Utilization of Agro-Industrial Residues in the Rearing and Nutritional Enrichment of Zophobas atratus Larvae: New Food Raw Materials
Abstract
1. Introduction
2. Results and Discussion
2.1. Nutritional Composition of Diets
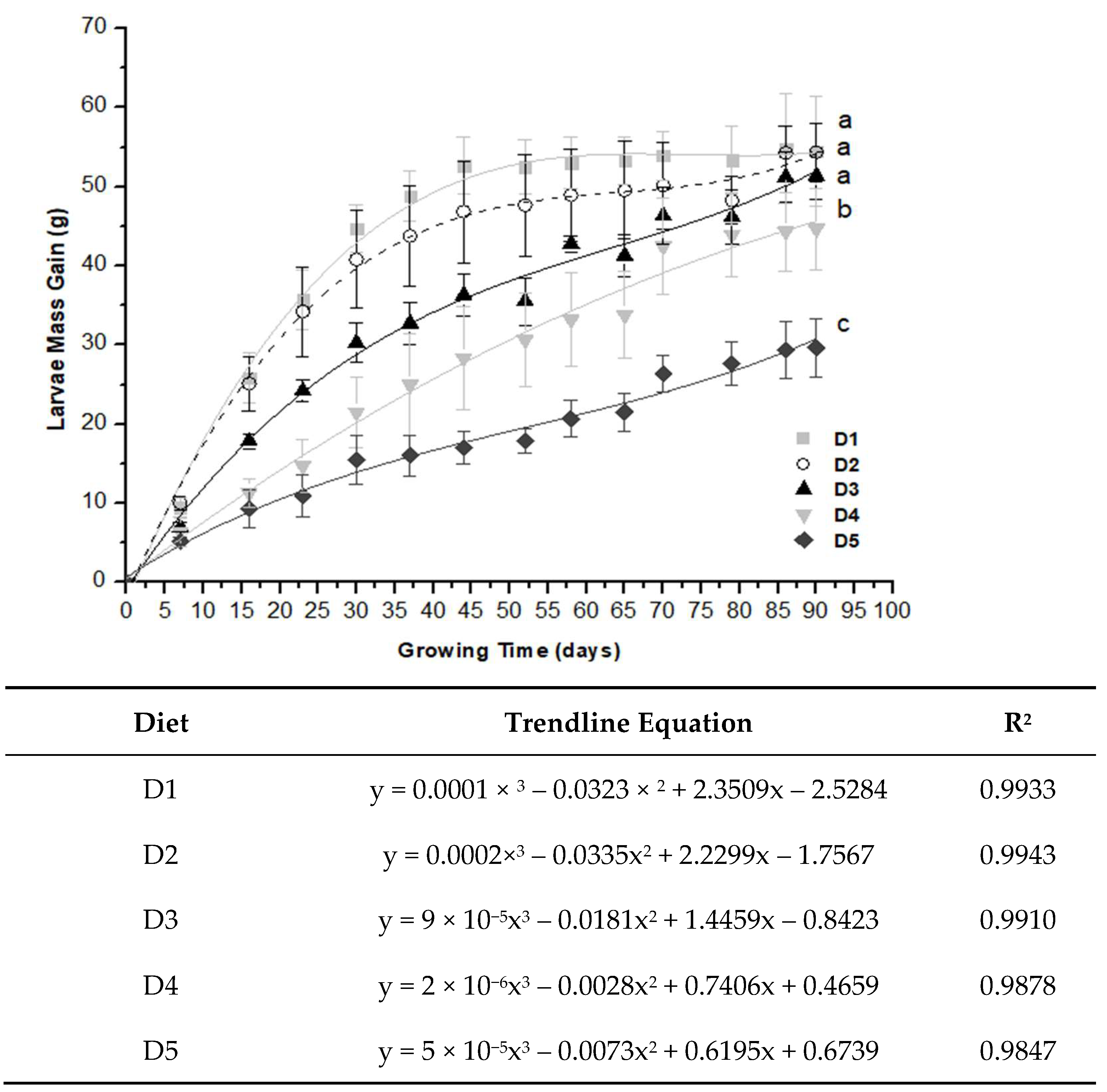
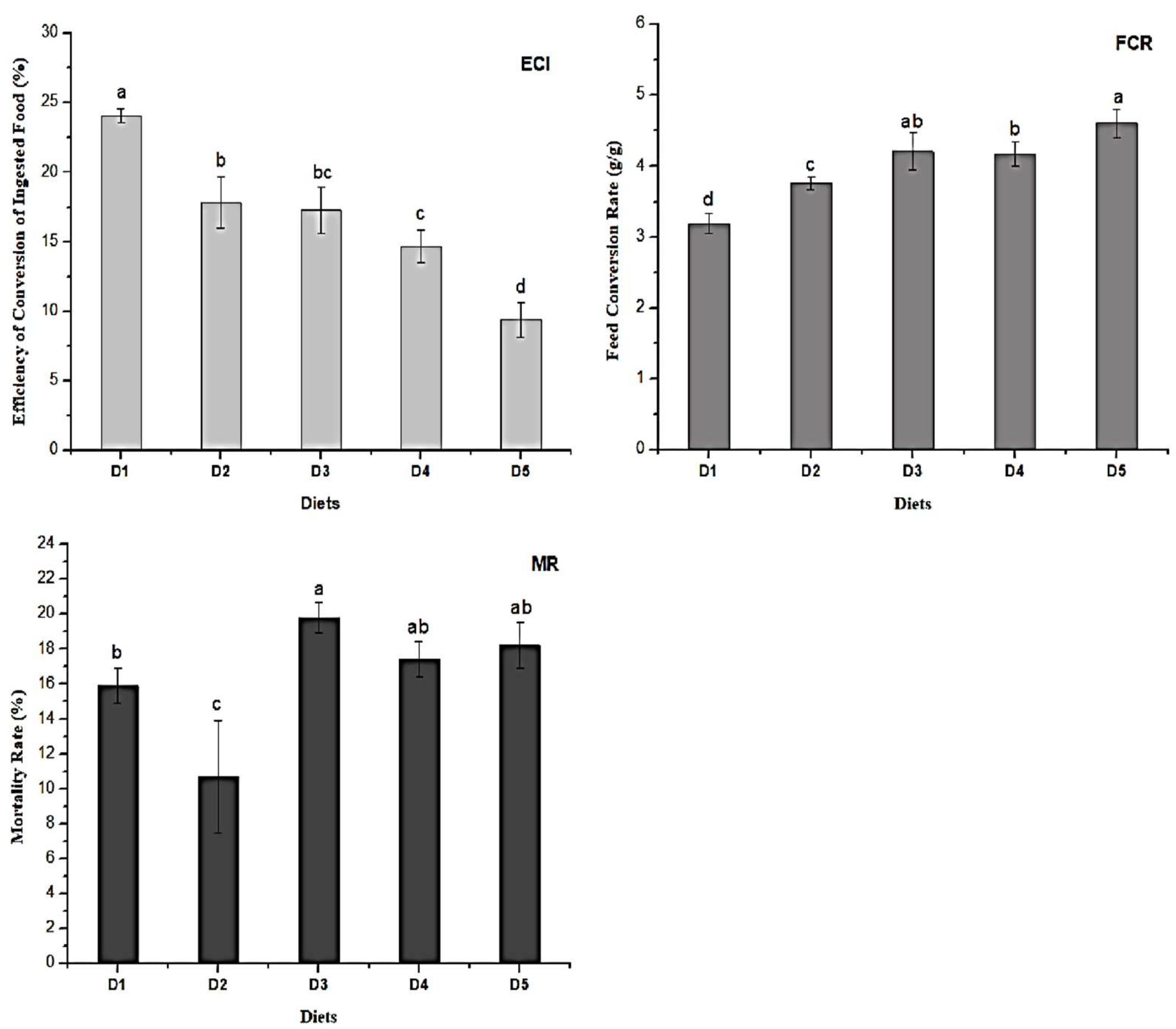
2.2. Larvae Growth Performance
2.3. Analysis of the Main Components of Nutritional Composition and Larval Performance
2.4. Larvae Physicochemical Characterization
2.5. Comparisons of Larvae Nutritional Properties
2.6. Composition of Metals and Non-Metals in Larvae
3. Materials and Methods
3.1. Biological Material
3.2. Diet Formulation
3.3. Larvae Cultivation
3.4. Physicochemical Analysis
3.5. Fatty Acid Profile
3.6. Larvae Growth Performance
3.7. Analysis of Metals and Non-Metals
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Van Huis, A.; van Itterbeeck, J.; Klunder, H.; Mertens, E.; Halloran, A.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Roma, Italy, 2013. [Google Scholar]
- Kim, K.; Yong, H.I.; Kim, B.; Kim, W.; Choi, S. Edible insects as a protein source: A review of public perception, processing technology, and research trends. Food Sci. Anim. Resour. 2019, 39, 521–540. [Google Scholar] [CrossRef] [PubMed]
- FAO. The State of Food and Agriculture 2010–2011; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- UN—United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects: The 2017 Revision, Volume I: Comprehensive Tables (ST/ESA/SER.A/399). 2017. Available online: https://esa.un.org/unpd/wpp/Publications/Files/WPP2017_Volume-I_Comprehensive-Tables.pdf. (accessed on 30 August 2022).
- Wu, R.A.; Ding, Q.; Yin, L.; Chi, X.; Sun, N.; He, R.; Luo, L.; Ma, H.; Li, Z. Comparison of the nutritional value of mysore thorn borer (Anoplophora chinensis) and mealworm larva (Tenebrio molitor): Amino acid, fatty acid, and element profiles. Food Chem. 2020, 323, 126818. [Google Scholar] [CrossRef] [PubMed]
- FAO/IFAD/WFP. The State of Food Insecurity in the World; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015. [Google Scholar]
- Eilenberg, J.; Van Loon, J.J.A. Insects: Key biological features. In Edible Insects in Sustainable Food Systems; Halloran, A., Flore, R., Vantomme, P., Roos, N., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Govorushko, S. Global status of insects as food and feed source: A review. Trends Food Sci. Technol. 2019, 91, 436–445. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; de Boer, I.J.M. Environmental impact of the production of mealworms as a protein source for humans—A life cycle assessment. PLoS ONE 2012, 7, e51145. [Google Scholar] [CrossRef]
- Bednářová, M.; Borkovcová, M.; Mlček, J.; Rop, O.; Zeman, L. Edible insects—Species suitable for entomophagy under condition of Czech Republic. Acta Univ. Agric. Silvic. Mendelianae Brun. 2013, 61, 587–593. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.G.; Yoon, H.J.; Lee, K.Y.; Kim, N.J. Nutritional analysis of alternative feed ingredients and their effects on the larval growth of Tenebrio molitor (Coleoptera: Tenebrionidae). Entomol. Res. 2017, 47, 194–202. [Google Scholar] [CrossRef]
- Nowak, V.; Persijn, D.; Rittenschober, D.; Charrondiere, U.R. Review of food composition data for edible insects. Food Chem. 2016, 193, 39–46. [Google Scholar] [CrossRef]
- Kosečková, P.; Zvěřina, O.; Pěchová, M.; Krulíková, M.; Duborská, E.; Borkovcová, M. Mineral profile of cricket powders, some edible insect species and their implication for gastronomy. J. Food Compos. Anal. 2022, 107, 104340. [Google Scholar] [CrossRef]
- Finke, M.D. Complete nutrient content of three species of wild caught insects, pallid-winged grasshopper, rhinoceros beetles and white-lined sphinx moth. J. Insects Food Feed. 2015, 1, 281–292. [Google Scholar] [CrossRef]
- van der Spiegel, M.; Noordam, M.Y.; van der Fels-Klerx, H.J. Safety of novel protein sources (insects, microalgae, seaweed, duckweed, and rapeseed) and legislative aspects for their application in food and feed production. Compr. Rev. Food Sci. Food Saf. 2013, 12, 662–678. [Google Scholar] [CrossRef]
- Kouřimská, L.; Adámková, A. Nutritional and sensory quality of edible insects. NFS J. 2016, 4, 22–26. [Google Scholar] [CrossRef]
- Payne, C.L.R.; Scarborough, P.; Rayner, M.; Nonaka, K. A systematic review of nutrient composition data available for twelve commercially available edible insects, and comparison with reference values. Trends Food Sci. Technol. 2016, 47, 69–77. [Google Scholar] [CrossRef]
- Ramos-Elorduy, J.; Gonzáles, E.A.; Hernández, A.R.; Pino, J.M. Use of Tenebrio molitor (Coleoptera: Tenebrionidae) to recycle organic wastes and as feed for broiler chickens. J. Econ. Entomol. 2002, 95, 214–220. [Google Scholar] [CrossRef]
- Yi, L.; Lakemond, C.M.M.; Sagis, L.M.C.; Eisner-Schadler, V.; Van Huis, A.; Van Boekel, M.A.J.S. Extraction and characterisation of protein fractions from five insect species. Food Chem. 2013, 141, 3341–3348. [Google Scholar] [CrossRef]
- Van Broekhoven, S.; Oonincx, D.G.A.B.; van Huis, A.; van Loon, J.J.A. Growth performance and feed conversion efficiency of three edible mealworm species (Coleoptera: Tenebrionidae) on diets composed of organic by-products. J. Insect Physiol. 2015, 73, 1–10. [Google Scholar] [CrossRef]
- EFSA; Turck, D.; Castenmiller, J.; Henauw, S.; Hirschernst, K.I.; Kearney, J.; Maciuk, A.; Mangelsdorf, I.; Mcardle, H.J.; Naska, A.; et al. Safety of dried yellow mealworm (Tenebrio molitor L.) as a novel food pursuant to Regulation (EU) 2015/2283. EFSA J. 2021, 19, 6343. [Google Scholar] [CrossRef]
- Mlček, J.; Adámková, A.; Adámek, M.; Borkovcová, M.; Bednářová, M.; Knížková, I. Fat from Tenebrionidae bugs—Sterols content, fatty acid profiles, and cardiovascular risk indexes. Pol. J. Food Nutr. Sci. 2019, 69, 247–254. [Google Scholar] [CrossRef]
- Veldkamp, T.; Van Duinkerken, G.; Van Huis, A.; Lakemond, C.M.M.; Ottevanger, E.; Bosch, G.; Van Boekel, M.A.J.S. Rapport 638—Wageningen Livestock Research. 2012. Available online: http://www.wageningenur.nl/en/show/Insects-as-a-sustainable-feed-ingredient-in-pig-and-poultry-diets-1.htm.August (accessed on 5 August 2022).
- Oonincx, D.G.A.B.; van Broekhoven, S.; van Huis, A.; van Loon, J.J.A. Feed conversion, survival and development, and composition of four insect species on diets composed of food by-products. PLoS ONE 2015, 10, e0144601. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, H.; Chen, G.; Qiao, L.; Li, J.; Liu, B.; Liu, Z.; Li, M.; Liu, X. Growth performance and nutritional profile of mealworms reared on corn stover, soybean meal, and distillers’ grains. Eur. Food Res. Technol. 2019, 245, 2631–2640. [Google Scholar] [CrossRef]
- Mancini, S.; Fratini, F.; Turchi, B.; Mattioli, S.; Dal Bosco, A.; Tuccinardi, T.; Nozic, S.; Paci, G. Former foodstuff products in Tenebrio molitor rearing: Effects on growth, chemical composition, microbiological load, and antioxidant status. Animals 2019, 9, 484. [Google Scholar] [CrossRef]
- Melis, R.; Braca, A.; Sanna, R.; Spada, S.; Mulas, G.; Fadda, M.L.; Sassu, M.M.; Serra, G.; Anedda, R. Metabolic response of yellow mealworm larvae to two alternative rearing substrates. Metabolomics 2019, 15, 113. [Google Scholar] [CrossRef]
- Ruschioni, S.; Loreto, N.; Foligni, R.; Mannozzi, C.; Raffaelli, N.; Zamporlini, F.; Pasquini, M.; Roncolini, A.; Cardinali, F.; Osimani, A.; et al. Addition of olive pomace to feeding substrate affects growth performance and nutritional value of mealworm (Tenebrio molitor L.) larvae. Foods 2020, 9, 317. [Google Scholar] [CrossRef]
- Kim, S.Y.; Kim, H.G.; Song, S.H.; Kim, N.J. Developmental characteristics of Zophobas atratus (Coleoptera: Tenebrionidae) larvae in different instars. Int. J. Indust. Entomol. 2015, 30, 45–49. [Google Scholar] [CrossRef]
- Chapman, R.F. The Insects: Structure and Function, 4th ed.; Cambridge University Press: Cambridge, UK, 1998. [Google Scholar]
- Li, L.; Zhao, Z.; Liu, H. Feasibility of feeding yellow mealworm (Tenebrio molitor L.) in bioregenerative life support systems as a source of animal protein for humans. Acta Astronaut. 2013, 92, 103–109. [Google Scholar] [CrossRef]
- de Vries, M.; de Boer, I.J.M. Comparing environmental impacts for livestock products: A review of life cucle assessments. Livest. Sci. 2010, 128, 1–11. [Google Scholar] [CrossRef]
- Wilkinson, J.M. Re-defining efficiency of feed use by livestock. Animal 2011, 5, 1014–1022. [Google Scholar] [CrossRef]
- Herrero, M.; Thornton, P.K. Livestock and global change: Emerging issues for sustainable food systems. Proc. Natl. Acad. Sci. USA 2013, 110, 20878–20881. [Google Scholar] [CrossRef]
- Lundy, M.E.; Parrella, M.P. Crickets are not a free lunch: Protein capture from scalable organic side-streams via high-density populations of Acheta domesticus. PLoS ONE 2015, 10, e0118785. [Google Scholar] [CrossRef]
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- da Silva, G.V.; Machado, B.A.S.; Oliveira, W.P.; Silva, C.F.G.; Quadros, C.P.; Druzian, J.I.; Ferreira, E.S.; Umsza-Guez, M.A. Effect of drying methods on bioactive compounds and antioxidant capacity in grape skin residues from the new hybrid variety “BRS Magna”. Molecules 2020, 25, 3701. [Google Scholar] [CrossRef]
- Lafka, T.-I.; Sinanoglou, V.; Lazos, E.S. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007, 104, 1206–1214. [Google Scholar] [CrossRef]
- Marques, T.R.; Caetano, A.A.; Alves, D.S.; Ramos, V.O.; Simao, A.A.; Carvalho, G.A.; Correa, A.D. Malpighia emarginata DC. bagasse acetone extract: Phenolic compounds and their effect on Spodoptera frugiperda (J.E. Smith) (Lepidoptera: Noctuidae). Chil. J. Agric. Res. 2016, 76, 55–61. [Google Scholar] [CrossRef]
- Pavela, R. The feeding effect of polyphenolic compounds on the Colorado potato beetle [Leptinotarsa decemlineata (Say)]. Pest Technol. 2007, 1, 81–84. Available online: http://www.globalsciencebooks.info/Online/GSBOnline/images/0706/PT_1(1)/PT_1(1)81-84o.pdf (accessed on 15 June 2022).
- Regnault-Roger, C.; Ribodeau, M.; Hamraoui, A.; Bareau, I.; Blanchard, P.; Gil-Munoz, M.I.; Barberan, F.T. Polyphenolic compounds of Mediterranean Lamiaceae and investigation of orientational effects on Acanthoscelides obtectus (Say). J. Stored Prod. Res. 2004, 40, 395–408. [Google Scholar] [CrossRef]
- Snart, C.J.; Hardy, I.C.; Barrett, D.A. Entometabolomics: Applications of modern analytical techniques to insect studies. Entomol. Exp. Appl. 2015, 155, 1–17. [Google Scholar] [CrossRef]
- Dreassi, E.; Cito, A.; Zanfini, A.; Materozzi, L.; Botta, M.; Francardi, V. Dietary fatty acids influence the growth and fatty acid composition of the yellow mealworm Tenebrio molitor (Coleoptera: Tenebrionidae). Lipids 2017, 52, 285–294. [Google Scholar] [CrossRef]
- Bjørge, J.D.; Overgaard, J.; Malte, H.; Gianotten, N.; Heckmann, L.H. Role of temperature on growth and metabolic rate in the tenebrionid beetles Alphitobius diaperinus and Tenebrio molitor. J. Insect Physiol. 2018, 107, 89–96. [Google Scholar] [CrossRef]
- Morales-Ramos, J.A.; Rojas, M.G.; Shapiro-Ilan, D.I.; Tedders, W.L. Self-selection of two diet components by Tenebrio molitor (Coleoptera: Tenebrionidae) larvae and its impact on fitness. Environ. Entomol. 2011, 40, 1285–1294. [Google Scholar] [CrossRef]
- Makkar, H.P.S.; Tran, G.; Heuzé, V.; Ankers, P. State-of-the-art on use of insects as animal feed. Anim. Feed Sci. Technol. 2014, 197, 1–33. [Google Scholar] [CrossRef]
- Klasing, K.C.; Thacker, P.T.; Lopez, M.A.; Calvert, C.C. Increasing the calcium content of mealworms (Tenebrio molitor) to improve their nutritional value for bone mineralization of growing chicks. J. Zoo Wildl. Med. 2000, 31, 512–517. [Google Scholar] [CrossRef]
- Finke, M.D. Estimate of chitin in raw whole insects. Zoo Biol. 2007, 26, 105–115. [Google Scholar] [CrossRef]
- Satija, A.; Hu, F.B. Cardiovascular benefits of dietary fiber. Curr. Atheroscler. Rep. 2012, 14, 505–514. [Google Scholar] [CrossRef]
- Cecchi, H.M. Fundamentos Teóricos e Práticos em Análise de Alimentos, 2nd ed.Editora da UNICAMP: Campinas, Brasil, 2003; 208p. [Google Scholar]
- Finke, M.D. Complete nutrient composition of commercially raised invertebrates used as food for insectivores. Zoo Biol. 2002, 21, 269–285. [Google Scholar] [CrossRef]
- Ravzanaadii, N.; Kim, S.H.; Choi, W.H.; Hong, S.J.; Kim, N.J. Nutritional value of mealworm, Tenebrio molitor as food source. Int. J. Indust. Entomol. 2012, 25, 93–98. [Google Scholar] [CrossRef]
- Yoo, J.; Hwang, J.S.; Goo, T.W.; Yun, E.Y. Comparative analysis of nutritional and harmful components in Korean and Chinese mealworms (Tenebrio molitor). J. Korean Soc. Food Sci. Nutr. 2013, 42, 249–254. [Google Scholar] [CrossRef]
- Gao, H.L.; Li, H.T.; Zhang, L.; Hao, M.J. Effects of Tenebrio molitor L. larva decomposing polystyrene foam. Adv. Mat. Res. 2010, 113–116, 1972–1975. [Google Scholar] [CrossRef]
- NEPA—UNICAMP. Tabela Brasileira de Composição de Alimentos, 4th ed.; NEPA—UNICAMP: São Paulo, Brazil, 2011; 164p. [Google Scholar]
- Tzompa-Sosa, D.A.; Yi, L.; Van Valenberg, H.J.F.; Van Boekel, M.A.J.S.; Lakemond, C.M.M. Insect lipid profile: Aqueous vs. organic solvent-based extraction methods. Food Res. Int. 2014, 62, 1087–1094. [Google Scholar] [CrossRef]
- Arrese, E.L.; Soulages, J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010, 55, 207–225. [Google Scholar] [CrossRef]
- Raksakantong, P.; Meeso, N.; Kubola, J.; Siriamornpun, S. Fatty acids and proximate composition of eight Thai edible terricolous insects. Food Res. Int. 2010, 43, 350–355. [Google Scholar] [CrossRef]
- Zielińska, E.; Baraniak, B.; Karaś, M.; Rybczyńska, K.; Jakubczyk, A. Selected species of edible insects as a source of nutrient composition. Food Res. Int. 2015, 77, 460–466. [Google Scholar] [CrossRef]
- Pavan, E.; Duckett, S.K. Fatty acid composition and interrelationships among eight retail cuts of grass-feed beef. Meat Sci. 2013, 93, 371–377. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Frederich, M.; Megido, R.C.; Alabi, T.; Malik, P.; Uyttenbroeck, R.; Francis, F.; Blecker, C.; Haubruge, E.; Lognay, G.; et al. Insect fatty acids: A comparison of lipids from three Orthopterans and Tenebrio molitor L. larvae. J. Asia Pac. Entomol. 2017, 20, 337–340. [Google Scholar] [CrossRef]
- Siemianowska, E.; Kosewska, A.; Aljewicz, M.; Skibniewska, K.A.; Polak-Juszczak, L.; Jarocki, A.; Jędras, M. Larvae of mealworm (Tenebrio molitor L.) as European novel food. J. Agric. Sci. 2013, 4, 287–291. [Google Scholar] [CrossRef]
- Ortiz, J.; Romero, N.; Robert, P.; Araya, J.; Lopez-Hernández, J.; Bozzo, C.; Navarrete, E.; Osorio, A.; Rios, A. Dietary fiber, amino acid, fatty acid and tocopherol contents of the edible seaweeds Ulva lactuca and Durvillaea Antarctica. Food Chem. 2006, 99, 98–104. [Google Scholar] [CrossRef]
- Barroso, F.G.; Haro, C.; Sánchez-Muros, M.J.; Venegas, E.; Martínez-Sánchez, A.; Pérez-Bañón, C. The potential of various insect species for use as food for fish. Aquaculture 2014, 422–423, 193–201. [Google Scholar] [CrossRef]
- Ramos-Bueno, R.P.; González-Fernández, M.J.; Sánchez-Muros-Lozano, M.J.; García-Barroso, F.; Guil-Guerrero, J.L. Fatty acid profiles and cholesterol content of seven insect species assessed by several extraction systems. Eur. Food Res. Technol. 2016, 242, 1471–1477. [Google Scholar] [CrossRef]
- De Carvalho, N.M.; Madureira, A.R.; Pintado, M.E. The potential of insects as food sources—A review. Crit. Ver. Food Sci. Nutr. 2020, 60, 3642–3652. [Google Scholar] [CrossRef]
- Delgado, M.C.; Chambers IV, E.; Carbonell-Barrachina, A.; Noguera Artiaga, L.; Vidal Quintanar, R.; Burgos Hernandez, A. Consumer acceptability in the USA, Mexico, and Spain of chocolate chip cookies made with partial insect powder replacement. J. Food Sci. 2020, 85, 1621–1628. [Google Scholar] [CrossRef]
- Sogari, G.; Dagevos, H.; Amato, M.; Taufik, D. Consumer Perceptions and Acceptance of Insects as Feed and Food: Current Findings and Future Outlook. In Novel Foods and Edible Insects in the European Union; Springer: Cham, Switzerland, 2022; pp. 147–169. [Google Scholar] [CrossRef]
- Da Silva, J.K.; Cazarin, C.B.B.; Correa, L.C.; Batista, Â.G.; Furlan, C.P.B.; Biasoto, A.C.T.; Pereira, G.E.; Camargo, A.C.; Maróstica Junior, M.R. Bioactive compounds of juices from two Brazilian grape cultivars. J. Sci. Food Agric. 2016, 96, 1990–1996. [Google Scholar] [CrossRef]
- AOAC—Official Methods of Analysis; AOAC International: Rockville, MD, USA, 2005.
- Bligh, E.G.; Dyer, W.J. A rapid method of total lipid extraction and purification. Can. J. Biochem. Physiol. 1959, 37, 911–917. [Google Scholar] [CrossRef]
- Brasil. RDC N° 360, de 23 de dezembro de 2003. Regulamento Técnico Sobre Rotulagem Nutricional De Alimentos Embalados; Diário Oficial da União; Poder Executivo: Brasília, Brasil, 2003. [Google Scholar]
- Atwater, W.O.; Woods, C.D. The Chemical Composition of American Food Materials; Government Printing Office: Washington, DC, USA, 1896. [Google Scholar]
- Mendonça, S. Efeito Hipocolesterolemizante da Proteína de Amaranto (Amranthus cruentus BRS-Alegria) em Hamsters. Ph.D. Thesis, Faculdade de Saúde Pública, Universidade de São Paulo, São Paulo, Brasil, 2006; 206p. [Google Scholar]
- Janssen, R.; Vincken, J.; van den Broek, L.; Fogliano, V.; Lakemond, C. Nitrogen-to-protein conversion factor for three edible insects: Tenebrio molitor, Alphitobius diaperinus and Hermetia illucens. J. Agric. Food Chem. 2017, 65, 2275–2278. [Google Scholar] [CrossRef]
- Souza, C.O.; Leite, M.E.Q.; Lasekan, J.; Baggs, G.; Pinho, L.S.; Druzian, J.I.; Ribeiro, T.C.M.; Mattos, Â.P.; Menezes-Filho, J.A.; Costa-Ribeiro, H. Milk proteins-based formulas containing different oils affect fatty acids balance in term infants: A randomized blinded crossover clinical trial. Lipids Health Dis. 2017, 16, 78. [Google Scholar] [CrossRef]
- Ulbricht, T.L.V.; Southgate, D.A.T. Coronary heart disease: Seven dietary factors. Lancet 1991, 338, 985–992. [Google Scholar] [CrossRef]

| Parameter * (g/100 g) | Diet ** | ||||
|---|---|---|---|---|---|
| D1 * | D2 | D3 | D4 | D5 | |
| Moisture | 8.46 ± 0.05 e | 9.74 ± 0.03 d | 11.02 ± 0.02 c | 12.31 ± 0.03 b | 13.59 ± 0.05 a |
| Carbohydrate | 73.24 ± 0.13 a | 69.50 ± 0.09 b | 65.77 ± 0.06 c | 62.03 ± 0.03 d | 58.30 ± 0.01 e |
| Protein | 10.66 ± 0.04 a | 8.96 ± 0.03 b | 7.06 ± 0.02 c | 5.56 ± 0.02 d | 3.86 ± 0.03 e |
| Lipid | 1.44 ± 0.02 e | 2.23 ± 0.02 d | 3.02 ± 0.03 c | 3.81 ± 0.04 b | 4.60 ± 0.06 a |
| Ashes | 3.72 ± 0.03 e | 3.83 ± 0.03 d | 3.95 ± 0.03 c | 4.06 ± 0.03 b | 4.17 ± 0.03 a |
| Fiber | 2.49 ± 0.01 e | 5.74 ± < 0.01 d | 8.99 ± < 0.01 c | 12.23 ± 0.01 b | 15.48 ± 0.02 a |
| WA | 0.710 ± < 0.01 a | 0.600 ± < 0.01 b | 0.560 ± 0.01 c | 0.520 ± < 0.01 d | 0.460 ± < 0.01 e |
| Energy (kcal/100 g) | 348.53 ± 0.23 a | 333.90 ± 0.15 b | 319.27 ± 0.26 c | 304.64 ± 0.43 d | 290.01 ± 0.61 e |
| Mass Gain (g/Day) | Time Course (Day) | Diet | ||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | ||
| DMG | 0 to 30 | 1.49 ± 0.10 aA | 1.36 ± 0.21 aA | 1.01 ± 0.08 bA | 0.72 ± 0.15 cA | 0.51 ± 0.10 dA |
| DMG | 31 to 60 | 0.14 ± 0.02 aB | 0.14 ± 0.03 aB | 0.18 ± 0.06 aB | 0.02 ± 0.04 cC | 0.09 ± 0.04 bB |
| DMG | 61 to 90 | 0.02 ± 0.06 aC | 0.06 ± 0.08 aB | 0.12 ± 0.02 aB | 0.13 ± 0.02 aB | 0.10 ± 0.04 aB |
| Parameter 1 (g/100 g) | Diet | ||||
|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | |
| Carbohydrate 2 | 3.58 ± 0.24 bc | 2.45 ± 0.57 c | 4.91 ± 0.38 b | 5.49 ± 1.26 ab | 7.37 ± 2.88 a |
| Protein | 41.90 ± 0.30 ab | 42.60 ± 0.62 a | 42.26 ± 0.40 a | 40.90 ± 1.24 ab | 39.58 ± 2.69 b |
| Lipid | 43.91 ± 0.37 b | 45.58 ± 0.03 a | 44.04 ± 0.05 b | 43.13 ± 0.04 c | 40.50 ± 0.05 d |
| Ashes | 3.02 ± 0.09 c | 3.04 ± 0.05 c | 3.06 ± 0.04 c | 3.43 ± 0.02 b | 3.93 ± 0.22 a |
| Fiber | 7.60 ± 0.28 b | 6.33 ± 0.04 d | 5.72 ± 0.04 e | 7.05 ± 0.03 c | 8.63 ± 0.04 a |
| Water activity 3 | 0.060 ± 0.004 a | 0.047 ± 0.002 b | 0.063 ± 0.003 a | 0.052 ± 0.002 b | 0.046 ± 0.001 b |
| Energy (kcal/100 g) | 577.06 ± 1.98 c | 590.42 ± 0.23 a | 585.07 ± 0.37 b | 573.72 ± 0.10 d | 552.27 ± 0.87 e |
| Fatty Acids (%) | Diet 1 | |||||
|---|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | ||
| Saturated | C8:0 | 0.36 ± 0.02 b | 0.37 ± 0.01 b | 0.48 ± 0.05 a | 0.48 ± 0.01 a | 0.46 ± 0.02 a |
| C10:0 | 0.07 ± 0.01 a | 0.10 ± 0.02 a | 0.09 ± 0.01 a | 0.09 ± 0.01 a | 0.09 ± 0.01 a | |
| C11:0 | 0.15 ± 0.01 b | 0.17 ± 0.02 ab | 0.19 ± 0.01 a | 0.17 ± 0.01 ab | 0.17 ± 0.01 ab | |
| C12:0 | 0.08 ± 0.01 a | 0.08 ± 0.01 a | 0.07 ± 0.01 ab | 0.04 ± 0.01 b | 0.05 ± 0.01 b | |
| C14:0 | 1.18 ± 0.05 a | 1.18 ± 0.03 a | 1.14 ± 0.04 a | 0.78 ± 0.02 c | 1.02 ± 0.01 b | |
| C15:0 | 0.15 ± 0.01 c | 0.18 ± 0.01 b | 0.16 ± 0.01 bc | 0.17 ± 0.01 b | 0.22 ± 0.01 a | |
| C16:0 | 34.37 ± 0.36 a | 32.91 ± 0.27 b | 32.40 ± 0.26 b | 29.90 ± 0.27 c | 28.22 ± 0.09 d | |
| C17:0 | 0.34 ± 0.04 c | 0.41 ± 0.03 bc | 0.40 ± 0.03 bc | 0.45 ± 0.03 b | 0.56 ± 0.01 a | |
| C18:0 | 6.59 ± 0.17 c | 7.40 ± 0.12 c | 7.15 ± 0.74 c | 8.38 ± 0.25 b | 9.59 ±0.06 a | |
| Monounsaturated | C16:1n7 | 0.85 ± 0.03 a | 0.82 ± 0.03 a | 0.72 ± 0.04 ab | 0.54 ± 0.03 b | 0.60 ± 0.16 b |
| C17:1n7 | 0.14 ± 0.04 a | 0.14 ± 0.03 a | 0.12 ± 0.01 ab | 0.07 ± 0.01 b | 0.10 ± 0.02 ab | |
| C18:1n-9 | 33.81 ± 0.25 a | 32.42 ± 0.62 a | 32.47 ± 0.51 a | 29.19 ± 1.05 b | 27.89 ± 0.42 b | |
| Polyunsaturated | C18:2n-6 | 19.40 ± 0.13 c | 21.12 ± 0.15 b | 21.78 ± 1.25 b | 26.83 ± 0.54 a | 27.79 ± 0.16 a |
| C18:3n-3 | 1.40 ± 0.01 c | 1.71 ± 0.07 b | 1.78 ± 0.15 b | 1.91 ± 0.04 b | 2.17 ± 0.08 a | |
| ∑ SFA | 43.28 ± 0.42 a | 42.80 ± 0.19 a | 42.08 ± 0.84 a | 40.46 ± 0.50 b | 40.38 ± 0.08 b | |
| ∑ MUFA | 34.80 ± 0.25 a | 33.38 ± 0.63 a | 33.31 ± 0.53 a | 29.80 ± 1.05 b | 28.59 ± 0.26 b | |
| ∑ PUFA | 20.80 ± 0.12 c | 22.82 ± 0.09 b | 23.56 ± 1.38 b | 28.74 ± 0.50 a | 29.96 ± 0.16 a | |
| n6/n3 | 13.88 ± 0.18 a | 12.45 ± 0.63 b | 12.28 ± 0.47 b | 14.08 ± 0.62 a | 12.89 ± 0.47 ab | |
| Other Fatty Acids | 1.15 ± 0.15 a | 1.04 ± 0.40 a | 1.08 ± 0.10 a | 1.02 ± 0.10 a | 1.16 ± 0.03 a | |
| IA | 0.76 ± 0.03 a | 0.74 ± 0.02 a | 0.71 ± < 0.01 b | 0.63 ± 0.02 d | 0.67 ± 0.01 c | |
| IT | 1.58 ± 0.06 a | 1.59 ± 0.04 a | 1.53 ± 0.03 a | 1.42 ± 0.03 b | 1.60 ± 0.04 a | |
| Metal and Non-Metal (mg/100 g) | Diet | ||||
|---|---|---|---|---|---|
| D1 | D2 | D3 | D4 | D5 | |
| Calcium | 49.69 ± 0.40 a | 49.36 ± 0.07 ab | 48.23 ± 0.06 bc | 47.52 ± 0.06 cd | 46.78 ± 0.94 d |
| Potassium | 1058.83 ± 7.88 e | 1125.45 ± 4.54 d | 1354.59 ± 4.96 c | 1495.82 ± 7.44 b | 1611.40 ± 2.76 a |
| Phosphor | 555.3 ± 5.51 e | 607.94 ± 2.99 d | 648.84 ± 7.29 c | 701.41 ± 2.63 b | 731.51 ± 2.34 a |
| Magnesium | 133.57 ± 3.27 c | 144.77 ± 1.96 b | 146.78 ± 0.57 b | 160.04 ± 0.82 a | 161.71 ± 0.85 a |
| Manganese | 0.9 ± 0.01 a | 0.90 ± 0.01 a | 0.91 ± 0.02 a | 0.91 ± 0.01 a | 0.91 ± 0.01 a |
| Iron | 15.74 ± 0.23 a | 15.71 ± 0.21 a | 15.69 ± 0.04 a | 15.70 ± 0.02 a | 15.68 ± 0.02 a |
| Selenium * | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 |
| Sodium | 2545.96 ± 4.77 a | 2512.86 ± 8.50 b | 2412.76 ± 14.17 c | 2352.47 ± 8.50 d | 2301.85 ± 18.21 e |
| Zinc | 7.14 ± 0.11 a | 6.92 ± 0.08 b | 6.95 ± 0.05 b | 6.85 ± 0.02 bc | 6.72 ± 0.03 c |
| Arsenic * | <1.50 | <1.50 | <1.50 | <1.50 | <1.50 |
| Cadmium * | <1.00 | <1.00 | <1.00 | <1.00 | <1.00 |
| Cobalt * | <1.50 | <1.50 | <1.560 | <1.50 | <1.50 |
| Copper * | <1.50 | <1.50 | <1.50 | <1.50 | <1.50 |
| Nickel * | <2.00 | <2.00 | <2.00 | <2.00 | <2.00 |
| Feedstock | Diet (%; Mass/Mass) | ||||
|---|---|---|---|---|---|
| D1 * | D2 | D3 | D4 | D5 | |
| Grape juice production residue | 0 | 25 | 50 | 75 | 100 |
| Mixture of 70 g/100 g commercial poultry feed ** and 30 g/100 g commercial ground maize grain | 100 | 75 | 50 | 25 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nascimento, R.Q.; Di Mambro Ribeiro, C.V.; Colauto, N.B.; da Silva, L.; Lemos, P.V.F.; de Souza Ferreira, E.; Linde, G.A.; Machado, B.A.S.; Tavares, P.P.L.G.; Biasoto, A.C.T.; et al. Utilization of Agro-Industrial Residues in the Rearing and Nutritional Enrichment of Zophobas atratus Larvae: New Food Raw Materials. Molecules 2022, 27, 6963. https://doi.org/10.3390/molecules27206963
Nascimento RQ, Di Mambro Ribeiro CV, Colauto NB, da Silva L, Lemos PVF, de Souza Ferreira E, Linde GA, Machado BAS, Tavares PPLG, Biasoto ACT, et al. Utilization of Agro-Industrial Residues in the Rearing and Nutritional Enrichment of Zophobas atratus Larvae: New Food Raw Materials. Molecules. 2022; 27(20):6963. https://doi.org/10.3390/molecules27206963
Chicago/Turabian StyleNascimento, Renata Quartieri, Cláudio Vaz Di Mambro Ribeiro, Nelson Barros Colauto, Larissa da Silva, Paulo Vitor França Lemos, Ederlan de Souza Ferreira, Giani Andrea Linde, Bruna Aparecida Souza Machado, Pedro Paulo Lordelo Guimarães Tavares, Aline Camarão Telles Biasoto, and et al. 2022. "Utilization of Agro-Industrial Residues in the Rearing and Nutritional Enrichment of Zophobas atratus Larvae: New Food Raw Materials" Molecules 27, no. 20: 6963. https://doi.org/10.3390/molecules27206963
APA StyleNascimento, R. Q., Di Mambro Ribeiro, C. V., Colauto, N. B., da Silva, L., Lemos, P. V. F., de Souza Ferreira, E., Linde, G. A., Machado, B. A. S., Tavares, P. P. L. G., Biasoto, A. C. T., Umsza Guez, M. A., Carvalho, N., de Jesus Assis, D., da Silva, J. B. A., & de Souza, C. O. (2022). Utilization of Agro-Industrial Residues in the Rearing and Nutritional Enrichment of Zophobas atratus Larvae: New Food Raw Materials. Molecules, 27(20), 6963. https://doi.org/10.3390/molecules27206963











