Quercetin’s Effects on Glutamate Cytotoxicity
Abstract
1. Introduction
2. Glutamate in the Central Nervous System
2.1. Role and Purpose
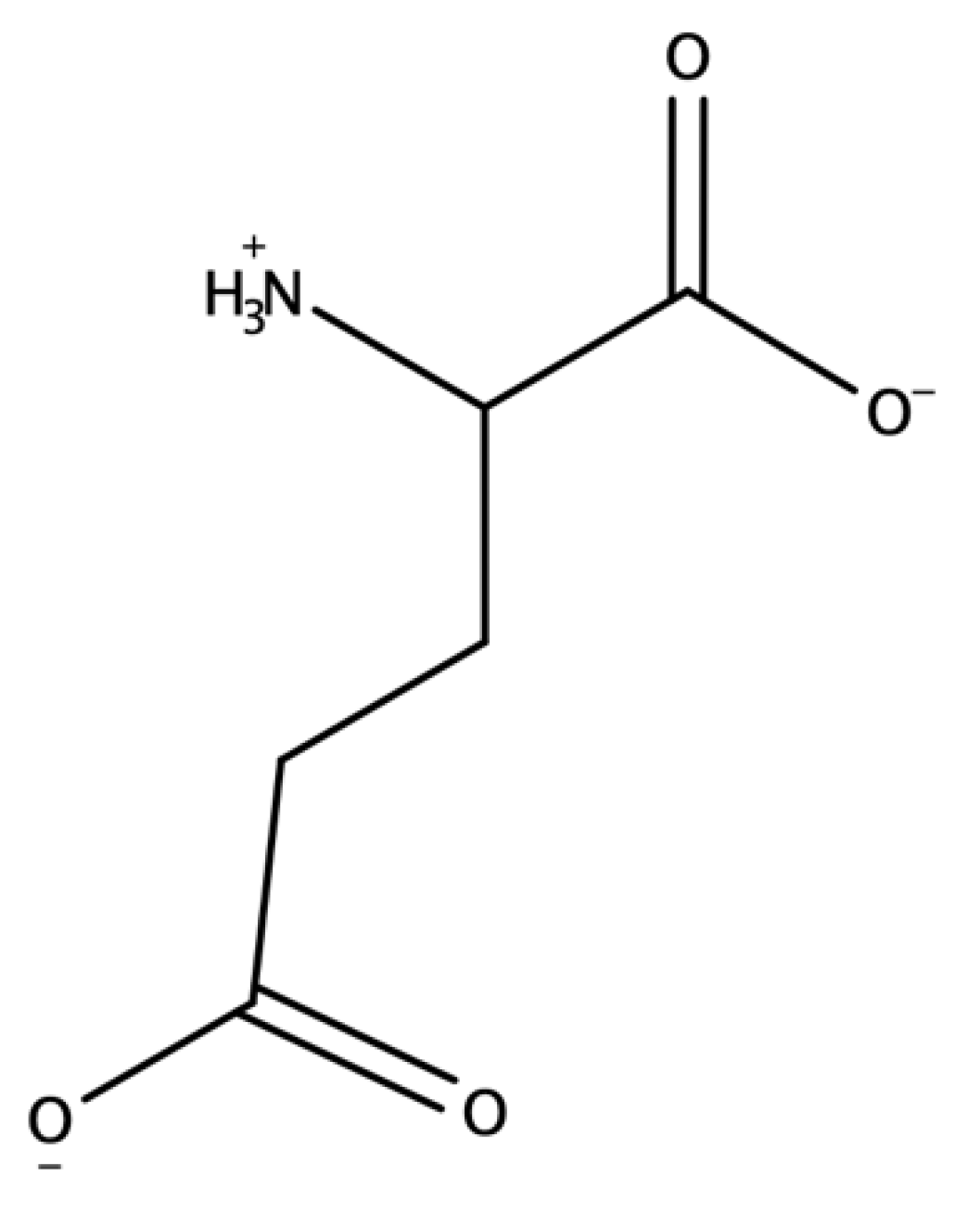
2.2. Metabolism
2.3. Receptors and Signaling
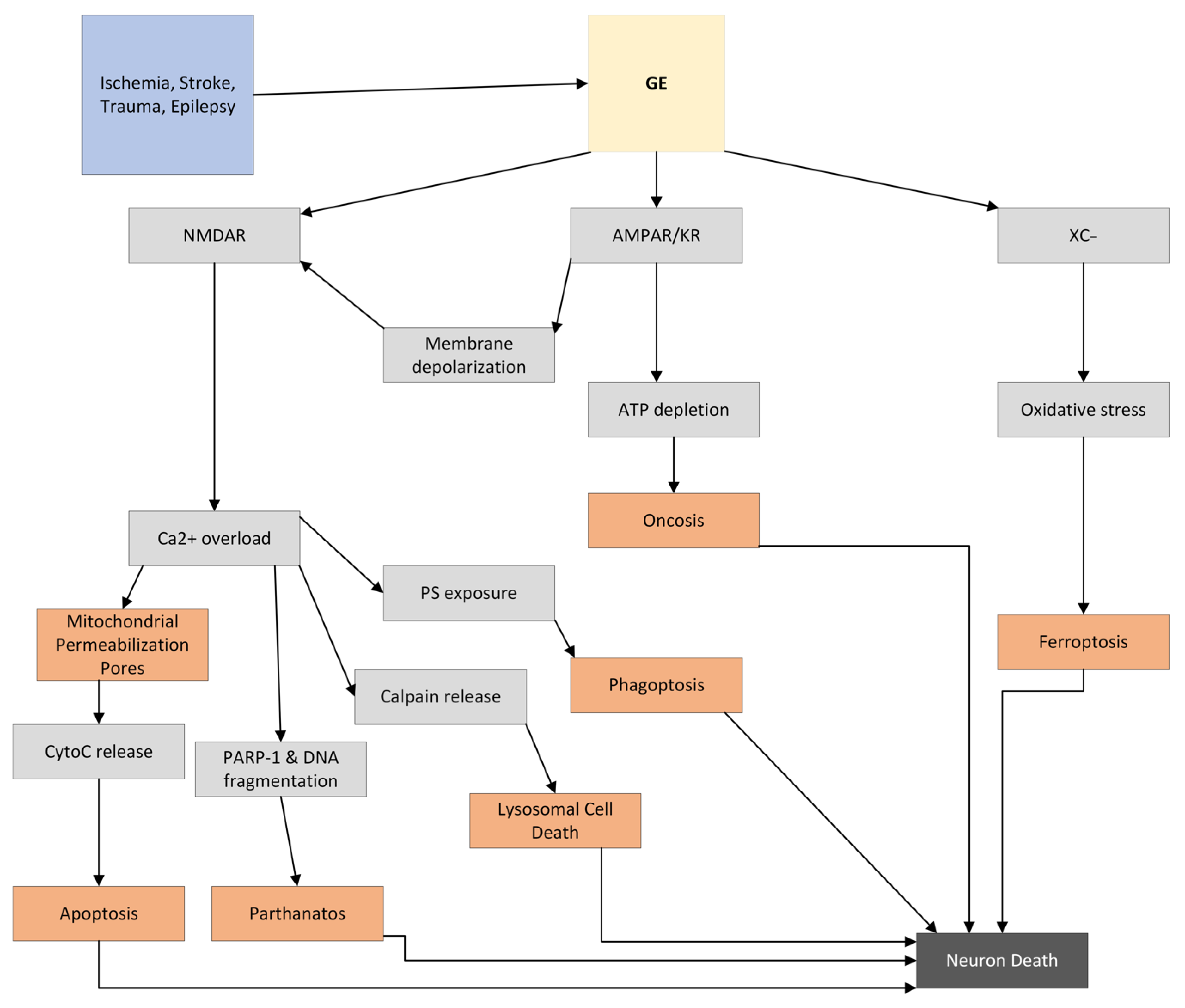
3. GE
3.1. Background
3.2. Importance of Calcium in GE
4. Quercetin
4.1. Background
4.2. Chemical Properties
4.3. Bioavailability and Metabolism
4.4. Quercetin’s Protective Mechanisms against Glutamate Excitotoxic Cell Death
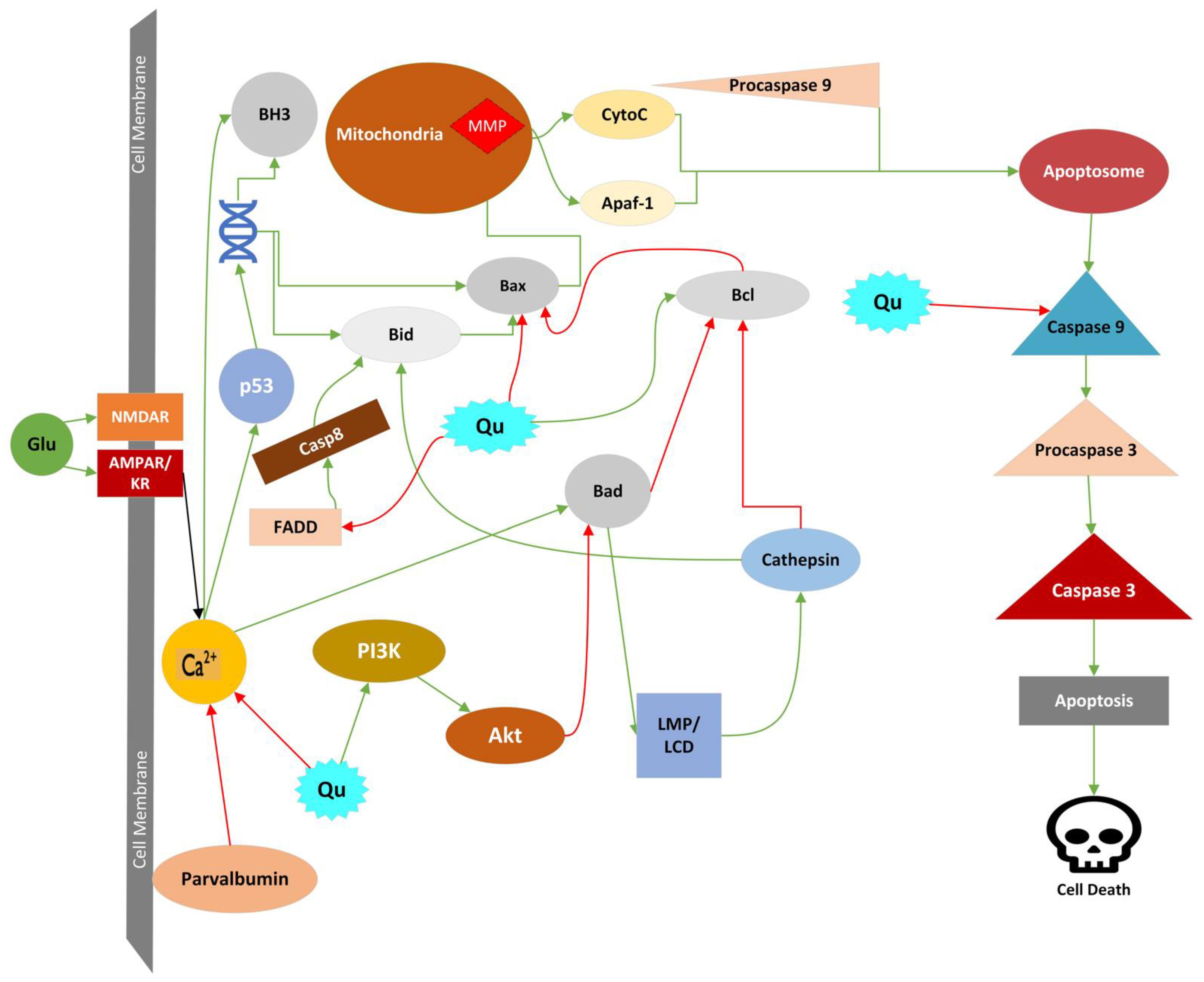
4.4.1. Apoptosis
4.4.2. Mitochondrial Permeability Transition
4.4.3. Lysosomal Cell Death (LCD)
4.4.4. Oncosis
4.4.5. Parthanatos
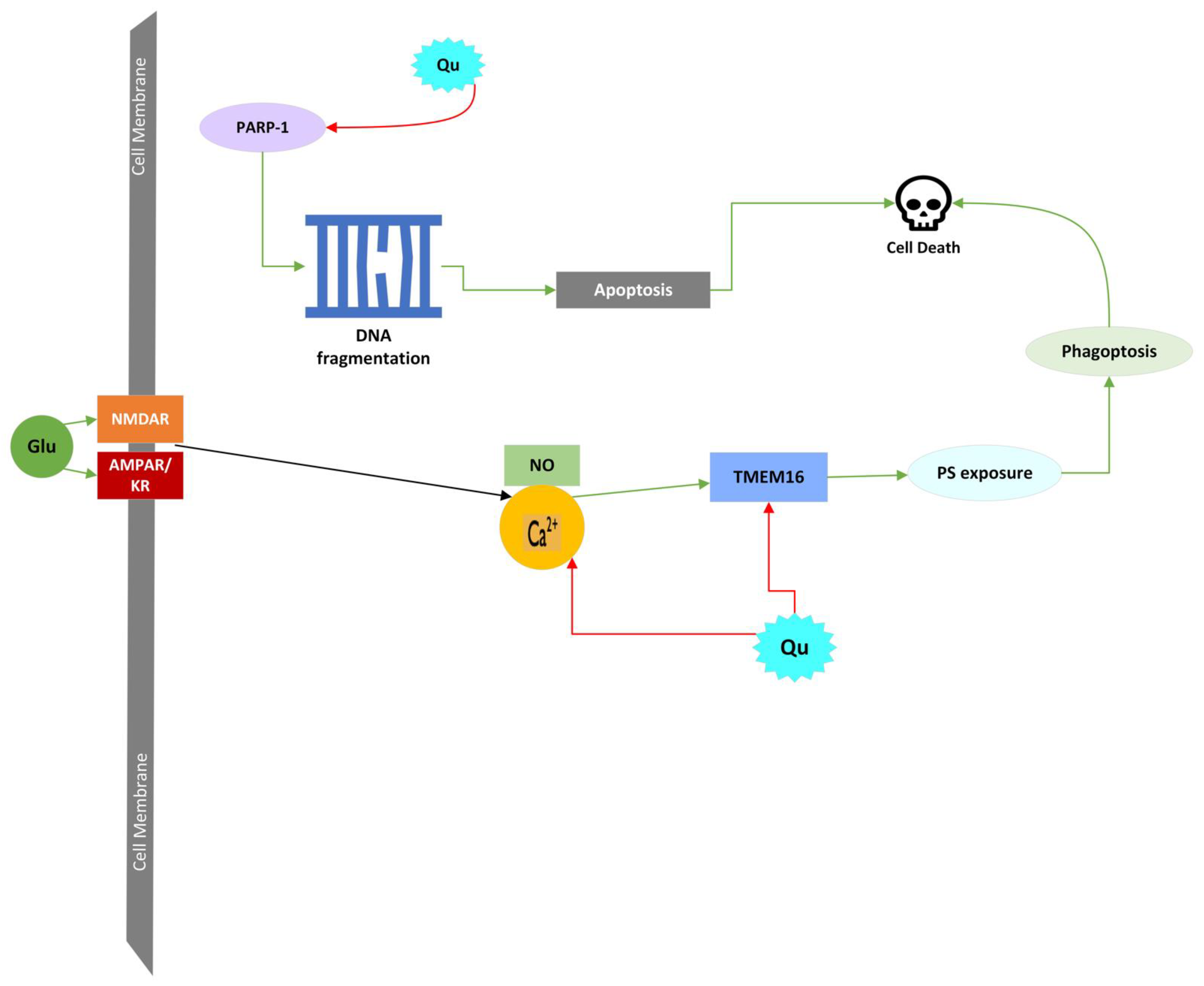
4.4.6. Phagoptosis
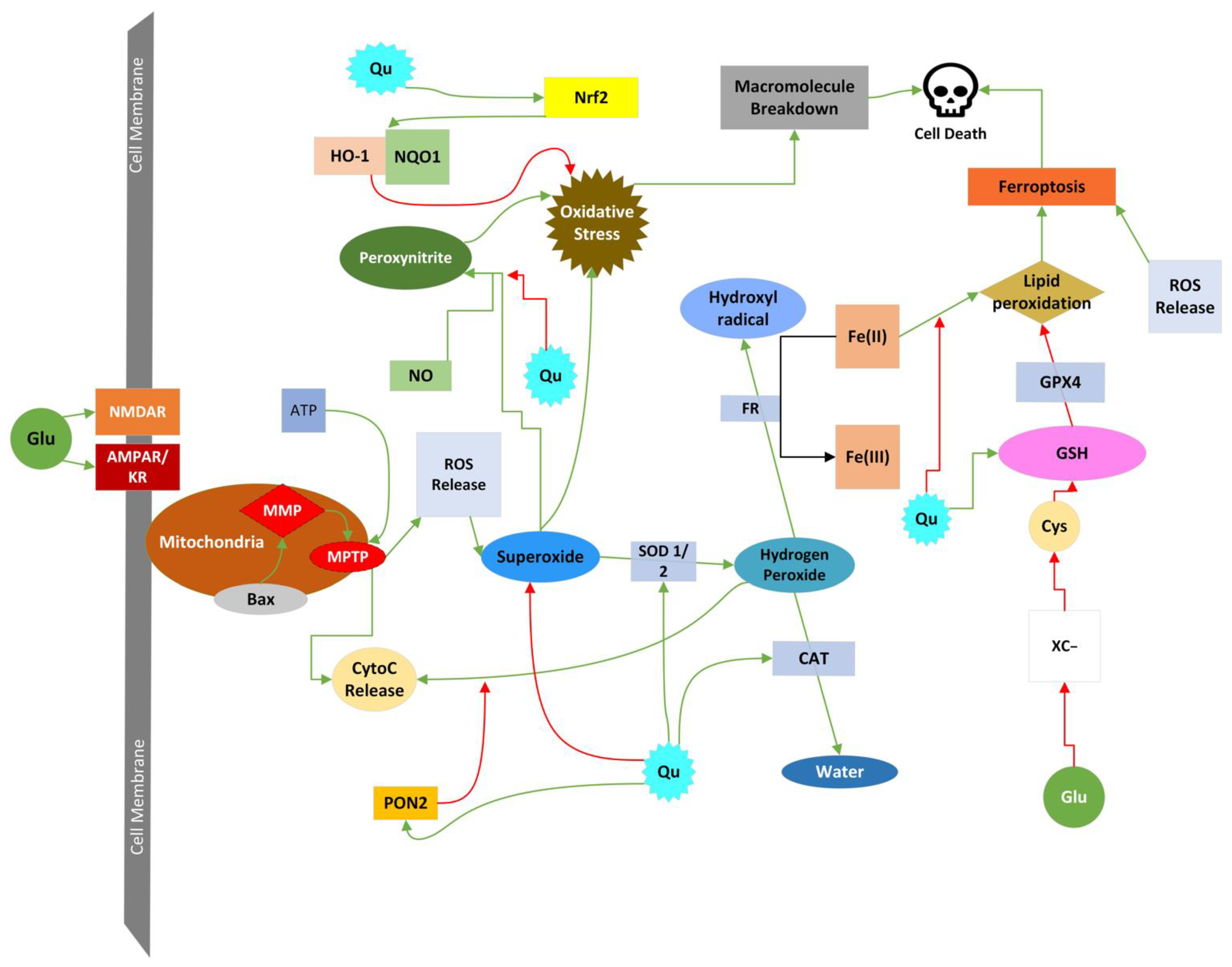
4.4.7. Ferroptosis
4.4.8. Cell Death Induced by ROS and RNS
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ishige, K.; Schubert, D.; Sagara, Y. Flavonoids Protect Neuronal Cells from Oxidative Stress by Three Distinct Mechanisms. Free Radic. Biol. Med. 2001, 30, 433–446. [Google Scholar] [CrossRef]
- Ezza, H.S.A.; Khadrawyb, Y.A. Glutamate Excitotoxicity and Neurodegeneration. J. Mol. Genet. Med. 2014, 8, 141. [Google Scholar] [CrossRef]
- McEntee, W.J.; Crook, T.H. Glutamate: Its Role in Learning, Memory, and the Aging Brain. Psychopharmacology 1993, 111, 391–401. [Google Scholar] [CrossRef]
- Mark, L.P.; Prost, R.W.; Ulmer, J.L.; Smith, M.M.; Daniels, D.L.; Strottmann, J.M.; Brown, W.D.; Hacein-Bey, L. Pictorial Review of Glutamate Excitotoxicity: Fundamental Concepts for Neuroimaging. AJNR Am. J. Neuroradiol. 2001, 22, 1813–1824. [Google Scholar] [PubMed]
- Watford, M. Glutamine and Glutamate: Nonessential or Essential Amino Acids? Anim. Nutr. 2015, 1, 119–122. [Google Scholar] [CrossRef] [PubMed]
- Luo, L. Principles of Neurobiology; Garland Science: New York, NY, USA, 2015; ISBN 978-0-8153-4492-6. [Google Scholar]
- Squire, L.R. (Ed.) Fundamental Neuroscience, 3rd ed.; Elsevier/Academic Press: Amsterdam, The Netherlands; Cambridge, MA, USA, 2008; ISBN 978-0-12-374019-9. [Google Scholar]
- Meriney, S.D.; Fanselow, E.E. Amino Acid Neurotransmitters. In Synaptic Transmission; Elsevier: Amsterdam, The Netherlands, 2019; pp. 399–419. ISBN 978-0-12-815320-8. [Google Scholar]
- Meldrum, B.S. Glutamate as a Neurotransmitter in the Brain: Review of Physiology and Pathology. J. Nutr. 2000, 130, 1007S–1015S. [Google Scholar] [CrossRef] [PubMed]
- Purves, D. (Ed.) Neuroscience, 5th ed.; Sinauer Associates: Sunderland, MA, USA, 2012; ISBN 978-0-87893-695-3. [Google Scholar]
- Jenner, P.; Caccia, C. The Role of Glutamate in the Healthy Brain and in the Pathophysiology of Parkinson’s Disease. Eur. Neurol. Rev. 2019, 14, 2–12. [Google Scholar]
- Andersen, J.V.; Markussen, K.H.; Jakobsen, E.; Schousboe, A.; Waagepetersen, H.S.; Rosenberg, P.A.; Aldana, B.I. Glutamate Metabolism and Recycling at the Excitatory Synapse in Health and Neurodegeneration. Neuropharmacology 2021, 196, 108719. [Google Scholar] [CrossRef]
- Meriney, S.D.; Fanselow, E.E. Ionotropic Receptors. In Synaptic Transmission; Elsevier: Amsterdam, The Netherlands, 2019; pp. 215–243. ISBN 978-0-12-815320-8. [Google Scholar]
- Dingledine, R.; Borges, K.; Bowie, D.; Traynelis, S.F. The Glutamate Receptor Ion Channels. Pharmacol. Rev. 1999, 51, 7. [Google Scholar]
- Lodge, D. The History of the Pharmacology and Cloning of Ionotropic Glutamate Receptors and the Development of Idiosyncratic Nomenclature. Neuropharmacology 2009, 56, 6–21. [Google Scholar] [CrossRef]
- Traynelis, S.F.; Wollmuth, L.P.; McBain, C.J.; Menniti, F.S.; Vance, K.M.; Ogden, K.K.; Hansen, K.B.; Yuan, H.; Myers, S.J.; Dingledine, R. Glutamate Receptor Ion Channels: Structure, Regulation, and Function. Pharmacol. Rev. 2010, 62, 405. [Google Scholar] [CrossRef] [PubMed]
- Cossart, R.; Epsztein, J.; Tyzio, R.; Becq, H.; Hirsch, J.; Ben-Ari, Y.; Crépel, V. Quantal Release of Glutamate Generates Pure Kainate and Mixed AMPA/Kainate EPSCs in Hippocampal Neurons. Neuron 2002, 35, 147–159. [Google Scholar] [CrossRef]
- Iacobucci, G.J.; Popescu, G.K. NMDA Receptors: Linking Physiological Output to Biophysical Operation. Nat. Rev. Neurosci. 2017, 18, 236–249. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, Y.; Qin, Z. Molecular Mechanisms of Excitotoxicity and Their Relevance to Pathogenesis of Neurodegenerative Diseases. Acta Pharmacol. Sin. 2009, 30, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Hollmann, M.; Hartley, M.; Heinemann, S. Ca2+ Permeability of KA-AMPA—Gated Glutamate Receptor Channels Depends on Subunit Composition. Science 1991, 252, 851–853. [Google Scholar] [CrossRef] [PubMed]
- Lucas, D.R.; Newhouse, J.P. The Toxic Effect of Sodium L-Glutamate on the Inner Layers of the Retina. AMA Arch. Ophthalmol. 1957, 58, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Park, E.; Bell, J.D.; Baker, A.J. Traumatic Brain Injury: Can the Consequences Be Stopped? Can. Med. Assoc. J. 2008, 178, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Tannenberg, R.; Scott, H.; Westphalen, R.; Dodd, P. The Identification and Characterization of Excitotoxic Nerve-Endings in Alzheimer Disease. CAR 2004, 1, 11–25. [Google Scholar] [CrossRef]
- Verma, M.; Wills, Z.; Chu, C.T. Excitatory Dendritic Mitochondrial Calcium Toxicity: Implications for Parkinson’s and Other Neurodegenerative Diseases. Front. Neurosci. 2018, 12, 523. [Google Scholar] [CrossRef]
- Warby, S.C.; Doty, C.N.; Graham, R.K.; Carroll, J.B.; Yang, Y.-Z.; Singaraja, R.R.; Overall, C.M.; Hayden, M.R. Activated Caspase-6 and Caspase-6-Cleaved Fragments of Huntingtin Specifically Colocalize in the Nucleus. Hum. Mol. Genet. 2008, 17, 2390–2404. [Google Scholar] [CrossRef]
- Girling, K.D.; Demers, M.-J.; Laine, J.; Zhang, S.; Wang, Y.T.; Graham, R.K. Activation of Caspase-6 and Cleavage of Caspase-6 Substrates Is an Early Event in NMDA Receptor-Mediated Excitotoxicity. J. Neurosci. Res. 2018, 96, 391–406. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P. Excitotoxic and Excitoprotective Mechanisms: Abundant Targets for the Prevention and Treatment of Neurodegenerative Disorders. NeuroMol. Med. 2003, 3, 65–94. [Google Scholar] [CrossRef]
- Belov Kirdajova, D.; Kriska, J.; Tureckova, J.; Anderova, M. Ischemia-Triggered Glutamate Excitotoxicity From the Perspective of Glial Cells. Front. Cell Neurosci. 2020, 14, 51. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.W. Excitotoxicity: Still Hammering the Ischemic Brain in 2020. Front. Neurosci. 2020, 14, 579953. [Google Scholar] [CrossRef]
- Gagliardi, R.J. Neuroprotection, Excitotoxicicity and Nmda Antagonists. Arq. Neuro-Psiquiatr. 2000, 58, 583–588. [Google Scholar] [CrossRef]
- Yang, D.; Wang, T.; Long, M.; Li, P. Quercetin: Its Main Pharmacological Activity and Potential Application in Clinical Medicine. Oxidative Med. Cell Longev. 2020, 2020, 8825387. [Google Scholar] [CrossRef]
- Marunaka, Y.; Marunaka, R.; Sun, H.; Yamamoto, T.; Kanamura, N.; Inui, T.; Taruno, A. Actions of Quercetin, a Polyphenol, on Blood Pressure. Molecules 2017, 22, 209. [Google Scholar] [CrossRef]
- Williamson, G.; Manach, C. Bioavailability and Bioefficacy of Polyphenols in Humans. II. Review of 93 Intervention Studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef]
- Bhagwat, S.; Haytowitz, D.B.; Holden, J.M. USDA Database for the Flavonoid Content of Selected Foods. Available online: https://data.nal.usda.gov/dataset/usda-database-flavonoid-content-selected-foods-release-32-november-2015 (accessed on 17 October 2022).
- Crozier, A.; Del Rio, D.; Clifford, M.N. Bioavailability of Dietary Flavonoids and Phenolic Compounds. Mol. Asp. Med. 2010, 31, 446–467. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.; Wang, S.; Liu, H.; Yin, Y. Quercetin, Inflammation and Immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic Insights and Perspectives Involved in Neuroprotective Action of Quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef] [PubMed]
- Graefe, E.U.; Derendorf, H.; Veit, M. Pharmacokinetics and Bioavailability of the Flavonol Quercetin in Humans. Int. J. Clin. Pharmacol. Ther. 1999, 37, 219–233. [Google Scholar] [PubMed]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara-Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from Shallots (Allium Cepa L. Var. Aggregatum) Is More Bioavailable Than Its Glucosides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ma, J.; Yang, F.; Li, S.; Ma, W.; Chang, X.; Yang, L. Neuroprotective Effects of Quercetin on Ischemic Stroke: A Literature Review. Front. Pharmacol. 2022, 13, 854249. [Google Scholar] [CrossRef]
- Kerr, J.F.R.; Wyllie, A.H.; Currie, A.R. Apoptosis: A Basic Biological Phenomenon with Wideranging Implications in Tissue Kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef]
- Yang, J.-L.; Mukda, S.; Chen, S.-D. Diverse Roles of Mitochondria in Ischemic Stroke. Redox Biol. 2018, 16, 263–275. [Google Scholar] [CrossRef]
- Fricker, M.; Tolkovsky, A.M.; Borutaite, V.; Coleman, M.; Brown, G.C. Neuronal Cell Death. Physiol. Rev. 2018, 98, 813–880. [Google Scholar] [CrossRef]
- Fujikawa, D.G. The Role of Excitotoxic Programmed Necrosis in Acute Brain Injury. Comput. Struct. Biotechnol. J. 2015, 13, 212–221. [Google Scholar] [CrossRef]
- Shen, Z.; Xiang, M.; Chen, C.; Ding, F.; Wang, Y.; Shang, C.; Xin, L.; Zhang, Y.; Cui, X. Glutamate Excitotoxicity: Potential Therapeutic Target for Ischemic Stroke. Biomed. Pharmacother. 2022, 151, 113125. [Google Scholar] [CrossRef]
- Li, W.; Chen, Z.; Yan, M.; He, P.; Chen, Z.; Dai, H. The Protective Role of Isorhamnetin on Human Brain Microvascular Endothelial Cells from Cytotoxicity Induced by Methylglyoxal and Oxygen-Glucose Deprivation. J. Neurochem. 2016, 136, 651–659. [Google Scholar] [CrossRef]
- Dewson, G.; Ma, S.; Frederick, P.; Hockings, C.; Tan, I.; Kratina, T.; Kluck, R.M. Bax Dimerizes via a Symmetric BH3:Groove Interface during Apoptosis. Cell Death Differ. 2012, 19, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Gavathiotis, E.; Reyna, D.E.; Davis, M.L.; Bird, G.H.; Walensky, L.D. BH3-Triggered Structural Reorganization Drives the Activation of Proapoptotic BAX. Mol. Cell 2010, 40, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.; Tu, H.-C.; Ren, D.; Takeuchi, O.; Jeffers, J.R.; Zambetti, G.P.; Hsieh, J.J.-D.; Cheng, E.H.-Y. Stepwise Activation of BAX and BAK by TBID, BIM, and PUMA Initiates Mitochondrial Apoptosis. Mol. Cell 2009, 36, 487–499. [Google Scholar] [CrossRef] [PubMed]
- D’Orsi, B.; Bonner, H.; Tuffy, L.P.; Dussmann, H.; Woods, I.; Courtney, M.J.; Ward, M.W.; Prehn, J.H.M. Calpains Are Downstream Effectors of Bax-Dependent Excitotoxic Apoptosis. J. Neurosci. 2012, 32, 1847–1858. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.K.W. Calpain and Caspase: Can You Tell the Difference? Trends Neurosci. 2000, 23, 20–26. [Google Scholar] [CrossRef]
- Jiang, X.; Li, L.; Ying, Z.; Pan, C.; Huang, S.; Li, L.; Dai, M.; Yan, B.; Li, M.; Jiang, H.; et al. A Small Molecule That Protects the Integrity of the Electron Transfer Chain Blocks the Mitochondrial Apoptotic Pathway. Mol. Cell 2016, 63, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Kanehisa, M. Toward Understanding the Origin and Evolution of Cellular Organisms. Protein Sci. 2019, 28, 1947–1951. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Ishiguro-Watanabe, M.; Tanabe, M. KEGG: Integrating Viruses and Cellular Organisms. Nucleic Acids Res. 2021, 49, D545–D551. [Google Scholar] [CrossRef]
- Park, D.-J.; Jeon, S.-J.; Kang, J.-B.; Koh, P.-O. Quercetin Reduces Ischemic Brain Injury by Preventing Ischemia-Induced Decreases in the Neuronal Calcium Sensor Protein Hippocalcin. Neuroscience 2020, 430, 47–62. [Google Scholar] [CrossRef]
- Pei, B.; Yang, M.; Qi, X.; Shen, X.; Chen, X.; Zhang, F. Quercetin Ameliorates Ischemia/Reperfusion-Induced Cognitive Deficits by Inhibiting ASK1/JNK3/Caspase-3 by Enhancing the Akt Signaling Pathway. Biochem. Biophys. Res. Commun. 2016, 478, 199–205. [Google Scholar] [CrossRef] [PubMed]
- Nakayama, M.; Aihara, M.; Chen, Y.-N.; Araie, M.; Tomita-Yokotani, K.; Iwashina, T. Neuroprotective Effects of Flavonoids on Hypoxia-, Glutamate-, and Oxidative Stress-Induced Retinal Ganglion Cell Death. Mol. Vis. 2011, 17, 1784–1793. [Google Scholar] [PubMed]
- Kook, D.; Wolf, A.H.; Yu, A.L.; Neubauer, A.S.; Priglinger, S.G.; Kampik, A.; Welge-Lu¨ssen, U.C. The Protective Effect of Quercetin against Oxidative Stress in the Human RPE In Vitro. Investig. Ophthalmol. Vis. Sci. 2008, 49, 1712. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-Y.; Chang, C.-Y.; Lin, S.-Y.; Wang, J.-D.; Wu, C.-C.; Chen, W.-Y.; Kuan, Y.-H.; Liao, S.-L.; Wang, W.-Y.; Chen, C.-J. Quercetin Protects against Cerebral Ischemia/Reperfusion and Oxygen Glucose Deprivation/Reoxygenation Neurotoxicity. J. Nutr. Biochem. 2020, 83, 108436. [Google Scholar] [CrossRef]
- Pandey, A.; Shukla, S.; Bhattacharya, P.; Patnaik, R. A Possible Therapeutic Potential of Quercetin through Inhibition of μ-Calpain in Hypoxia Induced Neuronal Injury: A Molecular Dynamics Simulation Study. Neural Regen. Res. 2016, 11, 1247. [Google Scholar] [CrossRef]
- Gardai, S.J.; McPhillips, K.A.; Frasch, S.C.; Janssen, W.J.; Starefeldt, A.; Murphy-Ullrich, J.E.; Bratton, D.L.; Oldenborg, P.-A.; Michalak, M.; Henson, P.M. Cell-Surface Calreticulin Initiates Clearance of Viable or Apoptotic Cells through Trans-Activation of LRP on the Phagocyte. Cell 2005, 123, 321–334. [Google Scholar] [CrossRef]
- Park, D.-J.; Kang, J.-B.; Shah, F.-A.; Koh, P.-O. Quercetin Attenuates the Reduction of Parvalbumin in Middle Cerebral Artery Occlusion Animal Model. Lab. Anim. Res. 2021, 37, 9. [Google Scholar] [CrossRef]
- Ghosh, A.; Sarkar, S.; Mandal, A.K.; Das, N. Neuroprotective Role of Nanoencapsulated Quercetin in Combating Ischemia-Reperfusion Induced Neuronal Damage in Young and Aged Rats. PLoS ONE 2013, 8, e57735. [Google Scholar] [CrossRef]
- Alberts, B. Molecular Biology of the Cell, 6th ed.; Garland Science, Taylor and Francis Group: New York, NY, USA, 2015; ISBN 978-0-8153-4432-2. [Google Scholar]
- Aits, S.; Jäättelä, M. Lysosomal Cell Death at a Glance. J. Cell Sci. 2013, 126, 1905–1912. [Google Scholar] [CrossRef]
- Fogarty, M.P.; McCormack, R.M.; Noonan, J.; Murphy, D.; Gowran, A.; Campbell, V.A. A Role for P53 in the β-Amyloid-Mediated Regulation of the Lysosomal System. Neurobiol. Aging 2010, 31, 1774–1786. [Google Scholar] [CrossRef]
- Gowran, A.; Campbell, V.A. A Role for P53 in the Regulation of Lysosomal Permeability by Δ 9-Tetrahydrocannabinol in Rat Cortical Neurones: Implications for Neurodegeneration. J. Neurochem. 2008, 105, 1513–1524. [Google Scholar] [CrossRef] [PubMed]
- Bové, J.; Martínez-Vicente, M.; Dehay, B.; Perier, C.; Recasens, A.; Bombrun, A.; Antonsson, B.; Vila, M. BAX Channel Activity Mediates Lysosomal Disruption Linked to Parkinson Disease. Autophagy 2014, 10, 889–900. [Google Scholar] [CrossRef] [PubMed]
- Windelborn, J.A.; Lipton, P. Lysosomal Release of Cathepsins Causes Ischemic Damage in the Rat Hippocampal Slice and Depends on NMDA-Mediated Calcium Influx, Arachidonic Acid Metabolism, and Free Radical Production. J. Neurochem. 2008, 106, 56–69. [Google Scholar] [CrossRef] [PubMed]
- Majno, G.; Joris, I. Apoptosis, Oncosis, and Necrosis. An Overview of Cell Death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar]
- Weerasinghe, P.; Buja, L.M. Oncosis: An Important Non-Apoptotic Mode of Cell Death. Exp. Mol. Pathol. 2012, 93, 302–308. [Google Scholar] [CrossRef]
- Luoma, J.I.; Kelley, B.G.; Mermelstein, P.G. Progesterone Inhibition of Voltage-Gated Calcium Channels Is a Potential Neuroprotective Mechanism against Excitotoxicity. Steroids 2011, 76, 845–855. [Google Scholar] [CrossRef]
- Fatokun, A.A.; Dawson, V.L.; Dawson, T.M. Parthanatos: Mitochondrial-Linked Mechanisms and Therapeutic Opportunities: Players in Parthanatos. Br. J. Pharmacol. 2014, 171, 2000–2016. [Google Scholar] [CrossRef]
- Alano, C.C.; Garnier, P.; Ying, W.; Higashi, Y.; Kauppinen, T.M.; Swanson, R.A. NAD+ Depletion Is Necessary and Sufficient ForPoly(ADP-Ribose) Polymerase-1-Mediated Neuronal Death. J. Neurosci. 2010, 30, 2967–2978. [Google Scholar] [CrossRef]
- Andrabi, S.A.; Kim, N.S.; Yu, S.-W.; Wang, H.; Koh, D.W.; Sasaki, M.; Klaus, J.A.; Otsuka, T.; Zhang, Z.; Koehler, R.C.; et al. Poly(ADP-Ribose) (PAR) Polymer Is a Death Signal. Proc. Natl. Acad. Sci. USA 2006, 103, 18308–18313. [Google Scholar] [CrossRef]
- Eliasson, M.J.L.; Sampei, K.; Mandir, A.S.; Hurn, P.D.; Traystman, R.J.; Bao, J.; Pieper, A.; Wang, Z.-Q.; Dawson, T.M.; Snyder, S.H.; et al. Poly(ADP-Ribose) Polymerase Gene Disruption Renders Mice Resistant to Cerebral Ischemia. Nat. Med. 1997, 3, 1089–1095. [Google Scholar] [CrossRef]
- Park, D.-J.; Shah, F.-A.; Koh, P.-O. Quercetin Attenuates Neuronal Cells Damage in a Middle Cerebral Artery Occlusion Animal Model. J. Vet. Med. Sci. 2018, 80, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Khan, M.; Hoda, M.; Raza, S.S.; Khan, M.B.; Javed, H.; Ishrat, T.; Ashafaq, M.; Ahmad, M.; Safhi, M.M.; et al. Quercetin Protects Against Oxidative Stress Associated Damages in a Rat Model of Transient Focal Cerebral Ischemia and Reperfusion. Neurochem. Res. 2011, 36, 1360–1371. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Imanishi, E.; Nagata, S. Xkr8 Phospholipid Scrambling Complex in Apoptotic Phosphatidylserine Exposure. Proc. Natl. Acad. Sci. USA 2016, 113, 9509–9514. [Google Scholar] [CrossRef] [PubMed]
- Segawa, K.; Kurata, S.; Yanagihashi, Y.; Brummelkamp, T.R.; Matsuda, F.; Nagata, S. Caspase-Mediated Cleavage of Phospholipid Flippase for Apoptotic Phosphatidylserine Exposure. Science 2014, 344, 1164–1168. [Google Scholar] [CrossRef]
- Suzuki, J.; Fujii, T.; Imao, T.; Ishihara, K.; Kuba, H.; Nagata, S. Calcium-Dependent Phospholipid Scramblase Activity of TMEM16 Protein Family Members. J. Biol. Chem. 2013, 288, 13305–13316. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, J.; Umeda, M.; Sims, P.J.; Nagata, S. Calcium-Dependent Phospholipid Scrambling by TMEM16F. Nature 2010, 468, 834–838. [Google Scholar] [CrossRef] [PubMed]
- Neher, J.J.; Neniskyte, U.; Zhao, J.-W.; Bal-Price, A.; Tolkovsky, A.M.; Brown, G.C. Inhibition of Microglial Phagocytosis Is Sufficient To Prevent Inflammatory Neuronal Death. J. Immunol. 2011, 186, 4973–4983. [Google Scholar] [CrossRef]
- Fricker, M.; Neher, J.J.; Zhao, J.-W.; Thery, C.; Tolkovsky, A.M.; Brown, G.C. MFG-E8 Mediates Primary Phagocytosis of Viable Neurons during Neuroinflammation. J. Neurosci. 2012, 32, 2657–2666. [Google Scholar] [CrossRef]
- Neher, J.J.; Emmrich, J.V.; Fricker, M.; Mander, P.K.; Théry, C.; Brown, G.C. Phagocytosis Executes Delayed Neuronal Death after Focal Brain Ischemia. Proc. Natl. Acad. Sci. USA 2013, 110, E4098–E4107. [Google Scholar] [CrossRef]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An Iron-Dependent Form of Nonapoptotic Cell Death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Yang, W.S.; Stockwell, B.R. Ferroptosis: Death by Lipid Peroxidation. Trends Cell Biol. 2016, 26, 165–176. [Google Scholar] [CrossRef] [PubMed]
- She, X.; Lan, B.; Tian, H.; Tang, B. Cross Talk Between Ferroptosis and Cerebral Ischemia. Front. Neurosci. 2020, 14, 776. [Google Scholar] [CrossRef] [PubMed]
- Bardestani, A.; Ebrahimpour, S.; Esmaeili, A.; Esmaeili, A. Quercetin Attenuates Neurotoxicity Induced by Iron Oxide Nanoparticles. J. Nanobiotechnol. 2021, 19, 327. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.-H.; Liu, Y.; Xue, N.-J.; Zheng, R.; Yan, Y.-Q.; Wang, Z.-X.; Li, Y.-L.; Ying, C.-Z.; Song, Z.; Tian, J.; et al. Quercetin Protects against MPP+/MPTP-Induced Dopaminergic Neuron Death in Parkinson’s Disease by Inhibiting Ferroptosis. Oxidative Med. Cell Longev. 2022, 2022, 7769355. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Borutaite, V. There Is No Evidence That Mitochondria Are the Main Source of Reactive Oxygen Species in Mammalian Cells. Mitochondrion 2012, 12, 1–4. [Google Scholar] [CrossRef]
- Brennan, A.M.; Won Suh, S.; Joon Won, S.; Narasimhan, P.; Kauppinen, T.M.; Lee, H.; Edling, Y.; Chan, P.H.; Swanson, R.A. NADPH Oxidase Is the Primary Source of Superoxide Induced by NMDA Receptor Activation. Nat. Neurosci. 2009, 12, 857–863. [Google Scholar] [CrossRef]
- Brennan-Minnella, A.M.; Shen, Y.; El-Benna, J.; Swanson, R.A. Phosphoinositide 3-Kinase Couples NMDA Receptors to Superoxide Release in Excitotoxic Neuronal Death. Cell Death Dis. 2013, 4, e580. [Google Scholar] [CrossRef]
- Reyes, R.C.; Brennan, A.M.; Shen, Y.; Baldwin, Y.; Swanson, R.A. Activation of Neuronal NMDA Receptors Induces Superoxide-Mediated Oxidative Stress in Neighboring Neurons and Astrocytes. J. Neurosci. 2012, 32, 12973–12978. [Google Scholar] [CrossRef]
- Bal-Price, A.; Matthias, A.; Brown, G.C. Stimulation of the NADPH Oxidase in Activated Rat Microglia Removes Nitric Oxide but Induces Peroxynitrite Production. J. Neurochem. 2002, 80, 73–80. [Google Scholar] [CrossRef]
- Chen, S.-H.; Oyarzabal, E.A.; Hong, J.-S. Critical Role of the Mac1/NOX2 Pathway in Mediating Reactive Microgliosis-Generated Chronic Neuroinflammation and Progressive Neurodegeneration. Curr. Opin. Pharmacol. 2016, 26, 54–60. [Google Scholar] [CrossRef]
- Mander, P.; Brown, G.C. Activation of Microglial NADPH Oxidase Is Synergistic with Glial INOS Expression in Inducing Neuronal Death: A Dual-Key Mechanism of Inflammatory Neurodegeneration. J. Neuroinflamm. 2005, 2, 20. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.A.; Cole, M.P. Oxidative and Nitrative Stress in Neurodegeneration. Neurobiol. Dis. 2015, 84, 4–21. [Google Scholar] [CrossRef] [PubMed]
- Gaschler, M.M.; Stockwell, B.R. Lipid Peroxidation in Cell Death. Biochem. Biophys. Res. Commun. 2017, 482, 419–425. [Google Scholar] [CrossRef] [PubMed]
- Neniskyte, U.; Fricker, M.; Brown, G.C. Amyloid β Induces Microglia to Phagocytose Neurons via Activation of Protein Kinase Cs and NADPH Oxidase. Int. J. Biochem. Cell Biol. 2016, 81, 346–355. [Google Scholar] [CrossRef] [PubMed]
- Descloux, C.; Ginet, V.; Clarke, P.G.H.; Puyal, J.; Truttmann, A.C. Neuronal Death after Perinatal Cerebral Hypoxia-ischemia: Focus on Autophagy—Mediated Cell Death. Int. J. Dev. Neurosci. 2015, 45, 75–85. [Google Scholar] [CrossRef]
- Boots, A.W.; Haenen, G.R.M.M.; Bast, A. Health Effects of Quercetin: From Antioxidant to Nutraceutical. Eur. J. Pharmacol. 2008, 585, 325–337. [Google Scholar] [CrossRef]
- Arredondo, F.; Echeverry, C.; Abin-Carriquiry, J.A.; Blasina, F.; Antúnez, K.; Jones, D.P.; Go, Y.-M.; Liang, Y.-L.; Dajas, F. After Cellular Internalization, Quercetin Causes Nrf2 Nuclear Translocation, Increases Glutathione Levels, and Prevents Neuronal Death against an Oxidative Insult. Free. Radic. Biol. Med. 2010, 49, 738–747. [Google Scholar] [CrossRef]
- Viswanatha, G.L.; Venkataranganna, M.V.; Prasad, N.B.L.; Hanumanthappa, S. Chemical Characterization and Cerebroprotective Effect of Methanolic Root Extract of Colebrookea Oppositifolia in Rats. J. Ethnopharmacol. 2018, 223, 63–75. [Google Scholar] [CrossRef]
- Viswanatha, G.L.; Venkataranganna, M.V.; Prasad, N.B.L.; Shylaja, H. Achyranthes Aspera Linn. Alleviates Cerebral Ischemia-Reperfusion-Induced Neurocognitive, Biochemical, Morphological and Histological Alterations in Wistar Rats. J. Ethnopharmacol. 2019, 228, 58–69. [Google Scholar] [CrossRef]
- Arikan, S.; Ersan, I.; Karaca, T.; Kara, S.; Gencer, B.; Karaboga, I.; Tufan, H.A. Quercetin Protects the Retina by Reducing Apoptosis Due to Ischemia-Reperfusion Injury in a Rat Model. Arq. Bras. Oftalmol. 2015, 78, 100–104. [Google Scholar] [CrossRef]
- Chen, L.; Sun, L.; Liu, Z.; Wang, H.; Xu, C. Protection Afforded by Quercetin against H2O2-Induced Apoptosis on PC12 Cells via Activating PI3K/Akt Signal Pathway. J. Recept. Signal Transduct. 2016, 36, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-C.; Yang, Y.-R.; Wang, P.S.; Wang, R.-Y. Quercetin Enhances Exercise-Mediated Neuroprotective Effects in Brain Ischemic Rats. Med. Sci. Sport. Exerc. 2014, 46, 1908–1916. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Shen, Y.-J.; Chen, M.; Zhao, J.-Y.; Chen, S.-H.; Zhang, W.; Song, J.-K.; Li, L.; Du, G.-H. Quercetin Attenuates Ischemia Reperfusion Injury by Protecting the Blood-Brain Barrier through Sirt1 in MCAO Rats. J. Asian Nat. Prod. Res. 2022, 24, 278–289. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Garrick, J.M.; Roquè, P.J.; Pellacani, C. Mechanisms of Neuroprotection by Quercetin: Counteracting Oxidative Stress and More. Oxidative Med. Cell Longev. 2016, 2016, 2986796. [Google Scholar] [CrossRef] [PubMed]
- Saw, C.L.L.; Guo, Y.; Yang, A.Y.; Paredes-Gonzalez, X.; Ramirez, C.; Pung, D.; Kong, A.-N.T. The Berry Constituents Quercetin, Kaempferol, and Pterostilbene Synergistically Attenuate Reactive Oxygen Species: Involvement of the Nrf2-ARE Signaling Pathway. Food Chem. Toxicol. 2014, 72, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Bernstock, J.D.; Nagaraja, N.; Ko, B.; Hallenbeck, J.M. Global SUMOylation Facilitates the Multimodal Neuroprotection Afforded by Quercetin against the Deleterious Effects of Oxygen/Glucose Deprivation and the Restoration of Oxygen/Glucose. J. Neurochem. 2016, 138, 101–116. [Google Scholar] [CrossRef]
- Le, K.; Song, Z.; Deng, J.; Peng, X.; Zhang, J.; Wang, L.; Zhou, L.; Bi, H.; Liao, Z.; Feng, Z. Quercetin Alleviates Neonatal Hypoxic-Ischemic Brain Injury by Inhibiting Microglia-Derived Oxidative Stress and TLR4-Mediated Inflammation. Inflamm. Res. 2020, 69, 1201–1213. [Google Scholar] [CrossRef]
- Gouédard, C.; Barouki, R.; Morel, Y. Dietary Polyphenols Increase Paraoxonase 1 Gene Expression by an Aryl Hydrocarbon Receptor-Dependent Mechanism. Mol. Cell Biol. 2004, 24, 5209–5222. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Kim, O.-H.; Sung, M.-K. Effects of Phenol-Depleted and Phenol-Rich Diets on Blood Markers of Oxidative Stress, and Urinary Excretion of Quercetin and Kaempferol in Healthy Volunteers. J. Am. Coll. Nutr. 2003, 22, 217–223. [Google Scholar] [CrossRef]
- Halliwell, B. Are Polyphenols Antioxidants or Pro-Oxidants? What Do We Learn from Cell Culture and in Vivo Studies? Arch. Biochem. Biophys. 2008, 476, 107–112. [Google Scholar] [CrossRef]
- Boesch-Saadatmandi, C.; Pospissil, R.; Graeser, A.-C.; Canali, R.; Boomgaarden, I.; Doering, F.; Wolffram, S.; Egert, S.; Mueller, M.; Rimbach, G. Effect of Quercetin on Paraoxonase 2 Levels in RAW264.7 Macrophages and in Human Monocytes—Role of Quercetin Metabolism. Int. J. Mol. Sci. 2009, 10, 4168–4177. [Google Scholar] [CrossRef] [PubMed]
- Costa, L.G.; Tait, L.; de Laat, R.; Dao, K.; Giordano, G.; Pellacani, C.; Cole, T.B.; Furlong, C.E. Modulation of Paraoxonase 2 (PON2) in Mouse Brain by the Polyphenol Quercetin: A Mechanism of Neuroprotection? Neurochem. Res. 2013, 38, 1809–1818. [Google Scholar] [CrossRef] [PubMed]
- Altenhöfer, S.; Witte, I.; Teiber, J.F.; Wilgenbus, P.; Pautz, A.; Li, H.; Daiber, A.; Witan, H.; Clement, A.M.; Förstermann, U.; et al. One Enzyme, Two Functions. J. Biol. Chem. 2010, 285, 24398–24403. [Google Scholar] [CrossRef] [PubMed]

| Source | Quercetin Amount (mg/100 mg) |
|---|---|
| Apples | 4.01 |
| Asparagus | 14.0 |
| Black tea | 2.5 mg/100 mL |
| Blueberry | 14.6 |
| Broccoli | 13.7 |
| Cherry | 17.4 |
| Chili pepper | 32.6 |
| Chives | 10.4 |
| Cranberry | 25.0 |
| Dill | 79.0 |
| Fennel leaves | 46.8 |
| Kale | 22.6 |
| Lettuce | 14.7 |
| Onions | 45.0 |
| Oregano | 42.0 |
| Red wine | 3.16 mg/100 mL |
| Spinach | 27.2 |
| Quercetin’s Effect (+/−) | Molecule/Protein Affected by Quercetin | Cell Death Pathway Affected |
|---|---|---|
| − | Intracellular Ca2+ increase | Apoptosis; LCD; Phagoptosis |
| + | Parvalbumin | Apoptosis |
| + | PI3K | Apoptosis; Parthanatos |
| − | NO | Oxidative Stress |
| − | PARP-1 | Parthanatos |
| − | Cytochrome c | Apoptosis |
| + | Bcl | Apoptosis |
| − | FADD | Apoptosis |
| − | Bax | Apoptosis |
| − | Casp9 | Apoptosis |
| + | Nrf-2 | Oxidative Stress |
| − | Peroxynitrite | Oxidative Stress |
| + | PON2 | Oxidative Stress |
| − | Superoxide | Oxidative Stress |
| + | Catalase | Oxidative Stress |
| + | SOD 1/2 | Oxidative Stress |
| − | Fe(II)/Lipid Peroxide | Ferroptosis |
| + | Glutathione | Oxidative Stress; Ferroptosis |
| − | TMEM16 | Phagoptosis |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riche, K.; Lenard, N.R. Quercetin’s Effects on Glutamate Cytotoxicity. Molecules 2022, 27, 7620. https://doi.org/10.3390/molecules27217620
Riche K, Lenard NR. Quercetin’s Effects on Glutamate Cytotoxicity. Molecules. 2022; 27(21):7620. https://doi.org/10.3390/molecules27217620
Chicago/Turabian StyleRiche, Kade, and Natalie R. Lenard. 2022. "Quercetin’s Effects on Glutamate Cytotoxicity" Molecules 27, no. 21: 7620. https://doi.org/10.3390/molecules27217620
APA StyleRiche, K., & Lenard, N. R. (2022). Quercetin’s Effects on Glutamate Cytotoxicity. Molecules, 27(21), 7620. https://doi.org/10.3390/molecules27217620







