Panchromatic Absorbers Tethered for Bioconjugation or Surface Attachment
Abstract
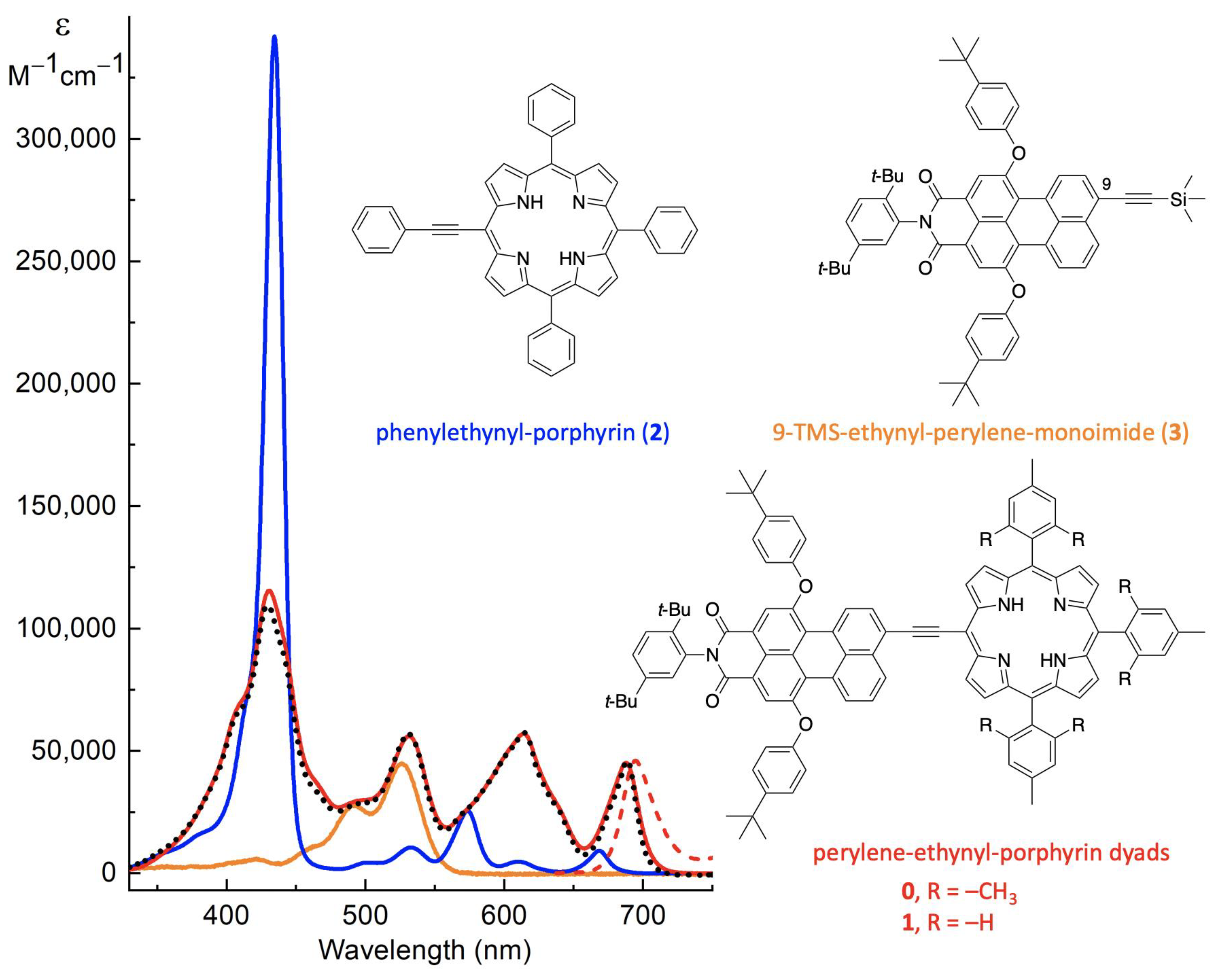
1. Introduction
2. Results and Discussion
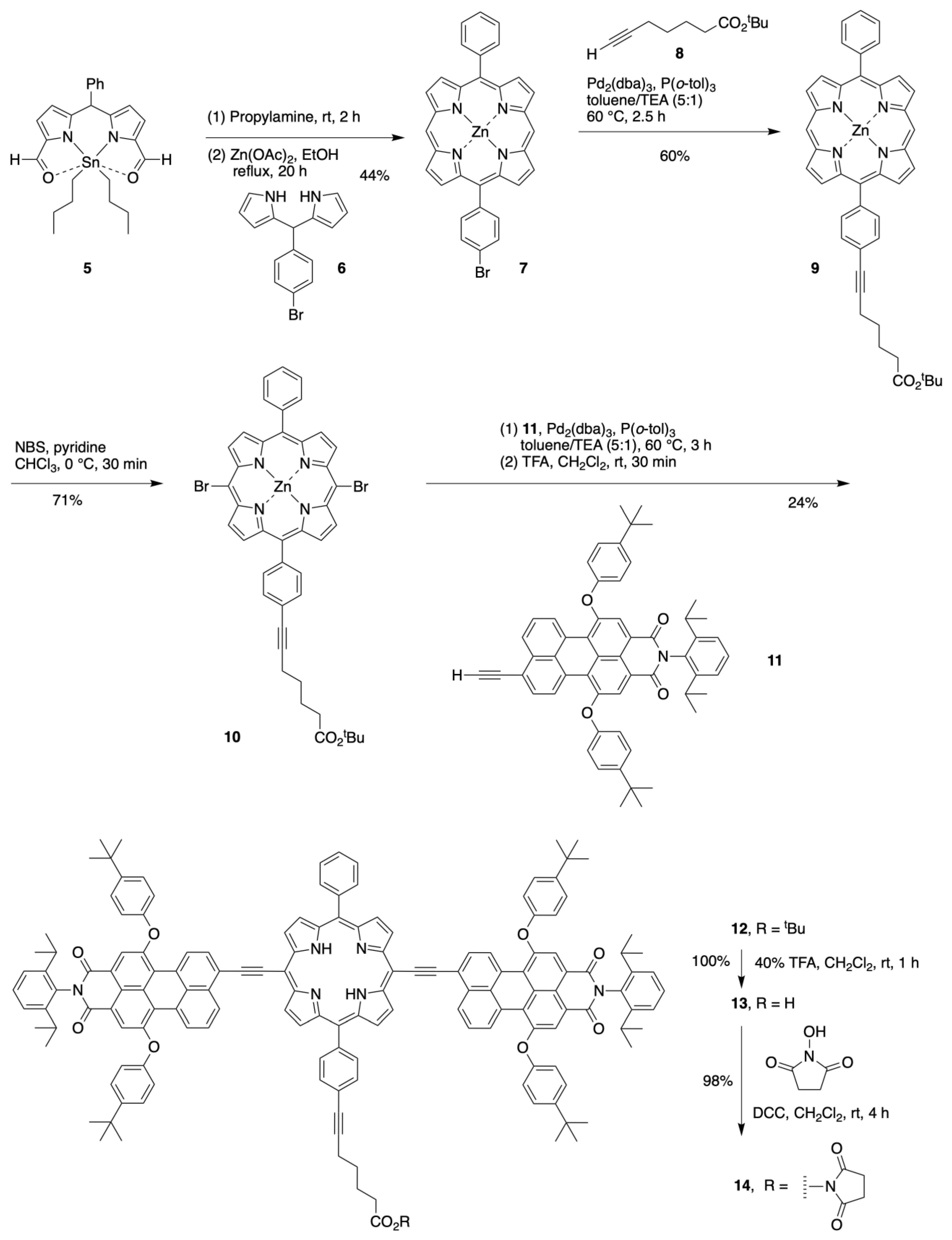
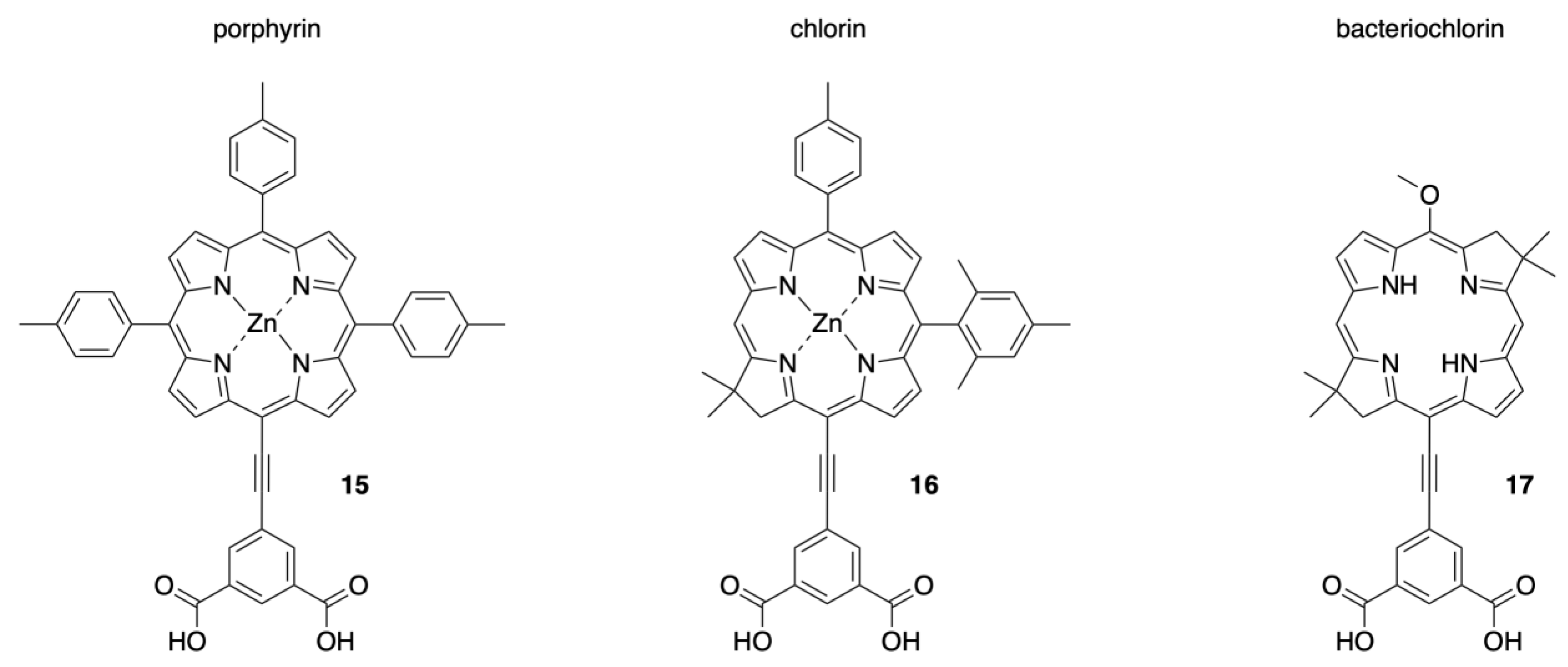
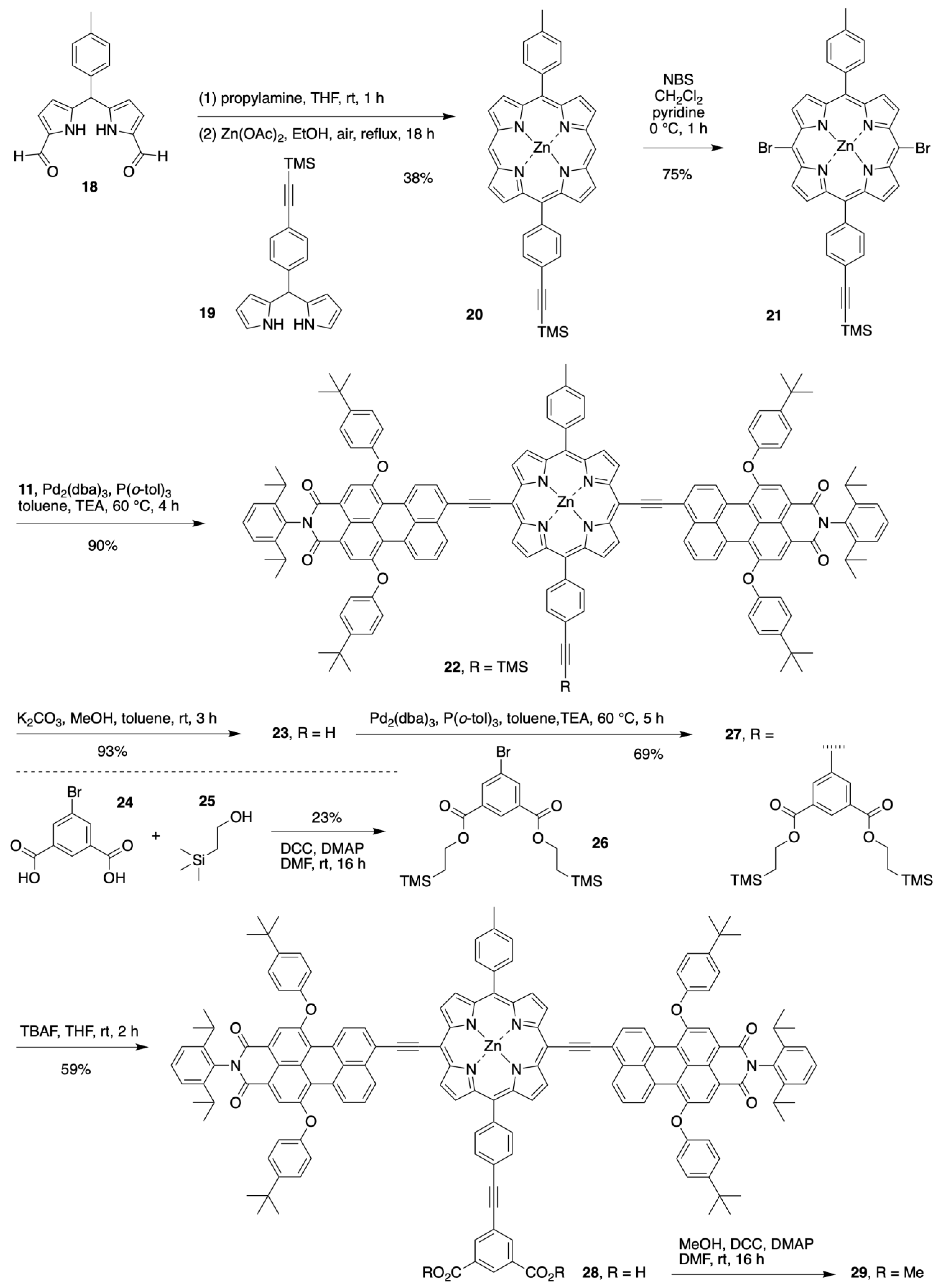
2.1. Synthesis of a Bioconjugatable Panchromatic Triad
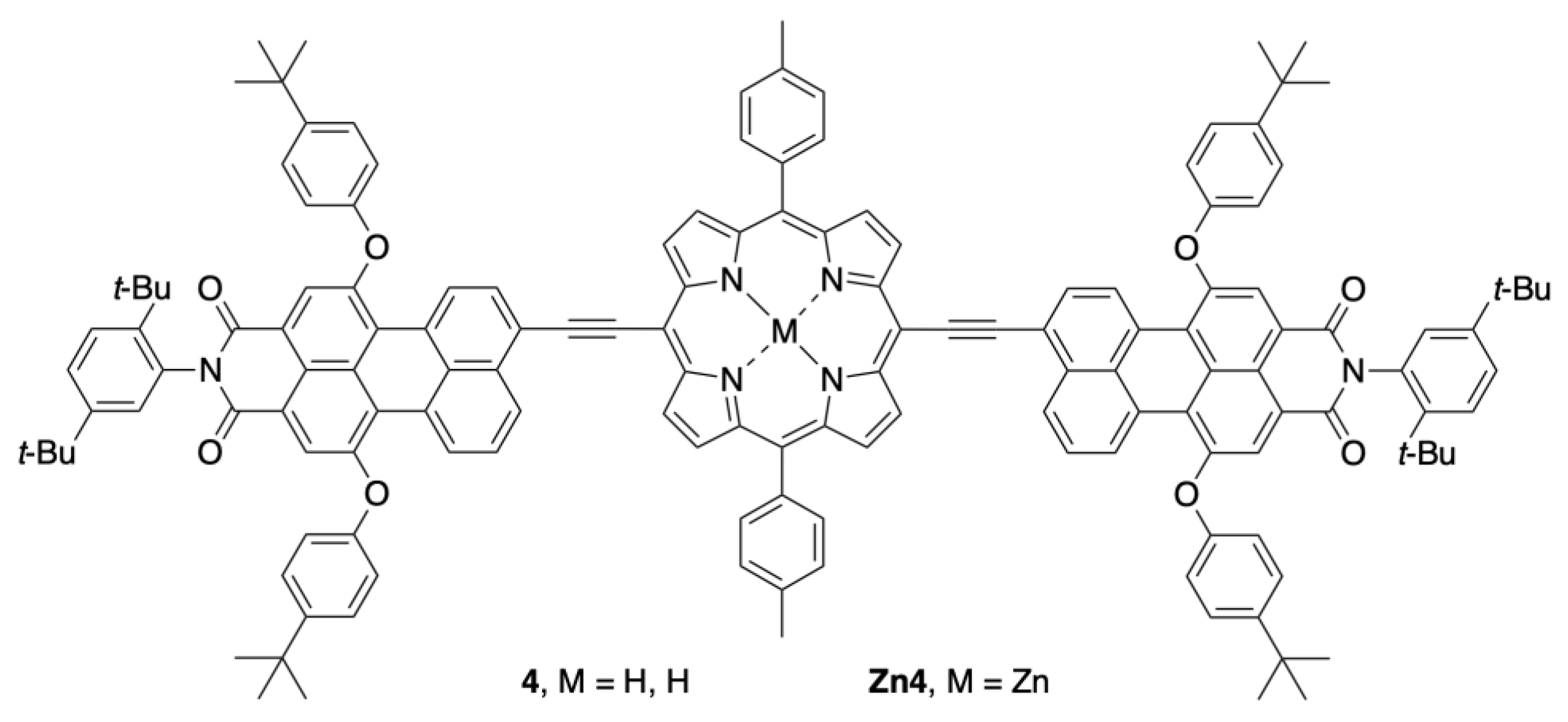
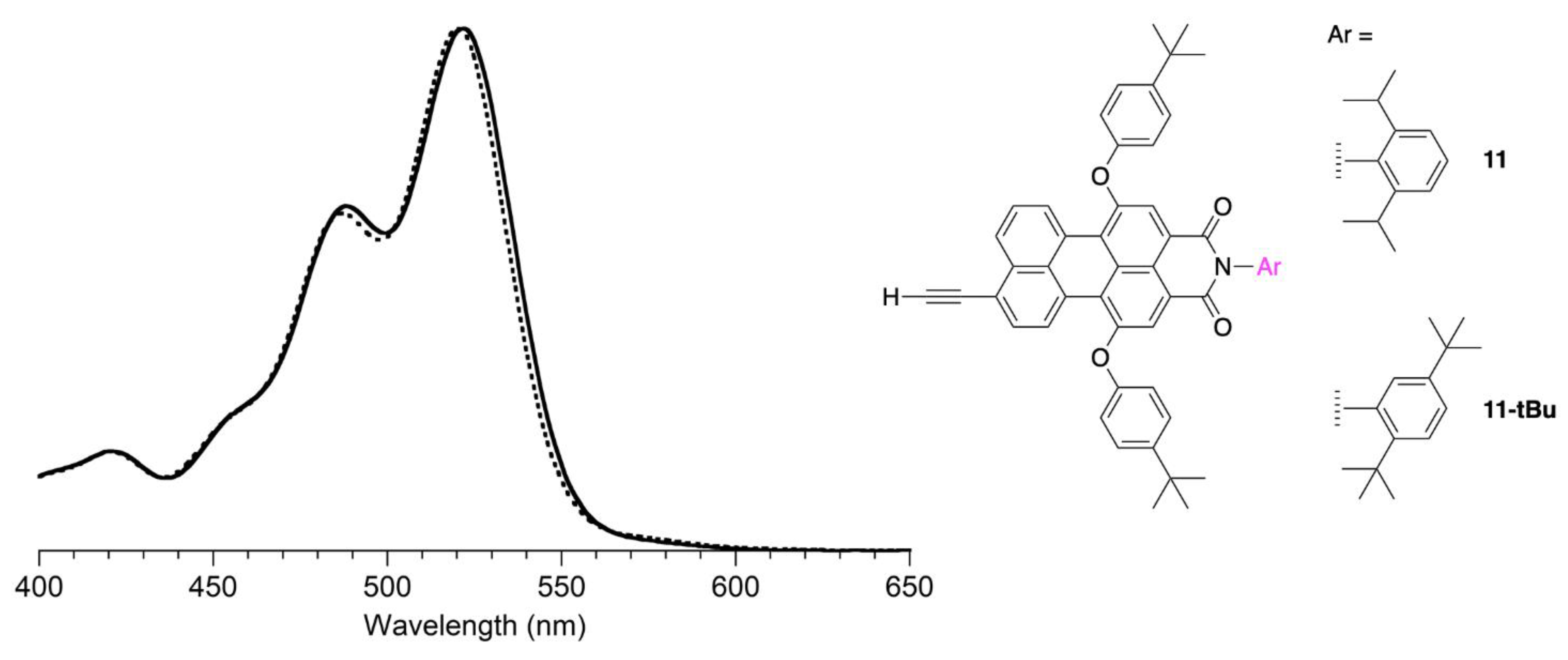
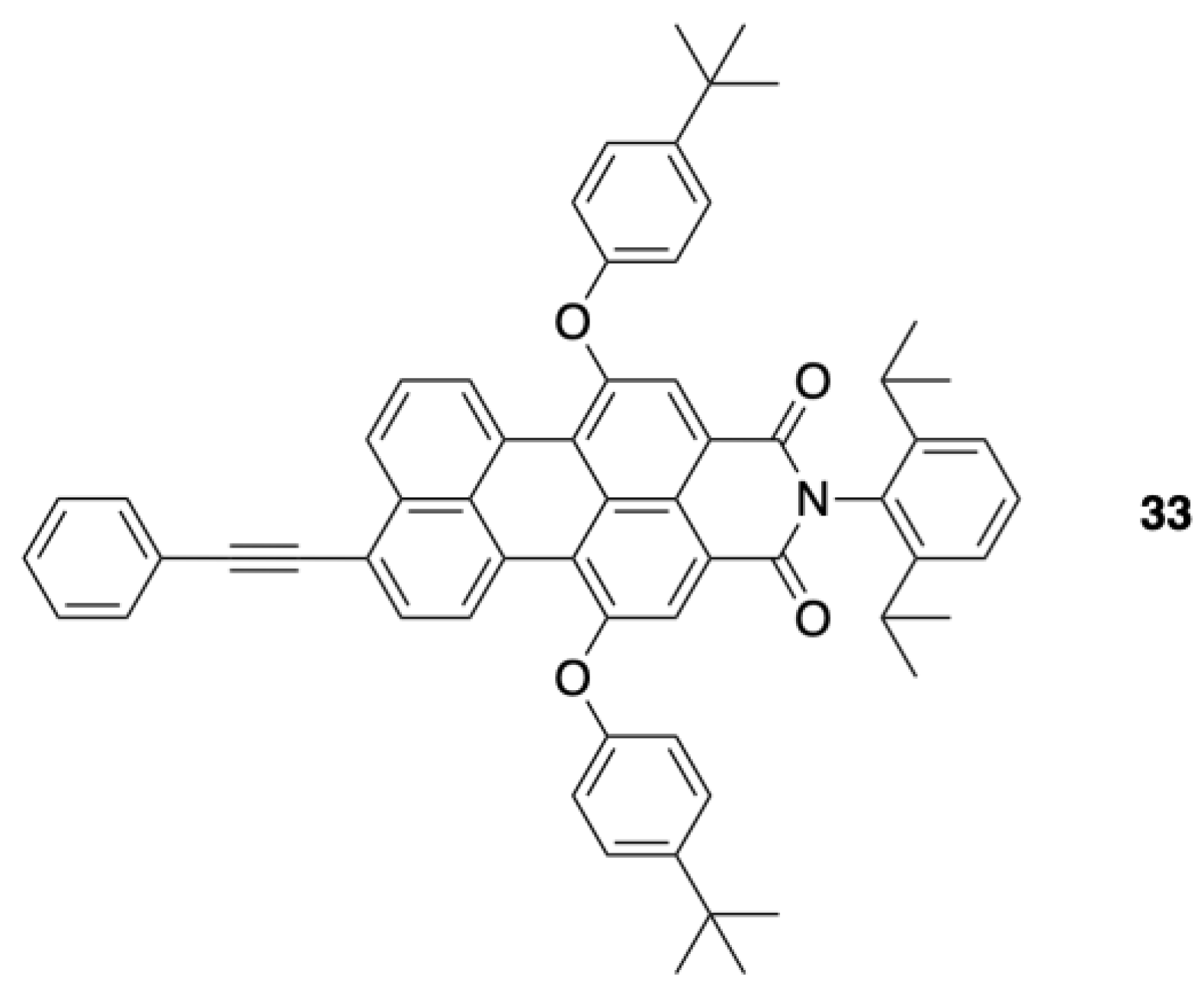
2.2. Synthesis of a Panchromatic Triad for Surface Attachment
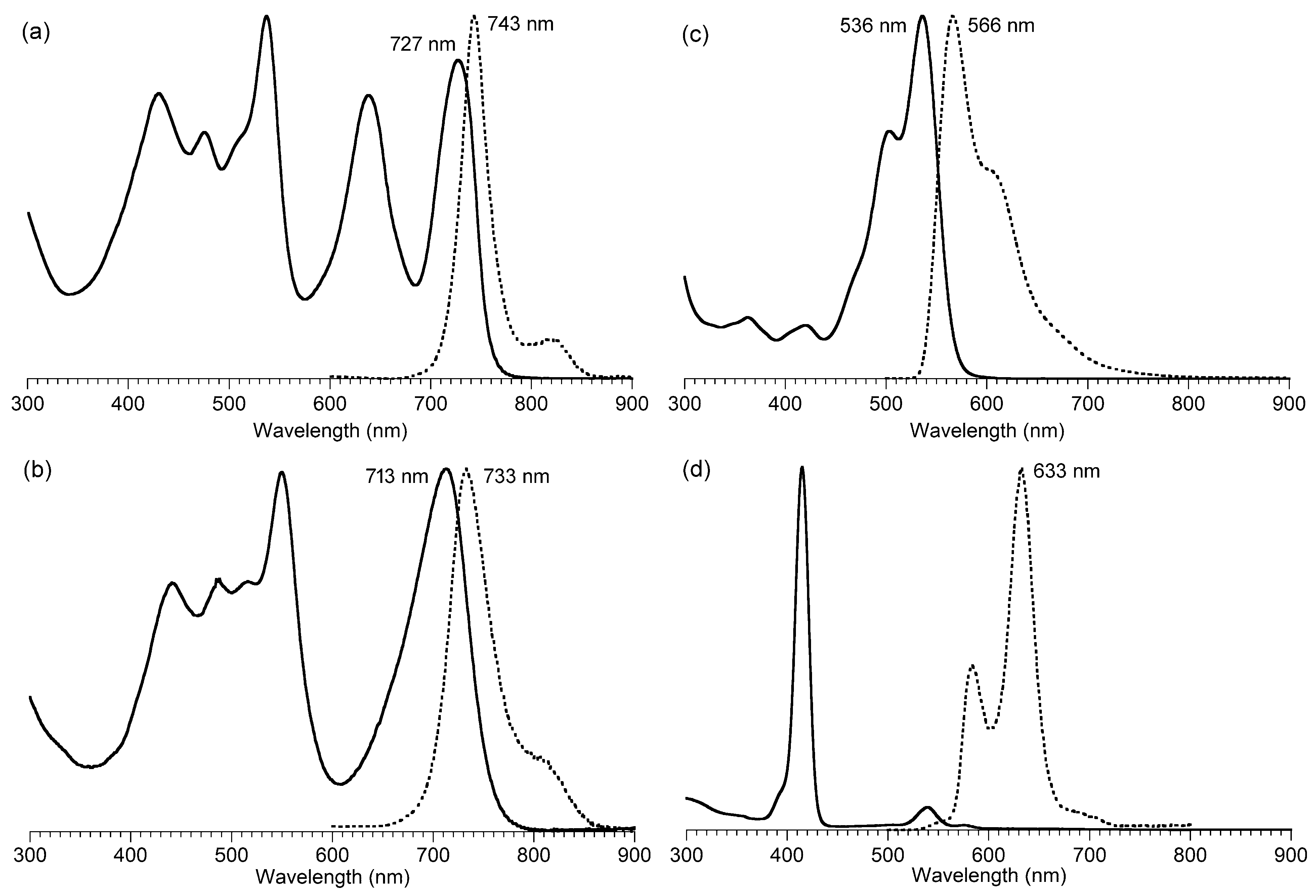
2.3. Chemical Characterization
3. Materials and Methods
3.1. General Methods
3.2. Purification Following Sonogashira Coupling Reactions
3.3. Synthesis and Characterization
3.4. Fluorescence Yield Determinations
4. Outlook
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Scheer, H. An Overview of Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications. In Chlorophylls and Bacteriochlorophylls: Biochemistry, Biophysics, Functions and Applications; Scheer, H., Grimm, B., Porra, R.J., Rüdiger, W., Scheer, H., Eds.; Springer: Dordrecht, The Netherlands, 2006; Volume 25, pp. 1–26. [Google Scholar]
- Blankenship, R.E. Molecular Mechanisms of Photosynthesis; Wiley-Blackwell: Chichester, UK, 2014. [Google Scholar]
- Arnold, D.P.; Johnson, A.W.; Mahendran, M. Some Reactions of meso-Formyloctaethylporphyrin. J. Chem. Soc. Perkin Trans. I 1978, 366–370. [Google Scholar] [CrossRef]
- Arnold, D.P.; Nitschinsk, L.J. Porphyrin Dimers Linked by Conjugated Butadiynes. Tetrahedron 1992, 48, 8781–8792. [Google Scholar] [CrossRef]
- Proess, G.; Pankert, D.; Hevesi, L. Synthesis of meso-Tetraalkynyl Porphyrins Using 1-Seleno-2-alkynyl Cation Precursors. Tetrahedron Lett. 1992, 33, 269–272. [Google Scholar] [CrossRef]
- Anderson, H.L. Meso-Alkynyl Porphyrins. Tetrahedron Lett. 1992, 33, 1101–1104. [Google Scholar] [CrossRef]
- Lin, V.S.-Y.; DiMagno, S.G.; Therien, M.J. Highly Conjugated, Acetylenyl Bridged Porphyrins: New Models for Light-Harvesting Antenna Systems. Science 1994, 264, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Rickhaus, M.; Jentzsch, A.V.; Tejerina, L.; Grübner, I.; Jirasek, M.; Claridge, T.D.W.; Anderson, H.L. Single-Acetylene Linked Porphyrin Nanorings. J. Am. Chem. Soc. 2017, 139, 16502–16505. [Google Scholar] [CrossRef]
- Angiolillo, P.J.; Lin, V.S.-Y.; Vanderkooi, J.M.; Therien, M.J. EPR Spectroscopy and Photophysics of the Lowest Photoactivated Triplet State of a Series of Highly Conjugated (Porphinato)Zn Arrays. J. Am. Chem. Soc. 1995, 117, 12514–12527. [Google Scholar] [CrossRef]
- Lin, V.S.-Y.; Therien, M.J. The Role of Porphyrin-to-Porphyrin Linkage Topology in the Extensive Modulation of the Absorptive and Emissive Properties of a Series of Ethynyl- and Butadiynyl-Bridged Bis- and Tris(porphinato)zinc Chromophores. Chem. Eur. J. 1995, 1, 645–651. [Google Scholar] [CrossRef]
- Kumble, R.; Palese, S.; Lin, V.S.-Y.; Therien, M.J.; Hochstrasser, R.M. Ultrafast Dynamics of Highly Conjugated Porphyrin Arrays. J. Am. Chem. Soc. 1998, 120, 11489–11498. [Google Scholar] [CrossRef]
- Shediac, R.; Gray, M.H.B.; Uyeda, H.T.; Johnson, R.C.; Hupp, J.T.; Angiolillo, P.J.; Therien, M.J. Singlet and Triplet Excited States of Emissive, Conjugated Bis(porphyrin) Compounds Probed by Optical and EPR Spectroscopic Methods. J. Am. Chem. Soc. 2000, 122, 7017–7033. [Google Scholar] [CrossRef]
- Fletcher, J.T.; Therien, M.J. Strongly Coupled Porphyrin Arrays Featuring Both π-Cofacial and Linear-π-Conjugative Interactions. Inorg. Chem. 2002, 41, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Rubtsov, I.V.; Susumu, K.; Rubtsov, G.I.; Therien, M.J. Ultrafast Singlet Excited-State Polarization in Electronically Asymmetric Ethyne-Bridged Bis[(porphinato)zinc(II)] Complexes. J. Am. Chem. Soc. 2003, 125, 2687–2696. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.V.; Susumu, K.; Sinks, L.E.; Therien, M.J. Exceptional Near-Infrared Fluorescence Quantum Yields and Excited-State Absorptivity of Highly Conjugated Porphyrin Arrays. J. Am. Chem. Soc. 2006, 128, 9000–9001. [Google Scholar] [CrossRef] [PubMed]
- Duncan, T.V.; Ishizuka, T.; Therien, M.J. Molecular Engineering of Intensely Near-Infrared Absorbing Excited States in Highly Conjugated Oligo(porphinato)zinc–(Polypyridyl)metal(II) Supermolecules. J. Am. Chem. Soc. 2007, 129, 9691–9703. [Google Scholar] [CrossRef]
- Fisher, J.A.N.; Susumu, K.; Therien, M.J.; Yodh, A.G. One- and Two-Photon Absorption of Highly Conjugated Multiporphyrin Systems in the Two-Photon Soret Transition Region. J. Chem. Phys. 2009, 130, 134506. [Google Scholar] [CrossRef]
- Duncan, T.V.; Frail, P.R.; Miloradovic, I.R.; Therien, M.J. Excitation of Highly Conjugated (Porphinato)palladium(II) and (Porphinato)platinum(II) Oligomers Produces Long-Lived, Triplet States at Unit Quantum Yield That Absorb Strongly over Broad Spectral Domains of the NIR. J. Phys. Chem. B 2010, 114, 14696–14702. [Google Scholar] [CrossRef] [PubMed]
- Singh-Rachford, T.N.; Nayak, A.; Muro-Small, M.L.; Goeb, S.; Therien, M.J.; Castellano, F.N. Supermolecular-Chromophore-Sensitized Near-Infrared-to-Visible Photon Upconversion. J. Am. Chem. Soc. 2010, 132, 14203–14211. [Google Scholar] [CrossRef] [PubMed]
- Susumu, K.; Therien, M.J. Design of Diethynyl Porphyrin Derivatives with High Near Infrared Fluorescence Quantum Yields. J. Porphyr. Phthalocyanines 2015, 19, 205–218. [Google Scholar] [CrossRef]
- Viere, E.J.; Qi, W.; Stanton, I.N.; Zhang, P.; Therien, M.J. Driving High Quantum Yield NIR Emission through Proquinoidal Linkage Motifs in Conjugated Supermolecular Arrays. Chem. Sci. 2020, 11, 8095–8104. [Google Scholar]
- Milgrom, L.R.; Yahioglu, G.; Bruce, D.W.; Morrone, S.; Henari, F.Z.; Blau, W.J. Mesogenic Zinc(II) Complexes of 5,10,15,20-Tetraarylethynyl-Substituted Porphyrins. Adv. Mater. 1997, 9, 313–316. [Google Scholar] [CrossRef]
- Sutton, J.M.; Boyle, R.W. First Synthesis of Porphyrin–Phthalocyanine Heterodimers With a Direct Ethynyl Linkage. Chem. Commun. 2001, 2014–2015. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.M.V.M.; Soares, A.R.M.; Calvete, M.J.F.; de la Torre, G. Recent Developments in the Synthesis of Homo- And Hetero-arrays of Porphyrins and Phthalocyanines. J. Porphyr. Phthalocyanines 2009, 13, 419–428. [Google Scholar] [CrossRef]
- Ke, H.; Li, W.; Zhang, T.; Zhu, X.; Tam, H.-L.; Hou, A.; Kwong, D.W.J.; Wong, W.-K. Acetylene Bridged Porphyrin–Monophthalocyaninato Ytterbium(III) Hybrids with Strong Two-Photon Absorption and High Singlet Oxygen Quantum Yield. Dalton Trans. 2012, 41, 4536–4543. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.L. Building Molecular Wires From the Colours of Life: Conjugated Porphyrin Oligomers. Chem. Commun. 1999, 2323–2330. [Google Scholar] [CrossRef]
- Maretina, I.A. Porphyrin–Ethynyl Arrays: Synthesis, Design, and Application. Russ. J. Gen. Chem. 2009, 79, 1544–1581. [Google Scholar] [CrossRef]
- Miller, M.A.; Lammi, R.K.; Prathapan, S.; Holten, D.; Lindsey, J.S. A Tightly Coupled Linear Array of Perylene, Bis(Porphyrin), and Phthalocyanine Units that Functions as a Photoinduced Energy-Transfer Cascade. J. Org. Chem. 2000, 65, 6634–6649. [Google Scholar] [CrossRef]
- Prathapan, S.; Yang, S.I.; Seth, J.; Miller, M.A.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Synthesis and Excited-State Photodynamics of Perylene-Porphyrin Dyads. 1. Parallel Energy and Charge Transfer via a Diphenylethyne Linker. J. Phys. Chem. B 2001, 105, 8237–8248. [Google Scholar] [CrossRef]
- Yang, S.I.; Prathapan, S.; Miller, M.A.; Seth, J.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Synthesis and Excited-State Photodynamics in Perylene-Porphyrin Dyads 2. Effects of Porphyrin Metalation State on the Energy-Transfer, Charge-Transfer, and Deactivation Channels. J. Phys. Chem. B 2001, 105, 8249–8258. [Google Scholar] [CrossRef]
- Yang, S.I.; Lammi, R.K.; Prathapan, S.; Miller, M.A.; Seth, J.; Diers, J.R.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Synthesis and Excited-State Photodynamics of Perylene–Porphyrin Dyads Part 3. Effects of Perylene, Linker, and Connectivity on Ultrafast Energy Transfer. J. Mater. Chem. 2001, 11, 2420–2430. [Google Scholar] [CrossRef]
- Ambroise, A.; Kirmaier, C.; Wagner, R.W.; Loewe, R.S.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Weakly Coupled Molecular Photonic Wires: Synthesis and Excited-State Energy-Transfer Dynamics. J. Org. Chem. 2002, 67, 3811–3826. [Google Scholar] [CrossRef]
- Kirmaier, C.; Yang, S.I.; Prathapan, S.; Miller, M.A.; Diers, J.R.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Synthesis and Excited-State Photodynamics in Perylene-Porphyrin Dyads. 4. Ultrafast Charge Separation and Charge Recombination Between Tightly Coupled Units in Polar Media. Res. Chem. Intermed. 2002, 28, 719–740. [Google Scholar] [CrossRef]
- Tomizaki, K.-Y.; Loewe, R.S.; Kirmaier, C.; Schwartz, J.K.; Retsek, J.L.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Synthesis and Photophysical Properties of Light-Harvesting Arrays Comprised of a Porphyrin Bearing Multiple Perylene-Monoimide Accessory Pigments. J. Org. Chem. 2002, 67, 6519–6534. [Google Scholar] [CrossRef] [PubMed]
- Loewe, R.S.; Tomizaki, K.-Y.; Youngblood, W.J.; Bo, Z.; Lindsey, J.S. Synthesis of Perylene–Porphyrin Building Blocks and Rod-Like Oligomers for Light-Harvesting Applications. J. Mater. Chem. 2002, 12, 3438–3451. [Google Scholar] [CrossRef]
- Loewe, R.S.; Tomizaki, K.-Y.; Chevalier, F.; Lindsey, J.S. Synthesis of Perylene–Porphyrin Dyads for Light-Harvesting Studies. J. Porphyr. Phthalocyanines 2002, 6, 626–642. [Google Scholar] [CrossRef]
- Muthukumaran, K.; Loewe, R.S.; Kirmaier, C.; Hinden, E.; Schwartz, J.K.; Sazanovich, I.V.; Diers, J.R.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Synthesis and Excited-State Photodynamics of A Perylene-Monoimide-Oxochlorin Dyad. A Light-Harvesting Array. J. Phys. Chem. B 2003, 107, 3431–3442. [Google Scholar] [CrossRef]
- Kirmaier, C.; Hinden, E.; Schwartz, J.K.; Sazanovich, I.V.; Diers, J.R.; Muthukumaran, K.; Taniguchi, M.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Synthesis and Excited-State Photodynamics of Perylene-Bis(imide)-Oxochlorin Dyads. A Charge-Separation Motif. J. Phys. Chem. B 2003, 107, 3443–3454. [Google Scholar] [CrossRef]
- Tomizaki, K.-Y.; Thamyongkit, P.; Loewe, R.S.; Lindsey, J.S. Practical Synthesis of Perylene-Monoimide Building Blocks That Possess Features Appropriate for Use in Porphyrin-Based Light-Harvesting Arrays. Tetrahedron 2003, 59, 1191–1207. [Google Scholar] [CrossRef]
- Kirmaier, C.; Song, H.-E.; Yang, E.K.; Schwartz, J.K.; Hindin, E.; Diers, J.R.; Loewe, R.S.; Tomizaki, K.-Y.; Chevalier, F.; Ramos, L.; et al. Excited-State Photodynamics of Perylene–Porphyrin Dyads. 5. Tuning Light-Harvesting Characteristics via Perylene Substituents, Connection Motif, and 3-Dimensional Architecture. J. Phys. Chem. B 2010, 114, 14249–14264. [Google Scholar] [CrossRef]
- Wang, J.; Yang, E.; Diers, J.R.; Niedzwiedzki, D.M.; Kirmaier, C.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Distinct Photophysical and Electronic Characteristics of Strongly Coupled Dyads Containing a Perylene Accessory Pigment and a Porphyrin, Chlorin, or Bacteriochlorin. J. Phys. Chem. B 2013, 117, 9288–9304. [Google Scholar] [CrossRef]
- Alexy, E.J.; Yuen, J.M.; Chandrashaker, V.; Diers, J.R.; Kirmaier, C.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Panchromatic Absorbers for Solar Light-Harvesting. Chem. Commun. 2014, 50, 14512–14515. [Google Scholar] [CrossRef]
- Muthiah, C.; Kee, H.L.; Diers, J.R.; Fan, D.; Ptaszek, M.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Synthesis and Excited-State Photodynamics of a Chlorin–Bacteriochlorin Dyad–Through-Space Versus Through-Bond Energy Transfer in Tetrapyrrole Arrays. Photochem. Photobiol. 2008, 84, 786–801. [Google Scholar] [CrossRef] [PubMed]
- Tomizaki, K.-Y.; Lysenko, A.B.; Taniguchi, M.; Lindsey, J.S. Synthesis of Phenylethyne-linked Porphyrin Dyads. Tetrahedron 2004, 60, 2011–2023. [Google Scholar] [CrossRef]
- Amanpour, J.; Hu, G.; Alexy, E.J.; Mandal, A.K.; Kang, H.S.; Yuen, J.M.; Diers, J.R.; Bocian, D.F.; Lindsey, J.S.; Holten, D. Tuning the Electronic Structure and Properties of Perylene–Porphyrin–Perylene Panchromatic Absorbers. J. Phys. Chem. A 2016, 120, 7434–7450. [Google Scholar] [CrossRef]
- Hu, G.; Liu, R.; Alexy, E.J.; Mandal, A.K.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Panchromatic Chromophore–Tetrapyrrole Light-Harvesting Arrays Constructed from Bodipy, Perylene, Terrylene, Porphyrin, Chlorin, and Bacteriochlorin Building Blocks. New J. Chem. 2016, 40, 8032–8052. [Google Scholar] [CrossRef]
- Mandal, A.K.; Diers, J.R.; Niedzwiedzki, D.M.; Hu, G.; Liu, R.; Alexy, E.J.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Tailoring Panchromatic Absorption and Excited-State Dynamics of Tetrapyrrole–Chromophore (Bodipy, Rylene) Arrays—Interplay of Orbital Mixing and Configuration Interaction. J. Am. Chem. Soc. 2017, 139, 17547–17564. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Kang, H.S.; Mandal, A.K.; Roy, A.; Kirmaier, C.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Synthesis of Arrays Containing Porphyrin, Chlorin, and Perylene-imide Constituents for Panchromatic Light-Harvesting and Charge Separation. RSC Adv. 2018, 8, 23854–23874. [Google Scholar] [CrossRef]
- Yuen, J.; Diers, J.R.; Alexy, E.J.; Roy, A.; Mandal, A.K.; Kang, H.S.; Niedzwiedzki, D.M.; Kirmaier, C.; Lindsey, J.S.; Bocian, D.F.; et al. Origin of Panchromaticity in Multichromophore–Tetrapyrrole Arrays. J. Phys. Chem. A 2018, 122, 7181–7201. [Google Scholar] [CrossRef]
- Rong, J.; Magdaong, N.C.M.; Taniguchi, M.; Diers, J.R.; Niedzwiedzki, D.M.; Kirmaier, C.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Electronic Structure and Excited-State Dynamics of Rylene–Tetrapyrrole Panchromatic Absorbers. J. Phys. Chem. A 2021, 125, 7900–7919. [Google Scholar] [CrossRef]
- Taniguchi, M.; Balakumar, A.; Fan, D.; McDowell, B.E.; Lindsey, J.S. Imine-Substituted Dipyrromethanes in the Synthesis of Porphyrins Bearing One or Two Meso Substituents. J. Porphyr. Phthalocyanines 2005, 9, 554–574. [Google Scholar] [CrossRef]
- Tamaru, S.-I.; Yu, L.; Youngblood, W.J.; Muthukumaran, K.; Taniguchi, M.; Lindsey, J.S. A Tin-Complexation Strategy for Use with Diverse Acylation Methods in the Preparation of 1,9-Diacyldipyrromethanes. J. Org. Chem. 2004, 69, 765–777. [Google Scholar] [CrossRef]
- Borbas, K.E.; Mroz, P.; Hamblin, M.R.; Lindsey, J.S. Bioconjugatable Porphyrins Bearing a Compact Swallowtail Motif for Water Solubility. Bioconjugate Chem. 2006, 17, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Liu, M.; Hood, D.; Chen, C.-Y.; MacNevin, C.J.; Holten, D.; Lindsey, J.S. Chlorophyll-Inspired Red-Region Fluorophores: Building Block Syntheses and Studies in Aqueous Media. Molecules 2018, 23, 130. [Google Scholar] [CrossRef] [PubMed]
- Chinchilla, R.; Nájera, C. The Sonogashira Reaction: A Booming Methodology in Synthetic Organic Chemistry. Chem. Rev. 2007, 107, 874–922. [Google Scholar] [CrossRef]
- Wagner, R.W.; Johnson, T.E.; Li, F.; Lindsey, J.S. Synthesis of Ethyne-Linked or Butadiyne-Linked Porphyrin Arrays Using Mild, Copper-Free, Pd-Mediated Coupling Reactions. J. Org. Chem. 1995, 60, 5266–5273. [Google Scholar] [CrossRef]
- Wagner, R.W.; Ciringh, Y.; Clausen, C.; Lindsey, J.S. Investigation and Refinement of Palladium-Coupling Conditions for the Synthesis of Diarylethyne-Linked Multiporphyrin Arrays. Chem. Mater. 1999, 11, 2974–2983. [Google Scholar] [CrossRef]
- Schmidt, I.; Jiao, J.; Thamyongkit, P.; Sharada, D.S.; Bocian, D.F.; Lindsey, J.S. Investigation of Stepwise Covalent Synthesis on a Surface Yielding Porphyrin-Based Multicomponent Architectures. J. Org. Chem. 2006, 71, 3033–3050. [Google Scholar] [CrossRef]
- Rademacher, A.; Märkle, S.; Langhals, H. Lösliche Perylen-Fluoreszenzfarbstoffe mit hoher Photostabilität. Chem. Ber. 1982, 115, 2927–2934. [Google Scholar] [CrossRef][Green Version]
- Langhals, H. Synthese von hochreinen Perylen-Fluoreszenzfarbstoffen in großen Mengen–gezielte Darstellung von Atrop-Isomeren. Chem. Ber. 1985, 118, 4641–4645. [Google Scholar] [CrossRef]
- Langhals, H.; Demmig, S.; Huber, H. Rotational Barriers in Perylene Fluorescent Dyes. Spectrochim. Acta 1988, 44A, 1189–1193. [Google Scholar] [CrossRef][Green Version]
- Demmig, S.; Langhals, H. Leichtlösliche, lichtechte Perylen-Fluoreszenzfarbstoffe. Chem. Ber. 1988, 121, 225–230. [Google Scholar] [CrossRef]
- Ebeid, E.-Z.M.; El-Daly, S.A.; Langhals, H. Emission Characteristics and Photostability of N,N′-Bis(2,5-di-tert-butylphenyl)-3,4:9,10-perylenebis(dicarboximide). J. Phys. Chem. 1988, 92, 4565–4568. [Google Scholar] [CrossRef]
- Feiler, L.; Langhals, H.; Polborn, K. Synthesis of Perylene-3,4-dicarboximides—Novel Highly Photostable Fluorescent Dyes. Liebigs Ann. 1995, 1995, 1229–1244. [Google Scholar] [CrossRef]
- Langhals, H. Cyclic Carboxylic Imide Structures as Structure Elements of High Stability. Novel Developments in Perylene Dye Chemistry. Heterocycles 1995, 40, 477–500. [Google Scholar] [CrossRef]
- Würthner, F. Perylene Bisimide Dyes as Versatile Building Blocks for Functional Supramolecular Architectures. Chem. Commun. 2004, 1564–1579. [Google Scholar] [CrossRef] [PubMed]
- Langhals, H. Control of the Interactions in Multichromophores: Novel Concepts. Perylene Bis-imides as Components for Larger Functional Units. Helv. Chim. Acta 2005, 88, 1309–1343. [Google Scholar] [CrossRef]
- Weil, T.; Vosch, T.; Hofkens, J.; Peneva, K.; Müllen, K. The Rylene Colorant Family—Tailored Nanoemitters for Photonics Research and Applications. Angew. Chem. Int. Ed. 2010, 49, 9068–9093. [Google Scholar] [CrossRef]
- Gosztola, D.; Niemczyk, M.P.; Wasielewski, M.R. Picosecond Molecular Switch Based on Bidirectional Inhibition of Photoinduced Electron Transfer Using Photogenerated Electric Fields. J. Am. Chem. Soc. 1998, 120, 5118–5119. [Google Scholar] [CrossRef]
- Odobel, F.; Séverac, M.; Pellegrin, Y.; Blart, E.; Fosse, C.; Cannizzo, C.; Mayer, C.R.; Elliott, K.J.; Harriman, A. Coupled Sensitizer–Catalyst Dyads: Electron-Transfer Reactions in a Perylene–Polyoxometalate Conjugate. Chem. Eur. J. 2009, 15, 3130–3138. [Google Scholar] [CrossRef] [PubMed]
- Boixel, J.; Blart, E.; Pellegrin, Y.; Odobel, F.; Perin, N.; Chiorboli, C.; Fracasso, S.; Ravaglia, M.; Scandola, F. Hole-Transfer Dyads and Triads Based on Perylene Monoimide, Quaterthiophene, and Extended Tetrathiafulvalene. Chem. Eur. J. 2010, 16, 9140–9153. [Google Scholar] [CrossRef]
- Wagner, R.W.; Johnson, T.E.; Lindsey, J.S. Soluble Synthetic Multiporphyrin Arrays. 1. Modular Design and Synthesis. J. Am. Chem. Soc. 1996, 118, 11166–11180. [Google Scholar] [CrossRef]
- Gorrea, E.; Carbajo, D.; Gutiérrez-Abad, R.; Illa, O.; Branchadell, V.; Royo, M.; Ortuño, R.M. Searching for New Cell-Penetrating Agents: Hybrid Cyclobutane–Proline γ,γ-Peptides. Org. Biomol. Chem. 2012, 10, 4050–4057. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Jiang, J.; Krayer, M.; Harris, M.A.; Springer, J.W.; Yang, E.; Jiao, J.; Niedzwiedzki, D.M.; Pandithavidana, D.; Parkes-Loach, P.S.; et al. Palette of Lipophilic Bioconjugatable Bacteriochlorins for Construction of Biohybrid Light-Harvesting Architectures. Chem. Sci. 2013, 4, 2036–2053. [Google Scholar] [CrossRef]
- Morisue, M.; Haruta, N.; Kalita, D.; Kobuke, Y. Efficient Charge Injection from the S2 Photoexcited State of Special-Pair Mimic Porphyrin Assemblies Anchored on a Titanium-Modified ITO Anode. Chem. Eur. J. 2006, 12, 8123–8135. [Google Scholar] [CrossRef] [PubMed]
- Muthiah, C.; Taniguchi, M.; Kim, H.-J.; Schmidt, I.; Kee, H.L.; Holten, D.; Bocian, D.F.; Lindsey, J.S. Synthesis and Photophysical Characterization of Porphyrin, Chlorin and Bacteriochlorin Molecules Bearing Tethers for Surface Attachment. Photochem. Photobiol. 2007, 83, 1513–1528. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-H.; Lindsey, J.S. One-Flask Synthesis of Meso-Substituted Dipyrromethanes and Their Application in the Synthesis of Trans-Substituted Porphyrin Building Blocks. Tetrahedron 1994, 50, 11427–11440. [Google Scholar] [CrossRef]
- Srinivasan, N.; Haney, C.A.; Lindsey, J.S.; Zhang, W.; Chait, B.T. Investigation of MALDI-TOF Mass Spectrometry of Diverse Synthetic Metalloporphyrins, Phthalocyanines, and Multiporphyrin Arrays. J. Porphyr. Phthalocyanines 1999, 3, 283–291. [Google Scholar] [CrossRef]
- Taniguchi, M.; Lindsey, J.S.; Bocian, D.F.; Holten, D. Comprehensive Review of Photophysical Parameters (ε, Φf, τS) of Tetraphenylporphyrin (H2TPP) and Zinc Tetraphenylporphyrin (ZnTPP)–Critical Benchmark Molecules in Photochemistry and Photosynthesis. J. Photochem. Photobiol. C Photochem. Rev. 2021, 46, 100401. [Google Scholar] [CrossRef]
- Lee, S.H.; Matula, A.J.; Hu, G.; Troiano, J.L.; Karpovich, C.J.; Crabtree, R.H.; Batista, V.S.; Brudvig, G.W. Strongly Coupled Phenazine−Porphyrin Dyads: Light-Harvesting Molecular Assemblies with Broad Absorption Coverage. ACS Appl. Mater. Interfaces 2019, 11, 8000–8008. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, R.; Rong, J.; Wu, Z.; Taniguchi, M.; Bocian, D.F.; Holten, D.; Lindsey, J.S. Panchromatic Absorbers Tethered for Bioconjugation or Surface Attachment. Molecules 2022, 27, 6501. https://doi.org/10.3390/molecules27196501
Liu R, Rong J, Wu Z, Taniguchi M, Bocian DF, Holten D, Lindsey JS. Panchromatic Absorbers Tethered for Bioconjugation or Surface Attachment. Molecules. 2022; 27(19):6501. https://doi.org/10.3390/molecules27196501
Chicago/Turabian StyleLiu, Rui, Jie Rong, Zhiyuan Wu, Masahiko Taniguchi, David F. Bocian, Dewey Holten, and Jonathan S. Lindsey. 2022. "Panchromatic Absorbers Tethered for Bioconjugation or Surface Attachment" Molecules 27, no. 19: 6501. https://doi.org/10.3390/molecules27196501
APA StyleLiu, R., Rong, J., Wu, Z., Taniguchi, M., Bocian, D. F., Holten, D., & Lindsey, J. S. (2022). Panchromatic Absorbers Tethered for Bioconjugation or Surface Attachment. Molecules, 27(19), 6501. https://doi.org/10.3390/molecules27196501







