In Situ Swelling Formulation of Glycerol-Monooleate-Derived Lyotropic Liquid Crystals Proposed for Local Vaginal Application
Abstract
:1. Introduction
2. Results and Discussion
2.1. Transition from Precursor Solution to Cubic Phase
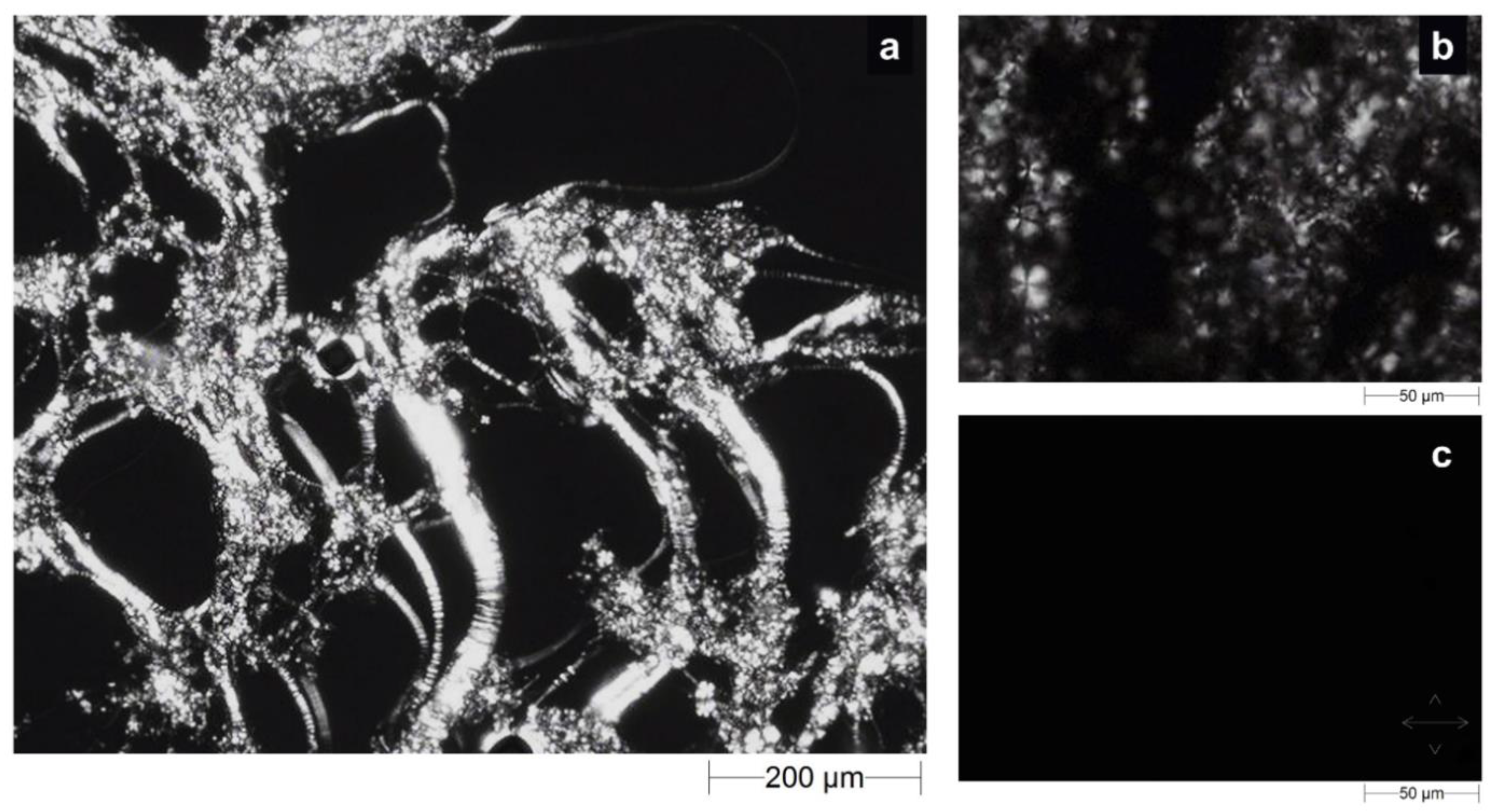
2.2. Morphological Investigation of LLCs
2.3. Stability Analysis and Rheological Behavior Studies
2.3.1. Precursor Solutions Stability over Time
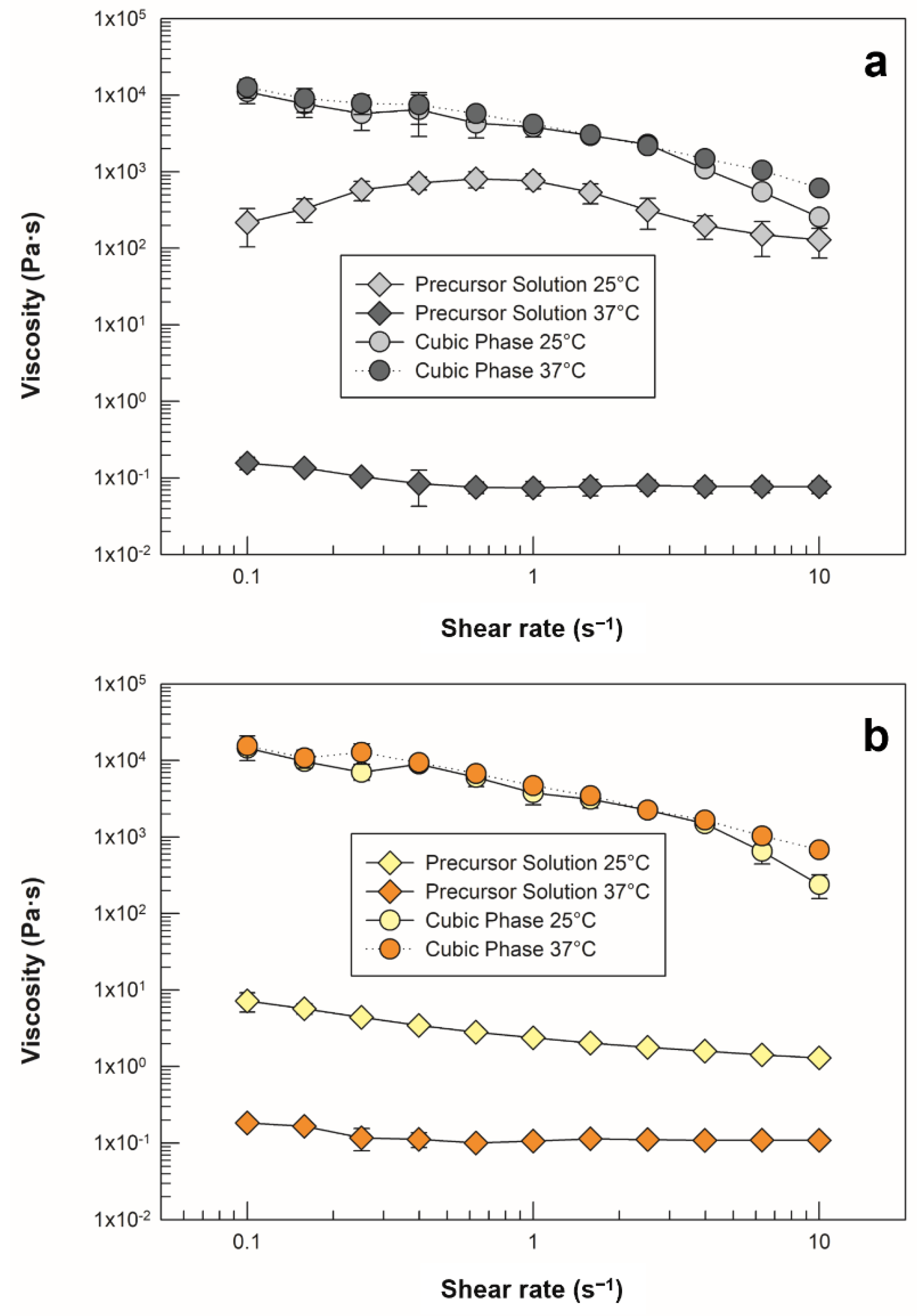
2.3.2. Viscosity Measurement with Kinexus Pro®
2.3.3. Mucoadhesion Potential of Cubic Phase
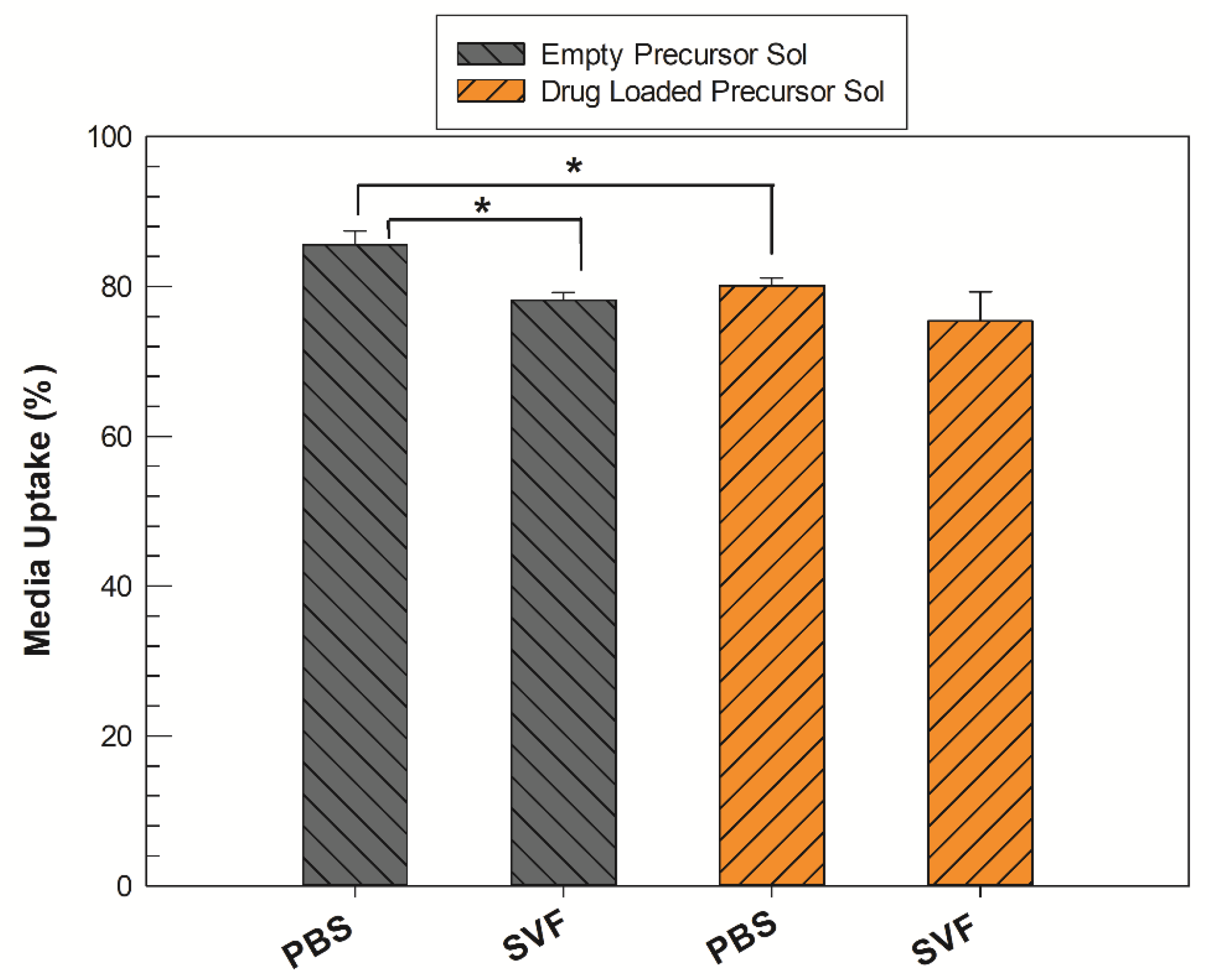
2.4. In Vitro Degradation and Drug Release Studies in SVF
3. Materials and Methods
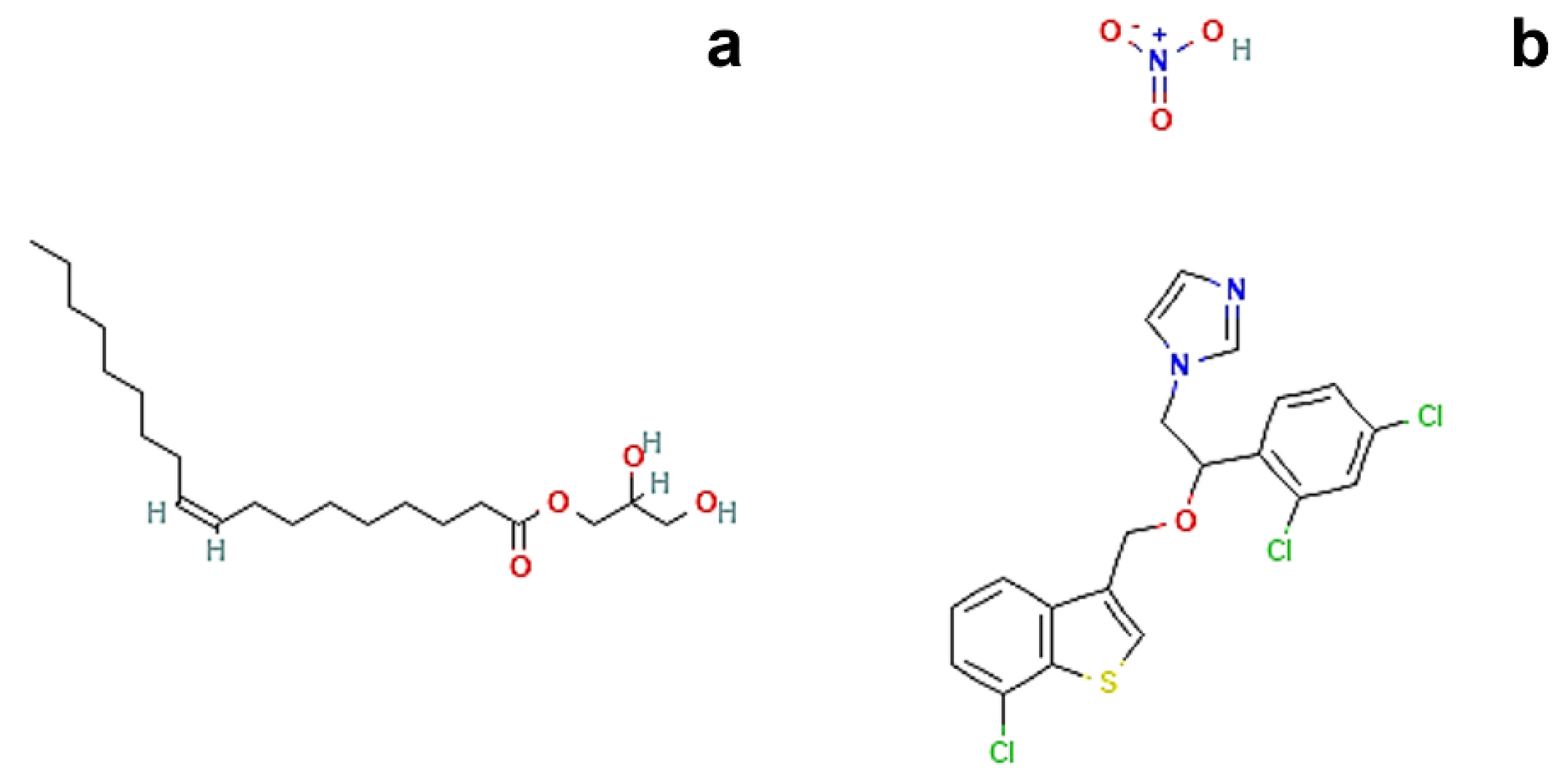
3.1. Materials
3.2. Preparation and Phase Transition Test of Precursor Solutions
3.3. In Vitro Simulated Media Uptake Studies
3.4. Phase Transition Studies
3.5. Rheological Behavior and Mucoadhesion Assessment
3.6. Turbiscan® Lab Expert Stability Evaluation
3.7. In Vitro Drug Release Kinetic Studies
3.8. In Vitro Degradation Studies
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Merabet, J.; Thompson, D.; Saul Levinson, R. Advancing Vaginal Drug Delivery. Expert Opin. Drug Deliv. 2005, 2, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Motwani, S.K.; Iqbal, Z.; Talegaonkar, S.; Ahmad, F.J.; Khar, R.K. Optimisation of Polyherbal Gels for Vaginal Drug Delivery by Box-Behnken Statistical Design. Eur. J. Pharm. Biopharm. 2007, 67, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Patel, P.; Patel, P. Formulation and Evaluation of Clindamycin HCL in Situ Gel for Vaginal Application. Int. J. Pharm. Investig. 2015, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- das Neves, J.; Bahia, M.F. Gels as Vaginal Drug Delivery Systems. Int. J. Pharm. 2006, 318, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Srikrishna, S.; Cardozo, L. The Vagina as a Route for Drug Delivery: A Review. Int. Urogynecol. J. Pelvic Floor Dysfunct. 2013, 24, 537–543. [Google Scholar] [CrossRef] [PubMed]
- Milak, S.; Zimmer, A. Glycerol Monooleate Liquid Crystalline Phases Used in Drug Delivery Systems. Int. J. Pharm. 2015, 478, 569–587. [Google Scholar] [CrossRef] [PubMed]
- Kaasgaard, T.; Drummond, C.J. Ordered 2-D and 3-D Nanostructured Amphiphile Self-Assembly Materials Stable in Excess Solvent. Phys. Chem. Chem. Phys. 2006, 8, 4957–4975. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Jahn, A.; Cho, S.J.; Kim, J.S.; Ki, M.H.; Kim, D.D. Lyotropic Liquid Crystal Systems in Drug Delivery: A Review. J. Pharm. Investig. 2015, 45, 1–11. [Google Scholar] [CrossRef]
- Torres, J.; Márquez, M.; Camps, F. Sertaconazole in the Treatment of Mycoses: From Dermatology to Gynecology. Int. J. Gynecol. Obstet. 2000, 71, 3–20. [Google Scholar] [CrossRef]
- Wang, P.H.; Chao, H.T.; Chen, C.L.; Yuan, C.C. Single-Dose Sertaconazole Vaginal Tablet Treatment of Vulvovaginal Candidiasis. J. Chin. Med. Assoc. 2006, 69, 259–263. [Google Scholar] [CrossRef]
- Dellenbach, P.; Thomas, J.L.; Guerin, V.; Ochsenbein, E.; Contet-Audonneau, N. Topical Treatment of Vaginal Candidosis with Sertaconazole and Econazole Sustained-Release Suppositories. Int. J. Gynecol. Obstet. 2000, 71, 47–52. [Google Scholar] [CrossRef]
- Carrillo-Muñoz, A.J.; Tur-Tur, C.; Giusiano, G.; Marcos-Arias, C.; Eraso, E.; Jauregizar, N.; Quindós, G. Sertaconazole: An Antifungal Agent for the Topical Treatment of Superficial Candidiasis. Expert Rev. Anti. Infect. Ther. 2013, 11, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Waghule, T.; Patil, S.; Rapalli, V.K.; Girdhar, V.; Gorantla, S.; Kumar Dubey, S.; Saha, R.N.; Singhvi, G. Improved Skin-Permeated Diclofenac-Loaded Lyotropic Liquid Crystal Nanoparticles: QbD-Driven Industrial Feasible Process and Assessment of Skin Deposition. Liq. Cryst. 2021, 48, 991–1009. [Google Scholar] [CrossRef]
- Mei, L.; Wang, H.; Jintian, C.; Zhang, Z.; Li, F.; Xie, Y.; Ying, H.; Tingting, P.; Guohua, C.; Xin, P.; et al. Self-Assembled Lyotropic Liquid Crystal Gel for Osteoarthritis Treatment via Anti-Inflammation and Cartilage Protection. Biomater. Sci. 2021, 9, 7205–7218. [Google Scholar] [CrossRef] [PubMed]
- Glycerol Monooleate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/5283468#section=2D-Structure (accessed on 19 September 2022).
- Sertaconazole Nitrate. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/200103#section=2D-Structure (accessed on 19 September 2022).
- Shoaib, A.; Mangla, B.; Javed, S.; Sultan, M.H.; Alqahtani, S.S.; Shakeel, F. Vicissitudes of Liquid Crystals for Solubility Enhancement of Poorly Soluble Drugs. J. Mol. Liq. 2021, 321, 114924. [Google Scholar] [CrossRef]
- Bubić Pajić, N.; Vucen, S.; Ilić, T.; O’Mahony, C.; Dobričić, V.; Savić, S. Comparative Efficacy Evaluation of Different Penetration Enhancement Strategies for Dermal Delivery of Poorly Soluble Drugs—A Case with Sertaconazole Nitrate. Eur. J. Pharm. Sci. 2021, 164, 105895. [Google Scholar] [CrossRef] [PubMed]
- Kracht, T.; Müller-Goymann, C.C. Antifungal Efficacy of Liquid Poloxamer 407-Based Emulsions Loaded with Sertaconazole Nitrate. Int. J. Pharm. 2020, 585, 119400. [Google Scholar] [CrossRef] [PubMed]
- Owen, D.H.; Katz, D.F. A Vaginal Fluid Stimulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- Marques, M.R.C.; Loebenberg, R.; Almukainzi, M. Simulated Biological Fluids with Possible Application in Dissolution Testing. Dissolution Technol. 2011, 18, 15–28. [Google Scholar] [CrossRef]
- Rochani, A.; Agrahari, V.; Chandra, N.; Singh, O.N.; McCormick, T.J.; Doncel, G.F.; Clark, M.R.; Kaushal, G. Development and Preclinical Investigation of Physically Cross-Linked and PH-Sensitive Polymeric Gels as Potential Vaginal Contraceptives. Polymers 2022, 14, 1728. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, N.; Galbis, E.; Valencia, C.; Díaz-Blanco, M.J.; Lacroix, B.; de-Paz, M.V. Biodegradable Double Cross-Linked Chitosan Hydrogels for Drug Delivery: Impact of Chemistry on Rheological and Pharmacological Performance. Int. J. Biol. Macromol. 2020, 165, 2205–2218. [Google Scholar] [CrossRef] [PubMed]
- Djekic, L.; Čalija, B.; Medarević, Đ. Gelation Behavior, Drug Solubilization Capacity and Release Kinetics of Poloxamer 407 Aqueous Solutions: The Combined Effect of Copolymer, Cosolvent and Hydrophobic Drug. J. Mol. Liq. 2020, 303, 112639. [Google Scholar] [CrossRef]
- Zhang, J.; Fu, M.; Zhang, M.; Xu, L.; Gao, Y. Synthesis of Oxidized Glycerol Monooleate-Chitosan Polymer and Its Hydrogel Formation for Sustained Release of Trimetazidine Hydrochloride. Int. J. Pharm. 2014, 465, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, Z. Phase Transition and Release Kinetics of Polyphenols Encapsulated Lyotropic Liquid Crystals. Int. J. Pharm. 2019, 565, 283–293. [Google Scholar] [CrossRef] [PubMed]
- Prasert, W.; Gohtani, S. Effect of Sucrose on Phase Behavior of Aqueous Phase/Polyoxyethylene Sorbitan Fatty Acid Ester (Tween Xx)/Vegetable Oil Systems and Food Nano-Emulsification Using Low-Energy Methods. J. Food Eng. 2016, 168, 119–128. [Google Scholar] [CrossRef]
- Huang, Y.; Gui, S. Factors Affecting the Structure of Lyotropic Liquid Crystals and the Correlation between Structure and Drug Diffusion. RSC Adv. 2018, 8, 6978–6987. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-palomo, A.; Lutz-bueno, V.; Guizar-sicairos, M.; Roland, K. Nanostructure and Anisotropy of 3D Printed Lyotropic Liquid Crystals Studied by Scattering and Birefringence Imaging. Addit. Manuf. 2021, 47, 102289. [Google Scholar] [CrossRef]
- Steck, K.; Dieterich, S.; Stubenrauch, C.; Giesselmann, F. Surfactant-Based Lyotropic Liquid Crystal Gels—The Interplay between Anisotropic Order and Gel Formation. J. Mater. Chem. C 2020, 8, 5335–5348. [Google Scholar] [CrossRef]
- Amar-yuli, I.; Garti, N. Transitions Induced by Solubilized Fat into Reverse Hexagonal Mesophases. Colloids Surf. B Biointerfaces 2005, 43, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.H.; Paradkar, A. Effect of HLB of Additives on the Properties and Drug Release from the Glyceryl Monooleate Matrices. Uropean, J. Pharm. Biopharm. 2007, 67, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Rizwan, S.B.; Hanley, T.; Boyd, B.J.; Rades, T.; Hook, S. Liquid Crystalline Systems of Phytantriol and Glyceryl Monooleate Containing a Hydrophilic Protein: Characterisation, Swelling and Release Kinetics. J. Pharm. Sci. 2009, 98, 4191–4204. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, A.; Cianflone, E.; Cristiano, M.C.; Salerno, N.; Tarsitano, M.; Marino, F.; Molinaro, C.; Fresta, M.; Torella, D.; Paolino, D. Lyotropic Liquid Crystals: A Biocompatible and Safe Material for Local Cardiac Application. Pharmaceutics 2022, 14, 452. [Google Scholar] [CrossRef] [PubMed]
- Cristiano, M.C.; Mancuso, A.; Giuliano, E.; Cosco, D.; Paolino, D.; Fresta, M. Etogel for Intra-Articular Drug Delivery: A New Challenge for Joint Diseases Treatment. J. Funct. Biomater. 2021, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Di Francesco, M.; Celia, C.; Cristiano, M.C.; D’Avanzo, N.; Ruozi, B.; Mircioiu, C.; Cosco, D.; Di Marzio, L.; Fresta, M. Doxorubicin Hydrochloride-Loaded Nonionic Surfactant Vesicles to Treat Metastatic and Non-Metastatic Breast Cancer. ACS Omega 2021, 6, 2973–2989. [Google Scholar] [CrossRef] [PubMed]
- Jiao, W.; Chen, W.; Mei, Y.; Yun, Y.; Wang, B.; Zhong, Q.; Chen, H.; Chen, W. Effects of Molecular Weight and Guluronic Acid/Mannuronic Acid Ratio on the Rheological Behavior and Stabilizing Property of Sodium Alginate. Molecules 2019, 24, 4374. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Cao, J.; Li, Z.; Chu, X. Cubic Liquid Crystalline Gels Based on Glycerol Monooleate for Intra-Articular Injection. AAPS PharmSciTech 2018, 19, 858–865. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.; Bodmeier, R. Low Viscosity Monoglyceride-Based Drug Delivery Systems Transforming into a Highly Viscous Cubic Phase. Int. J. Pharm. 1998, 173, 51–60. [Google Scholar] [CrossRef]
- Baker, F.C.; Siboza, F.; Fuller, A. Temperature Regulation in Women: Effects of the Menstrual Cycle. Temperature 2020, 7, 226–262. [Google Scholar] [CrossRef]
- dos Santos, A.M.; Carvalho, S.G.; Araujo, V.H.S.; Carvalho, G.C.; Gremião, M.P.D.; Chorilli, M. Recent Advances in Hydrogels as Strategy for Drug Delivery Intended to Vaginal Infections. Int. J. Pharm. 2020, 590, 119867. [Google Scholar] [CrossRef]
- Brannigan, R.P.; Khutoryanskiy, V.V. Progress and Current Trends in the Synthesis of Novel Polymers with Enhanced Mucoadhesive Properties. Macromol. Biosci. 2019, 19, 1900194. [Google Scholar] [CrossRef]
- Souza, C.; Watanabe, E.; Borgheti-Cardoso, L.N.; Carvalho De Abreu Fantini, M.; Guimarães Lara, M. Mucoadhesive System Formed by Liquid Crystals for Buccal Administration of Poly(Hexamethylene Biguanide) Hydrochloride. J. Pharm. Sci. 2014, 103, 3914–3923. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.S.; Schubert, L.; Hansen, J. Bioadhesive Drug Delivery Systems. Characterisation of Mucoadhesive Properties of Systems Based on Glyceryl Mono-Oleate and Glyceryl Monolinoleate. Eur. J. Pharm. Sci. 1998, 6, 231–239. [Google Scholar] [CrossRef]
- Hägerström, H.; Paulsson, M.; Edsman, K. Evaluation of Mucoadhesion for Two Polyelectrolyte Gels in Simulated Physiological Conditions Using a Rheological Method. Eur. J. Pharm. Sci. 2000, 9, 301–309. [Google Scholar] [CrossRef]
- Gouda, R.; Baishya, H.; Qing, Z. Application of Mathematical Models in Drug Release Kinetics of Carbidopa and Levodopa ER Tablets. J. Dev. Drugs 2017, 6, 2. [Google Scholar]
- Jommanee, N.; Chanthad, C.; Manokruang, K. Preparation of Injectable Hydrogels from Temperature and PH Responsive Grafted Chitosan with Tuned Gelation Temperature Suitable for Tumor Acidic Environment. Carbohydr. Polym. 2018, 198, 486–494. [Google Scholar] [CrossRef]
- Mancuso, A.; Tarsitano, M.; Udongo, B.P.; Cristiano, M.C.; Torella, D.; Paolino, D.; Fresta, M. A Comparison between Silicone Free and Silicone-Based Emulsions: Technological Features and in Vivo Evaluation. Int. J. Cosmet. Sci. 2022, 44, 14–529. [Google Scholar] [CrossRef]
- Xu, D.; Zhang, J.; Cao, Y.; Wang, J.; Xiao, J. Influence of Microcrystalline Cellulose on the Microrheological Property and Freeze-Thaw Stability of Soybean Protein Hydrolysate Stabilized Curcumin Emulsion. Lwt 2016, 66, 590–597. [Google Scholar] [CrossRef]
- Matusiak, J.; Grządka, E.; Bastrzyk, A.; Pasieczna-Patkowska, S. The influence of fucoidan on stability, adsorption and elec-trokinetic properties of ZnO and TiO2 suspensions. Appl. Nanosci. 2021, 4, 919–927. [Google Scholar]
- Nastaj, M.; Terpiłowski, K.; Sołowiej, B.G. The effect of native and polymerised whey protein isolate addition on surface and microstructural properties of processed cheeses and their meltability determined by Turbiscan. Int. J. Food Sci. Technol. 2020, 55, 2179–2187. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Khalil, I.A.; Elakkad, Y.E.; Eliwa, H.A.; Samir, T.M.; Al-Mokaddem, A.K. Formulation and Characterization of Sertaconazole Nitrate Mucoadhesive Liposomes for Vaginal Candidiasis. Int. J. Nanomed. 2020, 15, 4079–4090. [Google Scholar] [CrossRef]
- Muzaffar, F.; Singh, U.K. RP-HPLC and UV Spectrophotometric Methods for Estimation of Sertaconazole Nitrate in Microemulsion. J. Chem. Pharm. Res. 2016, 8, 740–745. [Google Scholar]


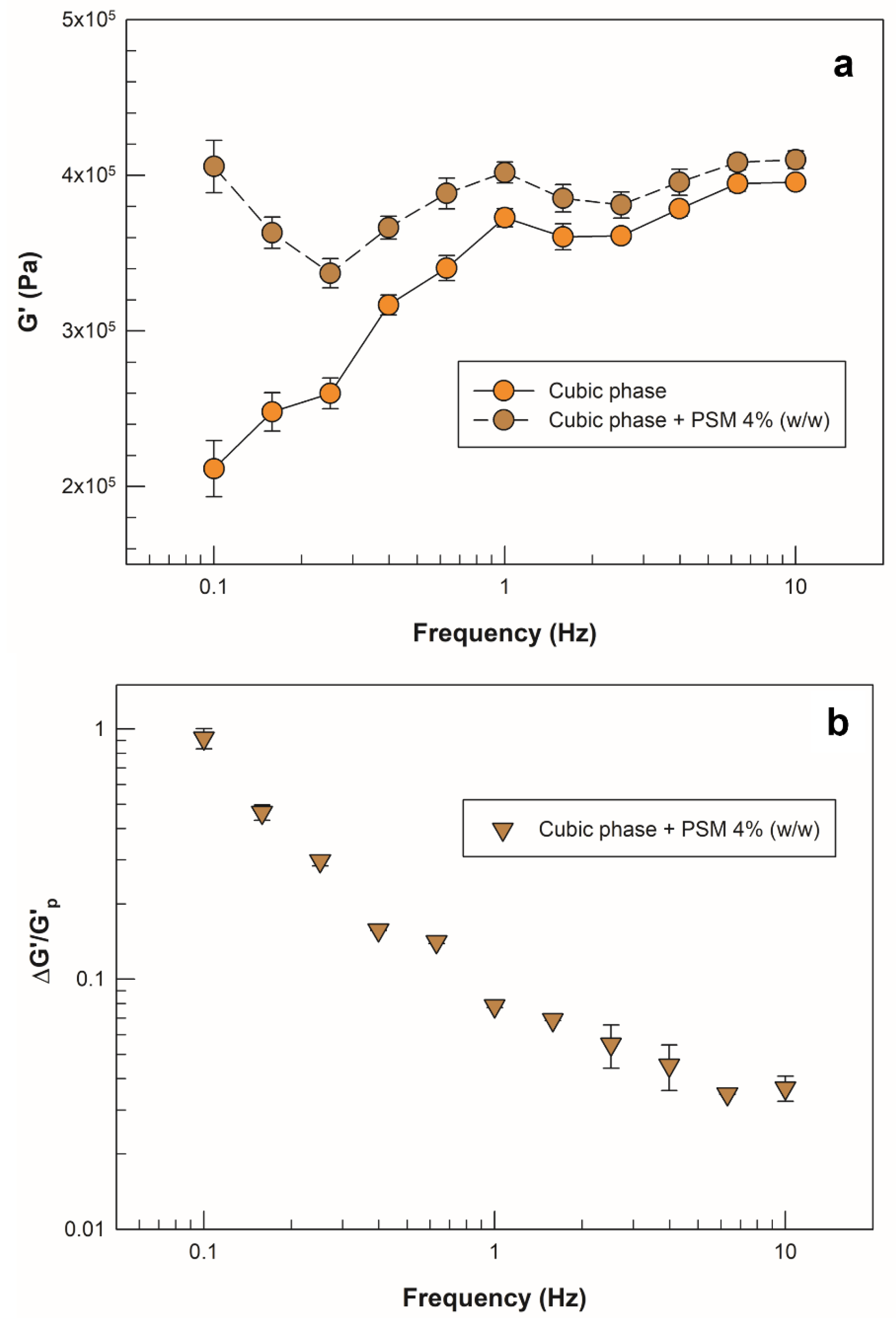

| Sample | Frequency (Hz) | G’cp (kPa) | G’mix (kPa) | ΔG’ (kPa) |
|---|---|---|---|---|
| Drug-loaded cubic phase + PSM 4%(w/w) | 0.1 | 211 ± 18 | 406 ± 17 | 194 ± 2 |
| 1 | 373 ± 6 | 402 ± 8 | 29 ± 1 | |
| 10 | 396 ± 4 | 410 ± 6 | 15 ± 2 |
| Kinetic Model Name | Representative Equation | Plot | Slope | Intercept | R2 |
|---|---|---|---|---|---|
| Zero Order | Cumulative % drug release vs. time | 0.1897 | 2.4178 | 0.8836 | |
| First Order | Log of cumulative % drug remaining vs. time | −0.0009 | 1.9894 | 0.8962 | |
| Higuchi | Cumulative % drug release vs. square root of time | 1.7521 | 0.1747 | 0.9859 | |
| Korsmeyer–Peppas | Log of cumulative % drug release vs. log of time | 0.5646 | 0.1754 | 0.9587 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarsitano, M.; Mancuso, A.; Cristiano, M.C.; Paolino, D.; Fresta, M. In Situ Swelling Formulation of Glycerol-Monooleate-Derived Lyotropic Liquid Crystals Proposed for Local Vaginal Application. Molecules 2022, 27, 6295. https://doi.org/10.3390/molecules27196295
Tarsitano M, Mancuso A, Cristiano MC, Paolino D, Fresta M. In Situ Swelling Formulation of Glycerol-Monooleate-Derived Lyotropic Liquid Crystals Proposed for Local Vaginal Application. Molecules. 2022; 27(19):6295. https://doi.org/10.3390/molecules27196295
Chicago/Turabian StyleTarsitano, Martine, Antonia Mancuso, Maria Chiara Cristiano, Donatella Paolino, and Massimo Fresta. 2022. "In Situ Swelling Formulation of Glycerol-Monooleate-Derived Lyotropic Liquid Crystals Proposed for Local Vaginal Application" Molecules 27, no. 19: 6295. https://doi.org/10.3390/molecules27196295
APA StyleTarsitano, M., Mancuso, A., Cristiano, M. C., Paolino, D., & Fresta, M. (2022). In Situ Swelling Formulation of Glycerol-Monooleate-Derived Lyotropic Liquid Crystals Proposed for Local Vaginal Application. Molecules, 27(19), 6295. https://doi.org/10.3390/molecules27196295











