An Azo-Group-Functionalized Porous Aromatic Framework for Achieving Highly Efficient Capture of Iodine
Abstract
1. Introduction
2. Results
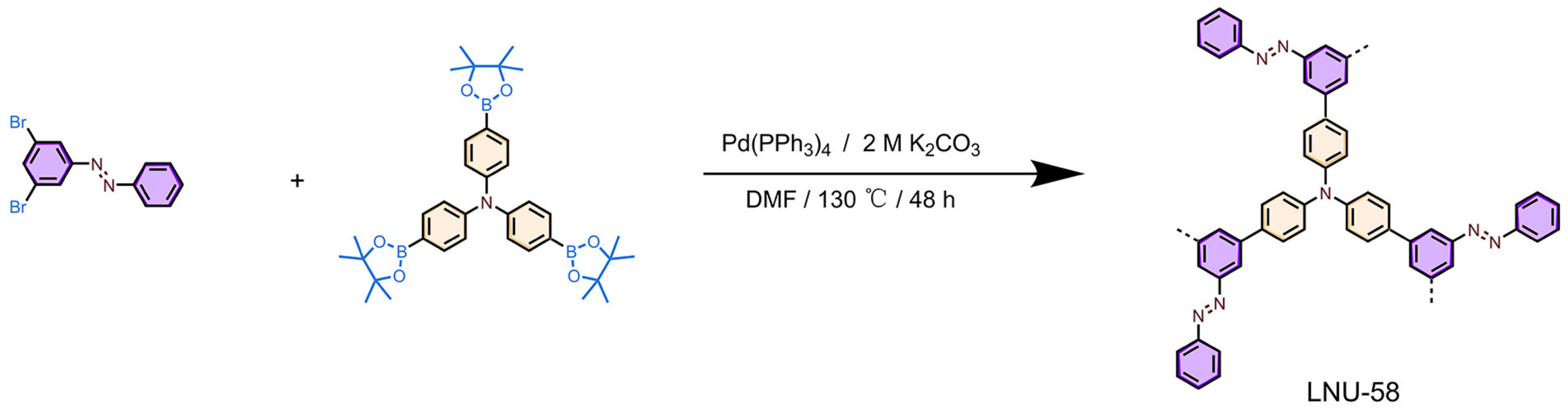
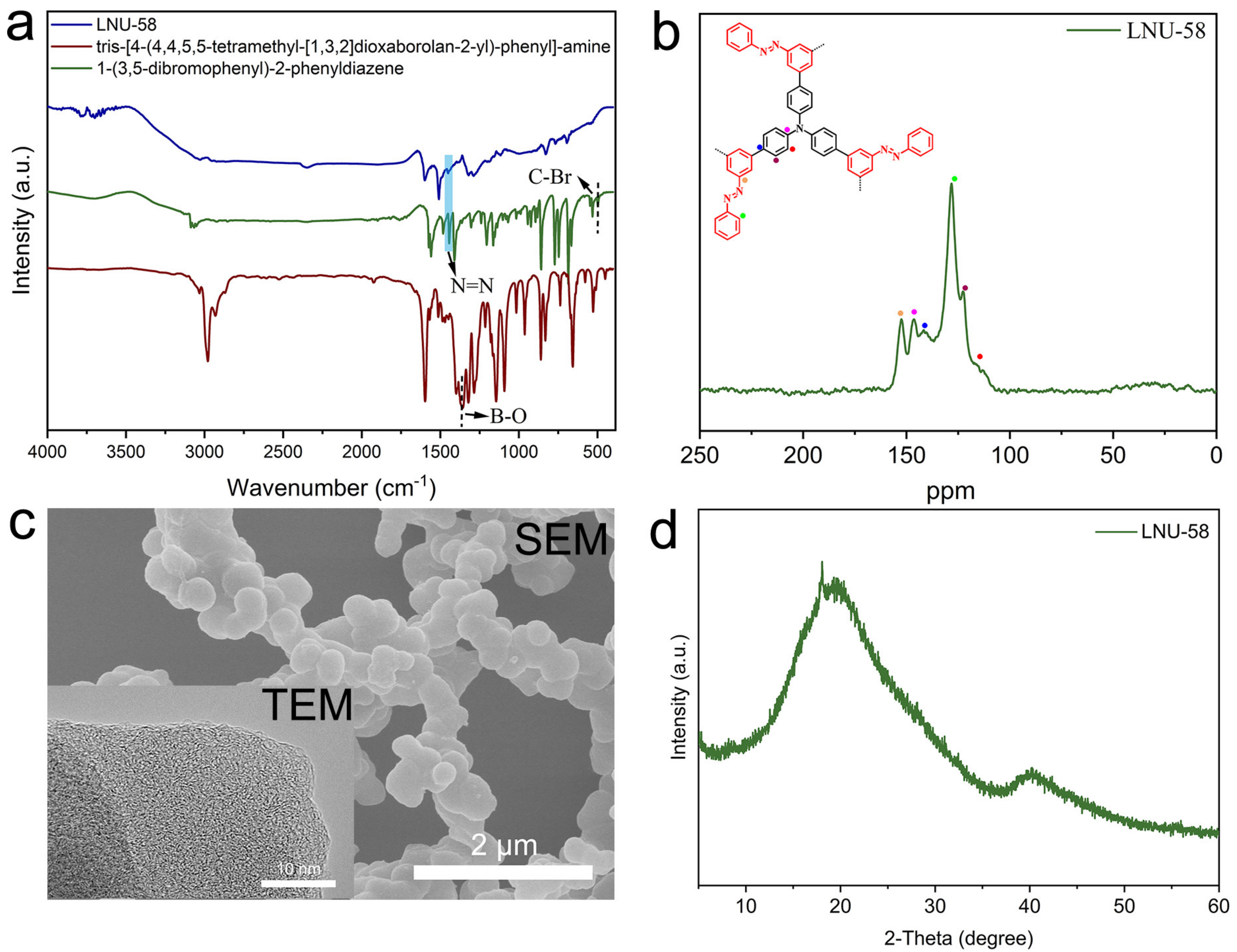
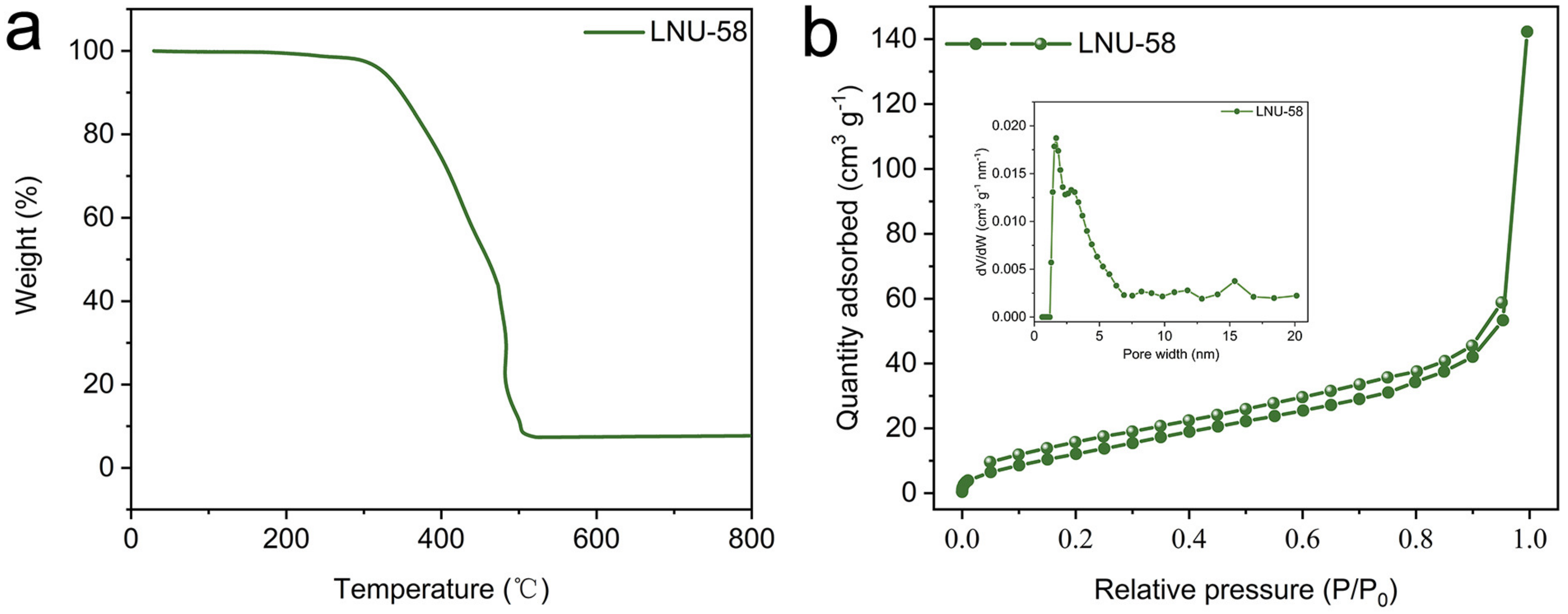
2.1. Structure Characterizations of LNU-58
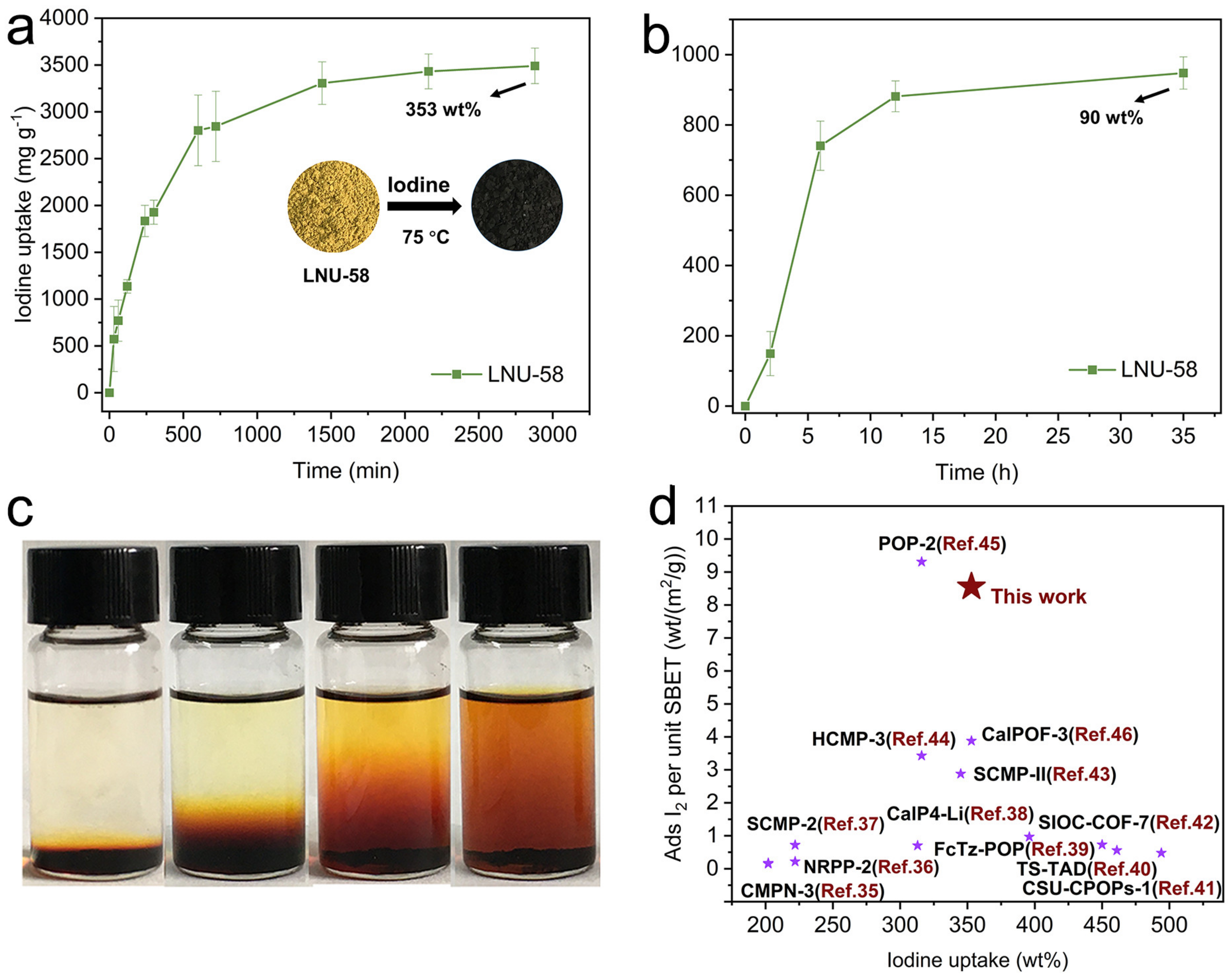
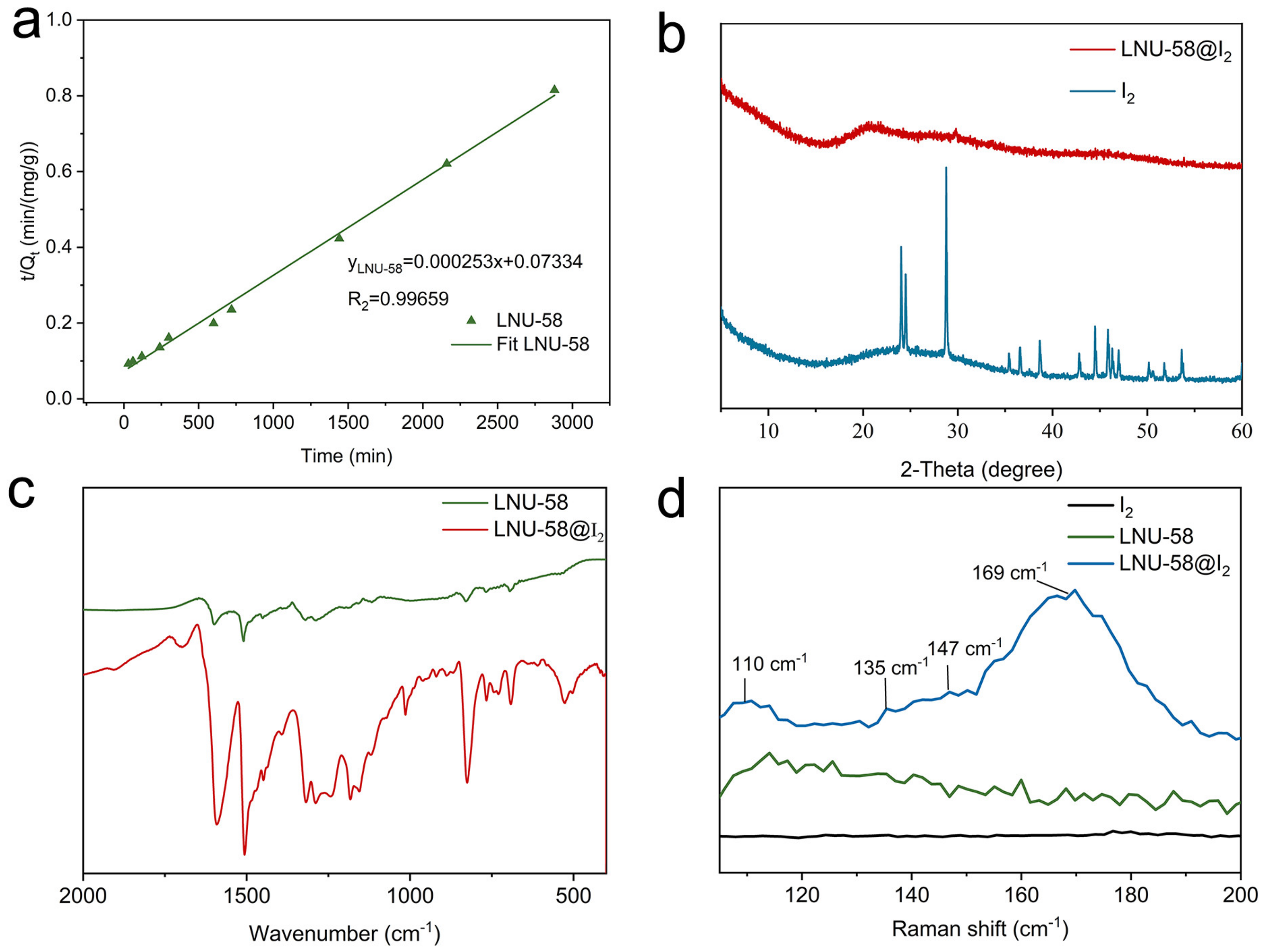
2.2. Iodine Adsorption Study
3. Experimental
3.1. Materials
3.2. Characterization
3.3. Synthesis of LNU-58
3.4. Iodine Adsorption and Release Experiment
3.4.1. Iodine Vapor Uptake Experiments
3.4.2. Dissoved Iodine Uptake Experiments
3.4.3. Iodine Desorption
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Sen, A.; Sharma, S.; Dutta, S.; Shirolkar, M.M.; Dam, G.K.; Let, S.; Ghosh, S.K. Functionalized ionic porous organic polymers exhibiting high iodine uptake from both the vapor and aqueous medium. ACS. Appl. Mater. Interfaces 2021, 13, 34188–34196. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Wang, Y.; Wei, C.; Ai, K.; Lu, L. Hydrogen bond-mediated strong adsorbent–I3− interactions enable high-efficiency radioiodine capture. Mater. Horiz. 2019, 6, 1517–1525. [Google Scholar] [CrossRef]
- Xie, W.; Cui, D.; Zhang, S.-R.; Xu, Y.-H.; Jiang, D.-L. Iodine capture in porous organic polymers and metal–organic frameworks materials. Mater. Horiz. 2019, 6, 1571–1595. [Google Scholar] [CrossRef]
- Shimamoto, Y.S.; Takahashi, Y.; Terada, Y. Formation of organic iodine supplied as iodide in a soil-water system in Chiba, Japan. Environ. Sci. Technol. 2011, 45, 2086–2092. [Google Scholar] [CrossRef] [PubMed]
- Sabri, M.A.; Al-Sayah, M.H.; Sen, S.; Ibrahim, T.H.; El-Kadri, O.M. Fluorescent aminal linked porous organic polymer for reversible iodine capture and sensing. Sci. Rep. 2020, 10, 15943. [Google Scholar] [CrossRef] [PubMed]
- Mushkacheva, G.; Rabinovich, E.; Privalov, V.; Povolotskaya, S.; Shorokhova, V.; Sokolova, S.; Turdakova, V.; Ryzhova, E.; Hall, P.; Schneider, A.B.; et al. Thyroid abnormalities associated with protracted childhood exposure to 131I from atmospheric emissions from the Mayak weapons facility in Russia. Radiat. Res. 2006, 166, 715–722. [Google Scholar] [CrossRef]
- Abrahams, B.F.; Moylan, M.; Orchard, S.D.; Robson, R. Zinc saccharate: A robust, 3D coordination network with two types of isolated, parallel channels, one hydrophilic and the other hydrophobic. Angew. Chem. Int. Ed. 2003, 42, 1848–1851. [Google Scholar] [CrossRef]
- Chaudhari, A.K.; Mukherjee, S.; Nagarkar, S.S.; Joarder, B.; Ghosh, S.K. Bi-porous metal–organic framework with hydrophilic and hydrophobic channels: Selective gas sorption and reversible iodine uptake studies. CrystEngComm 2013, 15, 9465–9471. [Google Scholar] [CrossRef]
- Sigen, A.; Zhang, Y.; Li, Z.; Xia, H.; Xue, M.; Liu, X.; Mu, Y. Highly efficient and reversible iodine capture using a metalloporphyrin-based conjugated microporous polymer. Chem. Commun. 2014, 50, 8495–8498. [Google Scholar]
- Feng, C.; Xu, G.; Xie, W.; Zhang, S.; Yao, C.; Xu, Y. Polytriazine porous networks for effective iodine capture. Polym. Chem. 2020, 11, 2786–2790. [Google Scholar] [CrossRef]
- Wang, J.; Wang, L.; Wang, Y.; Yang, F.; Li, J.; Guan, X.; Zong, J.; Zhou, F.; Huang, J.; Liu, Y.-N. Covalently connected core–shell NH2-UiO-66@Br-COFs hybrid materials for CO2 capture and I2 vapor adsorption. Chem. Eng. J. 2022, 438, 135555. [Google Scholar] [CrossRef]
- Zhou, M.; Li, Z.; Munyentwali, A.; Li, C.; Shui, H.; Li, H. Highly conjugated two-dimensional covalent organic frameworks for efficient iodine uptake. Chem. Asian J. 2022, 17, e202200358. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Zhu, G. Porous aromatic frameworks (PAFs). Chem. Rev. 2020, 120, 8934–8986. [Google Scholar] [CrossRef]
- Guo, X.-X.; Cai, Z.-T.; Muhammad, Y.; Zhang, F.-L.; Wei, R.-P.; Gao, L.-J.; Xiao, G.-M. Silver-anchored porous aromatic framework for efficient conversion of propargylic alcohols with CO2 at ambient pressure. Chin. Chem. Lett. 2022. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, L.; Shen, J.; Qi, L. Enzyme immobilization on a pH-responsive porous polymer membrane for enzymatic kinetics study. Chin. Chem. Lett. 2021, 32, 3195–3198. [Google Scholar] [CrossRef]
- Zhao, Y.; Yuan, Y.; Xu, Y.; Zheng, G.; Zhang, Q.; Jiang, Y.; Wang, Z.; Bu, N.; Xia, L.; Yan, Z. Fine-regulating ultramicropores in porous carbon via a self-sacrificial template route for high-performance supercapacitors. Nanoscale 2021, 13, 1961–1969. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Li, M.; Fa, Y.; Zhao, Z.; Cai, Y.; Liang, X.; Yu, Y.; Jiang, G. Porous covalent organic frameworks-improved solid phase microextraction ambient mass spectrometry for ultrasensitive analysis of tetrabromobisphenol—A analogs. Chin. Chem. Lett. 2021, 33, 3849–3852. [Google Scholar] [CrossRef]
- Han, Z.-Y.; Li, H.-K.; Zhu, Q.-Q.; Yuan, R.; He, H. An intriguing electrochemical impedance aptasensor based on a porous organic framework supported silver nanoparticles for ultrasensitively detecting theophylline. Chin. Chem. Lett. 2021, 32, 2865–2868. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, Y.; Sun, F.-X.; Zhu, G.-S. Targeted synthesis of novel porous aromatic frameworks with selective separation of CO2/CH4 and CO2/N2. Chin. Chem. Lett. 2014, 25, 1407–1410. [Google Scholar] [CrossRef]
- Cui, P.; Jing, X.-F.; Yuan, Y.; Zhu, G.-S. Synthesis of porous aromatic framework with Friedel–Crafts alkylation reaction for CO2 separation. Chin. Chem. Lett. 2016, 27, 1479–1484. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Z.; Wang, H.-G.; Cui, F.; Zhu, G. Integrated pyrazine-based porous aromatic frameworks/carbon nanotube composite as cathode materials for aqueous zinc ion batteries. Chem. Eng. J. 2022, 450, 138051. [Google Scholar] [CrossRef]
- You, B.; Tian, Y.; Wang, B.; Zhu, G. Au nanoparticles supported by porous aromatic frameworks—Efficient and recyclable catalysts for nitro reduction. Catalysis 2022, 12, 588. [Google Scholar] [CrossRef]
- Rangel-Rangel, E.; Verde-Sesto, E.; Rasero-Almansa, A.M.; Iglesias, M.; Sánchez, F. Porous aromatic frameworks (PAFs) as efficient supports for N-heterocyclic carbene catalysts. Catal. Sci. Technol. 2016, 6, 6037–6045. [Google Scholar] [CrossRef]
- Yu, W.J.; Li, H.Y.; Zhang, L.; Liu, J.; Kong, F.Y.; Wang, W. Preparation of magnetic porous aromatic framework for rapid and efficient removal of organic pollutants from water. Anal. Sci. 2020, 36, 1157–1163. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.Z.; Gao, F.; Wang, Z.; Liu, Y.A.; Hu, W.B.; Yang, H.; Wen, K. Highly Branched Pillar [5] arene-derived porous aromatic frameworks (PAFs) for removal of organic pollutants from water. ACS Appl. Mater. Interfaces 2021, 13, 16507–16515. [Google Scholar] [CrossRef]
- Yan, Z.; Cui, B.; Zhao, T.; Luo, Y.; Zhang, H.; Xie, J.; Li, N.; Bu, N.; Yuan, Y.; Xia, L. A carbazole-functionalized porous aromatic framework for enhancing volatile iodine capture via lewis electron pairing. Molecules 2021, 26, 5263. [Google Scholar] [CrossRef]
- Zhao, Y.; Bu, N.; Shao, H.; Zhang, Q.; Feng, B.; Xu, Y.; Zheng, G.; Yuan, Y.; Yan, Z.; Xia, L. A carbonized porous aromatic framework to achieve customized nitrogen atoms for enhanced supercapacitor performance. New J. Chem. 2019, 43, 18158–18164. [Google Scholar] [CrossRef]
- Yuan, R.; Ren, H.; He, H.; Jiang, L.; Zhu, G. Targeted synthesis of porous aromatic frameworks with stimuli-responsive adsorption properties. Sci. China Mater. 2015, 58, 38–43. [Google Scholar] [CrossRef][Green Version]
- Xia, L.; Yang, D.; Zhang, H.; Zhang, Q.; Bu, N.; Song, P.; Yan, Z.; Yuan, Y. Constructing “breathing” dynamic skeletons with extra pi-conjugated adsorption sites for iodine capture. RSC Adv. 2019, 9, 20852–20856. [Google Scholar] [CrossRef]
- An, D.; Li, L.; Zhang, Z.; Asiri, A.M.; Alamry, K.A.; Zhang, X. Amino-bridged covalent organic Polycalix [4] arenes for ultra efficient adsorption of iodine in water. Mater. Chem. Phys. 2020, 239, 122328. [Google Scholar] [CrossRef]
- Seo, S.; Chaikittisilp, W.; Koike, N.; Yokoi, T.; Okubo, T. Porous inorganic–organic hybrid polymers derived from cyclic siloxane building blocks: Effects of substituting groups on mesoporous structures. Micropor. Mesopor. Mater. 2019, 278, 212–218. [Google Scholar] [CrossRef]
- Du, W.; Qin, Y.; Ni, C.; Dai, W.; Zou, J. Efficient capture of volatile iodine by thiophene-containing porous organic polymers. ACS Appl. Polym. Mater. 2020, 2, 5121–5128. [Google Scholar] [CrossRef]
- Geng, T.; Zhang, W.; Zhu, Z.; Chen, G.; Ma, L.; Ye, S.; Niu, Q. A covalent triazine-based framework from tetraphenylthiophene and 2,4,6-trichloro-1,3,5-triazine motifs for sensing o-nitrophenol and effective I2 uptake. Polym. Chem. 2018, 9, 777–784. [Google Scholar] [CrossRef]
- Troschke, E.; Antonietti, M.; Thomas, A. Porous, covalent triazine-based frameworks prepared by ionothermal synthesis. Angew. Chem. Int. Ed. 2008, 47, 3450–3453. [Google Scholar]
- Chen, Y.; Sun, H.; Yang, R.; Wang, T.; Pei, C.; Xiang, Z.; Zhu, Z.; Liang, W.; Li, A.; Deng, W. Synthesis of conjugated microporous polymer nanotubes with large surface areas as absorbents for iodine and CO2 uptake. J. Mater. Chem. A 2015, 3, 87–91. [Google Scholar] [CrossRef]
- Abdelmoaty, Y.H.; Tessema, T.D.; Choudhury, F.A.; El-Kadri, O.M.; El-Kaderi, H.M. Nitrogen-rich porous polymers for carbon dioxide and iodine sequestration for environmental remediation. ACS Appl. Mater. Interfaces 2018, 10, 16049–16058. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Zhu, Z.Q.; Sun, H.X.; Ren, F.; Mu, P.; Liang, W.; Chen, L.; Li, A. Capture and reversible storage of volatile iodine by novel conjugated microporous polymers containing thiophene units. ACS Appl. Mater. Interfaces 2016, 8, 21063–21069. [Google Scholar] [CrossRef]
- Shetty, D.; Raya, J.; Han, D.S.; Asfari, Z.; Olsen, J.-C.; Trabolsi, A. Lithiated polycalix [4] arenes for efficient adsorption of iodine from solution and vapor phases. Chem. Mater. 2017, 29, 8968–8972. [Google Scholar] [CrossRef]
- Wang, Y.; Tao, J.; Xiong, S.; Lu, P.; Tang, J.; He, J.; Javaid, M.U.; Pan, C.; Yu, G. Ferrocene-based porous organic polymers for high-affinity iodine capture. Chem. Eng. J. 2020, 380, 122420. [Google Scholar] [CrossRef]
- Geng, T.; Chen, G.; Ma, L.; Zhang, C.; Zhang, W.; Xu, H. The spirobifluorene-based fluorescent conjugated microporous polymers for reversible adsorbing iodine, fluorescent sensing iodine and nitroaromatic compounds. Eur. Polym. J. 2019, 115, 37–44. [Google Scholar] [CrossRef]
- Xiong, S.; Tang, X.; Pan, C.; Li, L.; Tang, J.; Yu, G. Carbazole-bearing porous organic polymers with a mulberry-like morphology for efficient iodine capture. ACS Appl. Mater. Interfaces 2019, 11, 27335–27342. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.J.; Xu, S.Q.; Zhan, T.G.; Qi, Q.Y.; Wu, Z.Q.; Zhao, X. Ultrahigh volatile iodine uptake by hollow microspheres formed from a heteropore covalent organic framework. Chem. Commun. 2017, 53, 7266–7269. [Google Scholar] [CrossRef] [PubMed]
- Ren, F.; Zhu, Z.; Qian, X.; Liang, W.; Mu, P.; Sun, H.; Liu, J.; Li, A. Novel thiophene-bearing conjugated microporous polymer honeycomb-like porous spheres with ultrahigh iodine uptake. Chem. Commun. 2016, 52, 9797–9800. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Weber, J.; Mills, B.M.; Ren, Z.; Faul, C.F.J. Highly efficient and reversible iodine capture in hexaphenylbenzene-based conjugated microporous polymers. Macromolecules 2016, 49, 6322–6333. [Google Scholar] [CrossRef]
- Qian, X.; Wang, B.; Zhu, Z.Q.; Sun, H.X.; Ren, F.; Mu, P.; Ma, C.; Liang, W.D.; Li, A. Novel N-rich porous organic polymers with extremely high uptake for capture and reversible storage of volatile iodine. J. Hazard. Mater. 2017, 338, 224–232. [Google Scholar] [CrossRef]
- Su, K.; Wang, W.; Li, B.; Yuan, D. Azo-Bridged Calix [4] resorcinarene-Based Porous Organic Frameworks with Highly Efficient Enrichment of Volatile Iodine. ACS Sustain. Chem. Eng. 2018, 6, 17402–17409. [Google Scholar] [CrossRef]
- Song, S.; Shi, Y.; Liu, N.; Liu, F. C [double bond, length as m-dash] N linked covalent organic framework for the efficient adsorption of iodine in vapor and solution. RSC Adv. 2021, 11, 10512–10523. [Google Scholar] [CrossRef]
- Geng, T.; Chen, G.; Xia, H.; Zhang, W.; Zhu, Z.; Cheng, B. Poly{tris [4-(2-thienyl)phenyl]amine} and poly[tris(4-carbazoyl- 9-yl phenyl)amine] conjugated microporous polymers as absorbents for highly efficient iodine adsorption. J. Solid State Chem. 2018, 265, 85–91. [Google Scholar] [CrossRef]
- Li, X.; Chen, G.; Ma, J.; Jia, Q. Pyrrolidinone-based hypercrosslinked polymers for reversible capture of radioactive iodine. Sep. Purif. Technol. 2019, 210, 995–1000. [Google Scholar] [CrossRef]
- Anwar, N.; Willms, T.; Grimme, B.; Kuehne, A.J.C. Light-Switchable and Monodisperse Conjugated Polymer Particles. ACS Macro. Lett. 2013, 2, 766–769. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, Z.; Qiao, Y.; Wang, J.; Xie, J.; Cui, B.; Fu, Y.; Lu, J.; Yang, Y.; Bu, N.; Yuan, Y.; et al. An Azo-Group-Functionalized Porous Aromatic Framework for Achieving Highly Efficient Capture of Iodine. Molecules 2022, 27, 6297. https://doi.org/10.3390/molecules27196297
Yan Z, Qiao Y, Wang J, Xie J, Cui B, Fu Y, Lu J, Yang Y, Bu N, Yuan Y, et al. An Azo-Group-Functionalized Porous Aromatic Framework for Achieving Highly Efficient Capture of Iodine. Molecules. 2022; 27(19):6297. https://doi.org/10.3390/molecules27196297
Chicago/Turabian StyleYan, Zhuojun, Yimin Qiao, Jiale Wang, Jialin Xie, Bo Cui, Yu Fu, Jiawei Lu, Yajie Yang, Naishun Bu, Ye Yuan, and et al. 2022. "An Azo-Group-Functionalized Porous Aromatic Framework for Achieving Highly Efficient Capture of Iodine" Molecules 27, no. 19: 6297. https://doi.org/10.3390/molecules27196297
APA StyleYan, Z., Qiao, Y., Wang, J., Xie, J., Cui, B., Fu, Y., Lu, J., Yang, Y., Bu, N., Yuan, Y., & Xia, L. (2022). An Azo-Group-Functionalized Porous Aromatic Framework for Achieving Highly Efficient Capture of Iodine. Molecules, 27(19), 6297. https://doi.org/10.3390/molecules27196297








