Abstract
Hypericum lanuginosum is one of the traditional medicinal plants that grows in the arid area of the Al-Naqab desert in Palestine and is used by Bedouins to heal various communicable and non-communicable illnesses. The purpose of this investigation was to estimate the total phenolic, flavonoid, and tannin contents of aqueous, methanol, acetone, and hexane H. lanuginosum extracts and evaluate their cytotoxic, anti-oxidative, and antimicrobial properties. Qualitative phytochemical tests were used to identify the major phytochemical classes in H. lanuginosum extracts, while total phenol, flavonoid, and tannin contents were determined using Folin–Ciocalteu, aluminum chloride, and vanillin assays, respectively. Moreover, a microdilution test was employed to estimate the antimicrobial activity of H. lanuginosum extracts against several microbial species. At the same time, the cytotoxic and free radical scavenging effects were evaluated using 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium (MTS) and 2, 2-diphenyl-1-picryl-hydrazyl-hydrate (DPPH) assays, respectively. Quantitative examinations showed that the highest amounts of phenols, flavonoids, and tannins were noticed in the H. lanuginosum aqueous extract. Moreover, H. lanuginosum aqueous extract showed potent activity against methicillin-resistant Staphylococcus aureus even more than Amoxicillin and Ofloxacin antibiotics, with Minimum Inhibitory Concentrations (MICs) of 0.78 ± 0.01, 0, and 1.56 ± 0.03 µg/mL, respectively. Additionally, the aqueous extract exhibited the highest activity against Candida albicans and Epidermatophyton floccosum pathogens, with MIC values of 0.78 ± 0.01 µg/mL. Actually, the aqueous extract showed more potent antimold activity than Ketoconazole against E. floccosum with MICs of 0.78 ± 0.01 and 1.56 ± 0.02 µg/mL, respectively. Furthermore, all H. lanuginosum extracts showed potential cytotoxic effects against breast cancer (MCF-7), hepatocellular carcinoma (Hep 3B and Hep G2), and cervical adenocarcinoma (HeLa) tumor cell lines. In addition, the highest free radical scavenging activity was demonstrated by H. lanuginosum aqueous extract compared with Trolox with IC50 doses of 6.16 ± 0.75 and 2.23 ± 0.57 µg/mL, respectively. Studying H. lanuginosum aqueous extract could lead to the development of new treatments for diseases such as antibiotic-resistant microbes and cancer, as well as for oxidative stress-related disorders such as oxidative stress. H. lanuginosum aqueous extract may help in the design of novel natural preservatives and therapeutic agents.
1. Introduction
Not so many years ago, herbal remedies were the only effective weapon in the armory of physicians. Even today, naturally occurring remedies are of paramount importance in the fight against infectious and non-infectious illnesses [1]. In daily life, human beings are exposed to various types of pathogens such as bacteria, viruses, fungi, and parasites. In fact, many types of microbial infections harm human bodies, and in some instances, they can be fatal [2]. Therefore, pharmaceutical companies are working hard to develop different types of antimicrobial agents to overcome this problem [3].
Excessive oxidative stress triggers the formation of reactive oxygen species, which have been linked to several other recent fatal diseases, including diabetes, cancer, neurodegenerative, and cardiovascular diseases [4]. The genomic integrity of the cell is maintained by a balance between the levels of pro-oxidants and antioxidants [5]. If this balance is disrupted, the host immunity is modulated and the normal cellular signaling pathways are affected, leading to uncontrolled proliferation of the cells and ending in cancer, and macrophage polarization leads to the formation of atherogenic plaques [5,6].
Cancer is a broad term that describes the disease that results when cellular changes cause the uncontrolled growth and division of cells. Some types of cancer cause rapid cell growth, while others cause cells to grow and divide at a slower rate. Certain forms of cancer result in visible growths called tumors, while others, such as leukemia, do not [7]. Chemotherapy, hormonal therapy, immunotherapy, precision medicine, radiation therapy, stem cell transplant therapy, surgery, and targeted therapies are the most commonly used methods for the treatment of cancer disease [8].
The genus of the Hypericum plant compromises more than 460 species that are wildly and broadly distributed in all world regions [9]. Many researchers have documented several pharmacological properties of various Hypericum species, including antidepressant, antiproliferative, antimicrobial, antioxidant, anti-inflammatory, and neuroprotective effects [10]. In traditional medicine, different Hypericum species have been used in folk medicine in sore furuncle, bruises, wounds, stomatitis, sore throat, strangury, dysentery, and hepatitis remedies. Additionally, they are used as a treatment for various types of cancer, including nasopharyngeal, laryngeal, tonsil, lung, bladder, and prostatic carcinomas [10,11].
Hypericum lanuginosum Lam. is a perennial herb plant in the Hypericaceae family that can reach 0.8 m in height, with few green and terete stems. Its leaves are sub-glabrous on both surfaces, with whitish and thick veins. H. lanuginosum is found in the desert of Sinai (Egypt), Southern Turkey, Western parts of Syria, and arid areas of Palestine and Jordan [12].
Ebru reported that 41 molecules were recognized in the essential oil of H. lanuginosum aerial parts from Turkey, with spathulenol, caryophyllene oxide, α-pinene, and undecane [9].
In addition, Mahomoodallya et al. investigated the chemical components of H. lanuginosum organic extracts and detected quinic acid, flavonoid, acylquinic acids, and phenolic acids by the LC-HRMS technique [13]. Moreover, Odabas et al. reported that H. lanuginosum leaves methanol extract was abundant in quercitrin and neochlorogenic acid [14].
So far, only two phytochemical investigations and one biological study have been conducted on this species [9,13], while its antimicrobial and cytotoxic properties have not been investigated yet as far as the recent literature is concerned.
Therefore, the present study aims to conduct a qualitative and quantitative analysis of the cytotoxic, antibacterial, and antifungal characteristics of the n-hexane, acetone, methanol, and water extracts of the H. lanuginosum plant from Palestine.
2. Results
2.1. Qualitative Assessments
The conducted analytical tests identified the presence of different phytochemical classes of secondary and primary metabolites in the four solvent extracts of the H. lanuginosum plant. The results of the identification tests showed that the methanolic extract was the richest in bioactive molecules and contained seven metabolic phytochemical classes, including flavonoids, tannins, phenols, cardiac glycosides, monosaccharides, reducing sugars, and steroids (Table 1), followed by the aqueous extract, which contained flavonoids, tannins, phenols, proteins, cardiac glycosides, and steroids. On the other hand, flavonoids, tannins, and phenols were found in the acetone extract, and steroids were found in the H. lanuginosum hexane extract.

Table 1.
Identification tests results of H. lanuginosum extracts.
2.1.1. Estimation of Total Phenol Content
Quantitative analysis of phenol content in the four solvent extracts of the H. lanuginosum plant was performed using gallic acid as a reference compound, and the results are expressed as mg gallic acid equivalent per gram of dry weight for each extract (mg GAE/g DW). However, the total phenol contents were calculated from the following equation:
where y is the absorbance at 765 nm and x is the total phenol content of the plant extracts, which was obtained from the calibration curve that is given in the Supplementary Materials (Figure S1).
y = 0.0056x + 0.0495 R² = 0.981,
2.1.2. Assessment of Total Tannin Content
A quantitative analysis of tannin content in the four solvent extracts of H. lanuginosum was performed using catechin as a reference compound and expressed as catechin equivalent per gram of dry weight for each of the plant extracts (mg CAE/g DW). According to the standard catechin calibration curve (Figure S2), the following equation was utilized to estimate the total tannin content in the four solvent plant extracts.
where y is the absorbance at 510 nm and x is the total tannin content in each plant extract.
y = 0.0009x + 0.011, R2 = 0.9956,
2.1.3. Flavonoid Content
Total flavonoids content was calculated from the calibration curve of quercetin and expressed as milligram of Quercitin Equivalent per gram of extract (mg QUE/g extract).
From the quercetin’s calibration curve (Figure S3), the following equation was obtained to determine the total tannin content of the four plant extracts:
y = 0.0004x + 0.0011, R2 = 0.995.
The total contents of phenol, tannin, and flavonoid quantitative tests results of the hexane, acetone, methanol, and aqueous extracts of the H. lanuginosum plant are summarized in Table 2.

Table 2.
The total phenol, flavonoid, and tannin contents of the H. lanuginosum extracts.
2.2. Antimicrobial Activity
H. lanuginosum hexane, aqueous, acetone, and methanol extracts were tested for antimicrobial activity using the microdilution technique against C. albicans, E. floccosum, E. coli, P. aeruginosa, S. sonnie, E. faecium, S. aureus, and MRSA. Table 3 shows that most of the screened extracts have potential antimicrobial activity compared with the used positive control antibiotics in this study.

Table 3.
Antimicrobial activity MIC values (µg/mL) of methanol, aqueous, hexane, and acetone extracts of H. lanuginosum.
2.3. Cytotoxic Activity
In the present investigation, MTS was employed to estimate the cytotoxic effects of methanol, aqueous, hexane, and acetone extracts of H. lanuginosum on the cell proliferation of breast cancer (MCF-7), hepatocellular carcinoma (Hep 3B and Hep G2), and cervical adenocarcinoma (HeLa). Cells were exposed for 24 h to increasing concentrations of the tested extracts (0, 0.05, 0.1, 0.3, 0, 5, 1, and 2 µg/mL).
The results of the inhibitory effect of methanol, aqueous, hexane, and acetone extracts of H. lanuginosum in addition to Doxorubicin on cancer cell lines viability in the present study ranged from 15.66–88.1% at one µg/mL as shown in Figure 1, and the IC50 values are demonstrated in Table 4.
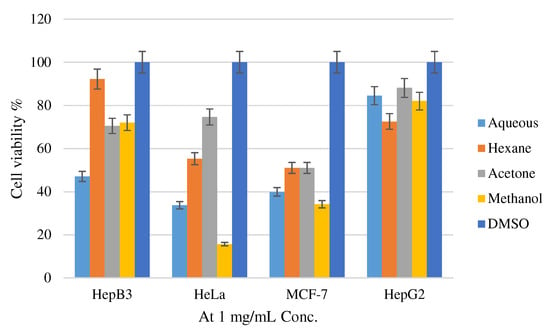
Figure 1.
Cell viability % of H. lanuginosum methanol, aqueous, hexane, and acetone extracts.

Table 4.
Cytotoxic activity IC50 values (µg/mL) of H. lanuginosum methanol, aqueous, hexane, and acetone extracts compared with the positive control chemotherapy drug doxorubicin.
2.4. Free Radical Scavenging Activity
Figure S4 depicts the free radical scavenging activity of various H. lanuginosum extracts and the standard compound Trolox. The results revealed that all the tested samples had free radical scavenging effects, but among the extractives, aqueous extract possessed the highest free radical scavenging influence. However, the antioxidant activity IC50 values of H. lanuginosum four extracts and Trolox are listed in Table 5, where the higher antioxidant effect is indicated by a lower IC50 value.

Table 5.
DPPH free radical scavenging activity of H. lanuginosum extracts and Trolox.
3. Discussion
Plants and their extracts have been utilized for hundreds of years in therapeutic remedies, food preservatives, and cosmetics. They have also been used for the treatment of various animal and human diseases, including infectious, systematic, and inflammatory diseases since the Hippocrates era (460–377 BC) [15].
3.1. Qualitative Phytochemical Screening
The identification tests of H. lanuginosum methanol, acetone, and water extracts detected the presence of therapeutically active secondary metabolic phytochemical groups such as cardiac glycosides, phenols, steroids, tannins, and flavonoids. A steroidal phytochemical group was the only detected phytochemical group in H. lanuginosum hexane extract.
Total Phenol, Flavonoid, and Tannin Contents
Plant secondary metabolites with an aromatic ring and at least one hydroxyl group are known as phenolic substances, such as flavonoids, simple phenols, and tannins. More than 8000 naturally occurring phenolic compounds from plants have been identified [16,17]. Studies on flavonoids and other phenolic compounds from medicinal plant species have grown significantly over the past several decades because of their numerous health advantages for humans [18]. Phenolic constituents have been reported for their effectiveness as anticancer, antibacterial, and antioxidant agents [19].
The results in the current study showed that the highest extraction yields were exhibited by polar solvents such as water, methanol, and acetone, and the lowest yield was observed in the non-polar hexane solvent. However, the yields of H. lanuginosum extracts were found to be 4.3 ± 0.22%, 2.2 ± 0.13%, 1.8 ± 0.03%, and 1.2 ± 0.02%, respectively. In the current investigation, total phenolic compounds were found in the acetone, methanol, and aqueous extracts of H. lanuginosum. The highest total phenol content was found in the aqueous (92.41 ± 1.54 mg GAE/g of plant extract) and methanol (32.82 ± 0.81 mg GAE/g plant extract) extracts, followed by the acetone extract (1.41 ± 0.25 mg of GAE/g of plant extract), whereas phenolic compounds were absent in the hexane extract. At the same time, the highest amounts of flavonoid content were estimated in the aqueous (76.7 ± 2.01 mg QUE/g of plant extract) and methanolic (28.21 ± 1.02 mg QUE/g of plant extract) H. lanuginosum extracts. In addition, aqueous H. lanuginosum extract contains the highest contents of tannins (15.11 ± 0.71 mg CAE/g of plant extract).
Only one study was found in the literature by Mahomoodally et al. that screened total phenols, tannins, and flavonoid contents in the H. lanuginosum from Turkey, which reported that the aqueous extract had the highest total phenol (168.56 mg GAE/g extract) and flavonoid (53.22 mg RE/g extract) contents among other investigated ethyl acetate and methanolic extracts. The methanol extract was in second place with a total phenol content of 72.03 mg GAE/g extract and a total flavonoid content of 30.96 mg RE/g extract [13]. However, to the best of our knowledge, no previous studies investigated total tannin content.
In fact, the current study outcomes agreed with the quantitative test results of Mahomoodally et al. As mentioned above, the highest total phenols and flavonoid contents were the abundant amounts in the aqueous H. lanuginosum extract.
As it is well known, herbs within the same species show significant variation in their chemical components based on their geographical locations associated with environmental interactions, including the type of soil, climate, rainfall changes, and growth stages [20].
3.2. Antimicrobial Activity
Current antimicrobial drugs have been used for the treatment of human and animal infections for less than a century. The development of drug-resistant pathogens has occurred rapidly, while the emergence of multi-drug resistant strains has increased exponentially in recent years [21].
The microdilution assay was used to evaluate the antimicrobial activity of the hexane, aqueous, acetone, and methanol solvent extracts of H. lanuginosum. The results revealed that the aqueous extract of H. lanuginosum exhibited the greatest antibacterial activity against the E. coli, P. aeruginosa, S. sonnie, E. faecium, S. aureus, and MRSA strains with MIC values of 3.13 ± 0.04, 1.56 ± 0.02, 1.56 ± 0.01, 0.78 ± 0.01, 0.78 ± 0.01, and 0.78 ± 0.01 µg/mL followed by the methanol extract with MIC value of 6.25 ± 0.88, 3.13 ± 0.01, 12.5 ± 0.41, 3.13 ± 0.01, 1.56 ± 0.21, and 3.13 ± 0.05 µg/mL, respectively. The hexane extract did not affect the growth of all the tested bacterial strains except against S. aureus (MIC = 3.13 ± 0.12 µg/mL). In comparison, the acetone extract inhibited the growth of most of the tested bacterial strains but with weak activities.
The aqueous and methanol extracts showed the highest anticandida and antimold activities against C. albicans and E. floccosum with MIC values of 0.78 ± 0.01 and 1.56 ± 0.02 µg/mL, respectively. Finally, the aqueous and methanol extracts exhibited the highest antibacterial and antifungal activities compared with Amoxicillin, Ofloxacin, and Ketoconazole antibiotics, which were used as a positive control. Against MRSA, the H. lanuginosum aqueous extract showed potent activity, even more than Amoxicillin and Ofloxacin antibiotics, with MICs of 0.78 ± 0.01, 0, and 1.56 ± 0.03 µg/mL, respectively. That could be seen as a good result since MRSA is one of the most dangerous hospital pathogens and affects many countries.
Concerning the antifungal activity of H. lanuginosum extracts, the results indicated that the aqueous extract exhibited the highest activity against C. albicans and E. floccosum pathogens, with MIC values of 0.78 ± 0.01 µg/mL followed by the methanol extract (MICs of 1.56 ± 0.02 µg/mL). Actually, the aqueous extract showed more potent antimold activity than Ketoconazole against E. floccosum with MICs of 0.78 ± 0.01 and 1.56 ± 0.02 µg/mL, respectively.
The antimicrobial activity of H. lanuginosum has not been investigated yet as far as the literature is concerned, and our study is the first to investigate this important biological activity.
As presented in the H. lanuginosum quantitative tests, the aqueous and methanolic extracts have the highest content of phenols, flavonoids, and tannins, while the qualitative tests show that these extracts are the richest ones with secondary metabolites. This shows that there is a positive correlation between the chemical constituents of the aqueous and methanolic extracts of the H. lanuginosum plant and antimicrobial activity. Several studies suggest that phytochemical classes such as tannins, flavonoids, and phenols have been known to be biologically active and thus responsible for the antimicrobial activities of plants [22,23].
3.3. Cytotoxic Effects
The term ”cancer” refers to a collection of over 100 diseases that can affect any organ of the body. These diseases are usually typically deadly and impose a colossal burden on society and the health care system [24]. Along with conventional medicine, some people choose other methods of cancer treatment such as complementary and alternative medicine or traditional medicine, particularly medicinal plants [25].
By employing the MTS colorimetric assay, the cell proliferation of MCF-7, Hep 3B, Hep G2, HeLa, and Hek293t cell lines were used to estimate H. lanuginosum methanol, aqueous, hexane, and acetone extracts’ cytotoxic effects. Cells were exposed for 24 h to increasing concentrations of the tested extracts (0, 0.05, 0.1, 0.3, 0, 5, 1, and 2 µg/mL).
Results indicated that all the tested plant extracts exerted cytotoxic activity against all the cancer cell lines utilized in this investigation. However, the highest cytotoxic effect against the Hep 3B cell line was noticed by the H. lanuginosum aqueous extract, with an IC50 value of 46.9 ± 1.01 µg/mL. The methanol extract had the highest cytotoxic effect against HeLa and MCF7 cells, with IC50 doses of 16.07 ± 0.25 and 33.92 ± 0.91 µg/mL, respectively. In addition, the highest cytotoxic effect against the Hep G2 cancer cell line was noticed by H. lanuginosum hexane extract followed by aqueous extract, with IC50 values of 73 ± 0.57 and 85.02 ± 0.52 µg/mL, respectively. All the obtained results were compared with Doxorubicin, the potent chemotherapeutic agent. The cytotoxic effects of the H. lanuginosum extracts were also evaluated against the normal cell line (Hek293t), and the results showed that the evaluated H. lanuginosum methanol, aqueous, hexane, and acetone extracts are not toxic, and the IC50 values were 331.87 ± 3.12, 190.16 ± 2.67, 176.02 ± 1.99 and 288.96 ± 1.55 µg/mL, respectively, compared with Doxorubicin, which was highly cytotoxic (IC50 = 0.58 ± 0.07 µg/mL).
Based on these facts, H. lanuginosum methanol, aqueous, hexane, and acetone extracts contain several phytochemical molecules, including flavonoids, phenols, and tannins, which have cytotoxic effects as previously reported in the literature. In addition, many research teams consider the synergic effect of these compounds important, so flavonoids, phenols, and tannins-rich plant extracts show different potential cytotoxic effects on various cancer cell lines [23].
3.4. DPPH-Free Radical Scavenging Activity
The DPPH-free radical test is extensively used to assess a compound’s or extract’s potential to act as free radical scavengers and hydrogen providers. It is a quick, easy, and low-cost approach for assessing antioxidant capacity [26,27].
The current experiment revealed that H. lanuginosum methanol, aqueous, hexane, and acetone extracts scavenged DPPH free radicals in a concentration-dependent manner. In fact, the aqueous extract exhibited the most potent free radical scavenging activity, followed by the methanolic extract with IC50 dosages of 6.16 ± 0.75 and 52.48 ± 1.31 µg/mL, respectively, in comparison to the positive control Trolox (IC50 = 2.23 ± 0.05 µg/mL), which is a potent antioxidant medication. Meanwhile, the acetone extract was a weak free radical scavenger, and the hexane extract was almost inactive.
The study by Mahomoodally et al. showed that the methanol and aqueous extracts of the H. lanuginosum plant collected from Turkey had an antioxidant activity with IC50 doses of 105.93 ± 2.07 and 354.60 ± 7.29 mg TE/g, respectively [13].
All these outcomes may be attributed to the presence of a variety of polyphenols in the H. lanuginosum plant polar extracts. As reported in the literature, the H. lanuginosum polar extract contains twenty-one phenolic compounds, including bioflavonoids, flavonoids, acylquinic acids, and phenolic acids, based on the chemical components of H. lanuginosum aqueous and methanol extracts that were identified [13].
The powerful antioxidant activity of these extracts seems to depend on the presence of carboxylic acid (COOH) in acylquinic and phenolic acids, as well as two hydroxyl (OH) groups in the ortho position of the benzene ring in flavonoids and bioflavonoids [28].
Advanced quantitative and qualitative tests using LC-MS, HPLC-DAD, or GC-MS techniques are required in order to identify the molecules responsible for the biological activities. Moreover, in vivo studies are needed to elucidate the potential of the H. lanuginosum aqueous and methanolic extracts as anticancer, antimicrobial, and antioxidant agents, and their possible mechanisms of action, in addition to the toxicological investigations needed to prove their safety.
4. Materials and Methods
4.1. Chemical Reagents
Methanol, n-hexane, and Acetone (Loba chemie, Mumbai, India) were used to prepare the solvent extracts. Dimethyl sulphoxide (DMSO; Riedel-de Haën, Seelze, Germany) and Mueller–Hinton broth (Himedia, Maharashtra, India) were used for the antimicrobial screening experiments.
Reagents used for the quantitative and qualitative assessments (ninhydrin solution, Benedict’s reagent, Millon’s reagent, magnesium ribbon, iodine, sulphuric acid, acetic acid, and Molish’s reagent) were obtained from Alfa Aesar (Lancashire, UK). NaHCO3, FeCl3, vanillin, HCl, gallic acid, quercetin, sodium nitrite, AlCl3, and chloroform were obtained from Sigma-Aldrich (Schnelldorf, Germany). Trolox was purchase from Sigma-Aldrich (St. Louis, MO, USA) was ((s)-6-hydroxy-2,5,7,8-tetramethyl chroman-2-carboxylic acid)), while DPPH reagent (2,2-diphenyl-1-1-picrylhydrazyl) was purchased from Sigma-Aldrich (Schnelldorf, Germany). For the cytotoxicity test, RPMI-1640 (Roswell Park Memorial Institute) medium was brought from Sigma-Norwich (Norfolk, UK). Pen-Strep solution, which consists of streptomycin and penicillin in concentrations of 10 mg/mL and 10,000 units/mL, respectively, was brought from BI (New Delhi, India), the L-glutamine solution was brought from Sigma (Welwyn Garden, UK), and the MTS reagent was purchased from Promega (Madison, WI, USA).
4.2. Instruments
A UV-visible spectrophotometer (Jenway 7315, Staffordshire, UK), balance (Radw, Gmina Górzyca, Poland), filter paper (Whatman no. 1, Marlborough, MA, USA), sonicator (MRC, 2014-207, Banbury, UK), water bath (LabTech, 2011051806, Namyangju, Korea), incubator (nüve, 06-3376, Ankara, Turkey), vortex (Heidolph, 090626691, Schwabach, Germany), autoclave (MRC, A13182, Banbury, UK), shaker incubator apparatus (Memmert, Büchenbach, Germany), lyophilizer (Mill-Rock Technology-BT85, Shanghai, China), rotary-evaporator (Heidolph, VV2000, Schwabach, Germany), grinder (Molineux I, Paris, France), microplate reader (Agilent BioTek, Winooski, VT, USA), and micro-broth plate from (Greiner/Bio-One, Ocala, FL, USA) were used in this study.
4.3. Plant Material
The aerial parts, including leaves and stems, of the H. lanuginosum plant were collected in May 2021 from the southern regions of the Hebron governorate of Palestine. Botanical determination was carried out in the Herbal Products Laboratory at An-Najah National University by a pharmacognosist, Dr. Nidal Jaradat. In the same laboratory, the plant sample was deposited under the voucher specimen code (Pharm-PCT-1239). The fresh parts were washed many times using tap water to remove soil and dust contamination and then washed again using distilled water. The clean parts were dried in the shade at 24 ± 3 °C and 55 ± 5 relative humidity for seven days. The prepared dry parts were then pulverized and kept in cotton bags for further use.
4.4. Preparation of H. Lanuginosum Extracts
The powdered plant material was sequentially extracted by the use of different solvents of increasing polarities to increase the extraction efficiency of phytochemicals, including the non-polar (hexane), polar aprotic (acetone), and polar protic solvents (methanol and water). In a special Pyrex® glass bottle, 50 g of the grounded plant material was steeped in 0.5 L of four different solvents (water, methanol, acetone, and n-hexane) separately. Each bottle was placed in a sonicator device for 3 h at room temperature and then macerated for five days. Following this, each of the organic extracts was filtered and concentrated under a vacuum with a rotary evaporator. Finally, the aqueous extract was dried using a lyophilizer. The yields of the H. lanuginosum hexane, acetone, methanol, and aqueous extracts were found to be 1.2 ± 0.02%, 1.8 ± 0.03%, 2.2 ± 0.13%, and 4.3 ± 0.22%, respectively. The obtained extracts were kept at 5 °C for further analysis.
4.5. Phytochemical Qualitative Analysis
The H. lanuginosum extracts were qualitatively screened to detect the presence of secondary metabolic products comprising tannins, steroids, saponins, phenols, cardiac glycosides, alkaloids, and flavonoids. Qualitative screening for primary metabolites, including carbohydrates, protein, reducing sugars, and starch, was also performed. All the identification tests were performed according to the standard analytical assays as follows:
A combination of Fehling’s solutions A and B in equal amounts was added to plant extracts. A red-colored precipitate indicates the presence of reducing sugars. Next, 2 mL of Molisch’s solution was mixed with plant extract. The presence of carbohydrates is shown by the appearance of a brown ring in the test tube’s interphase. A FeCl3 solution was added to the extract. Tannins were detected by their black or blue-green color. When 5 mL of distilled water was added to a crude plant extract and rapidly shaken, saponins were detected via the foam formation. Folin reagent was used to detect the presence of phenol, which appears in a dark blue color. Then, 2 mL of concentrated HCl was mixed with the plant extract. The mixture was then cooled, and 2 mL of KOH was added. A pink-red color indicated the presence of glycosides. Then, 2 mL of alkaloidal precipitating reagent mixed with plant extract, color change, or appearance of precipitate prove the presence of the alkaloids. Pieces of magnesium ribbon and concentrated HCl were mixed with crude plant extracts. After a few minutes, a pink-colored scarlet appeared that indicated the presence of flavonoids. The 2 mL of Millon’s reagent mixed with the plant extracts appeared as a white precipitate, which upon gentle heating turned into a red color, which indicated the presence of protein. A mixture of acetic acid glacial (2 mL) with two drops of 2% FeCl3 solution was added to the plant extract, and H2SO4 was concentrated. A brown ring was produced between the layers, which indicated the presence of cardiac glycosides. Then, 2 mL of chloroform and H2SO4 concentrated were mixed with the plant extracts. The lower chloroform layer produced a red color that indicated the presence of steroids [29].
4.6. Quantitative Chemical Analyses
4.6.1. Total Phenol Content
The total phenol content was determined using the previous technique. [29]. In brief, a 1 mg/mL stock solution of gallic acid was made by dissolving 0.1 g gallic acid in 100 mL of methanol as a solvent in a volumetric flask, and then a series of concentrations (10, 20, 30, 40, 50, and 100 μg/mL) were prepared from this solution. The working mixture was prepared by mixing 0.5 mL of each methanolic solution, 2.5 mL of 10% Folin–Ciocalteu’s reagent diluted in water, and 2.5 mL of 7.5% NaHCO3. The samples were then stored in a thermostat at 30 °C for 1.5 h. The absorbance was measured utilizing a UV-Vis-spectrophotometer at a λmax of 765 nm. The total phenol content was calculated using a calibration curve with gallic acid. The H. lanuginosum extracts were expressed as milligrams of gallic acid equivalents per gram of plant extract (mg GAE/g of plant extract).
4.6.2. Total Tannin Content
Tannin content was estimated following the procedure of [30] utilizing catechin as a reference compound. Briefly, 100 µg/mL of aqueous stock solutions of methanolic extract were prepared then several dilutions (10, 30, 50, 70, and 100 μg/mL) were prepared. Thereafter, 1 mL from each dilution was added to 3 mL 4% vanillin (in methanol) solution and 1.5 mL of concentrated HCl. The absorbance was measured at 510 nm after 15 min of incubation. Total tannin content was expressed as milligrams of catechin equivalents per gram of plant extract (mg CAE/g of each plant extract).
4.6.3. Total Flavonoid Content
The total flavonoid content of H. lanuginosum extracts was determined according to the procedure of [31]. It was calculated from the calibration curve of quercetin and expressed as milligrams of Quercitin Equivalent per gram of extract (mg QUE/g extract). Quercitin (100 mg) was dissolved in 10 mL of distilled water and diluted to 100 mL. Subsequently, the stock solution was diluted to provide a series of concentrations (10, 30, 50, 70, and 100 µg/mL). From each solution (0.5 mL) was mixed with 3 mL methanol, 0.2 mL of 10% AlCl3, 0.2 mL potassium acetate 1 M, and 5 mL distilled water and then incubated at room temperature for 30 min. Furthermore, absorbance was measured at 415 nm wavelength, and distilled water with methanol, 10% AlCl3, and potassium acetate was used as a blank.
4.7. Antimicrobial Activities
The microbial species utilized in the current investigation were obtained from the American Type Culture Collection (ATCC) and a selected methicillin-resistant Staphylococcus aureus (MRSA) strain, which was isolated in clinical settings in our region and exhibited multidrug resistance. The isolates included three Gram-positive strains: MRSA, Enterococcus faecium (ATCC 700221), and Staphylococcus aureus (ATCC 25923). The Gram-negative species Shigella sonnie (ATCC 25931), Pseudomonas aeruginosa (ATCC 27853), and Escherichia coli (ATCC 25922) were also used in the current investigation. Finally, the effect of the four extracts of H. lanuginosum on the fungal species Candida albicans (ATCC 90028) and Epidermatophyton floccosum (ATCC 10231) was also estimated. The extracts of H. lanuginosum were dissolved in DMSO to a concentration of 1 mg/mL. A broth microdilution test was used to determine Minimum Inhibitory Concentrations (MICs). Briefly, solutions were serially micro-diluted (2-fold) 10 times in sterile Mueller–Hinton broth. The dilution procedure was carried out in 96-well plates under aseptic conditions. In the micro-wells designated to test the antibacterial activity of plant extracts. A plant-free Mueller-Hinton broth was used as a positive control for microbial growth in micro-well number eleven. Plant-and microbe-free Mueller–Hinton broth was used as a negative control for microbial growth in micro-well number twelve. Micro-wells 1–11 were aseptically inoculated with the test microbes. All the inoculated plates were incubated at 35 °C. Regarding the fungal strains; the same method was used but utilized the RPMI media instead of Mueller–Hinton broth. The incubation period lasted for approximately 18–24 h for those plates inoculated with the test bacterial strains and for about 48 h for those plates inoculated with the Candida strain. The lowest concentration of H. lanuginosum extracts with no visible microbial growth was considered to be the MIC. The H. lanuginosum extracts’ antimicrobial activity was assessed in triplicate. For bacteria and fungi tested in the current work, two antibiotics and one antifungal were used as positive controls [32].
4.8. Cytotoxicity Analyzes
Breast cancer (MCF-7), hepatocellular carcinoma (Hep 3B and Hep G2), and cervical adenocarcinoma (HeLa) in addition to the normal human cell line (Hek293T) were obtained from American Type Culture Collection, Manassas, VA, USA (ATCC codes of HTB-22, HB-8064, HB-8065, CRM-CCL-2, CRL-3216, respectively). The cells were cultivated in an RPMI-1640 medium containing 10% fetal bovine serum, 1% streptomycin/penicillin antibiotics, and 1% l-glutamine. Cells were grown at 37 °C in a humidified environment containing 5% CO2. In a 96-well plate, screened cells were seeded at a density of 2.6 × 104 cells per well. Following 48 h and all screened cancer and normal cells were cultured for 24 h with varying doses of the tested materials. Cell viability was determined according to the manufacturer’s directions by employing the CellTilter 96® Aqueous One Solution Cell Proliferation (MTS) Assay (Promega Corporation, Madison, WI, USA). At the end of the treatment, each well was received 20 µL of MTS solution per 100 µL of medium and was incubated at 37 °C for 2 h. At 490 nm, absorbance was determined for all samples [33].
4.9. Free Radical Scavenging Property
The DPPH free radical scavenging activity of the H. lanuginosum four extracts and Trolox was performed following the method of [29]. A stock solution of a concentration of 1 mg/mL in methanol was prepared for the plant extract and Trolox. The working solutions of the following (2, 5, 10, 20, 50, and 100 μg/mL) concentrations were prepared by serial dilution with methanol from the stock solution. DPPH was freshly prepared at a concentration of 0.002% w/v. The DPPH solution was mixed with methanol and the above-prepared working concentration in a ratio of 1:1:1, respectively. The spectrophotometer was zeroed using methanol as a blank solution. The first solution of the series concentration was DPPH with methanol only. The solutions were incubated in the dark for 30 min at room temperature before the absorbance readings were recorded at 517 nm. The DPPH inhibitory percentages of the plant extracts and the Trolox standard were calculated using the following formula:
where: A = Absorbance of the blank, B = Absorbance of the sample.
DPPH inhibition (%) = (A − B)/A × 100%
The antioxidant half-maximal inhibitory concentration (IC50) for the plant samples and the positive control (Trolox) were calculated using BioDataFit edition 1.02 (data fit for biologist) https://www.changbioscience.com/stat/ec50.html (accessed on 1 June 2021).
4.10. Statistical Analyses
The statistical analyses were carried out using the SPSS software (Version 11.5, SPSS Inc., Chicago, IL, USA). All of the measurements were carried out in triplicate. The results were represented as mean values ± SDs. p < 0.05 was considered to be significant.
5. Conclusions
The quantitative phytochemical analysis of H. lanuginosum aqueous and methanolic extracts revealed that these extracts are rich in flavonoids and phenols. Moreover, these extracts demonstrated remarkable antibacterial and antifungal activities, especially against MRSA and C. albicans. The antimicrobial results showed that the aqueous extract has more potent activity against these two species, even more than the positive control antibiotics. In addition, H. lanuginosum polar extracts exhibited potential cytotoxic activity. All these outcomes make H. lanuginosum aqueous and methanol extracts good choices for developing novel antimicrobial, antioxidant, and antitumor medications. For these reasons, the present study has provided initial data that will ensure the importance of H. lanuginosum in conventional and traditional medicines.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27144574/s1, Figure S1. Gallic acid standard calibration curve; Figure S2. Catechin standard calibration curve; Figure S3. Quercetin reference calibration curve; Figure S4. In vitro free radical scavenging activity of H. lanuginosum extracts and Trolox.
Funding
This research did not receive any external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data is contained within the article.
Acknowledgments
The author would like to acknowledge An-Najah National University.
Conflicts of Interest
The author declares no conflict of interest.
Sample Availability
Sample of the Hypericum lanuginosum are available from the author.
References
- Welz, A.N.; Emberger-Klein, A.; Menrad, K. Why people use herbal medicine: Insights from a focus-group study in Germany. BMC Complement. Med. Ther. 2018, 18, 92. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, J.; Kessel, K.V.; Snippe, H. Immune Response in Human Pathology: Infections Caused by Bacteria, Viruses, Fungi, and Parasites. In Nijkamp and Parnham’s Principles of Immunopharmacology; Springer: Berlin, Germany, 2019; pp. 165–178. [Google Scholar]
- Miethke, M.; Pieroni, M.; Weber, T.; Brönstrup, M.; Hammann, P.; Halby, L.; Arimondo, P.B.; Glaser, P.; Aigle, B.; Bode, H.B. Towards the sustainable discovery and development of new antibiotics. Nat. Rev. Chem. 2021, 5, 726–749. [Google Scholar] [CrossRef] [PubMed]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef] [PubMed]
- Jain, A.K.; Mehra, N.K.; Swarnakar, N.K. Role of antioxidants for the treatment of cardiovascular diseases: Challenges and opportunities. Curr. Pharm. Des. 2015, 21, 4441–4455. [Google Scholar] [CrossRef]
- Khosravi, M.; Poursaleh, A.; Ghasempour, G.; Farhad, S.; Najafi, M. The effects of oxidative stress on the development of atherosclerosis. Biol. Chem. 2019, 400, 711–732. [Google Scholar] [CrossRef]
- Muresanu, C.; Khalchitsky, S. Updated Understanding of the Causes of Cancer, and a New Theoretical Perspective of Combinational Cancer Therapies, a Hypothesis. DNA Cell Biol. 2022, 41, 342–355. [Google Scholar] [CrossRef]
- Debela, D.T.; Muzazu, S.G.; Heraro, K.D.; Ndalama, M.T.; Mesele, B.W.; Haile, D.C.; Kitui, S.K.; Manyazewal, T. New approaches and procedures for cancer treatment: Current perspectives. SAGE Open Med. 2021, 9, 20503121211034366. [Google Scholar] [CrossRef]
- Ebru, Y. Analysis of the Essential Oils of two Hypericum species H. Lanuginosum var. lanuginosum Lam. and H. perforatum L. from Turkey. Hacettepe J. Biol. Chem. 2016, 44, 29–34. [Google Scholar]
- Zhang, R.; Ji, Y.; Zhang, X.; Kennelly, E.J.; Long, C. Ethnopharmacology of Hypericum species in China: A comprehensive review on ethnobotany, phytochemistry and pharmacology. J. Ethnopharmacol. 2020, 254, 112686. [Google Scholar] [CrossRef]
- Ji, Y.-Y.; Adam, N.; Edward, K.J.; Long, C.-L. Chemical constituents of Hypericum stellatum and their antioxidant bioactivities. Zhongguo Zhong Yao Za Zhi = Zhongguo Zhongyao Zazhi = China J. Chin. Mater. Med. 2018, 43, 3701–3707. [Google Scholar]
- Feinbrun-Dothan, N. Flora Palaestina; Academy of Sciences and Human: Jerusalem, Israel, 1986; Volume 4. [Google Scholar]
- Mahomoodally, M.F.; Zengin, G.; Zheleva-Dimitrova, D.; Mollica, A.; Stefanucci, A.; Sinan, K.I.; Aumeeruddy, M.Z. Metabolomics profiling, bio-pharmaceutical properties of Hypericum lanuginosum extracts by in vitro and in silico approaches. Ind. Crops Prod. 2019, 133, 373–382. [Google Scholar] [CrossRef]
- Odabas, M.S.; Radusiene, J.; Ivanauskas, L.; Jakstas, V.; Camas, N.; Kayikci, S. Secondary metabolites in Hypericum species and their distribution in different plant parts. Žemdirbystė 2016, 103, 193–198. [Google Scholar] [CrossRef] [Green Version]
- Dias, D.A.; Urban, S.; Roessner, U. A historical overview of natural products in drug discovery. Metabolites 2012, 2, 303–336. [Google Scholar] [CrossRef] [Green Version]
- Kumar, S.; Pandey, A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013, 2013, 162750. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.I.; Hayat, M.Q.; Tahir, M.; Mansoor, Q.; Ismail, M.; Keck, K.; Bates, R.B. Pharmacologically active flavonoids from the anticancer, antioxidant and antimicrobial extracts of Cassia angustifolia Vahl. BMC Complement. Med. Ther. 2016, 16, 460. [Google Scholar] [CrossRef] [Green Version]
- Tungmunnithum, D.; Thongboonyou, A.; Pholboon, A.; Yangsabai, A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines 2018, 5, 93. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, Y.; Guo, L.; Qiu, Z.; Huang, L.; Qu, X. Differences in chemical constituents of Artemisia annua L from different geographical regions in China. PLoS ONE 2017, 12, e0183047. [Google Scholar] [CrossRef] [Green Version]
- Hutchings, M.I.; Truman, A.W.; Wilkinson, B. Antibiotics: Past, present and future. Curr. Opin. Microbiol. 2019, 51, 72–80. [Google Scholar] [CrossRef]
- Wintola, O.A.; Afolayan, A.J. The antibacterial, phytochemicals and antioxidants evaluation of the root extracts of Hydnora africanaThunb. used as antidysenteric in Eastern Cape Province, South Africa. BMC Complement. Med. Ther. 2015, 15, 307. [Google Scholar] [CrossRef] [Green Version]
- Medini, F.; Fellah, H.; Ksouri, R.; Abdelly, C. Total phenolic, flavonoid and tannin contents and antioxidant and antimicrobial activities of organic extracts of shoots of the plant Limonium delicatulum. J. Taibah Univ. Sci. 2014, 8, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Bell, K.; Ristovski-Slijepcevic, S. Cancer survivorship: Why labels matter. J. Clin. Oncol. 2013, 31, 409–411. [Google Scholar] [CrossRef]
- Buckner, C.; Lafrenie, R.; Dénommée, J.; Caswell, J.; Want, D. Complementary and alternative medicine use in patients before and after a cancer diagnosis. Curr. Oncol. 2018, 25, 275–281. [Google Scholar] [CrossRef] [Green Version]
- Baliyan, S.; Mukherjee, R.; Priyadarshini, A.; Vibhuti, A.; Gupta, A.; Pandey, R.P.; Chang, C.-M. Determination of Antioxidants by DPPH Radical Scavenging Activity and Quantitative Phytochemical Analysis of Ficus religiosa. Molecules 2022, 27, 1326. [Google Scholar] [CrossRef]
- Al-Rimawi, F.; Jaradat, N.; Qneibi, M.; Hawash, M.; Emwas, N. Free radicals and enzymes inhibitory potentials of the traditional medicinal plant Echium angustifolium. Eur. J. Integr. Med. 2020, 38, 101196. [Google Scholar] [CrossRef]
- Alves, M.J.; Ferreira, I.C.; Froufe, H.J.; Abreu, R.; Martins, A.; Pintado, M. Antimicrobial activity of phenolic compounds identified in wild mushrooms, SAR analysis and docking studies. J. Appl. Microbiol. 2013, 115, 346–357. [Google Scholar] [CrossRef]
- Jaradat, N.A.; Abualhasan, M. Comparison of phytoconstituents, total phenol contents and free radical scavenging capacities between four Arum species from Jerusalem and Bethlehem. Pharm. Sci. 2016, 22, 120. [Google Scholar] [CrossRef] [Green Version]
- Sun, B.; Ricardo-da-Silva, J.M.; Spranger, I. Critical factors of vanillin assay for catechins and proanthocyanidins. J. Agric. Food Chem. 1998, 46, 4267–4274. [Google Scholar] [CrossRef]
- Jaradat, N.; Hussen, F.; Al Ali, A. Preliminary phytochemical screening, quantitative estimation of total flavonoids, total phenols and antioxidant activity of Ephedra alata Decne. J. Mater. Environ. Sci 2015, 6, 1771–1778. [Google Scholar]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [Green Version]
- Hegazy, M.G.; Imam, A.M.; Abdelghany, B.E. Evaluation of cytotoxic and anticancer effect of Orobanche crenata methanolic extract on cancer cell lines. Tumor Biol. 2020, 42, 1010428320918685. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).