Carboxymethyl Chitosan and Gelatin Hydrogel Scaffolds Incorporated with Conductive PEDOT Nanoparticles for Improved Neural Stem Cell Proliferation and Neuronal Differentiation
Abstract
1. Introduction
2. Results and Discussion
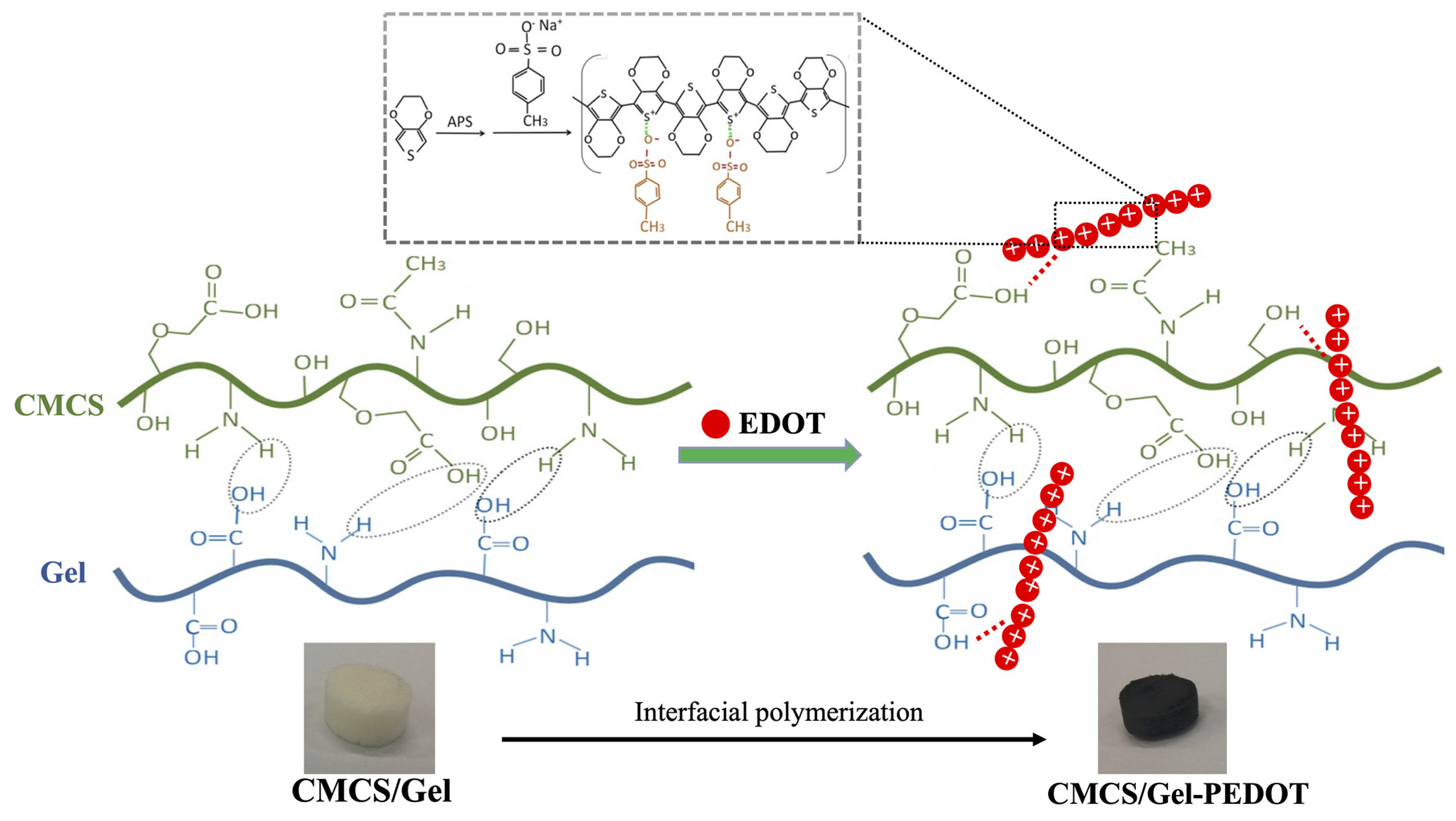
2.1. Synthesis of CMCS/Gel-PEDOT Conductive Hydrogels
2.2. FTIR Spectra Analysis of Hydrogels and PEDOT Nanoparticles
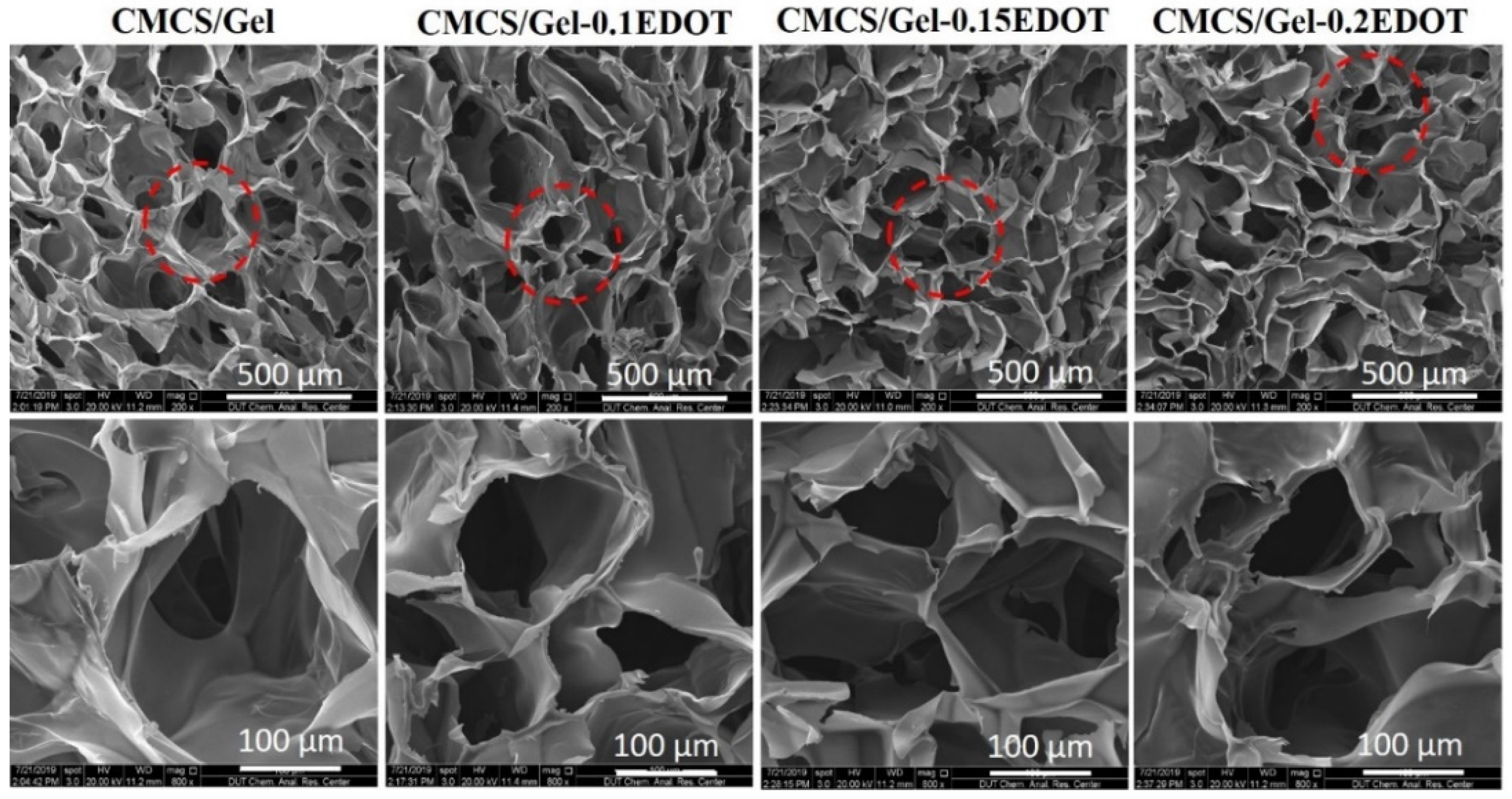
2.3. Morphology of Hydrogels
2.4. Electrical Conductivity of Hydrogels
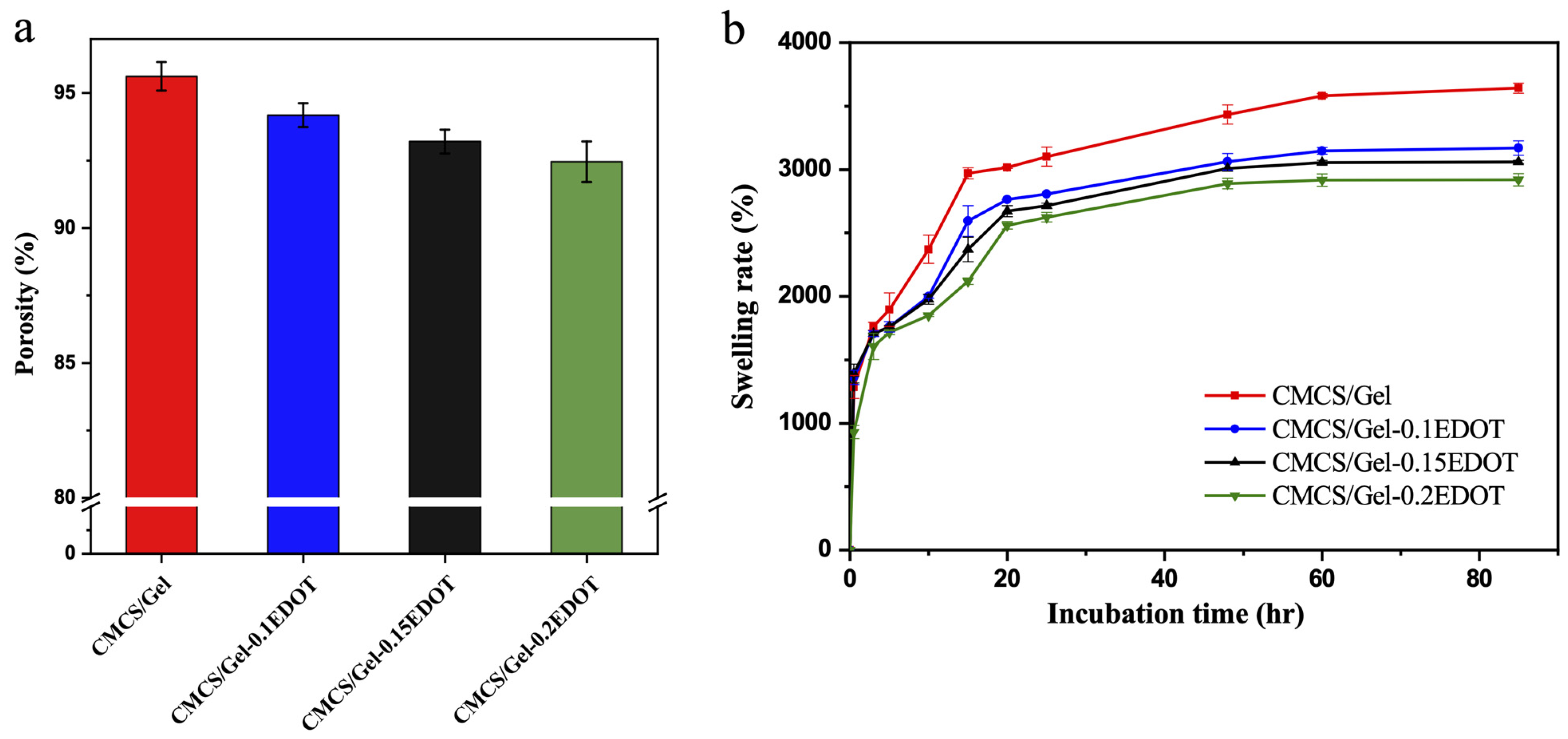
2.5. Porosity and Swelling Rate of Hydrogels
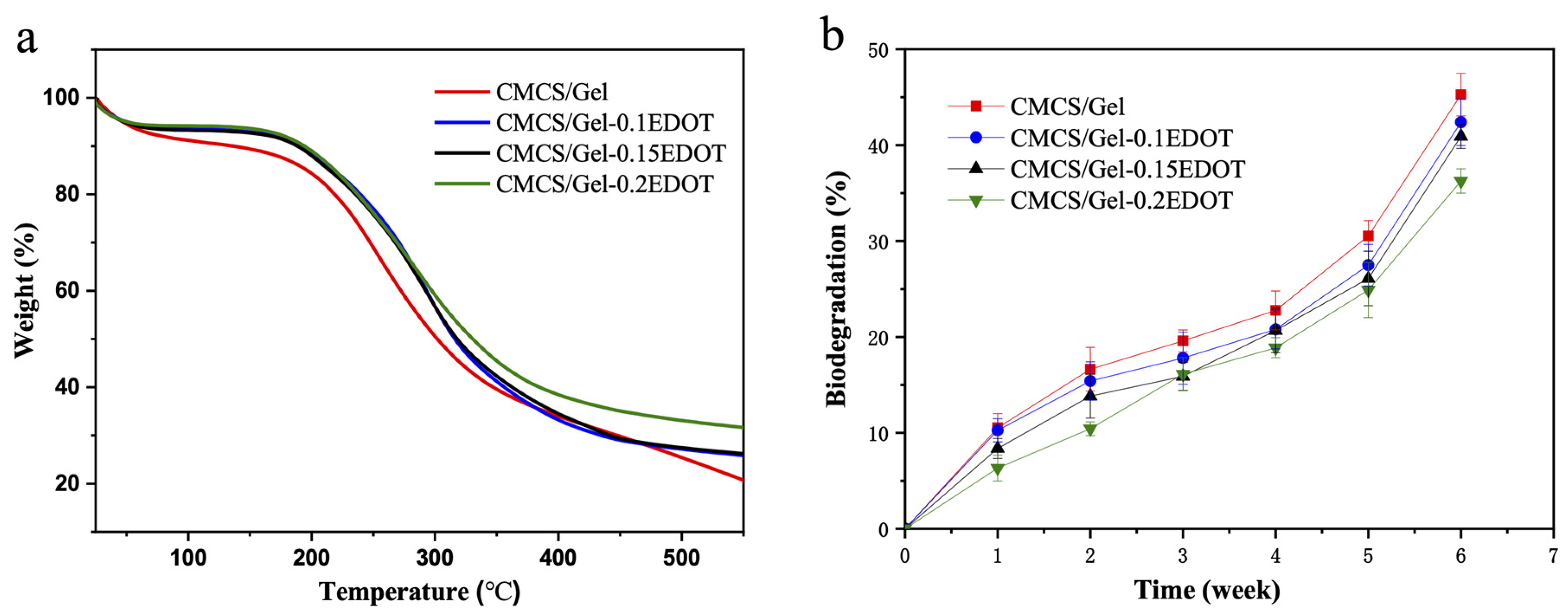
2.6. Thermal Stability and Biodegradation of Hydrogels
2.7. Mechanical Properties of Hydrogels
2.8. The Adhesion and Viability of NSCs in the CMCS/Gel-PEDOT Hydrogels
2.9. The Differentiation of NSCs in the CMCS/Gel-PEDOT Hydrogels
3. Materials and Methods
3.1. Materials
3.2. Preparation of CMCS
3.3. Preparation of CMCS/Gel-PEDOT Conductive Hydrogels
3.4. Fourier Transform Infrared Spectroscopy
3.5. Scanning Electron Microscope
3.6. Electrical Conductivity
3.7. Porosity
3.8. Swelling Rate
3.9. Thermogravimetric Analysis (TGA)
3.10. In Vitro Enzymatic Biodegradation
3.11. Compressive Modulus
3.12. NSC Culture
3.13. Cell Viability Assay
3.14. Immunocytochemistry Analysis
3.15. Gene Expression Analysis
3.16. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Thrivikraman, G.; Boda, S.K.; Basu, B. Unraveling the mechanistic effects of electric field stimulation towards directing stem cell fate and function: A tissue engineering perspective. Biomaterials 2018, 150, 60–86. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.W.; Tong, W.Y.; Pang, S.W.; Voelcker, N.H.; Lam, Y.W. Deconstructing, replicating, and engineering tissue microenvironment for stem cell differentiation. Tissue Eng. Part B-Rev. 2020, 26, 540–554. [Google Scholar] [CrossRef] [PubMed]
- Lindvall, O.; Kokaia, Z.; Martinez-Serrano, A. Stem cell therapy for human neurodegenerative disorders-how to make it work. Nat. Med. 2004, 10 (Suppl. S1), 42–50. [Google Scholar] [CrossRef] [PubMed]
- Martino, G.; Pluchino, S. The therapeutic potential of neural stem cells. Nat. Rev. Neurosci. 2006, 7, 395–406. [Google Scholar] [CrossRef]
- Holzapfel, B.M.; Reichert, J.C.; Schantz, J.T.; Gbureck, U.; Rackwitz, L.; Nöth, U.; Jakob, F.; Rudert, M.; Groll, J.; Hutmacher, D.W. How smart do biomaterials need to be? A translational science and clinical point of view. Adv. Drug Deliv. Rev. 2013, 65, 581–603. [Google Scholar] [CrossRef]
- Place, E.S.; Evans, N.D.; Stevens, M.M. Complexity in biomaterials for tissue engineering. Nat. Mater. 2009, 8, 457–470. [Google Scholar] [CrossRef]
- Khaing, Z.Z.; Thomas, R.C.; Geissler, S.A.; Schmidt, C.E. Advanced biomaterials for repairing the nervous system: What can hydrogels do for the brain? Mater. Today 2014, 17, 332–340. [Google Scholar] [CrossRef]
- Zimmermann, J.A.; Schaffer, D.V. Engineering biomaterials to control the neural differentiation of stem cells. Brain Res. Bull. 2019, 150, 50–60. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Saeb, M.R.; Shojaei, S.; Zarrin, N.K.; Thomas, S.; Ramakrishna, S. Conductive biomaterials as substrates for neural stem cells differentiation towards neuronal lineage cells. Macromol. Biosci. 2021, 21, e2000123. [Google Scholar] [CrossRef]
- Niari, S.A.; Rahbarghazi, R.; Geranmayeh, M.H.; Karimipour, M. Biomaterials patterning regulates neural stem cells fate and behavior: The interface of biology and material science. J. Biomed. Mater. Res. Part A 2022, 110, 725–737. [Google Scholar] [CrossRef]
- Bierman-Duquette, R.D.; Safarians, G.; Huang, J.; Rajput, B.; Chen, J.Y.; Wang, Z.Z.; Seidlits, S.K. Engineering tissues of the central nervous system: Interfacing conductive biomaterials with neural stem/progenitor cells. Adv. Healthc. Mater. 2021, 22, e2101577. [Google Scholar] [CrossRef]
- Guilak, F.; Cohen, D.M.; Estes, B.T.; Gimble, J.M.; Liedtke, W.; Chen, C.S. Control of stem cell fate by physical interactions with the extracellular matrix. Cell Stem. Cell 2009, 5, 17–26. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Gilbert, P.M.; Blau, H.M. Designing materials to direct stem cell fate. Nature 2009, 462, 433–441. [Google Scholar] [CrossRef]
- Panda, A.K.; Basu, B. Biomaterials-based bioengineering strategies for bioelectronic medicine. Mater. Sci. Eng. R-Rep. 2021, 146, 100630. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, Y.; Yue, H.; Fan, Y. Electrical stimulation promotes stem cell neural differentiation in tissue engineering. Stem. Cells Int. 2021, 20, 6697574. [Google Scholar] [CrossRef]
- Zhu, R.; Sun, Z.; Li, C.; Ramakrishna, S.; Chiu, K.; He, L. Electrical stimulation affects neural stem cell fate and function in vitro. Exp. Neurol. 2019, 319, 112963. [Google Scholar] [CrossRef] [PubMed]
- Prenassi, M.; Arlotti, M.; Borellini, L.; Bocci, T.; Cogiamanian, F.; Locatelli, M.; Rampini, P.; Barbieri, S.; Priori, A.; Marceglia, S. The relationship between electrical energy delivered by deep brain stimulation and levodopa-induced dyskinesias in Parkinson’s disease: A retrospective preliminary analysis. Front. Neurol. 2021, 12, 643841. [Google Scholar] [CrossRef] [PubMed]
- Zamproni, L.N.; Mundim, M.T.V.V.; Porcionatto, M.A. Neurorepair and regeneration of the brain: A decade of bioscaffolds and engineered microtissue. Front. Cell Dev. Biol. 2021, 9, 649891. [Google Scholar] [CrossRef] [PubMed]
- Martino, S.; D’Angelo, F.; Armentano, I.; Kenny, J.M.; Orlacchio, A. Stem cell-biomaterial interactions for regenerative medicine. Biotechnol. Adv. 2012, 30, 338–351. [Google Scholar] [CrossRef]
- Uto, K.; Arakawa, C.K.; DeForest, C.A. Next-generation biomaterials for culture and manipulation of stem cells. Cold Spring Harb. Perspect. Biol. 2020, 12, a035691. [Google Scholar] [CrossRef]
- Du, J.; Zhen, G.; Chen, H.; Zhang, S.; Qing, L.; Yang, X.; Lee, G.; Mao, H.; Jia, X. Optimal electrical stimulation boosts stem cell therapy in nerve regeneration. Biomaterials 2018, 181, 347–359. [Google Scholar] [CrossRef] [PubMed]
- Song, S.; Amores, D.; Chen, C.; McConnell, K.; Oh, B.; Poon, A.; George, P.M. Controlling properties of human neural progenitor cells using 2D and 3D conductive polymer scaffolds. Sci. Rep. 2019, 9, 19565. [Google Scholar] [CrossRef] [PubMed]
- Guimard, N.K.; Gomez, N.; Schmidt, C.E. Conducting polymers in biomedical engineering. Prog. Polym. Sci. 2007, 32, 876–921. [Google Scholar] [CrossRef]
- Balint, R.; Cassidy, N.J.; Cartmell, S.H. Conductive polymers: Towards a smart biomaterial for tissue engineering. Acta Biomater. 2014, 10, 2341–2353. [Google Scholar] [CrossRef] [PubMed]
- Green, R.; Abidian, M.R. Conducting polymers for neural prosthetic and neural interface applications. Adv. Mater. 2015, 27, 7620–7637. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Sun, C.K.; Guan, S.; Li, W.F.; Xu, J.Q.; Ge, D.; Zhuang, M.L.; Liu, T.Q.; Ma, X.H. Chitosan/gelatin porous scaffolds assembled with conductive poly(3,4-ethylenedioxythiophene) nanoparticles for neural tissue engineering. J. Mater. Chem. B 2017, 5, 4774–4788. [Google Scholar] [CrossRef]
- Xu, C.; Guan, S.; Wang, S.P.; Gong, W.T.; Liu, T.Q.; Ma, X.H.; Sun, C.K. Biodegradable and electroconductive poly(3,4-ethylenedioxythiophene)/carboxymethyl chitosan hydrogels for neural tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 84, 32–43. [Google Scholar] [CrossRef]
- Kim, Y.S.; Cho, K.; Lee, H.J.; Chang, S.; Lee, H.; Kim, J.H.; Koh, W.G. Highly conductive and hydrated PEG-based hydrogels for the potential application of a tissue engineering scaffold. React. Funct. Polym. 2016, 109, 15–22. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, B.; Lee, B.T.; Choi, J.S.; Yim, J.H. Fabrication of an electroconductive, flexible, and soft poly(3,4-ethylenedioxythiophene)-thermoplastic polyurethane hybrid scaffold by in situ vapor phase polymerization. J. Mater. Chem. B 2018, 6, 4082–4088. [Google Scholar] [CrossRef]
- Wang, W.Q.; Meng, Q.Y.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan derivatives and their application in biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef] [PubMed]
- Stevens, K.R.; Einerson, N.J.; Burmania, J.A.; Kao, W.J. In vivo biocompatibility of gelatin-based hydrogels and interpenetrating networks. J. Biomater. Sci. Polym. Ed. 2002, 13, 1353–1366. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Dehiya, B.S.; Sindhu, A. Comparative study of chitosan and chitosan–gelatin scaffold for tissue engineering. Int. Nano Lett. 2017, 7, 285–290. [Google Scholar] [CrossRef]
- Suo, H.R.; Zhang, D.M.; Yin, J.; Qian, J.; Wu, Z.L.; Fu, J.Z. Interpenetrating polymer network hydrogels composed of chitosan and photocrosslinkable gelatin with enhanced mechanical properties for tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 92, 612–620. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Rittman, L.; Vesely, I. Novel geometries for tissue engineered tendonous collagen constructs. Tissue Eng. 2006, 12, 2601–2609. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.; Zhang, X.L.; Lin, X.M.; Liu, T.Q.; Ma, X.H.; Cui, Z.F. Chitosan/gelatin porous scaffolds containing hyaluronic acid and heparan sulfate for neural tissue engineering. J. Biomater. Sci. Polym. Ed. 2013, 24, 999–1014. [Google Scholar] [CrossRef]
- Wang, S.P.; Guan, S.; Zhu, Z.B.; Li, W.F.; Liu, T.Q.; Ma, X.H. Hyaluronic acid doped-poly(3,4-ethylenedioxythiophene)/chitosan/gelatin (PEDOT-HA/Cs/Gel) porous conductive scaffold for nerve regeneration. Mater. Sci. Eng. C-Mater. Biol. Appl. 2017, 71, 308–316. [Google Scholar] [CrossRef]
- Kołodziejska, M.; Jankowska, K.; Klak, M.; Wszoła, M. Chitosan as an underrated polymer in modern tissue engineering. Nanomaterials 2021, 11, 3019. [Google Scholar] [CrossRef] [PubMed]
- Shariatinia, Z. Carboxymethyl chitosan: Properties and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 1406–1419. [Google Scholar] [CrossRef]
- Bu, Y.; Xu, H.X.; Li, X.; Xu, W.J.; Yin, Y.X.; Dai, H.L.; Wang, X.B.; Huang, Z.J.; Xu, P.H. A conductive sodium alginate and carboxymethyl chitosan hydrogel doped with polypyrrole for peripheral nerve regeneration. RSC Adv. 2018, 8, 10806–10817. [Google Scholar] [CrossRef]
- Lu, G.Y.; Sheng, B.Y.; Wang, G.; Wei, Y.J.; Gong, Y.D.; Zhang, X.F.; Zhang, L.H. Controlling the degradation of covalently cross-linked carboxymethyl chitosan utilizing bimodal molecular weight distribution. J. Biomater. Appl. 2009, 23, 435–451. [Google Scholar] [CrossRef]
- Wang, G.; Lu, G.Y.; Ao, Q.; Gong, Y.D.; Zhang, X.F. Preparation of cross-linked carboxymethyl chitosan for repairing sciatic nerve injury in rats. Biotechnol. Lett. 2010, 32, 59–66. [Google Scholar] [CrossRef]
- Jiang, Z.; Song, Y.A.; Qiao, J.; Yang, Y.; Zhang, W.; Liu, W.S.; Han, B.Q. Rat sciatic nerve regeneration across a 10-mm defect bridged by a chitin/CM-chitosan artificial nerve graft. Int. J. Biol. Macromol. 2019, 129, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Jamal, R.; Zhang, L.; Wang, M.C.; Abdiryim, T. The structure and properties of PEDOT synthesized by template-free solution method. Nanoscale Res. Lett. 2014, 9, 557. [Google Scholar] [CrossRef]
- Carvalho, I.C.; Mansur, H.S.; Leonel, A.G.; Mansur, A.A.P.; Lobato, Z.I.P. Soft matter polysaccharide-based hydrogels as versatile bioengineered platforms for brain tissue repair and regeneration. Int. J. Biol. Macromol. 2021, 182, 1091–1111. [Google Scholar] [CrossRef] [PubMed]
- Kamata, H.; Li, X.; Chung, U.; Sakai, T. Design of hydrogels for biomedical applications. Adv. Healthc. Mater. 2015, 4, 2360–2374. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Gaillez, M.P.; Rothe, R.; Hauser, S.; Voigt, D.; Pietzsch, J.; Zhang, Y.X. Conductive hydrogels with dynamic reversible networks for biomedical applications. Adv. Healthc. Mater. 2021, 10, e2100012. [Google Scholar] [CrossRef]
- Guo, B.L.; Ma, P.X. Conducting polymers for tissue engineering. Biomacromolecules 2018, 19, 1764–1782. [Google Scholar] [CrossRef]
- Nie, S.S.; Li, Z.F.; Yao, Y.Y.; Jin, Y.Z. Progress in synthesis of conductive polymer poly(3,4-ethylenedioxythiophene). Front. Chem. 2021, 9, 803509. [Google Scholar] [CrossRef]
- Catoira, M.C.; Fusaro, L.; Francesco, D.D.; Ramella, M.; Boccafoschi, F. Overview of natural hydrogels for regenerative medicine applications. J. Mater. Sci. Mater. Med. 2019, 30, 115. [Google Scholar] [CrossRef]
- Distler, T.; Kretzschmar, L.; Schneidereit, D.; Girardo, S.; Goswami, R.; Friedrich, O.; Detsch, R.; Guck, J.; Boccaccini, A.R.; Budday, S. Mechanical properties of cell- and microgel bead-laden oxidized alginate-gelatin hydrogels. Biomater. Sci. 2021, 9, 3051–3068. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, H.; Wang, X.; Yu, X.; Li, H.; Tang, K.; Li, Q. Preparation of gelatin-based hydrogels with tunable mechanical properties and modulation on cell-matrix interactions. J. Biomater. Appl. 2021, 36, 902–911. [Google Scholar] [CrossRef] [PubMed]
- Alegret, N.; Dominguez-Alfaro, A.; Mecerreyes, D. 3D scaffolds based on conductive polymers for biomedical applications. Biomacromolecules 2019, 20, 73–89. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Wei, S.; Song, L.; Liu, H.; Wang, T. Conductive hydrogels-a novel material: Recent advances and future perspectives. J. Agric. Food Chem. 2020, 68, 7269–7280. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Guan, S.; Xu, J.Q.; Li, W.F.; Ge, D.; Sun, C.K.; Liu, T.Q.; Ma, X.H. Neural stem cell proliferation and differentiation in the conductive PEDOT-HA/Cs/Gel scaffold for neural tissue engineering. Biomater. Sci. 2017, 5, 2024–2034. [Google Scholar] [CrossRef]
- Wang, S.P.; Guan, S.; Li, W.F.; Ge, D.; Xu, J.Q.; Sun, C.K.; Liu, T.Q.; Ma, X.H. 3D culture of neural stem cells within conductive PEDOT layer-assembled chitosan/gelatin scaffolds for neural tissue engineering. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 93, 890–901. [Google Scholar] [CrossRef]
- Svendsen, C. Stem cells and Parkinson’s disease: Toward a treatment, not a cure. Cell Stem. Cell 2008, 2, 412–413. [Google Scholar] [CrossRef][Green Version]
- Roper, S.N.; Steindler, D.A. Stem cells as a potential therapy for epilepsy. Exp. Neurol. 2013, 244, 59–66. [Google Scholar] [CrossRef]
- Xu, B.; Bai, T.; Sinclair, A.; Wang, W.; Wu, Q.; Gao, F.; Jia, H.Z.; Jiang, S.Y.; Liu, W.G. Directed neural stem cell differentiation on polyaniline-coated high strength hydrogels. Mater. Today Chem. 2016, 1, 15–22. [Google Scholar] [CrossRef]
- Bordoni, M.; Scarian, E.; Rey, F.; Gagliardi, S.; Carelli, S.; Pansarasa, O.; Cereda, C. Biomaterials in neurodegenerative disorders: A promising therapeutic approach. Int. J. Mol. Sci. 2020, 21, 3243. [Google Scholar] [CrossRef]
- Yamada, M.; Tanemura, K.; Okada, S.; Iwanami, A.; Nakamura, M.; Mizuno, H.; Ozawa, M.; Ohyama-Goto, R.; Kitamura, N.; Kawano, M.; et al. Electrical stimulation modulates fate determination of differentiating embryonic stem cells. Stem. Cells 2007, 25, 562–570. [Google Scholar] [CrossRef]
- Ostrakhovitch, E.A.; Byers, J.C.; O’Neil, K.D.; Semenikhin, O.A. Directed differentiation of embryonic P19 cells and neural stem cells into neural lineage on conducting PEDOT-PEG and ITO glass substrates. Arch. Biochem. Biophys. 2012, 528, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.B.; Wang, L.; Guo, B.L.; Shao, Y.P.; Ma, P.X. Electroactive biodegradable polyurethane significantly enhanced Schwann cells myelin gene expression and neurotrophin secretion for peripheral nerve tissue engineering. Biomaterials 2016, 87, 18–31. [Google Scholar] [CrossRef] [PubMed]
- Esfandiarpour-Boroujeni, S.; Bagheri-Khoulenjani, S.; Mirzadeh, H. Modeling and optimization of degree of folate grafted on chitosan and carboxymethyl-chitosan. Prog. Biomater. 2016, 5, 1–8. [Google Scholar] [CrossRef] [PubMed]




| Hydrogel | Composition (M) | Reaction System (mL) | Conductivity (S·cm−1) | ||
|---|---|---|---|---|---|
| pTS-Na | APS | EDOT | |||
| CMCS/Gel | 0.1 | 0.1 | 0 | 2 | (3.14 ± 0.36) × 10−6 |
| CMCS/Gel-0.1EDOT | 0.1 | 0.1 | 0.1 | 2 | (8.67 ± 0.27) × 10−5 |
| CMCS/Gel-0.15EDOT | 0.1 | 0.1 | 0.15 | 2 | (5.54 ± 0.73) × 10−4 |
| CMCS/Gel-0.2EDOT | 0.1 | 0.1 | 0.2 | 2 | (1.52 ± 0.15) × 10−3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, S.; Wang, Y.; Xie, F.; Wang, S.; Xu, W.; Xu, J.; Sun, C. Carboxymethyl Chitosan and Gelatin Hydrogel Scaffolds Incorporated with Conductive PEDOT Nanoparticles for Improved Neural Stem Cell Proliferation and Neuronal Differentiation. Molecules 2022, 27, 8326. https://doi.org/10.3390/molecules27238326
Guan S, Wang Y, Xie F, Wang S, Xu W, Xu J, Sun C. Carboxymethyl Chitosan and Gelatin Hydrogel Scaffolds Incorporated with Conductive PEDOT Nanoparticles for Improved Neural Stem Cell Proliferation and Neuronal Differentiation. Molecules. 2022; 27(23):8326. https://doi.org/10.3390/molecules27238326
Chicago/Turabian StyleGuan, Shui, Yangbin Wang, Feng Xie, Shuping Wang, Weiping Xu, Jianqiang Xu, and Changkai Sun. 2022. "Carboxymethyl Chitosan and Gelatin Hydrogel Scaffolds Incorporated with Conductive PEDOT Nanoparticles for Improved Neural Stem Cell Proliferation and Neuronal Differentiation" Molecules 27, no. 23: 8326. https://doi.org/10.3390/molecules27238326
APA StyleGuan, S., Wang, Y., Xie, F., Wang, S., Xu, W., Xu, J., & Sun, C. (2022). Carboxymethyl Chitosan and Gelatin Hydrogel Scaffolds Incorporated with Conductive PEDOT Nanoparticles for Improved Neural Stem Cell Proliferation and Neuronal Differentiation. Molecules, 27(23), 8326. https://doi.org/10.3390/molecules27238326








