M.; Ponomareva, E.N.; Berberova, N.T. Catechol thioethers with physiologically active fragments: Electrochemistry, antioxidant and cryoprotective activities. Bioorganic Chem. 2019, 89, 103003. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, C.; Yang, X.; Pu, K. Photoactivatable protherapeutic nanomedicine for cancer. Adv. Mater. 2020, 32, 2002661. [Google Scholar] [CrossRef] [PubMed]
- Wan, W.L.; Tian, B.; Lin, Y.J.; Korupalli, C.; Lu, M.Y.; Cui, Q.; Wan, D.; Chang, Y.; Sung, H.W. Photosynthesis-inspired H 2 generation using a chlorophyll-loaded liposomal nanoplatform to detect and scavenge excess ROS. Nat. Commun. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Umeno, A.; Biju, V.; Yoshida, Y. In vivo ROS production and use of oxidative stress-derived biomarkers to detect the onset of diseases such as Alzheimer’s disease, Parkinson’s disease, and diabetes. Free Radic. Res. 2017, 51, 413–414. [Google Scholar] [CrossRef] [PubMed]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef] [PubMed]
- Gu, X.; Zhang, Y.; Sun, H.; Song, X.; Fu, C.; Dong, P. Mussel-inspired polydopamine coated iron oxide nanoparticles for biomedical application. J. Nanomater. 2015, 2015, 3. [Google Scholar] [CrossRef]
- Yu, R.; Zou, Y.; Liu, B.; Guo, Y.; Wang, X.; Han, M. Surface modification of pH-sensitive honokiol nanoparticles based on dopamine coating for targeted therapy of breast cancer. Colloids Surf. B Biointerfaces 2019, 177, 1–10. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, D.; Zeng, Y.; Wu, L.; Liu, X.; Liu, J. Nanocluster of superparamagnetic iron oxide nanoparticles coated with poly (dopamine) for magnetic field-targeting, highly sensitive MRI and photothermal cancer therapy. Nanotechnology 2015, 26, 115102. [Google Scholar] [CrossRef] [PubMed]
- Cervantes, O.; Casillas, N.; Knauth, P.; Lopez, Z.; Virgen-Ortiz, A.; Lozano, O.; Delgado-Enciso, I.; Sámano, A.H.; Rosales, S.; Martinez-Ceseña, L.; et al. An easily prepared ferrofluid with high power absorption density and low cytotoxicity for biomedical applications. Mater. Chem. Phys. 2020, 245, 122752. [Google Scholar] [CrossRef]
- Mai, T.; Hilt, J.Z. Functionalization of iron oxide nanoparticles with small molecules and the impact on reactive oxygen species generation for potential cancer therapy. Colloids Surf. A Physicochem. Eng. Asp. 2019, 576, 9–14. [Google Scholar] [CrossRef]
- Rosensweig, R.E. Heating magnetic fluid with alternating magnetic field. J. Magn. Magn. Mater. 2002, 252, 370–374. [Google Scholar] [CrossRef]
- Sámano, A.H.; Rosales, S.; Mazon, E.E.; Casillas, N.; Topete, A.; Paz, J.A.; Quintero, L.H.; Estrada, J.C.; Cano, M.E. A dynamic hysteresis meter for studying ferrofluids designed for magnetic hyperthermia. Meas. Sci. Technol. 2020, 31, 055902. [Google Scholar] [CrossRef]
- Elbeshir, E.I. On the gum arabic to improve the thermal properties of Fe3O4 nanoparticles. AIP Adv. 2021, 11, 045224. [Google Scholar] [CrossRef]
- Bhargava, D.; Rattanadecho, P.; Wessapan, T. The effect of metal objects on the SAR and temperature increase in the human head exposed to dipole antenna (numerical analysis). Case Stud. Therm. Eng. 2020, 22, 100789. [Google Scholar] [CrossRef]
- Hua, Y.; Wang, C.; Wang, S.; Xiao, J. Poly (catechol) modified Fe3O4 magnetic nanocomposites with continuous high Fenton activity for organic degradation at neutral pH. Environ. Sci. Pollut. Res. 2021, 28, 62690–62702. [Google Scholar] [CrossRef] [PubMed]
- Riaz, S.; Bashir, M.; Naseem, S. Iron oxide nanoparticles prepared by modified co-precipitation method. IEEE Trans. Magn. 2013, 50, 1–4. [Google Scholar] [CrossRef]
- Krishnan, K.M. Biomedical nanomagnetics: A spin through possibilities in imaging, diagnostics, and therapy. IEEE Trans. Magn. 2010, 46, 2523–2558. [Google Scholar] [CrossRef] [Green Version]
- Bohara, R.A.; Thorat, N.D.; Pawar, S.H. Role of functionalization: Strategies to explore potential nano-bio applications of magnetic nanoparticles. RSC Adv. 2016, 6, 43989–44012. [Google Scholar] [CrossRef]
- Samrot, A.V.; Sahithya, C.S.; Selvarani, J.; Purayil, S.K.; Ponnaiah, P. A review on synthesis, characterization and potential biological applications of superparamagnetic iron oxide nanoparticles. Curr. Res. Green Sustain. Chem. 2021, 4, 100042. [Google Scholar] [CrossRef]
- Kusigerski, V.; Illes, E.; Blanusa, J.; Gyergyek, S.; Boskovic, M.; Perovic, M.; Spasojevic, V. Magnetic properties and heating efficacy of magnesium doped magnetite nanoparticles obtained by co-precipitation method. J. Magn. Magn. Mater. 2019, 475, 470–478. [Google Scholar] [CrossRef]
- Mazario, E.; Sánchez-Marcos, J.; Menéndez, N.; Herrasti, P.; García-Hernández, M.; Muñoz-Bonilla, A. One-pot electrochemical synthesis of polydopamine coated magnetite nanoparticles. RSC Adv. 2014, 4, 48353–48361. [Google Scholar] [CrossRef]
- Scheibe, B.; Mrówczyński, R.; Michalak, N.; Załęski, K.; Matczak, M.; Kempiński, M.; Pietralik, Z.; Lewandowski, M.; Jurga, S.; Stobiecki, F. Anchoring Fe3O4 nanoparticles in a reduced graphene oxide aerogel matrix via polydopamine coating. Beilstein J. Nanotechnol. 2018, 9, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Wildeboer, R.R.; Southern, P.; Pankhurst, Q.A. On the reliable measurement of specific absorption rates and intrinsic loss parameters in magnetic hyperthermia materials. J. Phys. D Appl. Phys. 2014, 47, 495003. [Google Scholar] [CrossRef]
- Rosales, S.; Casillas, N.; Topete, A.; Cervantes, O.; González, G.; Paz, J.A.; Cano, M.E. Evaluating physical changes of iron oxide nanoparticles due to surface modification with oleic acid. Chin. Phys. B 2020, 29, 100502. [Google Scholar] [CrossRef]
- Thomas, R.G.; Moon, M.J.; Lee, H.; Sasikala, A.R.; Kim, C.S.; Park, I.K.; Jeong, Y.Y. Hyaluronic acid conjugated superparamagnetic iron oxide nanoparticle for cancer diagnosis and hyperthermia therapy. Carbohydr. Polym. 2015, 131, 439–446. [Google Scholar] [CrossRef]
- Lu, H.F.; Chen, H.F.; Kao, C.L.; Chao, I.; Chen, H.Y. A computational study of the Fenton reaction in different pH ranges. Phys. Chem. Chem. Phys. 2018, 20, 22890–22901. [Google Scholar] [CrossRef] [PubMed]
- Melin, V.; Henríquez, A.; Freer, J.; Contreras, D. Reactivity of catecholamine-driven Fenton reaction and its relationships with iron (III) speciation. Redox Rep. 2015, 20, 89–96. [Google Scholar] [CrossRef]
- Laffon, B.; Fernández Bertólez, N.; Costa, C.; Brandão, F.; Teixera, J.P.; Pásaro, E.; Valdiglesias, V. Cellular and Molecular Toxicity of Iron Oxide Nanoparticles. In Cellular and Molecular Toxicology of Nanoparticles; Saquib, Q., Faisal, M., Al-Khedhairy, A.A., Alatar, A.A., Eds.; Springer: Cham, Switzerland, 2018; Volume 1048, pp. 199–213. [Google Scholar] [CrossRef]
- Mazon, E.E.; Villa-Martínez, E.; Hernández-Sámano, A.; Córdova-Fraga, T.; Ibarra-Sánchez, J.J.; Calleja, H.A.; Leyva Cruz, J.A.; Barrera, A.; Estrada, J.C.; Paz, J.A.; et al. A high-resolution frequency variable experimental setup for studying ferrofluids used in magnetic hyperthermia. Rev. Sci. Instrum. 2017, 88, 084705. [Google Scholar] [CrossRef]
- Wydra, R.J.; Rychahou, P.G.; Evers, B.M.; Anderson, K.W.; Dziubla, T.D.; Hilt, J.Z. The role of ROS generation from magnetic nanoparticles in an alternating magnetic field on cytotoxicity. Acta Biomater. 2015, 25, 284–290. [Google Scholar] [CrossRef] [Green Version]
- Prasad, N.K.; Rathinasamy, K.; Panda, D.; Bahadur, D. Mechanism of cell death induced by magnetic hyperthermia with nanoparticles of γ-Mn x Fe2−xO3 synthesized by a single step process. J. Mater. Chem. 2007, 17, 5042–5051. [Google Scholar] [CrossRef]
- Sadhukha, T.; Niu, L.; Wiedmann, T.S.; Panyam, J. Effective elimination of cancer stem cells by magnetic hyperthermia. Mol. Pharm. 2013, 10, 1432–1441. [Google Scholar] [CrossRef] [PubMed]
- Babincova, M.; Altanerovam, V.; Bergemann, C.; Babinec, P. In vitro analysis of cisplatin functionalized magnetic nanoparticles in combined cancer chemotherapy and electromagnetic hyperthermia. IEEE Trans. Nanobiosci. 2008, 7, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, M.A.; Alhadlaq, H.A.; Ahmad, J.; Al-Khedhairy, A.A.; Musarrat, J.; Ahamed, M. Copper oxide nanoparticles induced mitochondria mediated apoptosis in human hepatocarcinoma cells. PLoS ONE 2013, 8, e69534. [Google Scholar] [CrossRef] [PubMed] [Green Version]



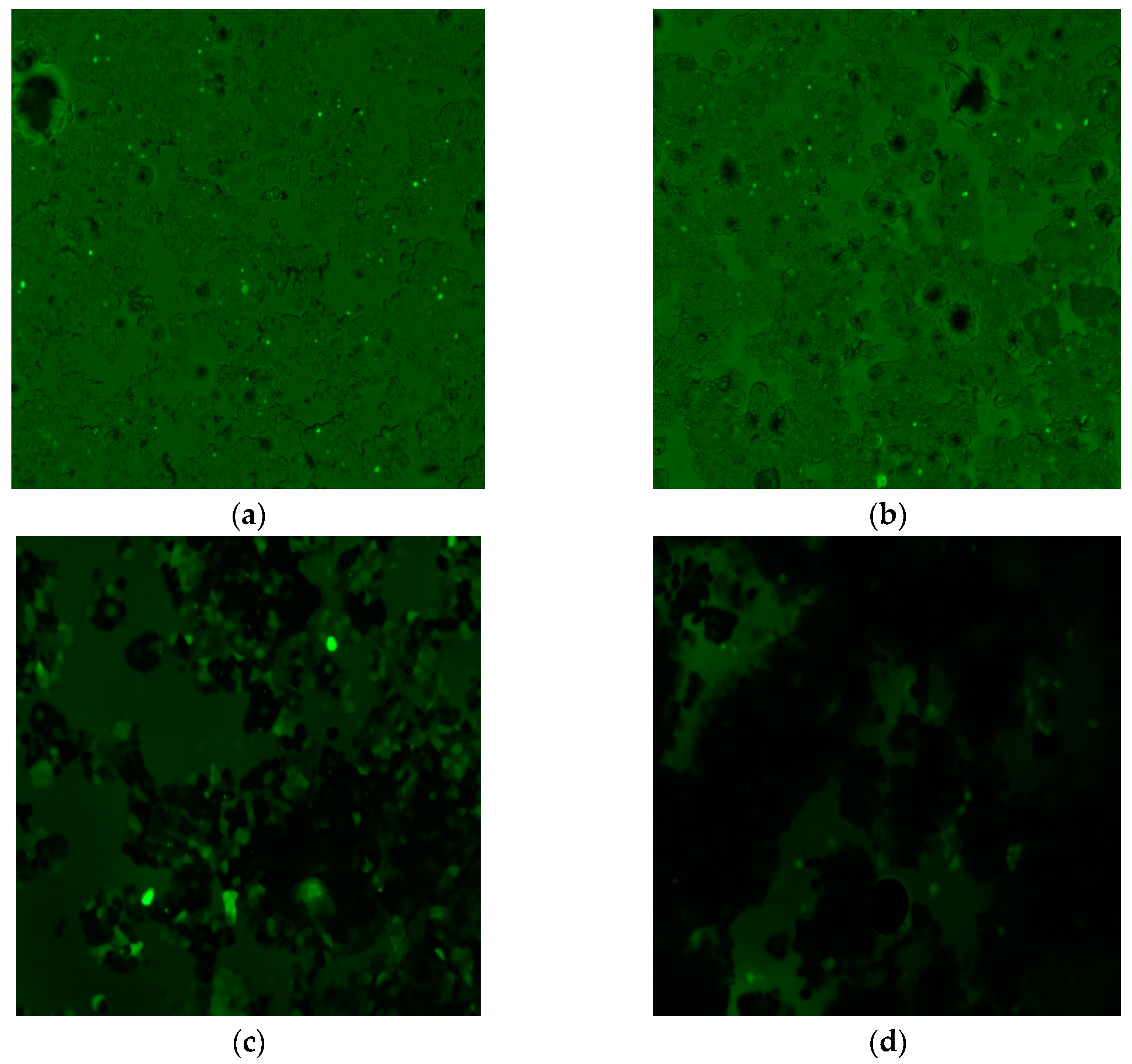
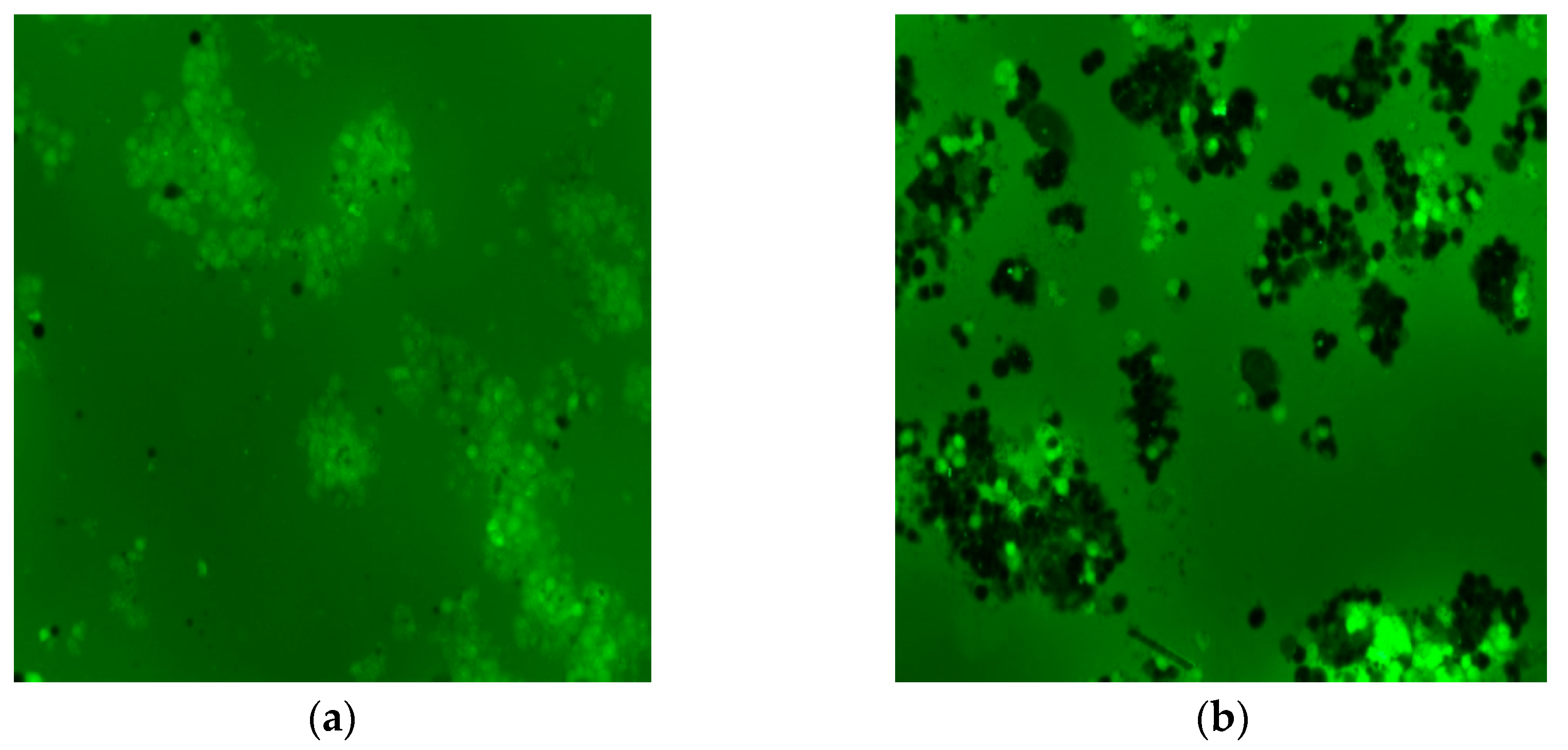

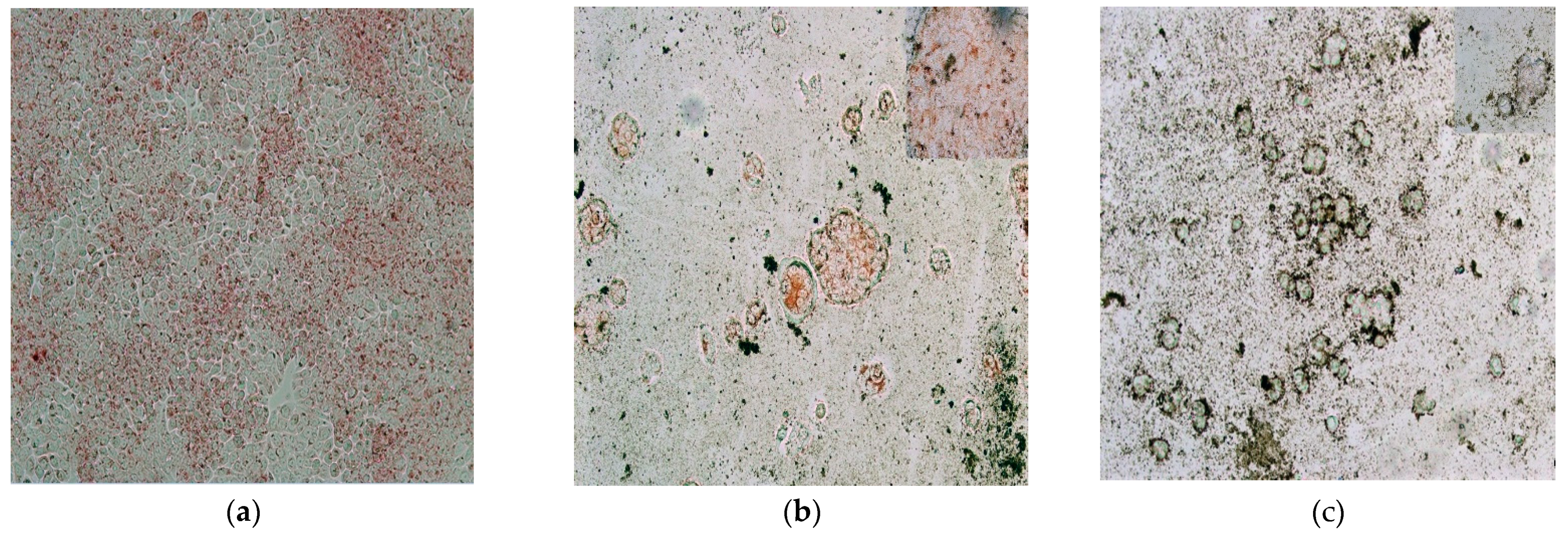
| Label | Content | ||||
|---|---|---|---|---|---|
| H2O | MB | H2O2 | UMNP | CMNP | |
| MBB | √ | √ | |||
| MB H2O2 | √ | √ | |||
| MB H2O2 UMNP | √ | √ | √ | √ | |
| MB H2O2 CMNP | √ | √ | √ | √ | |
| MB UMNP | √ | √ | √ | ||
| MB CMNP | √ | √ | √ |
| Label | Content | |||
|---|---|---|---|---|
| DCF | H2O2 | UMNP | CMNP | |
| NC | √ | |||
| PC | √ | √ | ||
| DCF H2O2 UMNP | √ | √ | √ | |
| DCF H2O2 CMNP | √ | √ | √ | |
| DCF UMNP | √ | √ | ||
| DCF CMNP | √ | √ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cervantes, O.; Lopez, Z.d.R.; Casillas, N.; Knauth, P.; Checa, N.; Cholico, F.A.; Hernandez-Gutiérrez, R.; Quintero, L.H.; Paz, J.A.; Cano, M.E. A Ferrofluid with Surface Modified Nanoparticles for Magnetic Hyperthermia and High ROS Production. Molecules 2022, 27, 544. https://doi.org/10.3390/molecules27020544
Cervantes O, Lopez ZdR, Casillas N, Knauth P, Checa N, Cholico FA, Hernandez-Gutiérrez R, Quintero LH, Paz JA, Cano ME. A Ferrofluid with Surface Modified Nanoparticles for Magnetic Hyperthermia and High ROS Production. Molecules. 2022; 27(2):544. https://doi.org/10.3390/molecules27020544
Chicago/Turabian StyleCervantes, Oscar, Zaira del Rocio Lopez, Norberto Casillas, Peter Knauth, Nayeli Checa, Francisco Apolinar Cholico, Rodolfo Hernandez-Gutiérrez, Luis Hector Quintero, Jose Avila Paz, and Mario Eduardo Cano. 2022. "A Ferrofluid with Surface Modified Nanoparticles for Magnetic Hyperthermia and High ROS Production" Molecules 27, no. 2: 544. https://doi.org/10.3390/molecules27020544
APA StyleCervantes, O., Lopez, Z. d. R., Casillas, N., Knauth, P., Checa, N., Cholico, F. A., Hernandez-Gutiérrez, R., Quintero, L. H., Paz, J. A., & Cano, M. E. (2022). A Ferrofluid with Surface Modified Nanoparticles for Magnetic Hyperthermia and High ROS Production. Molecules, 27(2), 544. https://doi.org/10.3390/molecules27020544








