Physicochemical Properties of a New Green Honey from Banggi Island, Sabah
Abstract
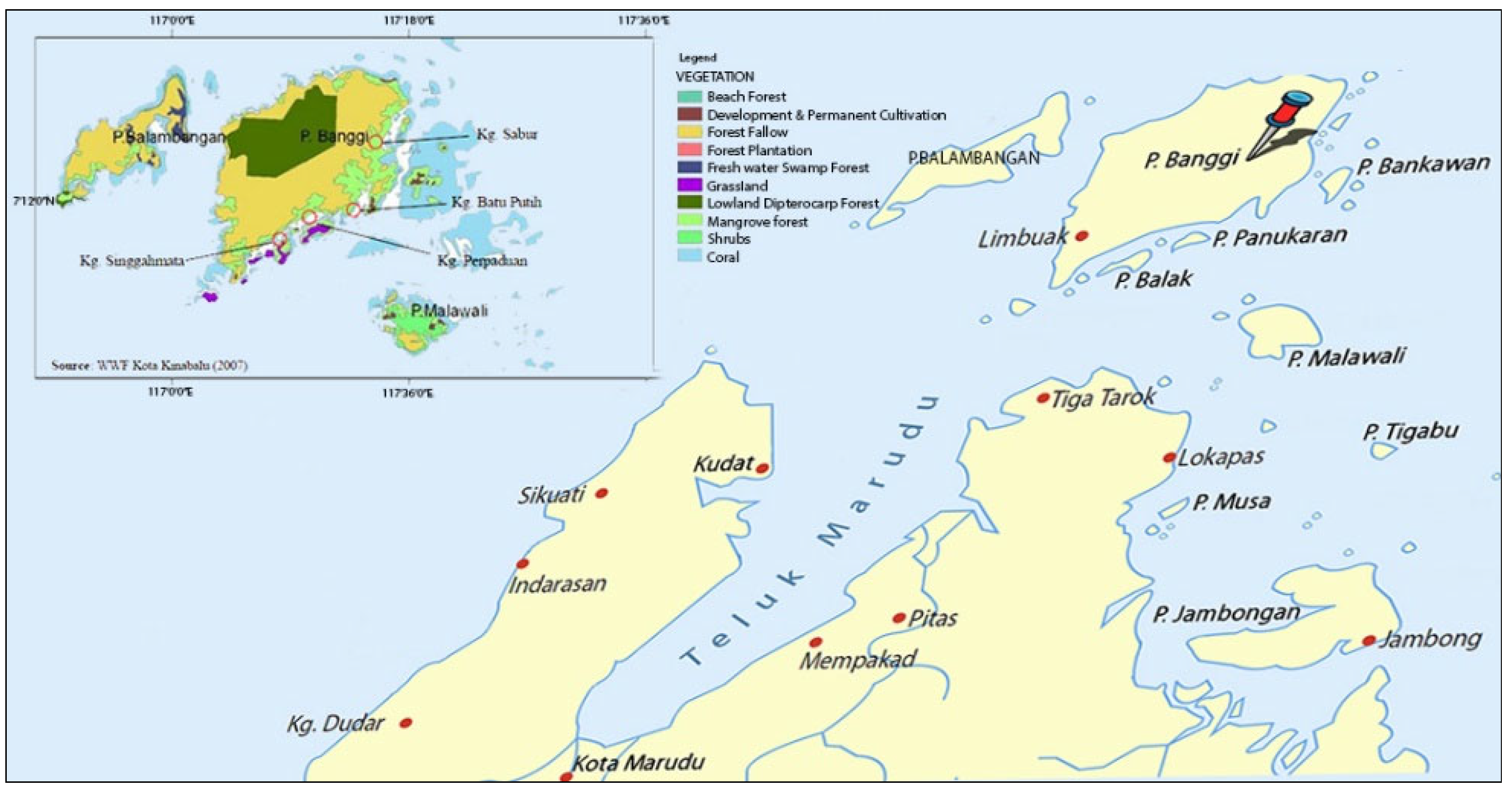
:1. Introduction
2. Results and Discussions
2.1. Physicochemical Properties
- A.
- Moisture Content
- B.
- pH
- C.
- Free acidity
- D.
- Ash content
- E.
- Electroconductivity
- F.
- Hydroxymethylfurfural (HMF)
- G.
- Total Phenolic Content (TPC)
- H.
- Total Flavonoid Content (TFC)
- I.
- TDPPH
- J.
- Chlorophyll
2.2. Sugar Content
2.3. Proteins and Amino Acids
2.4. Heavy Metals
| Type of Honeys | Raw Green | Processed Green | Raw Tualang | Tualang [4] | Gelam [70] | Kelulut [4] | Manuka |
|---|---|---|---|---|---|---|---|
| Total protein content (mg/kg) | 4000 ± 18 | 2000 ± 17 | 6000 ± 10 | 399.81 ± 0.10 | 644.08 ± 0.17 | 479.90 ± 0.24 | 1100 ± 0.31 |
| L–alanine (mg/kg) | ND | ND | ND | NA | 35.96 ± 0.26 | NA | 28.32 ± 0.20 |
| L–arginine (mg/kg) | ND | ND | 685.2 ± 0.26 | NA | ND | NA | 8.86 ± 0.23 |
| L–asparigine (mg/kg) | ND | ND | ND | NA | ND | NA | 19.15 ± 0.22 |
| L–aspartic acid (mg/kg) | 37.8 ± 0.25 | ND | ND | NA | ND | NA | 19.15 ± 0.20 |
| L–cysteine (mg/kg) | ND | ND | 392.8 | NA | 33.01 ± 0.22 | NA | ND |
| L–glutamic acid (mg/kg) | 36.1 ± 0.26 | 10.1 | ND | NA | 29.77 ± 0.23 | NA | 13.34 ± 0.22 |
| L–glutamine (mg/kg) | ND | ND | ND | NA | 29.32 ± 0.26 | NA | 13.34 ± 0.21 |
| L–glycine (mg/kg) | 82.8 ± 0.20 | 106.6 ± 0.21 | ND | NA | ND | NA | 11.58 ± 0.11 |
| L–histidine (mg/kg) | 57.1 ± 0.21 | ND | ND | NA | 17.82 ± 0.22 | NA | 16.13 ± 0.19 |
| L–isoleucine (mg/kg) | ND | ND | ND | NA | 6.38 ± 0.26 | NA | 8.36 ± 0.11 |
| L–leucine (mg/kg) | 125.8 ± 0.29 | 30.0 ± 0.20 | 403.7 ± 0.27 | NA | 44.50 ± 0.29 | NA | 7.68 ± 0.12 |
| L–lysine (mg/kg) | ND | 188.0 ± 0.23 | 172.9 ± 0.22 | NA | 14.67 ± 0.27 | NA | 40.86 ± 0.24 |
| L–methionine (mg/kg) | ND | ND | 137.9 ± 0.33 | NA | 19.49 ± 0.22 | NA | 0.67 ± 0.16 |
| L–phenylalanine (mg/kg) | ND | ND | 107.2 ± 0.36 | NA | 237.37 ± 0.22 | NA | 77.98 ± 0.36 |
| L–proline (mg/kg) | ND | ND | 1386.9 ± 0.44 | NA | 48.91 ± 0.19 | NA | 616.37 ± 0.21 |
| L–serine (mg/kg) | ND | ND | ND | NA | 19.07 ± 0.11 | NA | 21.60 ± 0.26 |
| L–threonine (mg/kg) | ND | ND | ND | NA | 7.07 ± 0.13 | NA | 9.23 ± 0.28 |
| L–tryptophan (mg/kg) | 842.1 ± 0.33 | 723.6 ± 0.41 | 131.0 ± 0.32 | NA | 19.05 ± 0.12 | NA | 3.33 ± 0.11 |
| L–tyrosine (mg/kg) | ND | ND | ND | NA | 19.57 ± 0.18 | NA | 16.38 ± 0.21 |
| L–valine (mg/kg) | ND | ND | ND | NA | 5.07 ± 0.16 | NA | 11.36 ± 0.26 |
| Trans-4-hydroxyl-proline (mg/kg) | 2960 ± 0.56 | 1102.6 ± 0.36 | 2972.2 ± 0.46 | NA | 29.63 ± 0.23 | NA | 9.53 ± 0.11 |
2.5. Volatile Organic Compound (VOC)
3. Materials and Methods
3.1. Honey Sample
3.2. Materials
3.3. Physicochemical Properties
- A.
- Moisture Content
- B.
- Free acidity
- C.
- Ash content
- D.
- Electroconductivity (EC)
- E.
- Sugar Content
- F.
- Protein Content
- G.
- Hydroxymethylfurfural (HMF) Content
- H.
- Total Phenolic Content (TPC)
- I.
- Total Flavonoid Content (TFC)
- J.
- DPPH Radical Scavenging Activity
- K.
- Amino acid profiling
- L.
- Chlorophyll
- M.
- Heavy metals
- N.
- Volatile Organic Compound (Gas chromatography–mass spectrometry).
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Teh, L.; Cabandan, A.S.; Sumaila, U.R. The reef fisheries of Pulau Banggi, Sabah: A preliminary profile and assessment of ecological and socio-economic sustainability. Fish. Res. 2005, 76, 359–367. [Google Scholar] [CrossRef]
- Bertoncelj, J.; Dobersek, U.; Jamnik, M.; Golob, T. Evaluation of the phenolic content, antioxidant activity and colour of Slovenian honey. Food Chem. 2007, 105, 822–828. [Google Scholar] [CrossRef]
- Szabó, R.T.; Mezes, M.; Szalai, T.; Zajácz, E.; Weber, M. Colour identification of honey and methodical development of its instrumental measuring. Columella J. Agric. Environ. Sci. 2016, 3, 29–36. [Google Scholar] [CrossRef]
- Ismail, N.I. Characterisation of Malaysian Honeys and Electrochemical Detection of Gallotannin for Pure Honey Identification. Ph.D. Thesis, Universiti Teknologi Malaysia, Johor Bahru, Malaysia, 2017. [Google Scholar]
- Kamal, M.A.; Klein, P. Determination of sugars in honey by liquid chromatography. Saudi J. Biol. Sci. 2011, 18, 17–21. [Google Scholar] [CrossRef] [Green Version]
- Fakhlaei, R.; Selamat, J.; Khatib, A.; Razis, A.; Sukor, R.; Ahmad, S.; Babadi, A.A. The toxic impact of honey adulteration: A review. Foods 2020, 9, 1538. [Google Scholar] [CrossRef]
- Rachineni, K.; Rao Kakita, V.M.; Awasthi, N.P.; Shirke, V.S.; Hosur, R.V.; Chandra, S.S. Identifying type of sugar adulterants in honey: Combined application of NMR spectroscopy and supervised machine learning classification. Curr. Res. Food Sci. 2022, 5, 272–277. [Google Scholar] [CrossRef]
- Codex STAN 12-1981; Codex Alimentarius Commission. Revised Codex Standard for Honey. Codex Alimentarius: Rome, Italy, 2001.
- Smith, F.G. The sucrose content of Western Australian Honey. J. Apicul. Res. 1965, 4, 177–184. [Google Scholar] [CrossRef]
- European Union (EU). Council directive 2001/110/EC of 20 December 2001 relating honey. Off. J. Eur. Communities 2002, 45, 10–47. [Google Scholar]
- Terrab, A.; Recamales, A.F.; Hernanz, D.; Heredia, F.J. Characterisation of Spanish thyme honeys by their physicochemical characteristics and mineral contents. Food Chem. 2004, 88, 537–542. [Google Scholar] [CrossRef]
- Acquarone, C.; Buera, P.; Elizalde, B. Pattern of pH and electrical conductivity upon honey dilution as a complementary tool for discriminating geographical origin of honeys. Food Chem. 2007, 101, 695–703. [Google Scholar] [CrossRef]
- Anand, S.; Pang, E.; Livanos, G.; Mantri, N. Characterization of physico-chemical properties and antioxidant capacities of bioactive honey produced from Australian grown Agastache rugosa and its correlation with colour and poly-phenol content. Molecules 2018, 23, 108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moniruzzaman, M.; Khalil, M.I.; Sulaiman, S.A.; Gan, S.H. Physicochemical and antioxidant properties of Malaysian honeys produced by Apis cerana, Apis dorsata and Apis mellifera. BMC Comp. Altern. Med. 2013, 13, 43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chirife, J.; Zamora, M.C.; Motto, A. The correlation between water activity and % moisture in honey: Fundamental aspects and application to Argentine honeys. J. Food Eng. 2006, 72, 287–292. [Google Scholar] [CrossRef] [Green Version]
- Downey, G.; Hussey, K.; Kelly, J.D.; Walshe, T.F.; Martin, P.G. Preliminary contribution to the characterisation of artisanal honey produced on the island of Ireland by palynological and physico-chemical data. Food Chem. 2005, 91, 347–354. [Google Scholar] [CrossRef]
- Singh, I.; Singh, S. Honey moisture reduction and its quality. J. Food Sci. Tech. 2018, 55, 3861–3871. [Google Scholar] [CrossRef]
- Saidan, N.H.; Roslan, N.; Baharuddin, N.R.M.; Hamil, M.S.R.; Krishnan, K.T. Compliance of selected stingless bee honey in Kelantan according to Malaysian Standard (MS). Malays. Appl. Biol. 2020, 49, 187–192. [Google Scholar] [CrossRef]
- Bogdanov, S.; Jurendic, T.; Sieber, R.; Gallmann, P. Honey for nutrition and health: A review. J. Amer. Coll. Nutr. 2008, 27, 677–689. [Google Scholar] [CrossRef]
- Silva, T.M.S.; Evangelista-Rodrigues, A.; Camara, C.A.; da Silva, E.M.S.; dos Santos, F.D.R.; dos Santos, F.P.; da Silva, G.S.; de Novais, G.S. Phenolic compounds, melissopalynological, physicochemical analysis and antioxidant activity of jandaira (Melipona subnitida) honey. J. Food Compost. Anal. 2013, 29, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Chuttong, B.; Chanbang, Y.; Sringarm, K.; Burgett, M. Physicochemical profiles of stingless bee (Apidae: Meliponini) honey from South East Asia (Thailand). Food Chem. 2016, 192, 149–155. [Google Scholar] [CrossRef]
- Hermanns, R.; Mateescu, C.; Thrasyvoulou, A.; Tananaki, C.; Wagener, F.A.D.T.G.; Cremers, N.A.J. Defining the standards for medical grade honey. J. Apicul. Res. 2020, 59, 125–135. [Google Scholar] [CrossRef]
- Suto, M.; Kawashima, H.; Nakamura, Y. Determination of organic acids in honey by liquid chromatography with tandem mass spectrometry. Food Anal. Methods 2020, 13, 2249–2257. [Google Scholar] [CrossRef]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Pharma. Res. 2017, 9, 121–127. [Google Scholar]
- Saxena, S.; Gautam, S.; Sharma, A. Physical, biochemical and antioxidant properties of some Indian honeys. Food Chem. 2010, 118, 391–397. [Google Scholar] [CrossRef]
- Majewska, E.; Druzynska, B.; Wołosiak, R. Determination of the botanical origin of honeybee honeys based on the analysis of their selected physicochemical parameters coupled with chemometric assays. Food Sci. Biotech. 2019, 28, 1307–1314. [Google Scholar] [CrossRef] [Green Version]
- Rysha, A.; Kastrati, G.; Biber, L.; Sadiku, V.; Rysha, A.; Zogaj, F.; Kabashi-Kastrati, E. Evaluating the physicochemical properties of some kosovo’s and imported honey samples. Appl. Sci. 2022, 12, 629. [Google Scholar] [CrossRef]
- Bouhlali, E.D.T.; Bammou, M.; Sellam, K.; El Midaoui, A.; Bourkhis, B.; Ennassir, J.; Alem, C.; Filali-Zegzouti, Y. Physicochemical properties of eleven monofloral honey samples produced in Morocco. Arab J. Basic Appl. Sci. 2019, 26, 476–487. [Google Scholar] [CrossRef] [Green Version]
- White, J.W.; Doner, L.W. Honey Composition and Properties: Beekeeping in the United States. In Agriculture Handbook No. 335; United States Department of Agriculture: Washington, DC, USA, 1980; pp. 82–91. [Google Scholar]
- Baloš, M.; Živkov, M.; Popov, N.; Vidaković, S.; Ljubojević, P.D.; Pelić, M.; Željko, M.; Jakšić, S. Electrical conductivity and acidity of honey. Arch. Vet. Medic. 2018, 11, 91–101. [Google Scholar] [CrossRef]
- Mato, I.; Huidobro, J.F.; Simal-Lozano, J.; Sancho, M.T. Analytical methods for the determination of organic acids in honey. Crit. Rev. Anal. Chem. 2006, 36, 3–11. [Google Scholar] [CrossRef]
- Shamsudin, S.; Jinap, S.; Maimunah, S.; Shamsul-Bahari, A.R.; Nuzul, N.J.; Zakbah, M.; Khatib, A. Influence of origins and bee species on physicochemical, antioxidant properties and botanical discrimination of stingless bee honey. Int. J. Food Prop. 2019, 22, 238–264. [Google Scholar] [CrossRef] [Green Version]
- Karabagias, I.K.; Badeka, A.V.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Botanical discrimination of Greek unifloral honeys with physico-chemical and chemometric analyses. Food Chem. 2014, 165, 181–190. [Google Scholar] [CrossRef]
- Tomczyk, M.; Tarapatskyy, M.; Dżugan, M. The influence of geographical origin on honey composition studied by Polish and Slovak honeys. Czech J. Food Sci. 2019, 37, 232–238. [Google Scholar] [CrossRef]
- Kaskoniene, V.; Venskutonis, P.; Ceksteryte, V. Carbohydrate composition and electrical conductivity of different origin honeys from Lithuania. LWT Food Sci. Tech. 2010, 43, 801–807. [Google Scholar] [CrossRef]
- White, J.W. The Role of HMF and diastase assays in honey quality evaluation. Bee World 1994, 75, 104–117. [Google Scholar] [CrossRef]
- Eshete, Y.; Eshete, T. A review on the effect of processing temperature and time duration on commercial honey quality. Madridge J. Food Technol. 2019, 4, 158–162. [Google Scholar] [CrossRef] [Green Version]
- Ranneh, Y.; Faisal, A.; Maryam, Z.; Abdah, M.A.; Hasiah, A.H.; Huzwah, K. Malaysian stingless bee and Tualang honeys: A comparative characterization of total antioxidant capacity and phenolic profile using liquid chromatography-mass spectrometry. LWT 2018, 89, 1–9. [Google Scholar] [CrossRef]
- Pant, P.; Pandey, S.; Dall′Acqua, S. The influence of environmental conditions on secondary metabolites in medicinal plants: A literature review. Chem. Biodiv. 2021, 18, e2100345. [Google Scholar] [CrossRef]
- Tenore, G.C.; Ritieni, A.; Campiglia, P.; Novellino, E. Nutraceutical potential of monofloral honeys produced by the Sicilian black honeybees (Apis mellifera ssp. sicula). Food Chem. Toxicol. 2012, 50, 1955–1961. [Google Scholar] [CrossRef]
- Wabaidur, S.M.; Obbed, M.S.; Alothman, Z.A.; Alfaris, N.A.; Badjah-Hadj-Ahmed, A.Y.; Siddiqui, M.R.; Al-Tamimi, J.Z.; Al-Dayel, T.S. Total phenolic acids and flavonoid contents determination in Yemeni honey of various floral sources: Folin-Ciocalteu and spectrophotometric approach. Food Sci. Technol. 2020, 40, 647–652. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef] [Green Version]
- Olas, B. Honey and its phenolic compounds as an effective natural medicine for cardiovascular diseases in humans? Nutrients 2020, 12, 283. [Google Scholar] [CrossRef] [Green Version]
- Meda, A.; Lamien, C.E.; Romito, M.; Millogo, J.; Nacoulma, O.G. Determination of the total phenolic, flavonoid and proline contents in Burkina Fasan honey, as well as their radical scavenging activity. Food Chem. 2005, 91, 571–577. [Google Scholar] [CrossRef]
- Liu, S.; Dandan, L.; Guanliang, M.; Jiahui, H.; Min, T.; Xin, Z. Tracing the origin of honey products based on metagenomics and machine learning. Food Chem. 2022, 371, 131066. [Google Scholar] [CrossRef] [PubMed]
- Manyi-Loh, C.E.; Ndip, R.N.; Clarke, A.M. Volatile compounds in honey: A review on their involvement in aroma, botanical origin determination and potential biomedical activities. Int. J. Mol. Sci. 2011, 12, 9514–9532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarei, M.; Fazlara, A.; Tulabifard, N. Effect of thermal treatment on physicochemical and antioxidant properties of honey. Heliyon 2019, 5, e01894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becerril-Sánchez, A.L.; Quintero-Salazar, B.; Dublán-García, O.; Escalona-Buendía, H.B. Phenolic compounds in honey and their relationship with antioxidant activity, botanical origin, and color. Antioxidants 2021, 10, 1700. [Google Scholar] [CrossRef] [PubMed]
- Beretta, G.; Granata, P.; Ferrero, M.; Orioli, M.; Facino, R.M. Standardization of antioxidant properties of honey by a combination of spectrophotometric/fluorimetric assays and chemometrics. Anal. Chim. Acta 2005, 533, 185–191. [Google Scholar] [CrossRef]
- Floegel, A.; Kim, D.-O.; Chung, S.-J.; Koo, S.I.; Chun, O.K. Comparison of ABTS/DPPH assays to measure antioxidant capacity in popular antioxidant-rich US foods. J. Food Compos. Anal. 2011, 24, 1043–1048. [Google Scholar] [CrossRef]
- Escuredo, O.; Irina, D.; María, F.G.; Seijo, M.C. Contribution of botanical origin and sugar composition of honeys on the crystallization phenomenon. Food Chem. 2014, 149, 84–90. [Google Scholar] [CrossRef]
- Tornuk, F.; Karaman, S.; Ozturk, I.; Toker, O.S.; Tastemur, B.; Sagdic, O.; Dogan, M.; Kayacier, A. Quality characterization of artisanal and retail Turkish blossom honeys: Determination of physicochemical, microbiological, bioactive properties and aroma profile. Ind. Crops Prod. 2013, 46, 124–131. [Google Scholar] [CrossRef]
- da Silva, P.M.; Gauche, C.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Honey: Chemical composition, stability and authenticity. Food Chem. 2016, 196, 309–323. [Google Scholar] [CrossRef]
- Gallego-Picó, A.; Garcinuño-Martínez, R.M.; Fernández-Hernando, P. Chapter 20-Honey authenticity and traceability. Compre. Analy. Chem. 2013, 60, 511–541. [Google Scholar]
- Zae, T.K.; Azlan, A.; Sajak, A.A.B.A.; Hock, E.K.; Nyuk, L.C.; Noh, M.D.; Mustafa, K.N. Comparison of selected local honey with Manuka honey based on their nutritional and antioxidant properties. Food Res. 2020, 4, 205–213. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S.; Paduret, S.; Todosi, E. Rheological analysis of honeydew honey adulterated with glucose, fructose, inverted sugar, hydrolysed inulin syrup and malt wort. LWT 2018, 95, 1–8. [Google Scholar] [CrossRef]
- Sohaimy, S.A.E.; Masry, S.H.D.; Shehata, M.G. Physicochemical characteristics of honey from different origins. Annals Agric. Sci. 2015, 60, 279–287. [Google Scholar] [CrossRef] [Green Version]
- Mohd Kamal, D.A.; Ibrahim, S.F.; Kamal, H.; Kashim, M.I.A.M.; Mokhtar, M.H. Physicochemical and medicinal properties of tualang, gelam and kelulut honeys: A comprehensive review. Nutrients 2021, 13, 197. [Google Scholar] [CrossRef]
- Chalcoff, V.R.; Aizen, M.A.; Galetto, L. Nectar concentration and composition of 26 species from the temperate forest of South America. Annal. Botany 2006, 97, 413–421. [Google Scholar] [CrossRef] [Green Version]
- Bogdanov, S. Book of Honey-Honey Composition. In Bee Product Science. 2019:1–9. Available online: http://fantasticflavour.com/yahoo_site_admin/assets/docs/CompositionHoney.20105942.pdf (accessed on 26 July 2012).
- Czipa, N.; Borbély, M.; Győri, Z. Proline content of different honey types. Acta Alimn. 2012, 41, 26–32. [Google Scholar] [CrossRef]
- Sommano, S.R.; Bhat, F.M.; Wongkeaw, M.; Sriwichai, T.; Sunanta, P.; Chuttong, B.; Burgett, M. Amino acid profiling and chemometric relations of black dwarf honey and bee pollen. Frontiers Nutr. 2020, 7, 283. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Khare, P.; Nagar, H.K.; Raghuwanshi, N.; Srivastava, R. Hydroxyproline: A potential biochemical marker and its role in the pathogenesis of different diseases. Curr. Protein Pept. Sci. 2016, 17, 596–602. [Google Scholar] [CrossRef]
- Richard, D.M.; Dawes, M.A.; Mathias, C.W.; Acheson, A.; Hill-Kapturczak, N.; Dougherty, D.M. L-Tryptophan: Basic metabolic functions, behavioral research and therapeutic indications. Int. J. Tryp. Res. 2009, 2, 45–60. [Google Scholar] [CrossRef] [Green Version]
- Viana, L.R.; Tobar, N.; Busanello, E.N.B.; Marques, A.C.; de Oliveira, A.G.; Lima, T.I.; Machado, G.; Castelucci, B.G.; Ramos, C.D.; Brunetto, S.Q.; et al. Leucine-rich diet induces a shift in tumour metabolism from glycolytic towards oxidative phosphorylation, reducing glucose consumption and metastasis in Walker-256 tumour-bearing rats. Sci. Rep. 2019, 9, 15529. [Google Scholar] [CrossRef] [PubMed]
- Holeček, M. Histidine in health and disease: Metabolism, physiological importance, and use as a supplement. Nutrients 2020, 12, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Danbolt, N.C. Glutamate as a neurotransmitter in the healthy brain. J. Neural Transmiss. 2014, 121, 799–817. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Yin, Y.-L.; Li, D.; Woo, K.S.; Wu, G. Amino acids and immune function. British J. Nutr. 2007, 98, 237. [Google Scholar] [CrossRef] [Green Version]
- Altun, K.S.; Dinç, H.; Paksoy, N.; Temamoğulları, F.K.; Savrunlu, M. Analyses of mineral content and heavy metal of honey samples from south and east region of turkey by using ICP-MS. Int. J. Anal. Chem. 2017, 2017, 6391454. [Google Scholar]
- Shamsudin, S.; Selamat, J.; Sanny, M.; AR, S.B.; Jambari, N.N.; Khatib, A. A Comparative Characterization of Physicochemical and Antioxidants Properties of Processed Heterotrigona itama Honey from Different Origins and Classification by Chemometrics Analysis. Molecules 2019, 24, 3898. [Google Scholar] [CrossRef] [Green Version]
- Nurul Syazana, M.S.; Gan, S.H.; Halim, A.S. Analysis of volatile compounds of Malaysian Tualang (Koompassia Excelsa) honey using gas chromatography mass spectrometry. Afr. J. Tradit. Compl. Altern. Med. 2013, 10, 180–188. [Google Scholar]
- Bauer, K.; Garbe, D.; Surburg, H. Common Fragrance and Flavor Materials Preparation, Properties and Uses, 4th ed.; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Sun, J. D-Limonene: Safety and clinical applications. Altern. Med. Rev. 2007, 12, 259–264. [Google Scholar]
- de Souza, M.C.; Vieira, A.J.; Beserra, F.P.; Pellizzon, C.H.; Nóbrega, R.H.; Rozza, A.L. Gastroprotective effect of limonene in rats: Influence on oxidative stress, inflammation and gene expression. Phytomedicine 2019, 53, 37–42. [Google Scholar] [CrossRef]
- Mizrahi, B.; Shapira, L.; Domb, A.J.; Houri-Haddad, Y. Citrus oil and MgCl2 as antibacterial and anti-inflammatory agents. J. Periodontol. 2006, 77, 963–968. [Google Scholar] [CrossRef]
- Jing, L.; Zhang, Y.; Fan, S.; Gu, M.; Guan, Y.; Lu, X.; Huang, C.; Zhou, Z. Preventive and ameliorating effects of citrus D-limonene on dyslipidemia and hyperglycemia in mice with high-fat diet-induced obesity. Eur. J. Pharmacol. 2012, 715, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Dar, B.A.; Sofi, S.N.; Bhat, B.A.; Qurishi, M.A. Foeniculum vulgare: A comprehensive review of its traditional use, phytochemistry, pharmacology, and safety. Arab. J. Chem. 2016, 9, S1574–S1583. [Google Scholar] [CrossRef] [Green Version]
- Badgujar, S.B.; Patel, V.V.; Bandivdekar, A.H. Foeniculum vulgare Mill: A review of its botany, phytochemistry, pharmacology, contemporary application, and toxicology. BioMed Res. Int. 2014, 2014, 842674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baroni, M.V.; Nores, M.L.; Díaz, M.P.; Chiabrando, G.A.; Fassano, J.P.; Costa, C.; Wunderlin, D.A. Determination of volatile organic compound patterns characteristics of five unifloral honey by solid-phase microextraction—Gas chromatography-mass spectrometry coupled to chemometrics. J. Agric. Food Chem. 2006, 54, 7235–7241. [Google Scholar] [CrossRef] [PubMed]
- Stanimirova, I.; Üstün, B.; Cajka, T.; Riddelova, K.; Hajslova, J.; Buydens, L.M.C.; Walczak, B. Tracing the geographical origin of honeys based on volatile compounds profiles assessment using patterns recognition techniques. Food Chem. 2010, 118, 171–176. [Google Scholar] [CrossRef]
- Sesta, G.; Lusco, L. Refractometric determination of water content in royal jelly. Apidologie 2008, 3, 225–232. [Google Scholar] [CrossRef] [Green Version]
- International Honey Commission (IHC). Harmonized Methods of the International Honey Commission. 2009. Available online: https://www.ihc-platform.net/ihcmethods2009.pdf (accessed on 16 April 2022).
- AOAC Official Method 998.12; C-4 Plant Sugars in Honey. International Standard Stable Carbon Isotope Ratio Method. AOAC: Rockville, MA, USA, 2005.
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 29, 10–18. [Google Scholar]
- Daves, J.W. Current Protocols in Food Analytical Chemistry; John. Wiley & Sons: Hoboken, NJ, USA, 2003; pp. 1073–1080. [Google Scholar]
- Alothman, M.; Bhat, R.; Karim, A.A. Antioxidant capacity and phenolic content of selected tropical fruits from Malaysia, extracted with different solvents. Food Chem. 2009, 115, 785–788. [Google Scholar] [CrossRef]
- Ferreira, I.C.F.R.; Aires, E.; Barreira, J.C.M.; Estevinho, L.M. Antioxidant activity of Portuguese honey samples: Different contributions of the entire honey and phenolic extract. Food Chem. 2009, 114, 1438–1443. [Google Scholar] [CrossRef]
- Kowalski, S.; Kopuncová, M.; Ciesarová, Z.; Kukurová, K. Free amino acids profile of Polish and Slovak honeys based on LC–MS/MS method without the prior derivatisation. J. Food Sci. Tech. 2017, 54, 3716–3723. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K.; Buschmann, C. Chlorophylls and carotenoids: Measurement and characterization by UV-VIS spectroscopy. Curr. Protoc. Food Anal. Chem. 2001, 1, F4.3.1–F4.3.8. [Google Scholar] [CrossRef]

| Honey Types | Moisture (g/100 g) | pH | Free Acidity (meq/kg) | Ash (g/100 g) | Electric Conductivity (mS/cm) | HMF (mg/kg) | Total Phenolic Content (mg GAE/100 g) | Total Flavonoid Content (mg QE/100 g) | Antioxidant (DPPH) (%) | Chlorophyll (mg/kg) |
|---|---|---|---|---|---|---|---|---|---|---|
| Raw green | 22.5 ± 0.55 | 3.4 ± 0.03 | 33.0 ± 6.145 | 0.08 ± 0.01 | 1.93 ± 0.02 | 0.01 ± 0.14 | 18.95 ± 0.08 | 7.6 ± 0.01 | 2.3 ± 0.02 | 539 ± 1.04 |
| Processed green | 12.7 ± 0.22 | 3.7 ± 0.02 | 28.0 ± 5.14 | 4.4 ± 0.01 | 0.85 ± 0.05 | 0.6 ± 0.25 | 15.57 ± 0.11 | 4.58 ± 0.01 | 2.0 ± 0.02 | 41 ± 0.44 |
| Raw tualang | 18.3 ± 0.21 | 3.5 ± 0.01 | 29.5 ± 6.14 | 1.3 ± 0.02 | 5.06 ± 0.02 | 6.4 ± 0.11 | 17.86 ± 0.02 | 6.92 ± 0.02 | 2.4 ± 0.03 | ND |
| Tualang [4] | 23.56 ± 0.32 | 3.2 ± 0.04 | 76.2 ± 4.14 | 0.15 ± 0.01 | 0.83 ± 0.01 | 46.54 ± 0.01 | 31.84 ± 0.76 | 40.5 ± 1.6 | 30.83 ± 0.01 | ND |
| Gelam [4] | 25.79 ± 0.21 | 3.3 ± 0.02 | 58.5 ± 4.14 | 0.28 ± 0.01 | 0.43 ± 0.01 | 45.42 ± 0.02 | 17.73 ± 0.86 | 58.3 ± 1.6 | 76.29 ± 0.02 | ND |
| Kelulut [4] | 28.3 ± 0.12 | 3.0 ± 0.03 | 448.10 ± 4.14 | 0.25 ± 0.01 | 1.05 ± 0.01 | 45.93 ± 4.72 | 42.6 ± 1.4 | 52.3 ± 3.4 | 71.93 ± 0.01 | ND |
| Manuka [13] | 19.5 ± 0.3 | 4.03 ± 0.2 | 50.0 ± 5.14 | 0.30 ± 0.01 | 0.53 ± 0.01 | 63.42 ± 0.01 | 1288.0 ± 102.8 | 37.64 ± 7.2 | 18.69 ± 0.9 | ND |
| Honey Types | Total Sugar (g/100 g) | Sucrose (g/100 g) | Glucose (g/100 g) | Fructose (g/100 g) | Maltose (g/100 g) |
|---|---|---|---|---|---|
| Raw green | 39.30 ± 0.02 | 14.55 ± 0.01 | 23.70 ± 0.05 | 11.35 ± 0.02 | <10 |
| Processed green | 52.84 ± 0.07 | 27.72 ± 0.02 | 17.52 ± 0.03 | 19.56 ± 0.03 | <10 |
| Raw tualang | 38.60 ± 0.02 | ND | 20.80 ± 0.01 | 26.9 ± 0.01 | ND |
| Tualang [55] | 63.40 ± 0.01 | 1.48 ± 0.01 | 28.79 ± 0.08 | 31.73 ± 0.14 | 0 |
| Gelam [55] | 62.74 ± 0.06 | 1.13 ± 0.03 | 29.17 ± 0.03 | 30.96 ± 0.03 | 1.48 ± 0.01 |
| Kelulut [55] | 62.70 ± 0.12 | 0.93 ± 0.04 | 29.72 ± 0.06 | 30.53 ± 0.01 | 1.52 ± 0.06 |
| Manuka [55] | 64.19 ± 2.69 | 1.32 ± 0.01 | 27.81 ± 1.20 | 34.14 ± 1.47 | 0.90 ± 0.05 |
| Type of Honey | Arsenic (mg/kg) | Lead (mg/kg) | Nickel (mg/kg) | Cadmium (mg/kg) | Copper (mg/kg) | Cobalt (mg/kg) |
|---|---|---|---|---|---|---|
| Raw green | <0.5 | <0.5 | <0.5 | ND | ND | ND |
| Processed green | <0.5 | <0.5 | <0.5 | ND | ND | ND |
| Tualang [14] | 0.062 ± 0.007 | 0.183 ± 0.007 | ND | ND | 1.25 ± 0.63 | 0.033 ± 0.002 |
| Gelam [14] | 0.064 ± 0.005 | 0.777 ± 0.012 | ND | 0.05 ± 0.07 | 2.21 ± 0.02 | 0.082 ± 0.005 |
| Kelulut [14] | 0.027 ± 0.001 | 0.691 ± 0.002 | ND | 0.78 ± 0.04 | ND | ND |
| Manuka [14] | ND | ND | ND | ND | ND | ND |
| Volatile Organic Compound | Processed Green Honey | Tualang Raw Honey | Tualang [71] |
|---|---|---|---|
| Ethyl Acetate (CH3COOC2H5) | Present | Present | Absent |
| Propanamide, N,N-dimethyl (C5H11NO) | Present | Absent | Absent |
| D-Limonene (C10H16) | Present | Absent | Absent |
| 1,4-Cyclohexadiene, 1-methyl-4-(1-methylethyl) (C10H16) | Present | Absent | Absent |
| Cyclohexene, 1-methyl-4-(1-methylethylidene) (C10H16) | Present | Absent | Absent |
| Benzene, (2-methyl-1-propenyl)-o-Isopropenyltoluene (C10H12) | Present | Absent | Absent |
| Cyclotetradecane (C14H28) | Absent | Absent | Present |
| Hexadecanoctadecane (C16H34) | Absent | Absent | Present |
| Cycloeicosane (C20H40) | Absent | Absent | Present |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rajindran, N.; Wahab, R.A.; Huda, N.; Julmohammad, N.; Shariff, A.H.M.; Ismail, N.I.; Huyop, F. Physicochemical Properties of a New Green Honey from Banggi Island, Sabah. Molecules 2022, 27, 4164. https://doi.org/10.3390/molecules27134164
Rajindran N, Wahab RA, Huda N, Julmohammad N, Shariff AHM, Ismail NI, Huyop F. Physicochemical Properties of a New Green Honey from Banggi Island, Sabah. Molecules. 2022; 27(13):4164. https://doi.org/10.3390/molecules27134164
Chicago/Turabian StyleRajindran, Nanthini, Roswanira Abdul Wahab, Nurul Huda, Norliza Julmohammad, Amir Husni Mohd Shariff, Norjihada Izzah Ismail, and Fahrul Huyop. 2022. "Physicochemical Properties of a New Green Honey from Banggi Island, Sabah" Molecules 27, no. 13: 4164. https://doi.org/10.3390/molecules27134164
APA StyleRajindran, N., Wahab, R. A., Huda, N., Julmohammad, N., Shariff, A. H. M., Ismail, N. I., & Huyop, F. (2022). Physicochemical Properties of a New Green Honey from Banggi Island, Sabah. Molecules, 27(13), 4164. https://doi.org/10.3390/molecules27134164









