Different Effects of Quercetin Glycosides and Quercetin on Kidney Mitochondrial Function—Uncoupling, Cytochrome C Reducing and Antioxidant Activity
Abstract
1. Introduction
2. Results
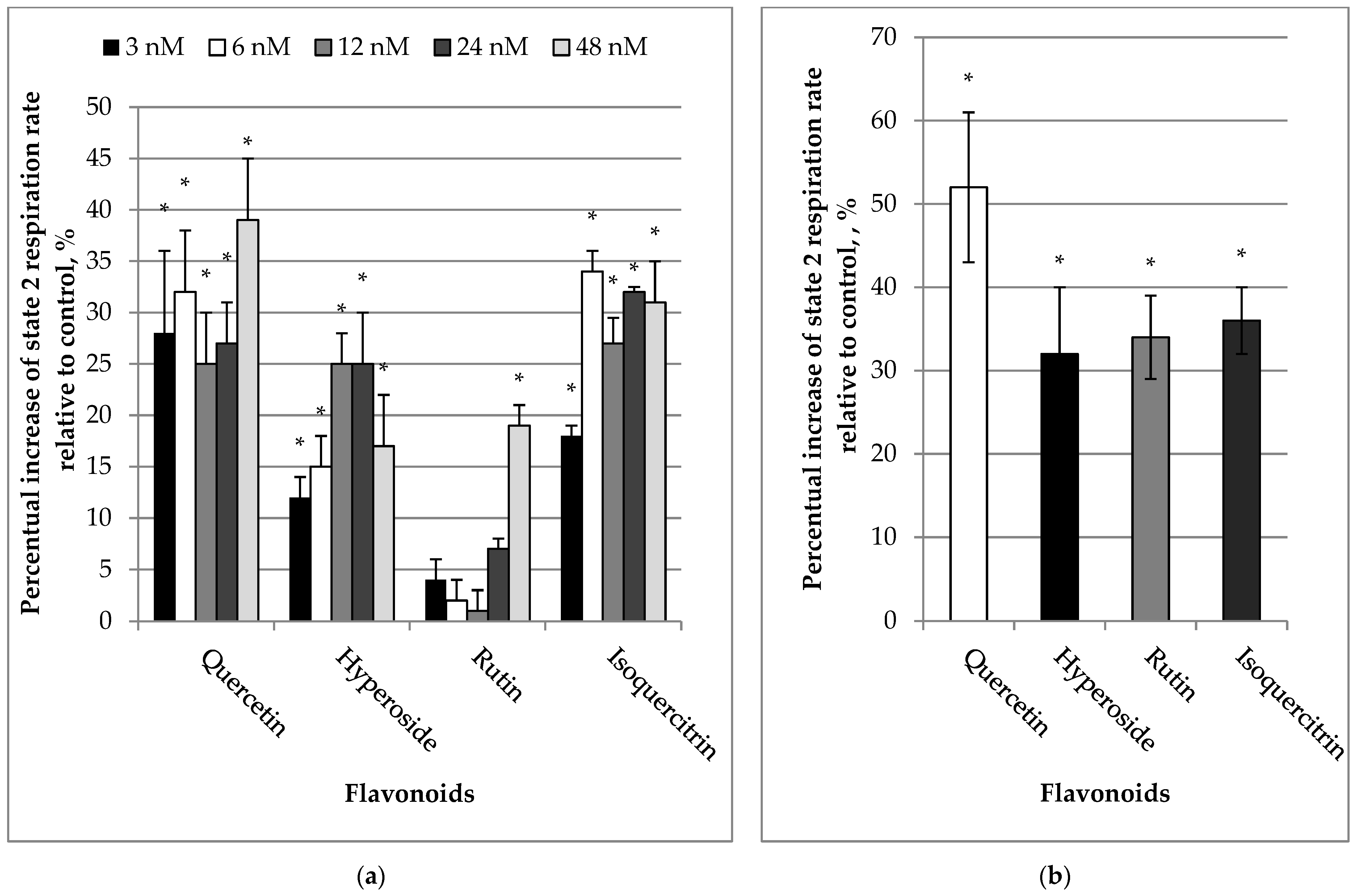
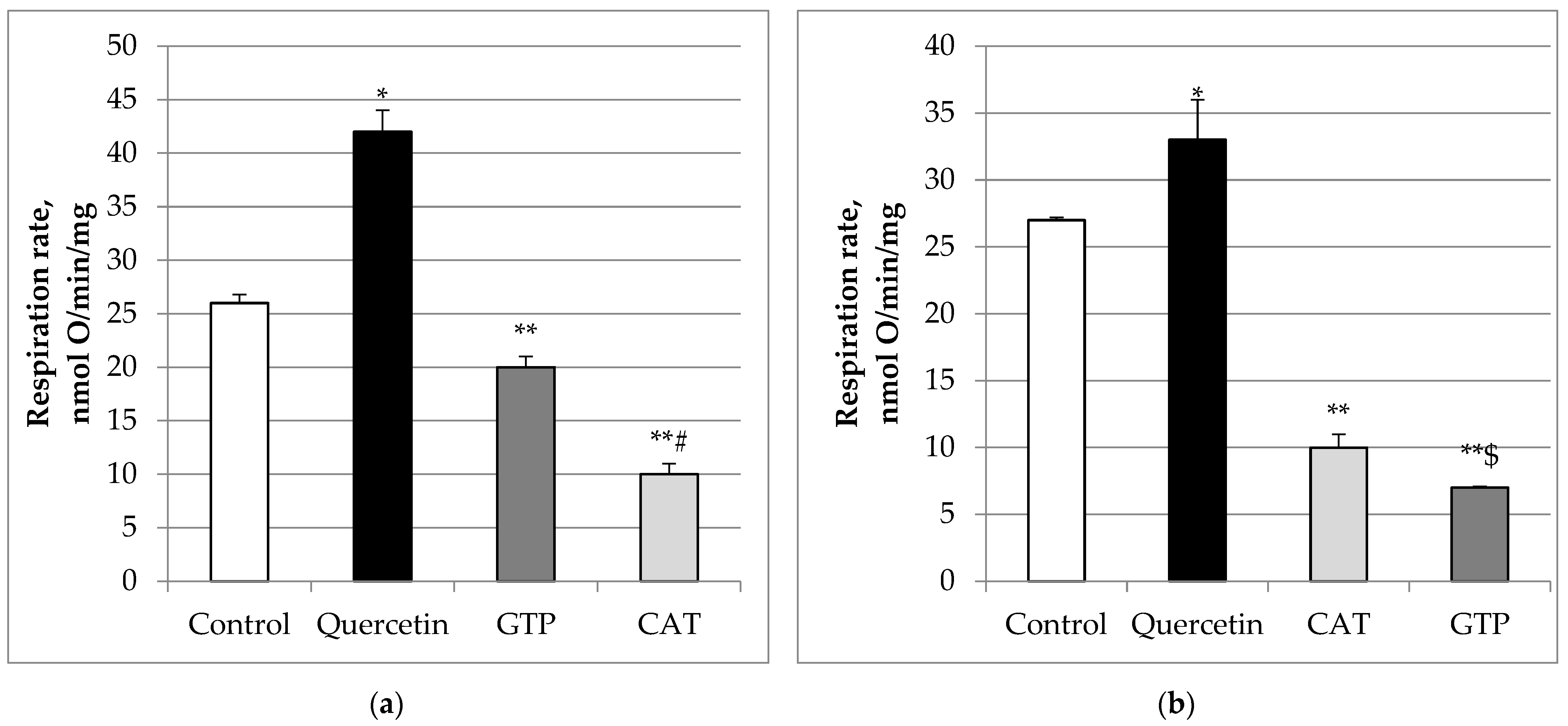
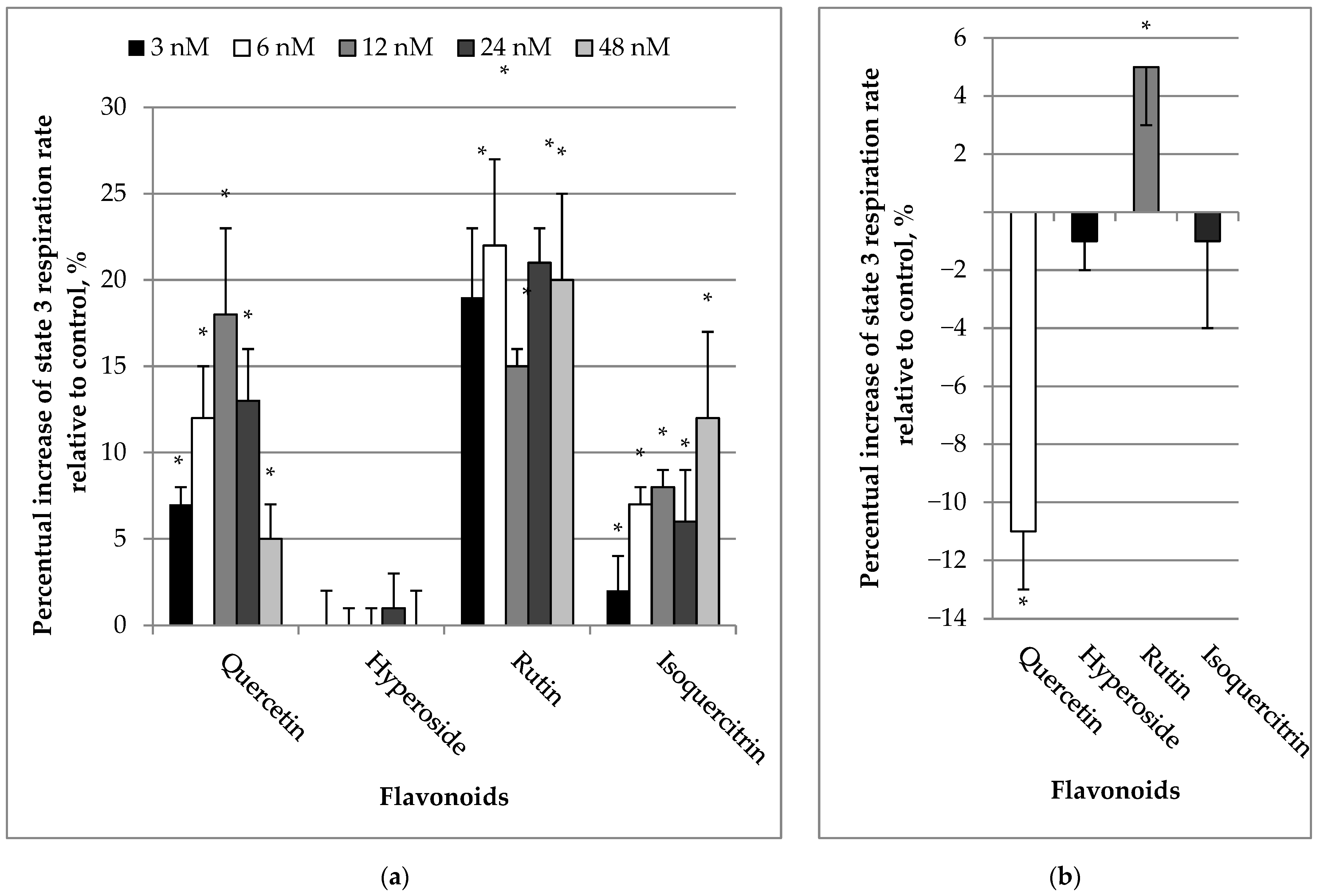
2.1. Effects of Quercetin and Its Glycosides on Mitochondrial Respiration Rates
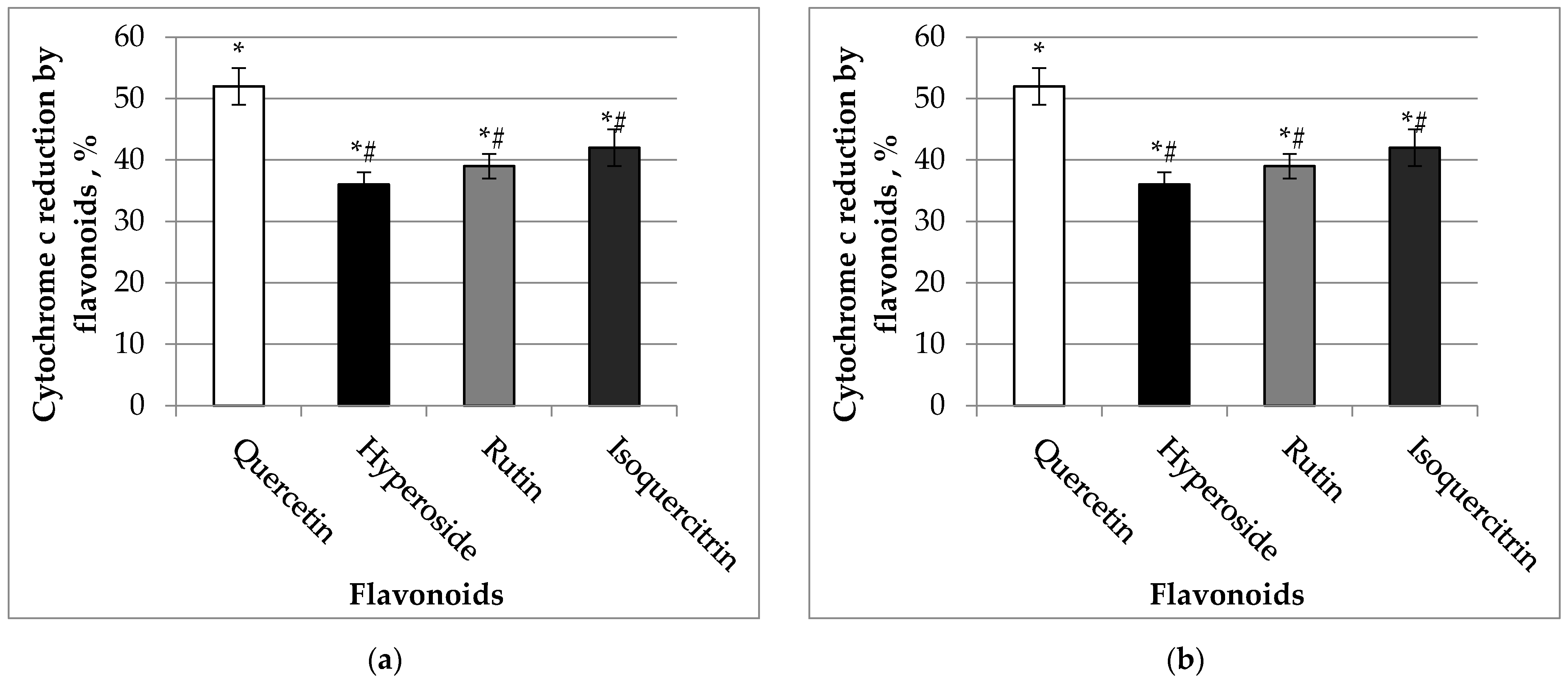
2.2. Effects of Quercetin and Its Glycosides on Reduction of Cytochrome C
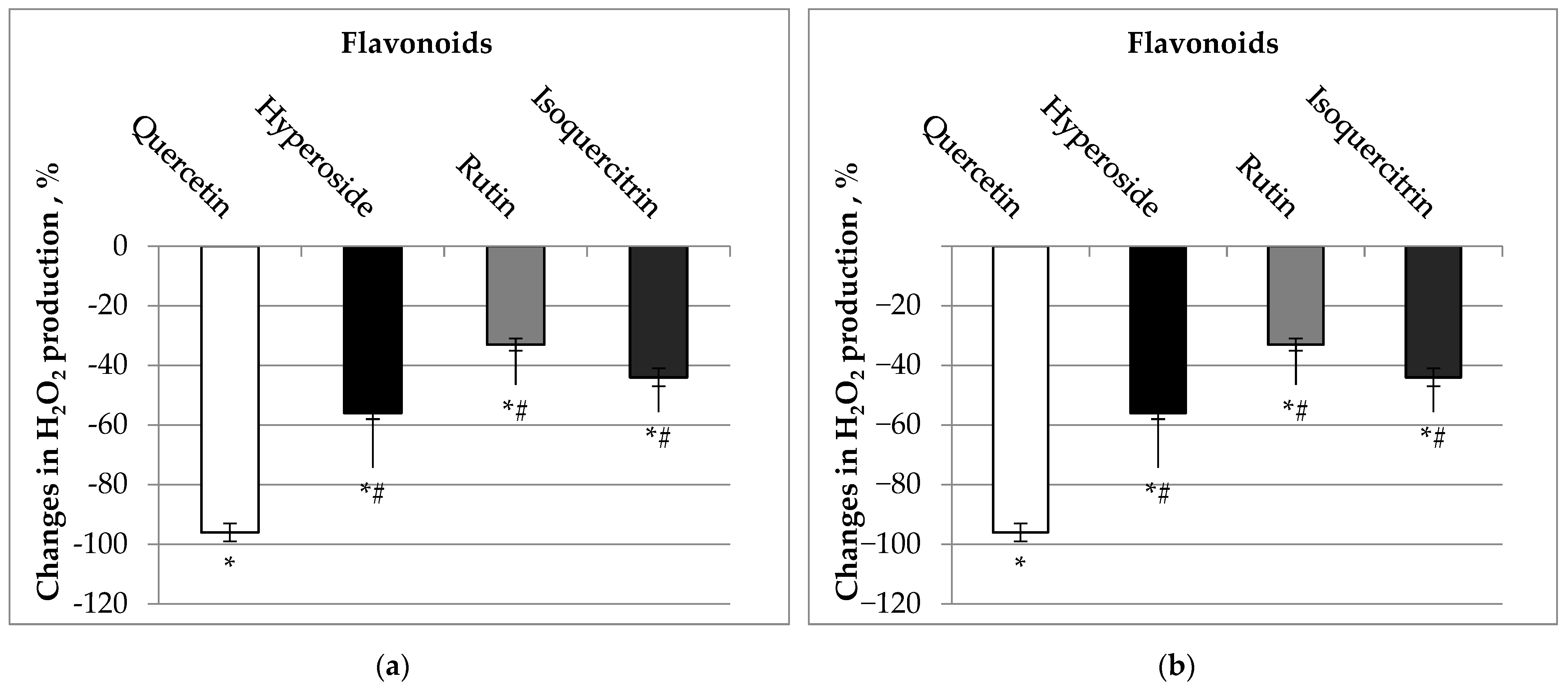
2.3. Effects of Quercetin and Its Glycosides on Mitochondrial Production of H2O2
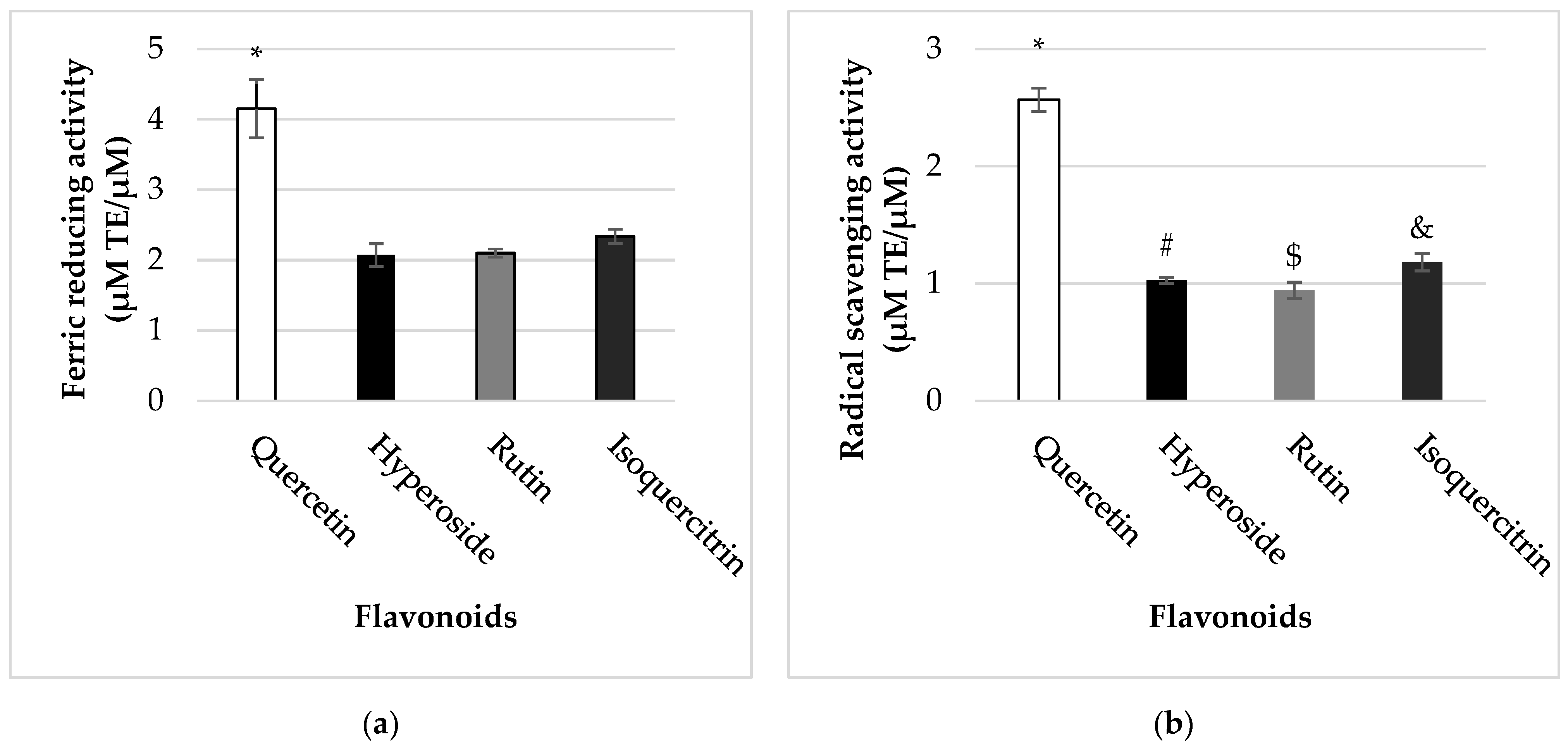
2.4. Radical Scavenging and Ferric Reducing Capacities of Quercetin and Its Glycosides
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of Isolated Kidney Mitochondria
4.3. Measurement of Mitochondrial Respiration
4.4. Measurement of Cytochrome C Reduction Level
4.5. Measurement of H2O2 Generation
4.6. On-Line Measurement of Flavonoids Antioxidant Activity Using HPLC-Post Column System
4.7. Statistical Data Processing Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xiao, J.; Kai, G.; Yamamoto, K.; Chen, X. Advance in Dietary Polyphenols as α-Glucosidases Inhibitors: A Review on Structure-Activity Relationship Aspect. Crit. Rev. Food Sci. Nutr. 2013, 53, 818–836. [Google Scholar] [CrossRef] [PubMed]
- Lamson, D.W.; Brignall, M.S. Antioxidants and Cancer, Part 3: Quercetin. Altern. Med. Rev. 2000, 5, 196–208. [Google Scholar] [PubMed]
- Hämäläinen, M.; Nieminen, R.; Vuorela, P.; Heinonen, M.; Moilanen, E. Anti-Inflammatory Effects of Flavonoids: Genistein, Kaempferol, Quercetin, and Daidzein Inhibit STAT-1 and NF-ΚB Activations, Whereas Flavone, Isorhamnetin, Naringenin, and Pelargonidin Inhibit Only NF-ΚB Activation along with Their Inhibitory Effect on INOS Expression and NO Production in Activated Macrophages. Mediat. Inflamm 2007, 2007, 45673. [Google Scholar] [CrossRef]
- García-Mediavilla, V.; Crespo, I.; Collado, P.S.; Esteller, A.; Sánchez-Campos, S.; Tuñón, M.J.; González-Gallego, J. The Anti-Inflammatory Flavones Quercetin and Kaempferol Cause Inhibition of Inducible Nitric Oxide Synthase, Cyclooxygenase-2 and Reactive C-Protein, and down-Regulation of the Nuclear Factor KappaB Pathway in Chang Liver Cells. Eur. J. Pharmacol. 2007, 557, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Oyewopo, A.O.; Adeleke, O.; Johnson, O.; Akingbade, A.; Olaniyi, K.S.; Areola, E.D.; Tokunbo, O. Regulatory Effects of Quercetin on Testicular Histopathology Induced by Cyanide in Wistar Rats. Heliyon 2021, 7, e07662. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, M.; Yu, L.; Zhao, Y.; He, N.; Yang, X. Antitumor Activities of Quercetin and Quercetin-5′,8-Disulfonate in Human Colon and Breast Cancer Cell Lines. Food Chem. Toxicol. 2012, 50, 1589–1599. [Google Scholar] [CrossRef]
- Shan, B.E.; Wang, M.X.; Li, R.Q. Quercetin Inhibit Human SW480 Colon Cancer Growth in Association with Inhibition of Cyclin D1 and Survivin Expression through Wnt/β-Catenin Signaling Pathway. Cancer Investig. 2009, 27, 604–612. [Google Scholar] [CrossRef]
- Hirai, I.; Okuno, M.; Katsuma, R.; Arita, N.; Tachibana, M.; Yamamoto, Y. Characterisation of Anti-Staphylococcus Aureus Activity of Quercetin. Int J Food Sci Technol 2010, 45, 1250–1254. [Google Scholar] [CrossRef]
- You, Q.; Chen, F.; Wang, X.; Jiang, Y.; Lin, S. Anti-Diabetic Activities of Phenolic Compounds in Muscadine against Alpha-Glucosidase and Pancreatic Lipase. LWT-Food Sci. Technol. 2012, 46, 164–168. [Google Scholar] [CrossRef]
- Jeong, S.M.; Kang, M.J.; Choi, H.N.; Kim, J.H.; Kim, J.I. Quercetin Ameliorates Hyperglycemia and Dyslipidemia and Improves Antioxidant Status in Type 2 Diabetic Db/Db Mice. Nutr. Res. Pract. 2012, 6, 201–207. [Google Scholar] [CrossRef]
- Kleemann, R.; Verschuren, L.; Morrison, M.; Zadelaar, S.; van Erk, M.J.; Wielinga, P.Y.; Kooistra, T. Anti-Inflammatory, Anti-Proliferative and Anti-Atherosclerotic Effects of Quercetin in Human in Vitro and in Vivo Models. Atherosclerosis 2011, 218, 44–52. [Google Scholar] [CrossRef] [PubMed]
- Annapurna, A.; Reddy, C.S.; Akondi, R.B.; Rao, S.R.C. Cardioprotective Actions of Two Bioflavonoids, Quercetin and Rutin, in Experimental Myocardial Infarction in Both Normal and Streptozotocin-Induced Type I Diabetic Rats. J. Pharm. Pharmacol. 2009, 61, 1365–1374. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.W.; Chou, H.C.; Lin, S.T.; Chen, Y.H.; Chang, Y.J.; Chen, L.; Chan, H.L. Cardioprotective Effects of Quercetin in Cardiomyocyte under Ischemia/Reperfusion Injury. Evidence-based Complement. Altern. Med. 2013, 2013, 364519. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Rodríguez, P.; Baquero, L.P.; Larrota, H.R. Flavonoids: Potential Therapeutic Agents by Their Antioxidant Capacity. In Bioactive Compounds: Health Benefits and Potential Applications; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Cai, Y.Z.; Sun, M.; Xing, J.; Luo, Q.; Corke, H. Structure-Radical Scavenging Activity Relationships of Phenolic Compounds from Traditional Chinese Medicinal Plants. Life Sci. 2006, 78, 2872–2888. [Google Scholar] [CrossRef]
- Russo, M.; Spagnuolo, C.; Tedesco, I.; Bilotto, S.; Russo, G.L. The Flavonoid Quercetin in Disease Prevention and Therapy: Facts and Fancies. Biochem. Pharmacol. 2012, 83, 6–15. [Google Scholar] [CrossRef]
- Chow, J.M.; Shen, S.C.; Huan, S.K.; Lin, H.Y.; Chen, Y.C. Quercetin, but Not Rutin and Quercitrin, Prevention of H2O2-Induced Apoptosis via Anti-Oxidant Activity and Heme Oxygenase 1 Gene Expression in Macrophages. Biochem. Pharmacol. 2005, 69, 1839–1851. [Google Scholar] [CrossRef]
- Brown, G.C.; Borutaite, V. Regulation of Apoptosis by the Redox State of Cytochrome c. Biochim. Biophys. Acta-Bioenerg. 2008, 1777, 877–881. [Google Scholar] [CrossRef]
- Trumbeckaite, S.; Bernatoniene, J.; Majiene, D.; Jakštas, V.; Savickas, A.; Toleikis, A. The Effect of Flavonoids on Rat Heart Mitochondrial Function. Biomed. Pharmacother. 2006, 60, 245–248. [Google Scholar] [CrossRef]
- Dorta, D.J.; Pigoso, A.A.; Mingatto, F.E.; Rodrigues, T.; Prado, I.M.R.; Helena, A.F.C.; Uyemura, S.A.; Santos, A.C.; Curti, C. The Interaction of Flavonoids with Mitochondria: Effects on Energetic Processes. Chem. Biol. Interact. 2005, 152, 67–78. [Google Scholar] [CrossRef]
- Dorta, D.J.; Pigoso, A.A.; Mingatto, F.E.; Rodrigues, T.; Pestana, C.R.; Uyemura, S.A.; Santos, A.C.; Curti, C. Antioxidant Activity of Flavonoids in Isolated Mitochondria. Phytother. Res. 2008, 22, 1213–1218. [Google Scholar] [CrossRef]
- Baliutyte, G.; Baniene, R.; Trumbeckaite, S.; Borutaite, V.; Toleikis, A. Effects of Ginkgo Biloba Extract on Heart and Liver Mitochondrial Functions: Mechanism(s) of Action. J. Bioenerg. Biomembr. 2010, 42, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lagoa, R.; Graziani, I.; Lopez-Sanchez, C.; Garcia-Martinez, V.; Gutierrez-Merino, C. Complex I and Cytochrome c Are Molecular Targets of Flavonoids That Inhibit Hydrogen Peroxide Production by Mitochondria. Biochim Biophys Acta Bioenerg 2011, 1807, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Heim, K.E.; Tagliaferro, A.R.; Bobilya, D.J. Flavonoid Antioxidants: Chemistry, Metabolism and Structure-Activity Relationships. J. Nutr. Biochem. 2002, 13, 572–584. [Google Scholar] [CrossRef]
- Amić, A.; Lučić, B.; Stepanić, V.; Marković, Z.; Marković, S.; Dimitrić Marković, J.M.; Amić, D. Free Radical Scavenging Potency of Quercetin Catecholic Colonic Metabolites: Thermodynamics of 2H+/2e− Processes. Food Chem. 2017, 218, 144–151. [Google Scholar] [CrossRef]
- Soobrattee, M.A.; Neergheen, V.S.; Luximon-Ramma, A.; Aruoma, O.I.; Bahorun, T. Phenolics as Potential Antioxidant Therapeutic Agents: Mechanism and Actions. Mutat. Res.-Fundam. Mol. Mech. Mutagen. 2005, 579, 200–213. [Google Scholar] [CrossRef]
- Baniene, R.; Trumbeckas, D.; Kincius, M.; Pauziene, N.; Raudone, L.; Jievaltas, M.; Trumbeckaite, S. Short Ischemia Induces Rat Kidney Mitochondria Dysfunction. J. Bioenerg. Biomembr. 2016, 48, 77–85. [Google Scholar] [CrossRef]
- Skemiene, K.; Rakauskaite, G.; Trumbeckaite, S.; Liobikas, J.; Brown, G.C.; Borutaite, V. Anthocyanins Block Ischemia-Induced Apoptosis in the Perfused Heart and Support Mitochondrial Respiration Potentially by Reducing Cytosolic Cytochrome c. Int. J. Biochem. Cell Biol. 2013, 45, 23–29. [Google Scholar] [CrossRef]
- Raudonis, R.; Raudone, L.; Jakstas, V.; Janulis, V. Comparative Evaluation of Post-Column Free Radical Scavenging and Ferric Reducing Antioxidant Power Assays for Screening of Antioxidants in Strawberries. J. Chromatogr. A 2012, 1233, 8–15. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zymone, K.; Benetis, R.; Trumbeckas, D.; Baseviciene, I.; Trumbeckaite, S. Different Effects of Quercetin Glycosides and Quercetin on Kidney Mitochondrial Function—Uncoupling, Cytochrome C Reducing and Antioxidant Activity. Molecules 2022, 27, 6377. https://doi.org/10.3390/molecules27196377
Zymone K, Benetis R, Trumbeckas D, Baseviciene I, Trumbeckaite S. Different Effects of Quercetin Glycosides and Quercetin on Kidney Mitochondrial Function—Uncoupling, Cytochrome C Reducing and Antioxidant Activity. Molecules. 2022; 27(19):6377. https://doi.org/10.3390/molecules27196377
Chicago/Turabian StyleZymone, Kristina, Raimondas Benetis, Darius Trumbeckas, Ingrida Baseviciene, and Sonata Trumbeckaite. 2022. "Different Effects of Quercetin Glycosides and Quercetin on Kidney Mitochondrial Function—Uncoupling, Cytochrome C Reducing and Antioxidant Activity" Molecules 27, no. 19: 6377. https://doi.org/10.3390/molecules27196377
APA StyleZymone, K., Benetis, R., Trumbeckas, D., Baseviciene, I., & Trumbeckaite, S. (2022). Different Effects of Quercetin Glycosides and Quercetin on Kidney Mitochondrial Function—Uncoupling, Cytochrome C Reducing and Antioxidant Activity. Molecules, 27(19), 6377. https://doi.org/10.3390/molecules27196377





