Abstract
Heracleum persicum Desf. ex Fischer seeds are a rich source of essential oils (EOs) with high antimicrobial and antioxidant effects. In order to determine the phytochemical variability in various Iranian H. persicum populations, seed samples were collected from 10 different climatic locations. The current study indicated that hexyl butyrate (20.9–44.7%), octyl acetate (11.2–20.3%), hexyl-2-methylbutyrate (4.81–8.64%), and octyl 2-methyl butyrate (3.41–8.91%) were the major components of the EOs. The maximum (44.7%) and the minimum (20.9%) content of hexyl butyrate were obtained from Kaleibar and Sari populations, respectively. Moreover, the octyl acetate content ranged from 2% (in Mahdasht) to 20.3% in Torghabeh population. The CA and PCA analysis divided the 10 Iranian H. persicum populations into three major groups. Populations from Khanghah, Kaleibar, Shebeilo, Showt, Mahdasht, and Amin Abbad showed a distinct separation in comparison with the other populations, having high contents of hexyl butyrate (39.8%) and low contents of octyl acetate (13.5%) (Chemotype II). According to correlation analysis, the highest correlation coefficient was among habitat elevation and hexyl butyrate content. In addition, the mean annual precipitation was negatively correlated with the content of hexyl butyrate. Although octyl acetate content showed high correlation with soil EC and mean annual temperature, it was not statistically significant. In general, in order to have plants with a high content of hexyl butyrate, it is recommended to harvest these plants from regions with high altitude and low rainfall such as Kaleibar.
1. Introduction
The genus Heracleum, with about 109 species in the world and predominantly in Asia, is one of the widespread members of the Apiaceae family [1]. Heracleum persicum Desf. ex Fischer is an aromatic spice, which is known as Golpar in Iran. Seeds and shoots of H. persicum are traditionally used as a flavoring agent and spice in food products. Furthermore, the leaves and fruits of the plant are used as carminative, antiseptic, analgesic, and digestive agents in Iranian traditional medicine [2,3,4]. Food microbial contamination is one of the most important unfavorable factors, which not only causes food deterioration but also induces many diseases. It has been reported that as many as 30% of people in industrialized countries suffer from food-borne diseases each year. Although application of synthetic antioxidants could control microbial contamination in foods, their application is restricted due to carcinogenicity. Thus, finding natural, safe, and effective antioxidants and antimicrobials has gained more attention for controlling food-borne pathogens [5,6,7,8,9,10,11]. Essential oils (EOs) are receiving a good deal of attention for use as food preservatives to reduce the oxidative reactions during food processing and storage [11]. H. persicum seeds are a rich source of EOs, which could be used as antibacterial and antioxidant agents in food and pharmaceutical industries. Mostaghim et al. [12] reported that the EO of H. persicum had potential for use in food industry to inhibit pathogen growth and improve food quality and safety. They reported that the main constituents of seed EO of H. persicum were hexyl butyrate (35.5%) and octyl acetate (27%). Hexyl butyrate is a fatty acid ester obtained from the formal condensation of hexanol with butyric acid. The aliphatic esters octyl acetate is formed from 1-octanol (octyl alcohol) and acetic acid. Hexyl esters, most notably hexyl butyrate, are widely used as fragrance and flavor in food and pharmaceutical industries [13]. In addition, Maggi et al. [14] showed that antioxidant and anticancer activities of hogweed (Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt) EO mainly depend on octyl acetate and octyl butyrate.
EO content, constituents, and biological effects can be significantly influenced by different exogenous and endogenous factors [11]. The exogenous factors are factors regulated by environmental conditions such as precipitation, light, growing site, and soil. In contrast, the endogenous factors are genetically related factors. Previous studies revealed that climatic conditions and soil properties influence the phytochemicals and antioxidant properties of aromatic plants [15]. For instance, Vokou et al. [16] reported that the higher content of EO in Origanum vulgare ssp. hirtum was achieved in higher altitude.
Thus, a comparative analysis of EO quantity and quality collected from various geographical locations would be vital for industrial purposes. Moreover, existing variation could be used in breeding programs for the development of cultivars satisfied levels of active ingredients, which in turn may promote the cultivation of this high value aromatic and spice plant [16]. The objective of the present study was to evaluate the phytochemical diversity of Iranian wild populations of H. persicum, which is important for the development of in situ and ex situ conservation programs and for the selection of preferable populations for breeding programs and industries.
2. Results and Discussion
2.1. EO Content
Analysis of variance showed that there were significant differences among H. persicum populations for EO content. Although the highest EO content (3.15%) was obtained from the plants collected from Amin Abbad, there were no statistically significant differences between samples obtained from this site and those harvested from Kaleibar (3.14%) and Khanghah (3.05%). The lowest EO content (2.16%) was found in the samples related to Sari. As shown in Figure 1, the highest content of EO is related to the plants originated from regions with higher altitude and harsher environmental conditions. Also, plants grown in regions with lower altitude and more suitable temperature and rainfall conditions, such as Sari, have a lower percentage of EO, which indicates the moderating effect of EO in dealing with environmental stress conditions. In fact, EO production was involved in protection against changing environments and stressful constraints during growth. Similar results were also reported by Mirza and Najafpour [17] and Radjabian et al. [18], both on Heracleum gorganicum Rech.f.
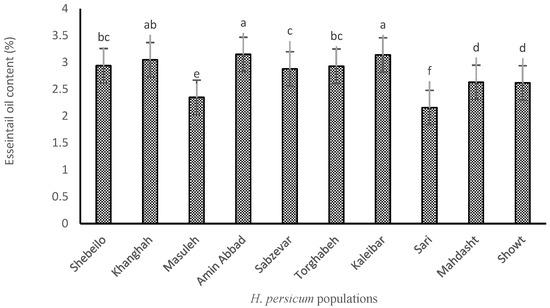
Figure 1.
EO content of different H. persicum populations. Means ± standard deviations with same letters do not have significant difference from each other.
2.2. EO Components Analysis
A total of 23 components were identified in the EOs representing 86.5–99.3% of the total identified components (Table 1). The GC−MS analysis revealed that hexyl butyrate (20.9–44.7%), octyl acetate (11.2–20.3%), hexyl-2-methylbutyrate (4.81–8.64%) and octyl 2-methyl butyrate (3.41–8.91%) were the major components, while butyl butyrate (0.82–2.22%), butyl 2-methylbutyrate (0.74–2.12%), hexyl isobutyrate (3.06–6.43%), n-decanal (0.36–3.14%), octyl isobutyrate (1.85–5.85%), n-hexyl hexanoate (3.32–7.98%), and n-octyl butyrate (2.33–5.65%) had lower contents in the EO of H. persicum.

Table 1.
The EO composition of the populations of H. persicum.
The maximum (44.7%) and minimum (20.9%) contents of hexyl butyrate were obtained from Kaleibar and Sari populations, respectively (Table 1). Moreover, the octyl acetate content ranged from 11.2% (in Mahdasht) to 20.3% in Torghabeh population. However, Masuleh population showed the highest contents of octyl isobutyrate (5.85%), n-hexyl hexanoate (7.98%), and n-octyl butyrate (5.65%) (Table 1).
The highest content of isopropyl 2-methylbutyrate (2.25%) and linalool (1.32%) were related to Sari population. Also, the samples collected from the Showt region showed the highest contents of isopropyl isovalerate, isobutyl 2-methylbutyrate, hexyl acetate, and limonene. Butyl butyrate content ranged from 1.1% to 2.4% in Sari and Torghabeh populations, respectively. The highest content of o-cymene (2.5%), γ-terpinene (0.81%), n-decanal (2.61%), and hexyl-2-methylbutyrate (8.64%) were obtained from Amin Abbad population.
The EO composition of H. persicum having different origins has been previously reported. The main constituents in some reports were hexyl butanoate (28.32%), heptyl isobutanoate (24.05%), hexyl 2-methylbutanoate (8.24%) [19] and octyl acetate and hexyl butyrate [20,21]. Our results revealed that EO content and composition of H. persicum significantly varied among the different populations which indicates the adaptability of the plant to the ecological conditions of the habitat. Sangwan et al. [22] demonstrated that genetics and ecological factors are two key factors influencing the EO variability. For example, Pouyanfar et al. [23] indicated that the higher amount of rainfall increases the caryophyllene oxide and bergamotol acetate contents in Melissa officinalis L. Diversity in plant active ingredients in response to ecological factors has been previously reported in Apiaceae [24] and Asteraceae families [25].
2.3. Classification of the H. persicum Populations
To determine the phytochemical variability and identify the different chemotypes of H. persicum, their EO components were submitted to principal component (PCA) and cluster analysis (CA). According to PCA, the first four components of the PCA explained 77.6% of the total variation (Table 2). The first PC (PC1) showed 26% of the total variation and had a positive correlation with isopropyl 2-methylbutyrate (0.71), hexyl-2-methylbutyrate (0.71), octyl isobutyrate (0.76%), n-hexyl hexanoate (0.56%) and n-octyl butyrate (0.73%). The second component (PC2) was formed of o-cymene, limonene, butyl 2-methylbutyrate, hexyl isobutyrate, octyl acetate, and hexyl isovalerate compounds. The third PC (PC3) had positive correlation with isobutyl butyrate and butyl butyrate, hexyl acetate, and n-decanal and a negative correlation with octyl 2-methyl butyrate. Finally, the fourth PC showed a positive and negative correlation with linalool and thymol, respectively (Table 2).

Table 2.
Eigenvectors of the first four principal component axes from PCA analysis of variables in studied H. persicum populations.
The biplots for PCA and dendrogram for cluster analysis showed similar patterns for populations grouping. The CA and PCA analysis divided the 10 Iranian H. persicum populations into three major groups, each representing a distinct chemotype. According to Figure 2 and Figure 3 and Table 1, chemotype I was made of two populations (including Sabzevar and Torghabeh), with hexyl butyrate (34.6%) and octyl acetate (19.6%) as the major compound of the EO. Populations from Khanghah, Kaleibar, Shebeilo, Showt, Mahdasht, and Amin Abbad showed a distinct separation in comparison with the other populations, having high contents of hexyl butyrate (39.8%) and low content of octyl acetate (13.5%) (Chemotype II). Chemotype III was comprised of Masuleh and Sari with isopropyl 2-methylbutyrate, octyl isobutyrate, and n-hexyl hexanoate (Table 1). Despite the fact that geographical and climatic conditions influence the distribution of populations into different chemotypes, it seems to be associated with the local selective forces. Hanover [26] reported that the appearance of chemotypes is strongly controlled by genetic factors. The results illustrated that geographic and ecological factors had a key role in the occurrence of different chemotypes such that populations with close and similar geographic distances may be subject to different chemotypes due to forces of ecological conditions.
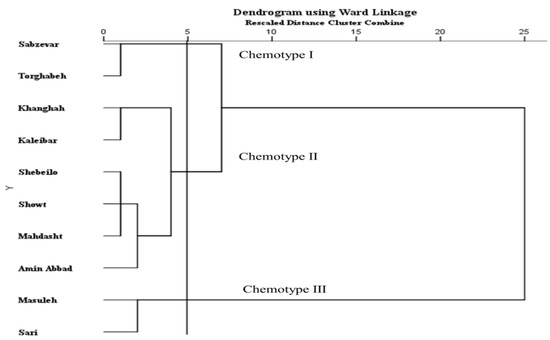
Figure 2.
Cluster analysis of 10 populations of H. persicum based on EO components. Populations were clustered using Ward Linkage algorithm.
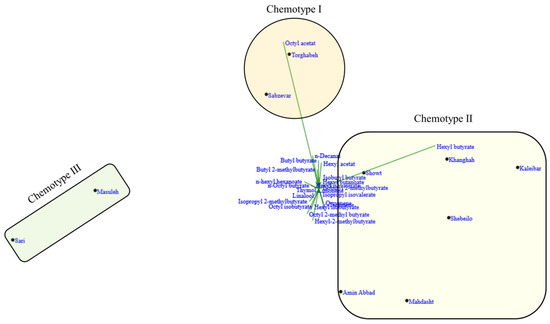
Figure 3.
PCA biplot analysis of H. persicum populations based on phytochemical traits.
2.4. Environmental Factors Affecting the Chemical Variability
Canonical correspondence analysis (CCA) was carried out according to matrix linking percentages of ten major constituents and six environmental factors to assess the environmental factors impact on chemical variability (Figure 4). The first CCA (CC1) variable in relation with collected sites and their climatic characteristics revealed that soil K content and altitude (positively) and soil EC, available P, MAP, and MAT (negatively) had a great share in the formation of this canonical variable. Coefficients of the first canonical variable in relation with major components revealed that hexyl butyrate (positively) and n-octyl butyrate, n-hexyl hexanoate, and hexyl-2-methylbutyrate (negatively) had a great share on the formation of this canonical variable. Furthermore, populations from Khanghah, Kaleibar, Shebeilo, Showt, Mahdasht, and Amin Abbad were positively, and those from Sabzevar, Torghabeh, Masuleh and Sari were negatively affected by the first canonical set (Table 3).
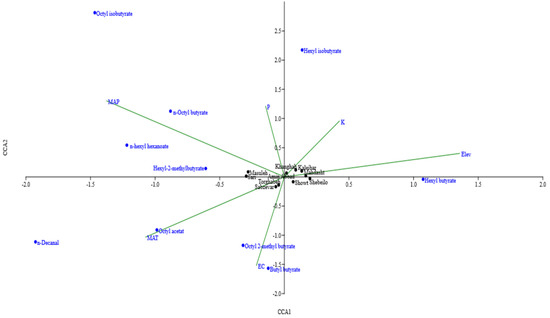
Figure 4.
Canonical correspondence analysis biplot of H. persicum populations, linking percentages of the major components, collected site and their climatic characteristics.

Table 3.
Canonical coefficients, eigen-values, estimated and cumulative variance for four CCA sets between phytochemicals with environmental factors.
2.5. Correlation Results
Correlation analysis was also carried out to confirm the results of CCA (Table 4). The octyl acetate content showed a positive and significant correlation with butyl butyrate and n-decanal contents. Although n-hexyl hexanoate content was positively correlated with n-octyl butyrate, a negative and significant correlation was found with hexyl butyrate content. In addition, the content of n-octyl butyrate was positively correlated with the octyl isobutyrate content. There was no significant coefficient between hexyl isobutyrate, hexyl-2-methylbutyrate and octyl 2-methyl butyrate contents with the other oil components. In the case of environmental factors and soil characteristics, the highest correlation coefficient was among habitat elevation and hexyl butyrate content. In addition, soil available P and EC positively correlated with hexyl isobutyrate and butyl butyrate contents, respectively. Other positive correlations were between mean annual temperature and octyl 2-methyl butyrate content. In addition, mean annual precipitation was negatively correlated with the content of hexyl butyrate, but a positive and significant correlation was found with octyl isobutyrate, n-hexyl hexanoate, and n-octyl butyrate contents. Maggi et al. [14] revealed that the cultivated varieties of Heracleum sphondylium had lower contents of octyl acetate than wild populations.

Table 4.
Correlation of major components of Heracleum persicum and environmental factors.
3. Materials and Methods
3.1. Plant Materials
The seeds of H. persicum wild populations were collected from 10 different geographical regions in Iran (Figure 5). Geographic coordinates, including altitude, latitude, and longitude of each region were recorded using Global Positioning System (GPS) and are presented in Table 5. In each studied site, the soil samples were taken from the root depth of the plants (0–30 cm) and were analyzed to determine the main physicochemical properties of the soil, including available phosphorus, potassium, and electrical conductivity (EC) (Table 5) [27]. Sampling was done using a random–systematic method along with the located transects. The plant seeds were collected at ripening stage. They were identified and recorded by Seed Bank of Medicinal Plants Institute, ACECR with the HP-436 code.
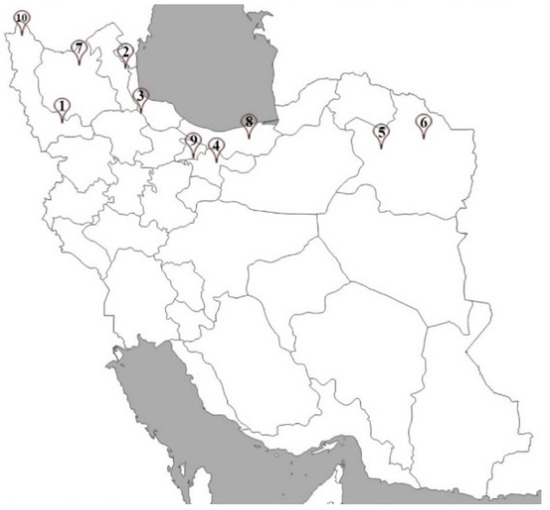
Figure 5.
Sample collection sites located in different provinces of Iran. Numbers showing the collection sites.

Table 5.
Origins, geographical characteristics, and soil properties of studied H. persicum populations.
3.2. Preparation of the EO
To extract the EO, the seed samples of H. persicum in three replications were ground to obtain a fine powder. Then, the EOs were extracted by hydro-distillation method for 3 h using a Clevenger-type apparatus. The EOs were dried over anhydrous sodium sulphate and kept at 4 °C until the analysis stage. After that, EO content (%) was measured based on mass of EOs (g) extracted from 40 g mass of seed samples using the following formula:
EO content (%) = [mass of EO (g)/mass of seed dry matter (g)] × 100
3.3. GC-MS Analysis
3.3.1. Gas Chromatography (GC)
Gas chromatography analysis was performed using a Younglin Acme6000 gas chromatograph equipped with BP-5 column (30 m, 0.25 mm internal diameter, and 0.25 µm film thickness). The column temperature program was set as follows: oven temperature was held at 50 °C for 5 min, from 50 to 240 °C at a rate of 3 °C per min, and to 300 °C at a rate of 15 °C per min and held at this temperature for 3 min. Injection and detection temperature was set at 290 and 300 °C, respectively. Helium was used as carrier gas with a linear velocity of 0.8 mL per min. All samples before injection were diluted in n-hexane (1:100). One microliter of the diluted sample was injected manually in the split mode 1:25 to the instrument. C8–C40 alkanes calibration std (Supelco, Bellefonte, PA, USA) was used to calculate the retention indices (RI). Identification of the individual copmounds of EOs was done by comparing their retention index (RI) with those of a computer library (Wiley, Adams and NIST05).
3.3.2. Gas Chromatography/Mass Spectroscopy (GC-MS)
GC-MS analyses were carried out with an Agilent 6890 system equipped with a mass spectrometer (Agilent 5973) detector and a BPX5 column (30 m, 0.25 mm internal diameter, and 0.25 µm film thicknesses). The carrier gas was helium at a flow rate of 0.5 mL per min. The column temperature was held at 50 °C for 5 min and was gradually increased from 50 to 240 °C with a rate of 3 °C per min and to 300 °C with a rate of 15 °C per min and held at this temperature for 3 min. For detection, an electron ionization system was used with ionization energy of 70 electron Volts. The injector and ionization source temperature were 290 and 220 °C, respectively [28].
3.4. Data Analysis
Analysis of variance appropriate to the experimental design was done using SAS software (ver. 9.1). Mean comparison of the traits was done using Duncan multiple range tests at p ≤ 0.05 significance level. The simple Pearson correlation coefficients were calculated to determine the relationships between the studied traits. Cluster analysis, based on EO components, was performed using the ward method. In order to determine the most variable characters among the populations, factor analysis using the principal component analysis (PCA) method was performed.
4. Conclusions
Our results showed that there were significant differences among H. persicum populations for EO content. Although the highest EO content was obtained from the plants collected from the Amin Abbad, there were no statistically significant differences between samples from this region and those harvested from Kaleibar and Khanghah. The current study indicated that the maximum and the minimum contents of hexyl butyrate were obtained from Kaleibar and Sari populations, respectively. Moreover, the octyl acetate content ranged from 11.2% (in Mahdasht) to 20.3% in Torghabeh population. The CA and PCA analysis divided the 10 Iranian H. persicum populations into three major groups. Populations from Khanghah, Kaleibar, Shebeilo, Showt, Mahdasht, and Amin Abbad showed a distinct separation in comparison to the other populations, having high contents of hexyl butyrate and low content of octyl acetate (Chemotype II). According to correlation analysis, the highest correlation coefficient was among habitat elevation and hexyl butyrate content. In addition, the mean annual precipitation was negatively correlated with the content of hexyl butyrate. Although octyl acetate content showed high correlation with soil EC and mean annual temperature, it was not statistically significant. In general, in order to have plants with a high content of hexyl butyrate, it is recommended to harvest these plants from regions with high altitude and low rainfall, such as Kaleibar.
Author Contributions
Conceptualization, S.H.M., A.A. and M.R.M.; methodology, S.H.M., M.P. and J.M.L.; validation, S.H.M. and A.A.; formal analysis, S.H.M. and A.A.; investigation, A.A., M.R.M. and J.M.L.; data curation, S.H.M. and M.P.; writing—original draft preparation, S.H.M., A.A. and M.R.M.; writing—review and editing, M.P. and J.M.L.; visualization, A.A., M.R.M. and J.M.L.; supervision, A.A.; project administration, A.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data used to support the findings of this study are available. Further inquiries can be directed to the corresponding authors.
Acknowledgments
Jose M. Lorenzo belongs to the competitive reference research group FunMeat (Axencia Galega de Innovación, GAININ607A2019/01).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are available from the authors.
References
- Pimenov, M.G.; Leonov, M.V. The Asian Umbelliferae biodiversity database (ASIUM) with particular reference to South-West Asian taxa. Turk. J. Botany 2004, 28, 139–145. [Google Scholar]
- Lorenzo, J.M.; Munekata, P.E.S. Phenolic compounds of green tea: Health benefits and technological application in food. Asian Pac. J. Trop. Biomed. 2016, 6, 709–719. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Alcántara, C.; Collado, M.C.; Garcia-Perez, J.V.; Saraiva, J.A.; Lopes, R.P.; Barba, F.J.; do Prado Silva, L.; Sant’Ana, A.S.; Fierro, E.M.; et al. Ethnopharmacology, phytochemistry and biological activity of Erodium species: A review. Food Res. Int. 2019, 126, 108659. [Google Scholar] [CrossRef]
- Alirezalu, A.; Ahmadi, N.; Salehi, P.; Sonboli, A.; Alirezalu, K.; Mousavi Khaneghah, A.; Barba, F.J.; Munekata, P.E.S.; Lorenzo, J.M. Physicochemical Characterization, Antioxidant Activity, and Phenolic Compounds of Hawthorn (Crataegus spp.) Fruits Species for Potential Use in Food Applications. Foods 2020, 9, 436. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Barba, F.J.; Domínguez, R.; Sant’Ana, A.S.; Mousavi Khaneghah, A.; Gavahian, M.; Gómez, B.; Lorenzo, J.M. Essential oils as natural additives to prevent oxidation reactions in meat and meat products: A review. Food Res. Int. 2018, 113, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Vargas, F.C.; Chincha, A.A.I.A.; Sant’Ana, A.S.; Strozzi, I.; Rocchetti, G.; Barba, F.J.; Domínguez, R.; Lucini, L.; do Amaral Sobral, P.J.; et al. Guarana seed extracts as a useful strategy to extend the shelf life of pork patties: UHPLC-ESI/QTOF phenolic profile and impact on microbial inactivation, lipid and protein oxidation and antioxidant capacity. Food Res. Int. 2018, 114, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef]
- Munekata, P.E.S.; Gullón, B.; Pateiro, M.; Tomasevic, I.; Domínguez, R.; Lorenzo, J.M. Natural Antioxidants from Seeds and Their Application in Meat Products. Antioxidants 2020, 9, 815. [Google Scholar] [CrossRef] [PubMed]
- Domínguez, R.; Gullón, P.; Pateiro, M.; Munekata, P.E.S.; Zhang, W.; Lorenzo, J.M. Tomato as potential source of natural additives for meat industry. A review. Antioxidants 2020, 9, 73. [Google Scholar] [CrossRef]
- Peñalver, R.; Lorenzo, J.M.; Ros, G.; Amarowicz, R.; Pateiro, M.; Nieto, G. Seaweeds as a Functional Ingredient for a Healthy Diet. Mar. Drugs 2020, 18, 301. [Google Scholar] [CrossRef] [PubMed]
- Pateiro, M.; Munekata, P.E.S.; Sant’Ana, A.S.; Domínguez, R.; Rodríguez-Lázaro, D.; Lorenzo, J.M. Application of essential oils as antimicrobial agents against spoilage and pathogenic microorganisms in meat products. Int. J. Food Microbiol. 2021, 337, 108966. [Google Scholar] [CrossRef] [PubMed]
- Nabi, S.; Toktam, M.; Afshin, A.; Issa, M.; Mehran, S.; Mohammad, R. Antibacterial Properties of Essential Oil of Heracleum persicum (Golpar) and Foodborne Pathogens. Int. J. Enteric. Pathog. 2017, 5, 41–44. [Google Scholar]
- Lanciotti, R.; Gianotti, A.; Patrignani, F.; Belletti, N.; Guerzoni, M.E.; Gardini, F. Use of natural aroma compounds to improve shelf-life and safety of minimally processed fruits. Trends Food Sci. Technol. 2004, 15, 201–208. [Google Scholar] [CrossRef]
- Maggi, F.; Quassinti, L.; Bramucci, M.; Lupidi, G.; Petrelli, D.; Vitali, L.A.; Papa, F.; Vittori, S. Composition and biological activities of hogweed [Heracleum sphondylium L. subsp. ternatum (Velen.) Brummitt] essential oil and its main components octyl acetate and octyl butyrate. Nat. Prod. Res. 2014, 28, 1354–1363. [Google Scholar] [CrossRef] [PubMed]
- Alirezalu, K.; Pateiro, M.; Yaghoubi, M.; Alirezalu, A.; Peighambardoust, S.H.; Lorenzo, J.M. Phytochemical constituents, advanced extraction technologies and techno-functional properties of selected Mediterranean plants for use in meat products. A comprehensive review. Trends Food Sci. Technol. 2020, 100, 292–306. [Google Scholar] [CrossRef]
- Vokou, D.; Kokkini, S.; Bessiere, J.M. Geographic variation of Greek oregano (Origanum vulgare ssp. hirtum) essential oils. Biochem. Syst. Ecol. 1993, 21, 287–295. [Google Scholar] [CrossRef]
- Mirza, M.; Najafpour Navaei, M. Comparative Study on Chemical Composition of Fruit Essential Oil of Heracleum Gorganicum Rech. F. in Different Altitudes. Iran. J. Med. Aromat. Plants 2012, 28, 324–329. [Google Scholar]
- Radjabian, T.; Rahmani, N.; Salimi, A.; Shahiri Tabarestani, F. Essential Oil Composition of Heracleum Gorganicum Rech. F. from Four Wild Populations Growing in Iran. Iran. J. Med. Aromat. Plants 2014, 27, 82–90. [Google Scholar]
- Gharachorloo, M.; Honarvar, M.; Mardani, S. Chemical compositions and antioxidant activity of Heracleum persicum essential oil. Braz. J. Pharm. Sci. 2018, 53, 260. [Google Scholar] [CrossRef]
- Mirza, M.; Najafpour Navaei, M.; Behrad, Z. Essential Oil Composition of Heracleum Pastinasifolium C. Koch. Seed in Different Altitudes of Arasbaran Region. Iran. J. Med. Aromat. Plants 2014, 2, 26–30. [Google Scholar]
- Radjabian, T.; Salimi, A.; Rahmani, N.; Shockravi, A.; Mozaffarian, V. Essential Oil Composition of Some Wild Populations of Heracleum persicum Desf. Ex Fischer Growing in Iran. J. Essent. Oil Bear. Plants 2013, 16, 841–849. [Google Scholar] [CrossRef]
- Sangwan, N.S.; Farooqi, A.H.A.; Shabih, F.; Sangwan, R.S. Regulation of essential oil production in plants. Plant Growth Regul. 2001, 34, 3–21. [Google Scholar] [CrossRef]
- Pouyanfar, E.; Hadian, J.; Akbarzade, M.; Hatami, M.; Kanani, M.R.; Ghorbanpour, M. Analysis of phytochemical and morphological variability in different wild-and agro-ecotypic populations of Melissa officinalis L. growing in northern habitats of Iran. Ind. Crops Prod. 2018, 112, 262–273. [Google Scholar] [CrossRef]
- Rahimmalek, M.; Maghsoudi, H.; Sabzalian, M.R.; Ghasemi Pirbalouti, A. Variability of Essential Oil Content and Composition of Different Iranian Fennel (Foeniculum vulgare Mill.) Accessions in Relation to Some Morphological and Climatic Factors. J. Agric. Sci. Technol. 2014, 16, 1365–1374. [Google Scholar]
- Haider, F.; Dwivedi, P.; Singh, S.; Naqvi, A.A.; Bagchi, G. Influence of transplanting time on essential oil yield and composition in Artemisia annua plants grown under the climatic conditions of sub-tropical north India. Flavour Fragr. J. 2004, 19, 51–53. [Google Scholar] [CrossRef]
- Hanover, J.W. Applications of terpene analysis in forest genetics. New For. 1992, 6, 159–178. [Google Scholar] [CrossRef]
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Sparkman, O.D. Identification of essential oil components by gas chromatography/mass spectroscopy Robert P. Adams. J. Am. Soc. Mass Spectrom. 1997, 8, 671–672. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).