The Effects of Grape Polysaccharides Extracted from Grape By-Products on the Chemical Composition and Sensory Characteristics of White Wines
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Polysaccharide Extracts
2.2. Effects of the Addition of Different Polysaccharide Extracts on Chemical Composition of the Wines
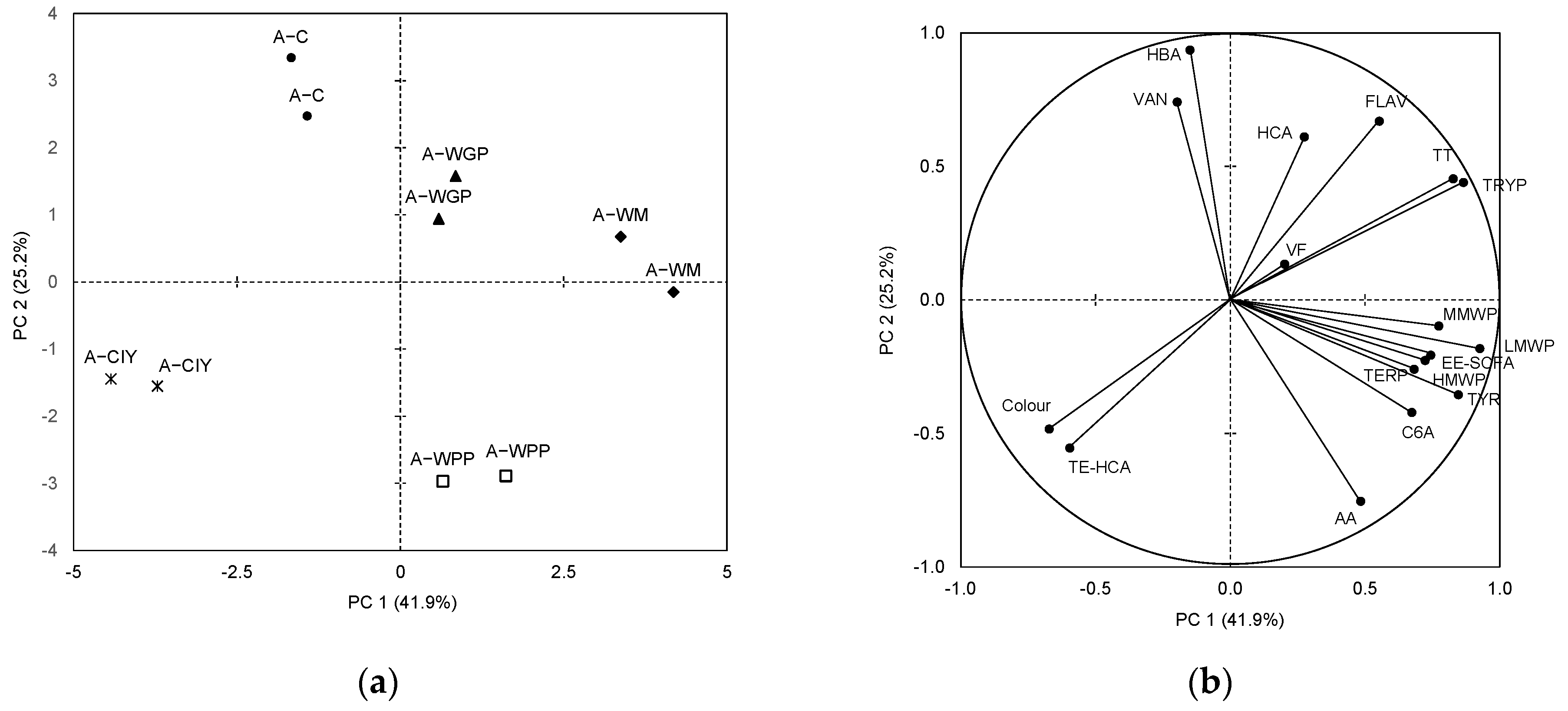
2.3. Multivariate Statistical Analyses
2.4. Effects of the Addition of Different Polysaccharide Extracts on Sensory Characteristics of the Wines
3. Materials and Methods
3.1. Polysaccharide Extractions
3.2. Winemaking and Treatments
3.3. Standards, Gases, and Chemical Reagents
3.4. Analytical Methods
3.5. Sensory Analysis
3.6. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Boussetta, N.; Lanoisellé, J.L.; Bedel-Cloutour, C.; Vorobiev, E. Extraction of soluble matter from grape pomace by high voltage electrical discharges for polyphenol recovery: Effect of sulphur dioxide and thermal treatments. J. Food Eng. 2009, 95, 192–198. [Google Scholar] [CrossRef]
- Muhlack, R.A.; Potumarthi, R.; Jeffery, D.W. Sustainable wineries through waste valorisation: A review of grape marc utilisation for value-added products. Waste Manag. 2018, 72, 99–118. [Google Scholar] [CrossRef]
- Xia, E.Q.; Deng, G.F.; Guo, Y.J.; Li, H.B. Biological activities of polyphenols from grapes. Int. J. Mol. Sci. 2010, 11, 622–646. [Google Scholar] [CrossRef] [PubMed]
- Ky, I.; Crozier, A.; Cros, G.; Teissedre, P.L. Polyphenols composition of wine and grape sub-products and potential effects on chronic diseases. Nutr. Aging 2014, 2, 165–177. [Google Scholar] [CrossRef] [Green Version]
- Dwyer, K.; Hosseinian, F.; Rod, M. The Market Potential of grape waste alternatives. J. Food Res. 2014, 3, 91–106. [Google Scholar] [CrossRef]
- García-Lomillo, J.; González-SanJosé, M.L. Applications of wine pomace in the food industry: Approaches and functions. Compr. Rev. Food Sci. Food Saf. 2017, 16, 3–22. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Ayestarán, B.; Williams, P.; Doco, T. Determination of must and wine polysaccharides by Gas Chromatography-Mass Spectrometry (GC-MS) and Size-Exclusion Chromatography (SEC). In Polysaccharides; Ramawat, K., Mérillon, J.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 1265–1297. [Google Scholar] [CrossRef] [Green Version]
- Ribéreau-Gayon, P.; Glories, Y.; Maujean, A.; Dubourdieu, D. Handbook of Enology: The Chemistry of Wine, Stabilization and Treatments; Wiley: New York, NY, USA, 2006; Volume 2. [Google Scholar]
- Gerbaud, V.; Gabas, N.; Laguerie, C.; Blouin, J.; Vidal, S.; Moutounet, M.; Pellerin, P. Effect of wine polysaccharides on the nucleation of potassium hydrogen tartrate in model solutions. Chem. Eng. Res. Des. 1996, 74, 782–789. [Google Scholar]
- Riou, V.; Vernhet, A.; Doco, T.; Moutounet, M. Aggregation of grape seed tannins in model wine-effect of wine polysaccharides. Food Hydrocoll. 2002, 16, 17–23. [Google Scholar] [CrossRef]
- Mateus, N.; Carvalho, E.; Luis, C.; de Freitas, V. Influence of the tannin structure on the disruption effect of carbohydrates on protein–tannin aggregates. Anal. Chim. Acta 2004, 513, 135–140. [Google Scholar] [CrossRef]
- Carvalho, E.; Mateus, N.; Plet, B.; Pianet, I.; Dufourc, E.; de Freitas, V. Influence of wine pectic polysaccharides on the interactions between condensed tannins and salivary proteins. J. Agric. Food Chem. 2006, 54, 8936–8944. [Google Scholar] [CrossRef] [PubMed]
- Poncet-Legrand, C.; Doco, T.; Williams, P.; Vernhet, A. Inhibition of grape seed tannin aggregation by wine mannoproteins: Effect of polysaccharide molecular weight. Am. J. Enol. Vitic. 2007, 58, 87–91. [Google Scholar]
- Vidal, S.; Francis, L.; Williams, P.; Kwiatkowski, M.; Gawel, R.; Cheynier, V.; Waters, E.J. The mouth-feel properties of polysaccharides and anthocyanins in a wine like medium. Food Chem. 2004, 85, 519–525. [Google Scholar] [CrossRef]
- Quijada-Morín, N.; Williams, P.; Rivas-Gonzalo, J.C.; Doco, T.; Escribano-Bailón, M.T. Polyphenolic, polysaccharide and oligosaccharide composition of Tempranillo red wines and their relationship with the perceived astringency. Food Chem. 2014, 154, 44–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lubbers, S.; Leger, B.; Charpentier, C.; Feuillat, M. Effet colloïde protecteur d’extraits de parois de levures sur la stabilité tartrique d’une solution hydroalcoolique modèle. J. Int. Sci. Vigne Du Vin 1993, 27, 13–22. [Google Scholar] [CrossRef] [Green Version]
- Waters, E.J.; Pellerin, P.; Brillouet, J.M. A Saccharomyces mannoprotein that protects wine from protein haze. Carbohydr. Polym. 1994, 23, 185–191. [Google Scholar] [CrossRef]
- Moine-Ledoux, V.; Dubourdieu, D. Role of yeast mannoproteins with regard to tartaric stabilisation of wines. Bull. OIV 2002, 75, 471–482. [Google Scholar]
- Lemos Junior, W.J.F.; Nadai, C.; Rolle, L.; da Silva Gulão, E.; Miguez da Rocha Leãoe, M.H.; Giacomini, A.; Corich, V.; Vincenzi, S. Influence of the mannoproteins of different strains of Starmenella bacillaris used in single and sequential fermentations on foamability, tartaric and protein stabilities of wines. OENO One 2020, 54, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Moine-Ledoux, V.; Dubourdieu, D. An invertase fragment responsible for improving the protein stability of dry white wines. J. Sci. Food Agric. 1999, 79, 537–543. [Google Scholar] [CrossRef]
- Dupin, I.V.S.; McKinnon, B.M.; Ryan, C.; Boulay, M.; Markides, A.J.; Jones, G.P.; Williams, P.J.; Waters , E.J. Saccharomyces cerevisiae mannoproteins that protect wine from protein haze: Their release during fermentation and lees contact and a proposal for their mechanism of action. J. Agric. Food Chem. 2000, 48, 3098–3105. [Google Scholar] [CrossRef]
- Waters, E.J.; Alexander, G.; Muhlack, R.; Pocock, K.F.; Colby, C.; O’Neill, B.K.; Hoj, P.B.; Jones, P. Preventing protein haze in bottled white wine. Aust. J. Grape Wine Res. 2005, 11, 215–225. [Google Scholar] [CrossRef]
- Schmidt, S.A.; Tan, E.L.; Brown, S.; Nasution, U.L.; Pettolino, F.; MacIntyre, O.J.; Lopes, M.B.; Waters, E.J.; Anderson, P.A. Hpf2 glycan structure is critical for protection against protein haze formation in white wine. J. Agric. Food Chem. 2009, 57, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.; Fernandes, C.; Nunes, F.M.; Filipe-Ribeiro, L.; Cosme, F. Influence of the structural features of commercial mannoproteins in white wine protein stabilization and chemical and sensory properties. Food Chem. 2014, 159, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Escot, S.; Feuillat, M.; Dulau, L.; Charpentier, C. Release of polysaccharides by yeasts and the influence of released polysaccharides on colour stability and wine astringency. Aust. J. Grape Wine Res. 2001, 7, 153–159. [Google Scholar] [CrossRef]
- Vidal, S.; Courcoux, P.; Francis, L.; Kwiatkowski, M.; Gawel, R.; Williams, P.; Waters, E.J.; Cheynier, V. Use of an experimental design approach for evaluation of key wine components on mouth-feel perception. Food Qual. Pref. 2004, 15, 209–217. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Martínez, L.; Ayestaraán, B. Yeast mannoproteins in red winemaking: Effect on polysaccharide, polyphenolic, and color composition. Am. J. Enol. Vitic. 2010, 61, 191–200. [Google Scholar]
- Lubbers, S.; Charpentier, C.; Feuillat, M.; Voilley, A. Influence of yeast cell walls on the behaviour of aroma compounds in a model wine. Am. J. Enol. Vitic. 1994, 45, 29–33. [Google Scholar]
- Chalier, P.; Angot, B.; Delteil, D.; Doco, T.; Gunata, Z. Interactions between aroma compounds and whole mannoprotein isolated from Saccharomyces cerevisiae strains. Food Chem. 2007, 100, 22–30. [Google Scholar] [CrossRef]
- Rodríguez-Bencomo, J.J.; Ortega-Heras, M.; Pérez-Magariño, S. Effect of alternative techniques to ageing on lees and use of non-toasted oak chips in alcoholic fermentation on the aromatic composition of a red wine. Eur. Food Res. Technol. 2010, 230, 485–496. [Google Scholar] [CrossRef]
- Del Barrio-Galán, R.; Ortega-Heras, M.; Sánchez-Iglesias, M.; Pérez-Magariño, S. Interactions of phenolic and volatile compounds with yeast lees, commercial yeast derivatives and non toasted chips in model solutions and young red wines. Eur. Food Res. Technol. 2012, 234, 231–244. [Google Scholar] [CrossRef]
- Rigou, P.; Mekoue, J.; Sieczkowski, N.; Doco, T.; Vernhet, A. Impact of industrial yeast derivative products on the modification of wine aroma compounds and sensorial profile. A review. Food Chem. 2021, 358, 129760. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Ayestarán, B. Effect of commercial mannoprotein addition on polysaccharide, polyphenolic, and color composition in red wines. J. Agric. Food Chem. 2008, 56, 9022–9029. [Google Scholar] [CrossRef] [PubMed]
- Del-Barrio-Galán, R.; Pérez-Magariño, S.; Ortega-Heras, M. Techniques for improving or replacing ageing on lees of oak aged red wines: The effects on polysaccharides and the phenolic composition. Food Chem. 2011, 127, 528–540. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, A.; Ricardo-Da-Silva, J.M.; Lucas, C.; Laureano, O. Effect of commercial mannoproteins on wine colour and tannins stability. Food Chem. 2012, 131, 907–914. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Martínez-Lapuente, L.; Bueno-Herrera, M.; Ortega-Heras, M.; Guadalupe, Z.; Ayestarán, B. Use of commercial dry yeast products rich in mannoproteins for white and rosé sparkling wine elaboration. J. Agric. Food Chem. 2015, 63, 5670–5681. [Google Scholar] [CrossRef] [PubMed]
- Del Barrio-Galán, R.; Pérez-Magariño, S.; Ortega-Heras, M.; Guadalupe, Z.; Ayestarán, B. Polysaccharide characterization of commercial dry yeast preparations and their effect on white and red wine composition. LWT−Food Sci. Technol. 2012, 48, 215–223. [Google Scholar] [CrossRef]
- Gawel, R.; Smith, P.A.; Waters, E.J. Influence of polysaccharides on the taste and mouthfeel of white wine. Aust. J. Grape Wine Res. 2016, 22, 350–357. [Google Scholar] [CrossRef]
- Canalejo, D.; Guadalupe, Z.; Martínez-Lapuente, L.; Ayestarán, B.; Pérez-Magariño, S. Optimization of a method to extract polysaccharides from white grape pomace by-products. Food Chem. 2021, 365, 130445. [Google Scholar] [CrossRef]
- Canalejo, D.; Guadalupe, Z.; Martínez-Lapuente, L.; Ayestarán, B.; Pérez-Magariño, S.; Doco, T. Characterization of polysaccharide extracts recovered from different grape and winemaking products. Food Res. Int. 2022, 157, 111480. [Google Scholar] [CrossRef]
- Martínez-Lapuente, L.; Apolinar-Valiente, R.; Guadalupe, Z.; Ayestarán, B.; Pérez-Magariño, S.; Williams, P.; Doco, T. Influence of grape maturity on complex carbohydrate composition of red sparkling wines. J. Agric. Food Chem. 2016, 64, 5020–5030. [Google Scholar] [CrossRef]
- Ortega-Regules, A.; Ros-García, J.M.; Bautista-Ortín, A.B.; López-Roca, J.M.; Gómez-Plaza, E. Differences in morphology and composition of skin and pulp cell walls from grapes (Vitis vinifera L.): Technological implications. Eur. Food Res. Technol. 2008, 227, 223–231. [Google Scholar] [CrossRef]
- Apolinar-Valiente, R.; Romero-Cascales, I.; López-Roca, J.M.; Gómez-Plaza, E.; Ros-García, J.M. Application and comparison of four selected procedures for the isolation of cell-wall material from the skin of grapes cv. Monastrell. Anal. Chim. Acta 2010, 660, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Gil-Cortiella, M.; Peña-Neira, A. Extraction of soluble polysaccharides from grape skins. Cienc. Investig. Agrar. 2017, 44, 83–93. [Google Scholar] [CrossRef] [Green Version]
- Spinei, M.; Oroian, M. The influence of extraction conditions on the yield and physico-chemical parameters of pectin from grape pomace. Polymers 2022, 14, 1378. [Google Scholar] [CrossRef]
- Razmkhab, S.; López-Toledano, A.; Ortega, J.M.; Mayen, M.; Mérida, J.; Medina, M. Adsorption of phenolic compounds and browning products in white wines by yeasts and their cell walls. J. Agric. Food Chem. 2002, 50, 7432–7437. [Google Scholar] [CrossRef] [PubMed]
- Márquez, T.; Millán, C.; Souquet, J.M.; Salmon, J.M. Effect of different yeast strains and their culture conditions on the prevention of wine model solution browning by yeast lees. J. Agric. Food Chem. 2009, 57, 3771–3779. [Google Scholar] [CrossRef]
- Comuzzo, P.; Tat, L.; Fenzi, D.; Brotto, L.; Battistutta, F.; Zironi, R. Interactions between yeast autolysates and volatile compounds in wine and model solution. Food Chem. 2011, 127, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Rapp, A.; Mandery, H. Wine aroma. Experientia 1986, 42, 873–884. [Google Scholar] [CrossRef]
- Antalick, G.; Perello, M.C.; de Revel, G. Esters in wines: New insight through the establishment of a database of French wines. Am. J. Enol. Vitic. 2014, 65, 293–304. [Google Scholar] [CrossRef]
- Del Barrio Galán, R.; Pérez Magariño, S.; Ortega Heras, M.; Williams, P.; Doco, T. Effect of aging on lees and of three different dry yeast derivative products on Verdejo white wine composition and sensorial characteristics. J. Agric. Food Chem. 2011, 59, 12433–12442. [Google Scholar] [CrossRef] [PubMed]
- Bautista, R.; Fernández, E.; Falqué, E. Effect of the contact with fermentation lees or commercial-lees on the volatile composition of white wines. Eur. Food Res. Technol. 2007, 224, 405–413. [Google Scholar] [CrossRef]
- Mitropoulou, A.; Hatzidimitriou, E.; Paraskevopoulou, A. Aroma release of a model wine solution as influenced by the presence of non-volatile components. Effect of commercial tannin extracts, polysaccharides and artificial saliva. Food Res. Int. 2011, 44, 1561–1570. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, J.; Chen, F.; Zhao, G. Effect of molecular structure of polyphenols on their noncovalent interactions with oat β-glucan. J. Agric. Food Chem. 2013, 61, 4533–4538. [Google Scholar] [CrossRef] [PubMed]
- Brandão, E.; Santos Silva, M.; García-Estévez, I.; Williams, P.; Mateus, N.; Doco, T.; de Freitas, V.; Soares, S. Inhibition mechanisms of wine polysaccharides on salivary protein precipitation. J. Agric. Food Chem. 2020, 68, 2955–2963. [Google Scholar] [CrossRef] [PubMed]
- Laguna, L.; Bartolomé, B.; Moreno-Arribas, M.V. Mouthfeel perception of wine: Oral physiology, components and instrumental characterization. Trends Food Sci. Technol. 2017, 59, 49–59. [Google Scholar] [CrossRef]
- Guadalupe, Z.; Martínez-Pinilla, O.; Garrido, Á.; Carrillo, J.; Ayestarán, B. Quantitative determination of wine polysaccharides by gas chromatography-mass spectrometry (GC-MS) and size exclusion chromatography (SEC). Food Chem. 2012, 131, 367–374. [Google Scholar] [CrossRef]
- OIV. International Organisation of Vine and Wine. In Compendium of International Methods of Wine and Must Analysis; International Organisation of Vine and Wine: Paris, France, 2016. [Google Scholar]
- Pérez-Magariño, S.; Ortega-Heras, M.; Cano-Mozo, E. Optimization of a solid-phase extraction method using copolymer sorbents for isolation of phenolic compounds in red wines and quantification by HPLC. J. Agric. Food Chem. 2008, 56, 11560–11570. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Magariño, S.; Bueno-Herrera, M.; López de la Cuesta, P.; González-Lázaro, M.; Martínez-Lapuente, L.; Guadalupe, Z.; Ayestarán, B. Volatile composition, foam characteristics and sensory properties of Tempranillo red sparkling wines elaborated using different techniques to obtain the base wines. Eur. Food Res. Technol. 2019, 245, 1047–1059. [Google Scholar] [CrossRef]
- del Barrio Galán, R.; del Valle-Herrero, H.; Bueno-Herrera, M.; López de la Cuesta, P.; Pérez-Magariño, S. Volatile and non-volatile characterization of white and rosé wines from different Spanish Protected Designations of Origin. Beverages 2021, 7, 49. [Google Scholar] [CrossRef]
- Jiménez-Martínez, M.D.; Bautista-Ortín, A.B.; Gil-Muñoz, R.; Gómez-Plaza, E. Fining with purified grape pomace. Effect of dose, contact time and varietal origin on the final wine phenolic composition. Food Chem. 2019, 271, 570–576. [Google Scholar] [CrossRef]



| Extracts1 | PRAG2 | RG-II2 | HG2 | MP2 | GP2 | TPS2 | HMWP3 | MMWP3 | LMWP3 |
|---|---|---|---|---|---|---|---|---|---|
| WGP | 36.9 (5.5) | 26.0 (2.8) | 24.8 (5.8) | 11.1 (0.8) | 193 (17.9) | 292 (19.8) | 45.5 | 0.0 | 54.5 |
| WM | 129 (16.3) | 33.0 (2.9) | 55.7 (8.4) | 273 (20.1) | 190 (13.5) | 681 (30.5) | 44.7 | 45.3 | 10.0 |
| WPP | 132 (6.9) | 670 (36.8) | 61.8 (4.4) | 3.7 (0.8) | 28.8 (5.3) | 897 (38.1) | 0.0 | 71.2 | 28.8 |
| CIY | nd 4 | nd | nd | 428 (17.7) | 189 (22.4) | 617 (28.6) | 31.6 | 0.0 | 68.4 |
| Parameters | Albillo Wines | Verdejo Wines |
|---|---|---|
| Alcohol degree (% etanol v/v) | 12.2 | 12.9 |
| Total acidity (g/L of tartaric acid) | 7.0 | 7.2 |
| pH | 3.10 | 3.05 |
| Acetic acid (g/L) | 0.13 | 0.37 |
| Malic acid (g/L) | 2.1 | 2.0 |
| Total SO2 (mg/L) | 40 | 54 |
| Albillo Wines | Verdejo Wines | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A-C | A-WM | A-WGP | A-WPP | A-CIY | V-C | V-WM | V-WGP | V-WPP | V-CIY | |
| Colour | 0.075±0.001 b | 0.077±0.001 c | 0.067±0.001 a | 0.078±0.001 c | 0.101±0.001 d | 0.113±0.001 c | 0.118±0.001 d | 0.130±0.001 e | 0.092±0.001 b | 0.083±0.001 a |
| TP | 129±1.7 | 131±0.5 | 130±2.1 | 127±2.4 | 128±1.0 | 172±0.7 c | 171±1.7 c | 168±0.4 c | 125±1.1 b | 122±0.8 a |
| TT | 84±0.6 bc | 87±0.5 c | 87±1.1 c | 82±2.5 b | 72±1.6 a | 161±2.7 cd | 170±2.9 d | 157±2.5 c | 107±1.9 b | 87±1.9 a |
| T-PS | 173±2.6 a | 277±9.7 d | 212±3.6 c | 210±9.7 bc | 191±7.1 b | 123±2.9 a | 223±4.0 c | 160±5.3 b | 158±6.5 b | 158±9.8 b |
| HMWP | 92.2±0.3 a | 123±0.4 c | 103±1.0 b | 103±2.0 b | 102±1.9 b | 61.3±2.2 a | 98.9±1.2 c | 80.0±1.5 b | 89.6±4.5 bc | 92.8±4.1 c |
| MMWP | 58.9±0.3 a | 100±6.2 c | 70.6±0.3 b | 68.4±1.0 b | 65.8±1.1 b | 43.8±1.1 a | 84.5±3.3 c | 56.0±1.3 b | 49.5±0.7 ab | 54.3±5.1 b |
| LMWP | 22.0±0.6 a | 54.5±5.0 c | 38.6±2.3 b | 38.9±6.8 b | 23.6±6.2 a | 18.0±0.4 b | 39.5±1.9 d | 23.7±2.5 c | 18.7±1.3 b | 11.2±0.5 a |
| HBA | 2.24±0.01 e | 1.93±0.01 c | 1.99±0.01 d | 1.77±0.01 a | 1.86±0.03 b | 10.9±0.07 d | 10.5±0.06 c | 10.9±0.01 d | 3.48±0.08 b | 2.05±0.04 a |
| HCA | 4.23±0.01 c | 4.29±0.01 d | 4.53±0.01 e | 4.06±0.01 a | 4.15±0.01 b | 11.4±0.01 d | 11.2±0.01 c | 11.5±0.03 e | 7.21±0.01 b | 6.44±0.01 a |
| TE-HCA | 3.44±0.06 b | 3.28±0.06 a | 3.29±0.01 a | 3.53±0.01 b | 3.51±0.01 b | 2.85±0.04 c | 2.80±0.01 c | 3.00±0.06 d | 2.53±0.01 b | 2.16±0.03 a |
| TYR | 26.7±0.59 a | 28.2±0.10 c | 27.1±0.33 ab | 27.7±0.10 bc | 26.7±0.17 a | 27.5±0.92 ab | 26.5±0.25 a | 26.7±0.10 a | 28.7±0.47 bc | 29.5±0.10 c |
| TRYP | 6.37±0.04 b | 6.54±0.03 c | 6.35±0.04 b | 6.30±0.08 b | 6.02±0.03 a | 1.43±0.07 c | 1.39±0.07 bc | 1.29±0.01 b | 0.66±0.03 a | 0.64±0.01 a |
| FLAV | 2.89±0.04 c | 2.86±0.01 bc | 2.85±0.01 bc | 2.83±0.01 b | 2.75±0.01 a | 1.43±0.01 d | 1.40±0.01 c | 1.47±0.01 e | 1.34±0.01 b | 1.26±0.01 a |
| HA | 336±11 | 341±12 | 341±6 | 330±11 | 332±6 | 272±7 | 271±6 | 268±6 | 281±7 | 283±5 |
| EE-SCFA | 3.32±0.017 b | 3.49±0.027 c | 3.44±0.032 c | 3.57±0.011 d | 3.00±0.032 a | 4.13±0.028 b | 4.30±0.004 b | 4.12±0.069 b | 3.18±0.041 a | 3.12±0.017 a |
| EE-BCFA | 0.042±0.001 | 0.039±0.007 | 0.039±0.007 | 0.042±0.004 | 0.036±0.005 | 0.044±0.002 a | 0.046±0.001 a | 0.041±0.004 a | 0.054±0.001 b | 0.064±0.001 c |
| AA | 1.204±0.004 a | 1.310±0.065 b | 1.313±0.006 b | 1.434±0.039 c | 1.269±0.047 ab | 1.075±0.052 b | 1.103±0.029 b | 1.152±0.032 b | 0.596±0.025 a | 0.551±0.010 a |
| FA | 16.6±0.159 | 16.5±0.079 | 16.4±0.055 | 16.7±0.031 | 16.4±0.065 | 17.4±0.081 ab | 17.8±0.132 b | 18.3±0.201 c | 17.3±0.030 a | 17.4±0.038 ab |
| C6A | 1.09±0.018 a | 1.22±0.070 bc | 1.25±0.015 c | 1.25±0.010 c | 1.13±0.033 ab | 1.36±0.026 bc | 1.47±0.152 c | 1.46±0.014 c | 1.26±0.041 ab | 1.08±0.014 a |
| TERP | 0.110±0.014 b | 0.128±0.001 c | 0.122±0.006 c | 0.138±0.001 c | 0.079±0.001 a | 0.115±0.001 d | 0.131±0.002 e | 0.078±0.007 c | 0.051±0.002 b | 0.021±0.002 a |
| VAN | 0.178±0.003 c | 0.162±0.002 bc | 0.142±0.024 b | 0.117±0.006 a | 0.154±0.001 bc | 0.205±0.008 bc | 0.226±0.034 cd | 0.244±0.025 d | 0.162±0.010 a | 0.199±0.009 b |
| VF | 0.677±0.048 c | 0.691±0.046 c | 0.515±0.035 a | 0.604±0.022 b | 0.600±0.020 b | 0.967±0.019 b | 1.026±0.098 b | 0.970±0.047 b | 0.568±0.007 a | 0.537±0.003 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pérez-Magariño, S.; Cano-Mozo, E.; Bueno-Herrera, M.; Canalejo, D.; Doco, T.; Ayestarán, B.; Guadalupe, Z. The Effects of Grape Polysaccharides Extracted from Grape By-Products on the Chemical Composition and Sensory Characteristics of White Wines. Molecules 2022, 27, 4815. https://doi.org/10.3390/molecules27154815
Pérez-Magariño S, Cano-Mozo E, Bueno-Herrera M, Canalejo D, Doco T, Ayestarán B, Guadalupe Z. The Effects of Grape Polysaccharides Extracted from Grape By-Products on the Chemical Composition and Sensory Characteristics of White Wines. Molecules. 2022; 27(15):4815. https://doi.org/10.3390/molecules27154815
Chicago/Turabian StylePérez-Magariño, Silvia, Estela Cano-Mozo, Marta Bueno-Herrera, Diego Canalejo, Thierry Doco, Belén Ayestarán, and Zenaida Guadalupe. 2022. "The Effects of Grape Polysaccharides Extracted from Grape By-Products on the Chemical Composition and Sensory Characteristics of White Wines" Molecules 27, no. 15: 4815. https://doi.org/10.3390/molecules27154815
APA StylePérez-Magariño, S., Cano-Mozo, E., Bueno-Herrera, M., Canalejo, D., Doco, T., Ayestarán, B., & Guadalupe, Z. (2022). The Effects of Grape Polysaccharides Extracted from Grape By-Products on the Chemical Composition and Sensory Characteristics of White Wines. Molecules, 27(15), 4815. https://doi.org/10.3390/molecules27154815







