Chemical Profile and Biological Activities of Essential Oil from Piper arboreum for Development and Improvement of Mouthwash
Abstract
1. Introduction
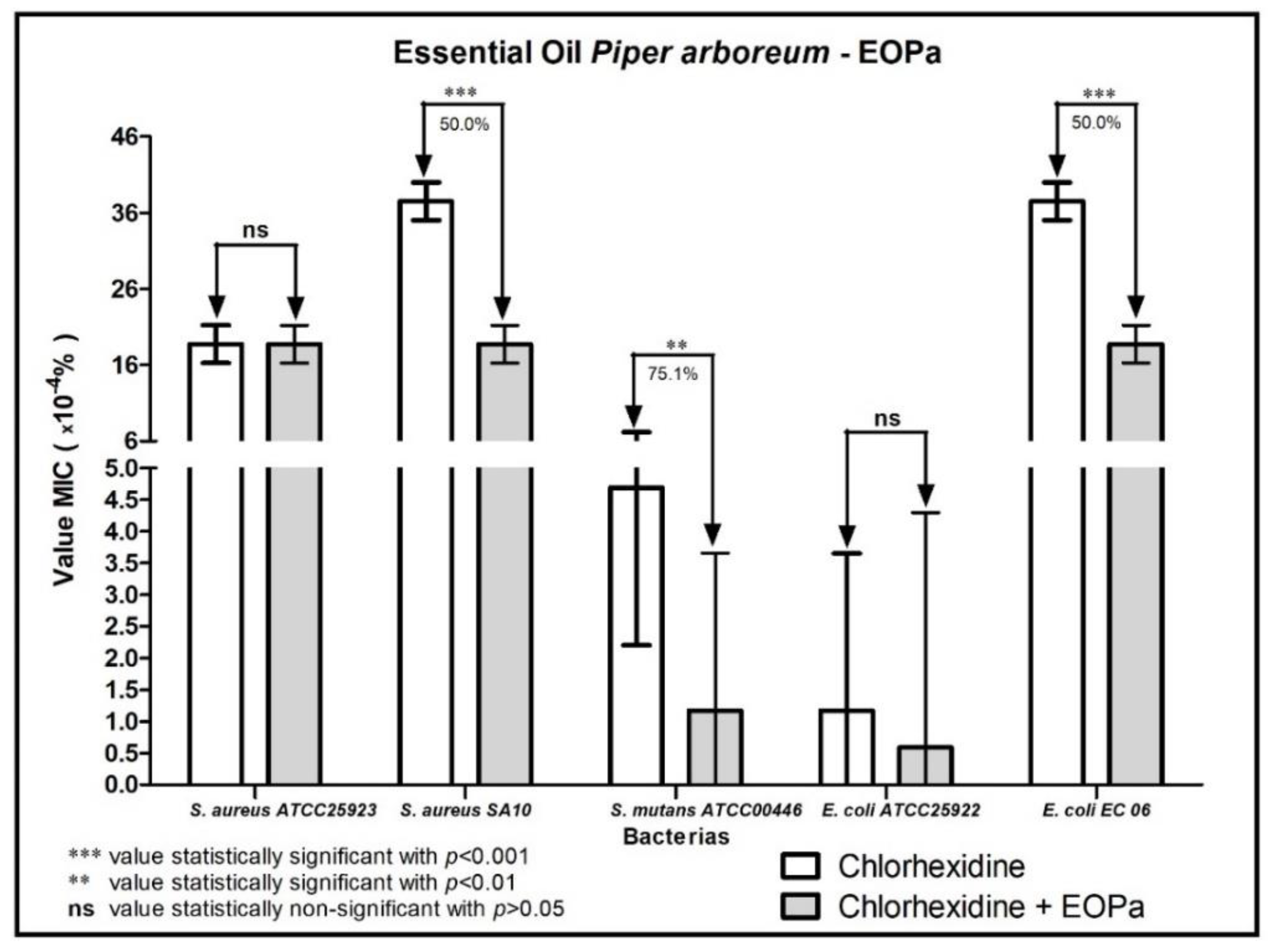
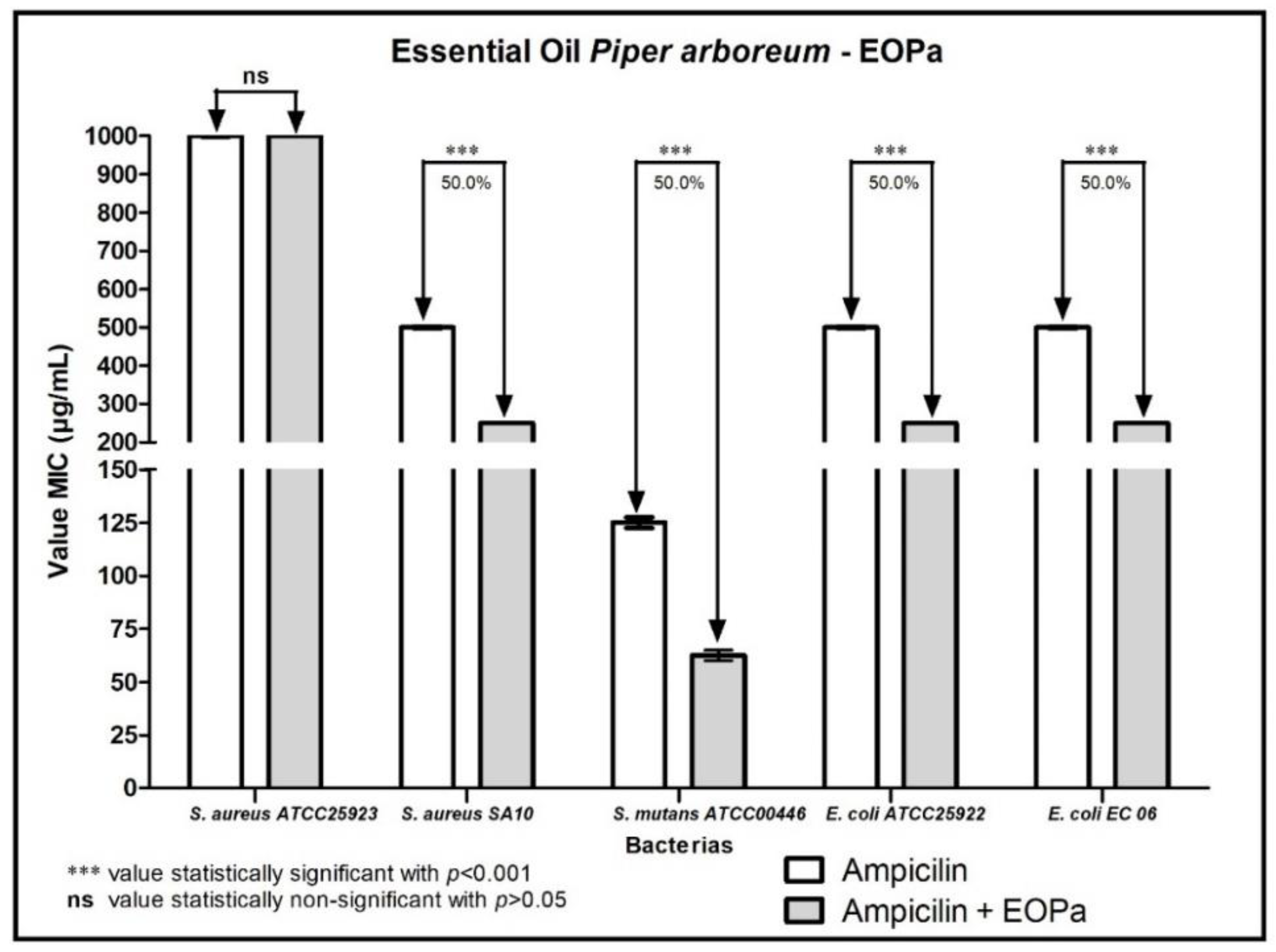
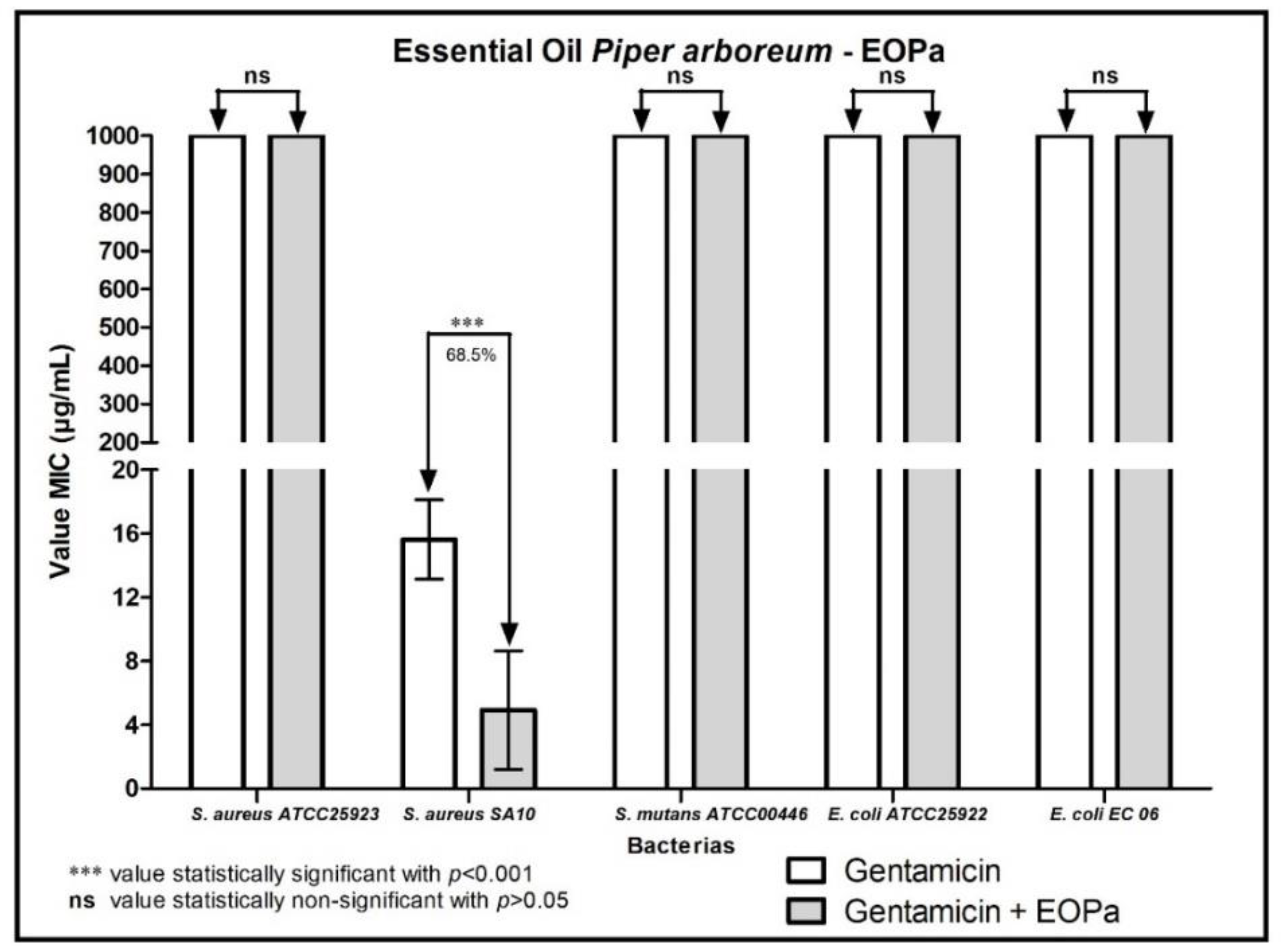
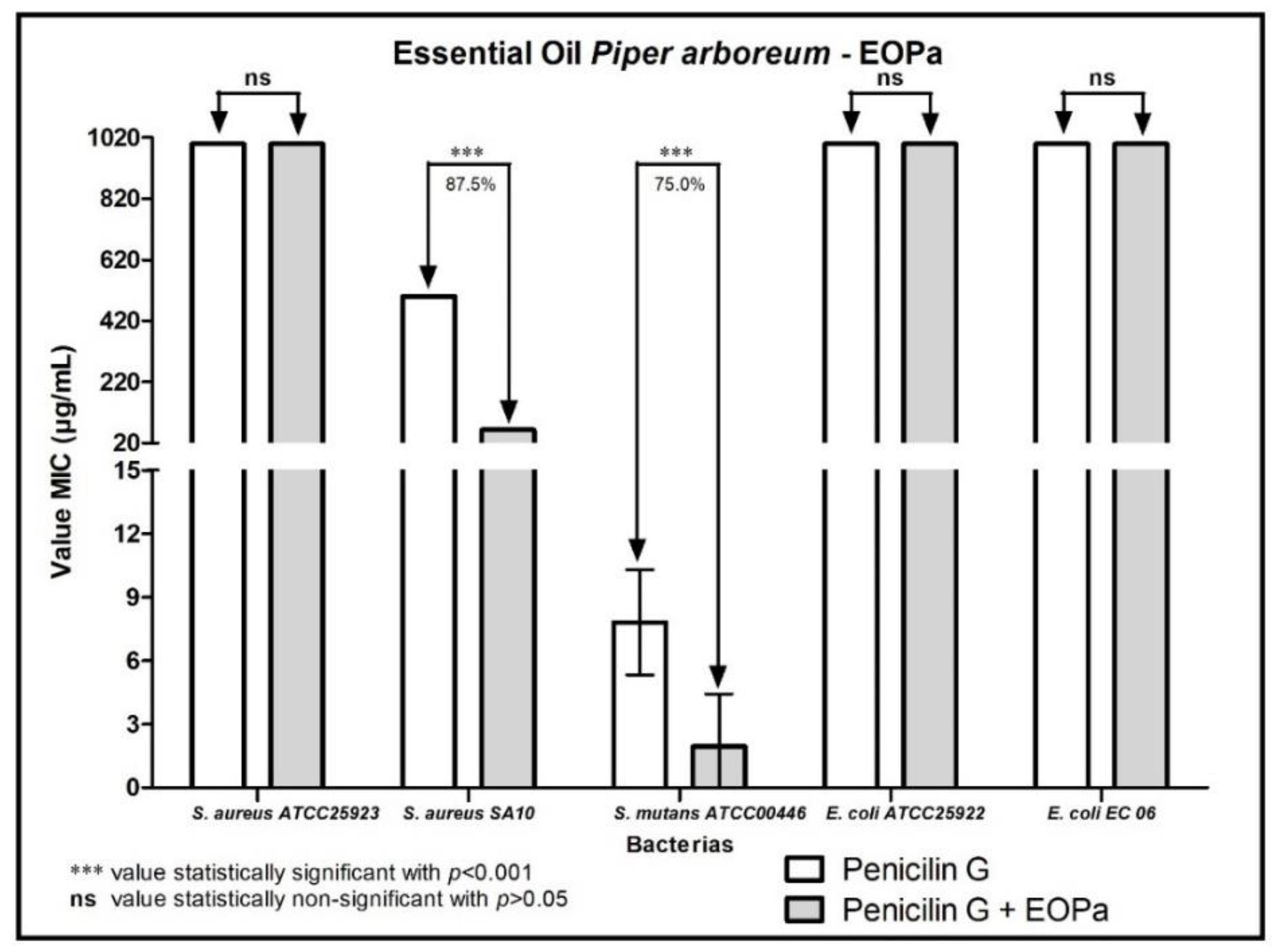
2. Results
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains
4.2. Preparation and Standardization of Bacterial Inoculum
4.3. Antibiotics and Mouthwash Solutions
4.4. Botanical Materials and Extraction of Essential Oils
4.5. Antibacterial Tests
4.5.1. Determination of Minimum Inhibitory Concentration (MIC) In Vitro by Direct Contact
4.5.2. Modulating Activity of In Vitro Antibiotic Action by Direct Contact
4.5.3. Statistical Analysis of Microbiological Tests
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Lima, M.J.S.; Silva, M.Y.C.; Melo, K.R.; Chagas, B.F.; Rolim, L.A.; Neto, P.J.R.; Silva, R.M.F. Caracterização do extrato seco de Libidibia ferrea para terapia antihiperglicemiante. Braz. J. Dev. 2020, 6, 97488–97506. [Google Scholar] [CrossRef]
- Vieira, D.S.; Oliveira, F.T.D.; Suarez, J.A.G.; Silva, D.P.D.; Bernardo, T.H.L.; Bastos, M.L.D.A. Atividades biológicas: Anti-infecciosa, antioxidante e cicatrizante da espécie vegetal Jatropha multifida. Rev. Bras. Enferm. 2021, 74, e20200451. [Google Scholar] [CrossRef] [PubMed]
- Arbos, K.; Nader, J.K.S.; Camelo, E.N.L. Atividade ansiolítica de extrato das folhas de Miconia albicans. Rev. Cient. Multidiscip. 2021, 2, 221–232. [Google Scholar] [CrossRef]
- Tankam, J.M.; Ito, M. Sedative, anxiolytic and antidepressant-like effects of inhalation of the essential oil of Ocimum gratissimum L. from Cameroon in mice. J. Pharmacogn. Phytochem. 2014, 2, 1–9. [Google Scholar]
- Silva, M.D.G.D.; Oliveira, F.D.S.; Diniz, M.D.F.F.M.; Takemura, O.S. Atividade antiinflamatória do extrato etanólico de Conocliniopsis prasiifolia RM King & H. Robinson na resposta celular de neutrófilos. Rev. Bras. Farmacog. 2008, 18, 569–572. [Google Scholar] [CrossRef]
- Lorenzoni, A.A.; Lusa, F.T.; Cavalli, A.P.; Gnoato, C.V.; Ferrari, B.; Graciani, P.C.; Corralo, V.S. Efeito hepatoprotetor de produtos naturais frente ao dano induzido por paracetamol. Acta Ambient. Catarin. 2014, 11, 43–52. [Google Scholar] [CrossRef]
- Cavalcante, G.M.; Barbosa, P.L.; Silva, A.A. Atividade antitumoral in vitro de Prosopis juliflora frente a células cancerígenas do tipo melanoma. Res. Soc. Dev. 2021, 10, e39510414095. [Google Scholar] [CrossRef]
- Gege-Adebayo, G.I.; Igbokwe, V.U.; Shafe, M.O.; Akintayo, C.O.; Mbaka, D.I. Anti-ulcer effect of Ocimum gratissimum on indomethacin induced ulcer and percentage of superoxide dismutase on wistar rats. J. Med. Sci. 2013, 4, 8–12. [Google Scholar]
- Ekoh, S.N.; Akubugwo, E.I.; Ude, V.C.; Edwin, N. Anti-hyperglycemic and anti-hyperlipidemic effect of spices (Thymus vulgaris, Murraya koenigii, Ocimum gratissimum and Piper guineense) in alloxan-induced diabetic rats. Int. J. Biosci. 2014, 4, 179–187. [Google Scholar]
- Matias, E.F.F.; Santos, K.K.A.; Almeida, T.S.; Costa, J.G.M.; Coutinho, H.D.M. Enhancement of antibiotic activity by Cordia verbenacea DC. Lat. Am. J. Pharm. 2010, 29, 1049–1052. [Google Scholar]
- Matasyoh, L.G.; Matasyoh, J.C.; Wachira, F.N.; Kinyua, M.G.; Muigai, A.W.T.; Mukiama, T.K. Chemical composition and antimicrobial activity of the essential oil of Ocimum gratissimum L. growing in Eastern Kenya. Afr. J. Biotechnol. 2007, 6, 760–765. [Google Scholar]
- Bankole, H.A.; Anjorin, A.A.; Kazeem, M.I.; Ogbeche, M.E.; Agbafor, U. Antibacterial activity of Ocimum gratissimum and Gongronema latifolium on Staphylococcus aureus and Salmonella typhi. Afr. J. Sci. Technol. Innov. Dev. 2012, 2, 114–128. [Google Scholar]
- Kpoviessi, B.G.H.K.; Ladekan, E.Y.; Kpoviessi, D.S.S.; Gbaguidi, F.; Yehouenou, B.; Quetin-Leclercq, J. Chemical Variation of Essential Oil Constituents of Ocimum gratissimum L. from Benin, and Impact on Antimicrobial Properties and Toxicity against Artemia salina Leach. Chem. Biodivers. 2012, 9, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Adesegun, A.S.; Samuel, F.O.; Anthony, O.B.; Nurudeen, O.A. Antioxidant and Inhibitory Properties of Essential Oil of Ocimum gratissimum against Extracellular Protease of Escherichia coli. IOSR J. Pharm. 2013, 3, 50–55. [Google Scholar] [CrossRef]
- Siani, A.C.; Sampaio, A.L.F.; Souza, M.C.; Henriques, M.G.M.O.; Ramos, M.F.S. Óleos essenciais: Potencial antiinflamatório. Biotecnolog. Cienc. Desenvolv. 2000, 16, 38–43. [Google Scholar]
- Teixeira, J.P.F.; Marques, M.O.M.; Pio, R.M. Caracterização dos óleos essenciais em frutos de nove genótipos de tangerina. Citrus. Res. Technol. 2017, 35, 1–10. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Setzer, W.N. Biological Activities and safety of Citrus spp. Essential Oils. Int. J. Mol. Sci. 2018, 19, 1966. [Google Scholar] [CrossRef]
- Benini, P.C.; Schwan-Estrada, K.R.F.; Klais, E.C.; Cruz, M.E.S.; Itako, A.T.; Mesquini, R.M.; Tolentino Júnior, J.B. Efeito in vitro do óleo essencial e extrato aquoso de Ocimum gratissimum colhido nas quatro estações do ano sobre fitopatógenos. Arq. Inst. Biol. 2021, 77, 677–683. [Google Scholar] [CrossRef]
- Suhr, K.I.; Nielsen, P.V. Antifungal activity of essential oils evaluated by two different application techniques against rye bread spoilage fungi. J. Appl. Microbiol. 2003, 94, 665–674. [Google Scholar] [CrossRef]
- Da Silva, A.C.A.; Matias, E.F.F.; Rocha, J.E.; Araújo, A.C.J.; Freitas, T.S.; Campina, F.F.; Costa, M.D.S.; Silva, L.E.; Amaral, W.D.; Maia, B.H.L.N.S.; et al. Gas chromatography coupled to mass spectrometry (GC-MS) characterization and evaluation of antibacterial bioactivities of the essential oils from Piper arboreum Aubl., Piper aduncum L. e Piper gaudichaudianum Kunth. Z. Nat. C J. Biosci. 2020, 76, 35–42. [Google Scholar] [CrossRef]
- Regasini, L.O.; Cotinguiba, V.; Morandim, A.A.; Kato, M.J.; Scorzoni, L.; Mendes-Giannini, M.J.S. Atividade antimicrobiana de Piper arboreum e Piper tuberculatum (Piperaceae) contra leveduras oportunistas. Afr. J. Biotechnol. 2009, 8, 2866–2870. [Google Scholar]
- Lima, R.N.; Ribeiro, A.S.; Santiago, G.M.; Costa, C.O.D.S.; Soares, M.B.; Bezerra, D.P.; Alves, P.B. Antitumor and Aedes aegypti Larvicidal Activities of Essential Oils from Piper klotzschianum, P. hispidum, and P. arboreum. Nat. Prod. Commun. 2019, 14, 1934578X19863932. [Google Scholar] [CrossRef]
- Zaze, A.C.S.F.; Oliveira, E.R.; Melão, M.J.A.S.; Alves, E. Eficácia de diferentes tipos de escovas dentais na remoção do biofilme bucal Arq. Ciênc. Saúde UNIPAR 2016, 20, 101–109. [Google Scholar]
- Lowy, A.; Ojo, R.; Stegeman, A.; Vellacott, I. Meeting women’s need for a flexible abortion service: Retrospective study of a specialist day-care unit. J. Public. Health. Med. 1998, 20, 449–454. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Naimi, T.S.; Ledell, K.H.; Como-Sabetti, K.; Borchardt, S.M.; Boxrud, D.J.; Etienne, J.; Johnson, S.K.; Vandenesch, F.; Fridkin, S.; O’Boyle, C.; et al. Comparison of community-and health care–associated methicillin-resistant Staphylococcus aureus infection. JAMA-J. Am. Med. Assoc. 2003, 290, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Weinstein, R.A.; Fridkin, S.K. Vancomycin-intermediate and-resistant Staphylococcus aureus: What the infectious disease specialist needs to know. Clin. Infect. Dis. 2001, 32, 108–115. [Google Scholar] [CrossRef]
- Wang, W.; Baloch, Z.; Jiang, T.; Zhang, C.; Peng, Z.; Li, F.; Fanning, S.; Ma, A.; Xu, J. Enterotoxigenicity and Antimicrobial Resistance of Staphylococcus aureus Isolated from Retail Food in China. Front. Microbiol. 2017, 8, 2256. [Google Scholar] [CrossRef]
- Wen, Z.T.; Yates, D.; Ahn, S.J.; Burne, R.A. Biofilm formation and virulence expression by Streptococcus mutans are altered when grown in dual-species model. BMC Microbiol. 2010, 10, 111. [Google Scholar] [CrossRef]
- Krzyściak, W.; Jurczak, A.; Kościelniak, D.A.; Bystrowska, B.; Skalniak, A. The virulence of Streptococcus mutans and the ability to form biofilms. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 499–515. [Google Scholar] [CrossRef]
- Matsumoto-Nakano, M. Role of Streptococcus mutans surface proteins for biofilm formation. Jpn. Dent. Sci. Rev. 2018, 54, 22–29. [Google Scholar] [CrossRef]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 435–448. [Google Scholar] [CrossRef] [PubMed]
- De Menezes, M.L.F.V.; De Macedo, Y.V.G.; Ferraz, N.M.P.; Matos, K.F.; Pereira, R.O.; Fontes, N.M.; Batista, M.I.H.M.; Paulino, M.R. A importância do controle do biofilme dentário: Uma revisão da literatura. Rev. Eletr. Acer. Saúde 2020, 55, e3698. [Google Scholar] [CrossRef]
- Hervella-Garcés, M.; García-Gavín, J.; Silvestre-Salvador, J.F. Actualización de la serie estándar española de pruebas alérgicas de contacto por el Grupo Español de Investigación en Dermatitis de Contacto y Alergia Cutánea (GEIDAC) para 2016. Actas. Dermo-Sifiliogr. 2016, 107, 559–566. [Google Scholar] [CrossRef]
- Hortense, S.R.; Carvalho, É.S.; Carvalho, F.S.; Silva, R.P.R.; Bastos, J.R.M.; Bastos, R.S. Uso da clorexidina como agente preventivo e terapêutico na odontologia. Rev. Odontol. Univ. Cid. São Paulo 2017, 22, 178–184. [Google Scholar] [CrossRef]
- Wand, M.E.; Bock, L.J.; Bonney, L.C.; Sutton, M.S. Mechanisms of increased resistance to chlorhexidine and cross-resistance to colistin following exposure of Klebsiella pneumoniae clinical isolates to chlorhexidine. Antimicrob. Agents Chemother. 2017, 61, e01162-16. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L. Chlorhexidine gel use in the oral district: A systematic review. Gels 2019, 5, 31. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Chen, Y.; Huang, L.; Lin, Z.; Cai, Z. Core–shell structured magnetic covalent organic framework nanocomposites for triclosan and triclocarban adsorption. ACS Appl. Mater. Interfaces 2019, 11, 22492–22500. [Google Scholar] [CrossRef]
- Halden, R.U.; Lindeman, A.E.; Aiello, A.E.; Andrews, D.; Arnold, W.A.; Patrícia, F.; Fuoco, R.E.; Geer, L.A.; Johnson, P.I.; Lohmann, R.; et al. The Florence statement on triclosan and triclocarban. Environ. Health Perspect. 2017, 125, 064501. [Google Scholar] [CrossRef]
- Jacob, B.; Nivedhitha, M.S. Comparative Assessment of the Antibacterial Efficacy of Natural Products and Chlorhexidine Mouthwash against Streptococcus Mutans: A Systematic Review. J. Clin. Diagn. Res. 2018, 12, 1–7. [Google Scholar] [CrossRef]
- Souza, E.E.D.; Vasconcelos, E.D.C.Z.; Oliveira, A.P.R.D.; Duarte, J.C.G.; Barbosa, A.S.; Barros, V.L.; Abboud, C.S. Banho com toalhas antissépticas com clorexidine 2% em pacientes críticos: Há impacto na redução de infecção primária da corrente sanguínea? J. Infect. Control. 2018, 7, 107–108. [Google Scholar]
- Saliasi, I.; Llodra, J.C.; Bravo, M.; Tramini, P.; Dussart, C.; Viennot, S.; Carrouel, F. Effect of a Toothpaste/Mouthwash Containing Carica papaya Leaf Extract on Interdental Gingival Bleeding: A Randomized Controlled Trial. Int. J. Environ. Res. Public Health 2018, 15, 2660. [Google Scholar] [CrossRef] [PubMed]
- Safrida, S.; Khairil, K.; Artika, W.; Rinaldi, R. Pandanus amaryllifolius Roxb. leaf extract prepared by nanoemulsion technique as a natural mouthwash. J. Phys. Conf. Ser. 2020, 1460, 012050. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry, 4th ed.; Allured publishing Corporation: Carol Stream, IL, USA, 2007; pp. 544–545. [Google Scholar]
- Thomford, N.E.; Senthebane, D.A.; Rowe, A.; Munro, D.; Seele, P.; Maroyi, A.; Dzobo, K. Natural products for drug discovery in the 21st century: Innovations for novel drug discovery. Int. J. Mol. Sci. 2018, 19, 1578. [Google Scholar] [CrossRef]
- Liang, X.; Luo, D.; Luesch, H. Advances in exploring the therapeutic potential of marine natural products. Pharmacol. Res. 2019, 147, 104373. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, F.S.; Silva, N.L.; Matias, E.F.F.; Brito, S.V.; Oliveira, F.G.; Costa, J.G.M.; Coutinho, H.D.M.; Almeida, W.O.; Alves, R.R.N. Potentiation of aminoglycoside antibiotic activity using the body fat from the snake Boa constrictor. Rev. Bras. Farmacogn. 2011, 21, 503–509. [Google Scholar] [CrossRef]
- Ambroz, M.; Smatová, M.; Sadibolová, M.; Pospíšilová, E.; Hadravská, P.; Kašparová, M.; Skarková, V.H.; Králová, V.; Skálová, L. Sesquiterpenes α-humulene and β-caryophyllene oxide enhance the efficacy of 5-fluorouracil and oxaliplatin in colon cancer cells. Acta Pharm. 2019, 69, 121–128. [Google Scholar] [CrossRef]
- Silva, J.O.S.D. Toxicidade do óleo Essential e Sazonalidade do aroma Floral de Ipomoea Asarifolia (Desr.) Roem. & Schult. E I. Setifera Poir; Universidade Federal Rural da Amazônia, Museu Paraense Emílio Goeldi: Belém, Brazil, 2021. [Google Scholar]
- Bao, H.; Muge, Q. Anticancer effect of myristicin on hepatic carcinoma and related molecular mechanism. Pharm. Biol. 2021, 59, 1124–1130. [Google Scholar] [CrossRef]
- Matias, E.F.F.; Alves, E.F.; Silva, M.K.N.; Carvalho, V.R.A.; Figueredo, F.G.; Ferreira, J.V.A.; Coutinho, H.D.M.; Silva, J.M.F.L.; Ribeiro-Filho, J.; Costa, J.G.M. Seasonal variation, chemical composition and biological activity of the essential oil of Cordia verbenacea DC (Boraginaceae) and the sabinene. Ind. Crops Prod. 2016, 87, 45–53. [Google Scholar] [CrossRef]
- Freitas, C.L.A.; Santos, F.F.P.; Dantas-Junior, O.M.; Inácio, V.V.; Matias, E.F.F.; Quintans-Júnior, L.J. Enhancement of antibiotic activity by phytocompounds of Turnera subulata. Nat. Prod. Res. 2020, 34, 2384–2388. [Google Scholar] [CrossRef]
- Arriaga, A.M.C.; Silva, F.R.L.; Texeira, M.V.S.; Pereira, I.G.; Silva, M.R.; Mafezoli, J.; Santiago, G.M.P.; Vasconcelos, J.N.E.; Braz-Filho, R.; Costa, J.G.M.; et al. Chemical Compounds and Antibacterial Activity of Tephrosia toxicaria Pers. Orient. J. Chem. 2017, 33, 2173–2178. [Google Scholar] [CrossRef]
- Udo, E.E.; Boswihi, S.S. Antibiotic Resistance Trends in Methicillin-Resistant Staphylococcus aureus Isolated in Kuwait Hospitals: 2011–2015. Med. Princ. Pract. 2017, 26, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.B.L.D.; Cruz, C.A.; Freitas, C.L.A.D.; Aguiar, J.J.S.; Nunes, P.L.W.S.; Lima, V.M.S.; Matias, E.F.F.; Muniz, D.F.; Coutinho, H.D.M. Chemical Profile, Antibacterial Activity and Antibiotic-Modulating Effect of the Hexanic Zea mays L. Silk Extract (Poaceae). Antibiotics 2019, 8, 22. [Google Scholar] [CrossRef] [PubMed]
- Javadpour, M.M.; Juban, M.M.; Lo, W.C.J.; Bishop, S.M.; Alberty, J.B.; Cowell, S.M.; Becker, C.L.; McLaughlin, M.L. De novo antimicrobial peptides with low mammalian cell toxicity. J. Med. Chem. 1996, 39, 3107–3113. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, O.R.; Magalhães, M.T. Essential oil of the bark and wood of Aniba canellila. Perf. Essent. Oil Rec. 1960, 51, 69–70. [Google Scholar]
- Van Den Dool, H.; Kratz, P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Sader, H.S.; Biedenbach, D.J.; Jones, R.N. Global patterns of susceptibility for 21 commonly utilized antimicrobial agents tested against 48,440 Enterobacteriaceae in the SENTRY Antimicrobial Surveillance Program (1997–2001). Diagn. Microbiol. Infect. Dis. 2003, 47, 361–364. [Google Scholar] [CrossRef]
- Palomino, J.C.; Martin, A.; Camacho, M.; Guerra, H.; Swings, J.; Portaels, F. Resazurin microtiter assay plate: Simple and inexpensive method for detection of drug resistance in Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2002, 46, 2720–2722. [Google Scholar] [CrossRef] [PubMed]

| No. | Constituents | RT (min) GC-MS (EOPa) | IAexp | IAlit | EOPa [%] |
|---|---|---|---|---|---|
| 1 | Linalool | 11.34 | 1097 | 1095 | 0.50 |
| 2 | δ-Elemene | 21.19 | 1331 | 1335 | 1.00 |
| 3 | α-Copaene | 22.86 | 1372 | 1374 | 2.00 |
| 4 | β-Elemene | 23.44 | 1386 | 1389 | 0.93 |
| 5 | E-Caryophyllene | 24.65 | 1417 | 1389 | 5.10 |
| 6 | α-Humulene | 26.08 | 1452 | 1452 | 0.96 |
| 7 | Germacrene D | 27.13 | 1478 | 1484 | 1.20 |
| 8 | β-Selinene | 27.44 | 1486 | 1484 | 1.52 |
| 9 | γ-Amorphene | 27.72 | 1492 | 1495 | 0.60 |
| 10 | α-Muurolene | 27.86 | 1495 | 1495 | 0.50 |
| 11 | α-Bulnesene | 27.97 | 1498 | 1500 | 0.55 |
| 12 | δ-Amorphene | - | 1515 | 1509 | - |
| 13 | trans-Calamenene | - | 1517 | 1511 | - |
| 14 | Myristicin | 28.71 | 1518 | 1517 | 7.00 |
| 15 | Elemol | 29.81 | 1549 | 1548 | 2.00 |
| 16 | E-Nerolidol | - | 1556 | 1561 | - |
| 17 | Spathulenol | 30.88 | 1577 | 1577 | 6.20 |
| 18 | Caryophyllene oxide | 31.08 | 1582 | 1582 | 30.50 |
| 19 | Humulene Epoxide II | 32.08 | 1608 | 1608 | 5.10 |
| 20 | 1,10-Di-epi-Cubenol | 32.34 | 1613 | 1618 | 1.87 |
| Identified total | 67.53 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matias, E.F.F.; Pereira, A.P.D.; Braz, A.V.d.O.; Rodrigues, M.C.; Silva, J.d.L.; Maia, P.A.A.; Santos, S.C.d.; Rebelo, R.A.; Begnini, I.M.; Silva, L.E.d.; et al. Chemical Profile and Biological Activities of Essential Oil from Piper arboreum for Development and Improvement of Mouthwash. Molecules 2022, 27, 6408. https://doi.org/10.3390/molecules27196408
Matias EFF, Pereira APD, Braz AVdO, Rodrigues MC, Silva JdL, Maia PAA, Santos SCd, Rebelo RA, Begnini IM, Silva LEd, et al. Chemical Profile and Biological Activities of Essential Oil from Piper arboreum for Development and Improvement of Mouthwash. Molecules. 2022; 27(19):6408. https://doi.org/10.3390/molecules27196408
Chicago/Turabian StyleMatias, Edinardo Fagner Ferreira, Ana Paula Dantas Pereira, Ana Valéria de Oliveira Braz, Mariana Carvalho Rodrigues, Jussara de Lima Silva, Philippe Alencar Araujo Maia, Sarah Castro dos Santos, Ricardo Andrade Rebelo, Ieda Maria Begnini, Luiz Everson da Silva, and et al. 2022. "Chemical Profile and Biological Activities of Essential Oil from Piper arboreum for Development and Improvement of Mouthwash" Molecules 27, no. 19: 6408. https://doi.org/10.3390/molecules27196408
APA StyleMatias, E. F. F., Pereira, A. P. D., Braz, A. V. d. O., Rodrigues, M. C., Silva, J. d. L., Maia, P. A. A., Santos, S. C. d., Rebelo, R. A., Begnini, I. M., Silva, L. E. d., Amaral, W. d., Kowalska, G., Rowiński, R., Hawlena, J., Kowalski, R., Coutinho, H. D. M., & Alencar, V. R. C. T. d. (2022). Chemical Profile and Biological Activities of Essential Oil from Piper arboreum for Development and Improvement of Mouthwash. Molecules, 27(19), 6408. https://doi.org/10.3390/molecules27196408






