The Signaling Pathways and Targets of Natural Compounds from Traditional Chinese Medicine in Treating Ischemic Stroke
Abstract
:1. Introduction
2. The Signaling Pathways of Active Compounds in the Treatment of IS
2.1. JAK/STAT Signaling Pathway
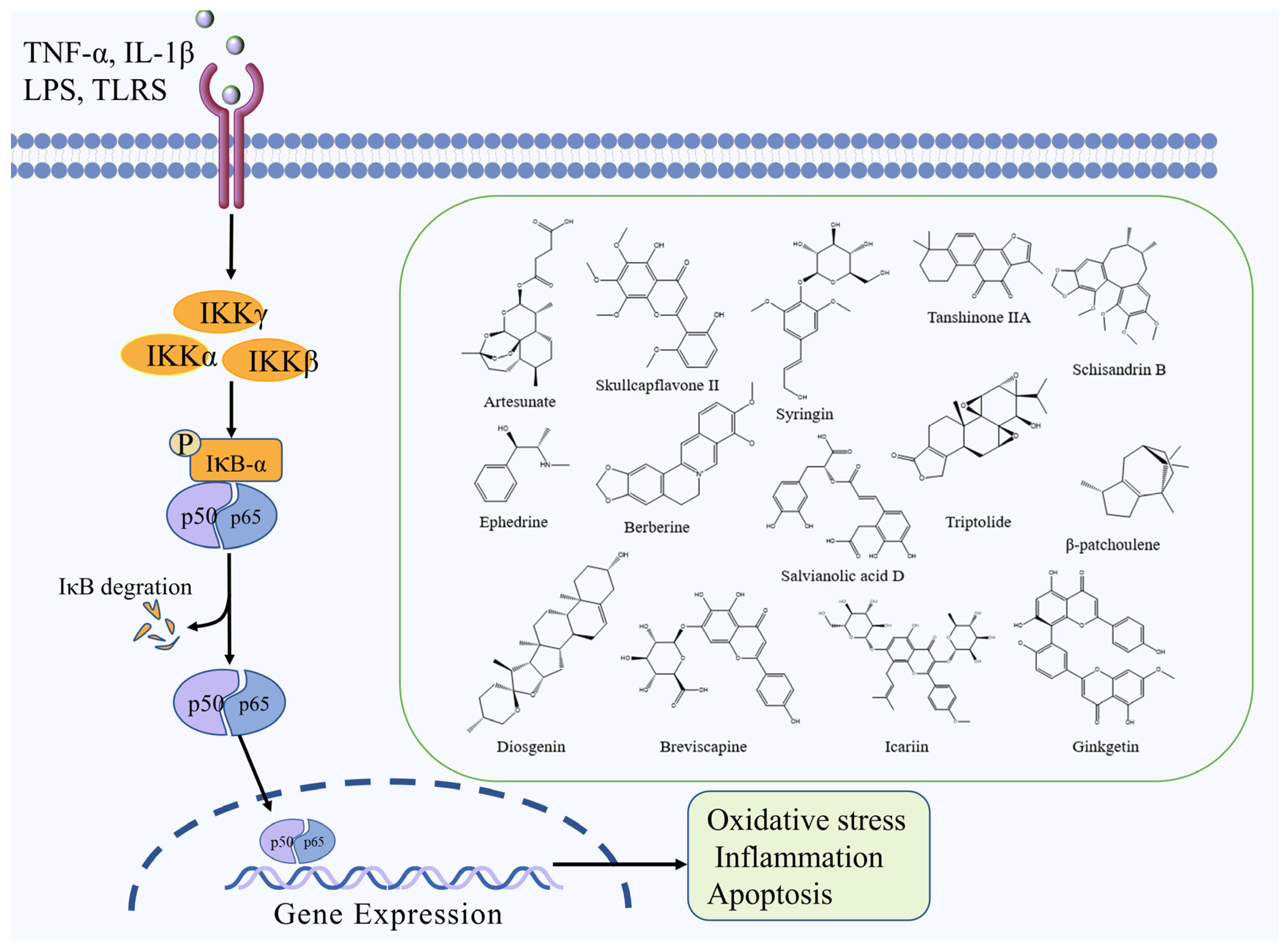
2.2. NF-κB Signaling Pathway
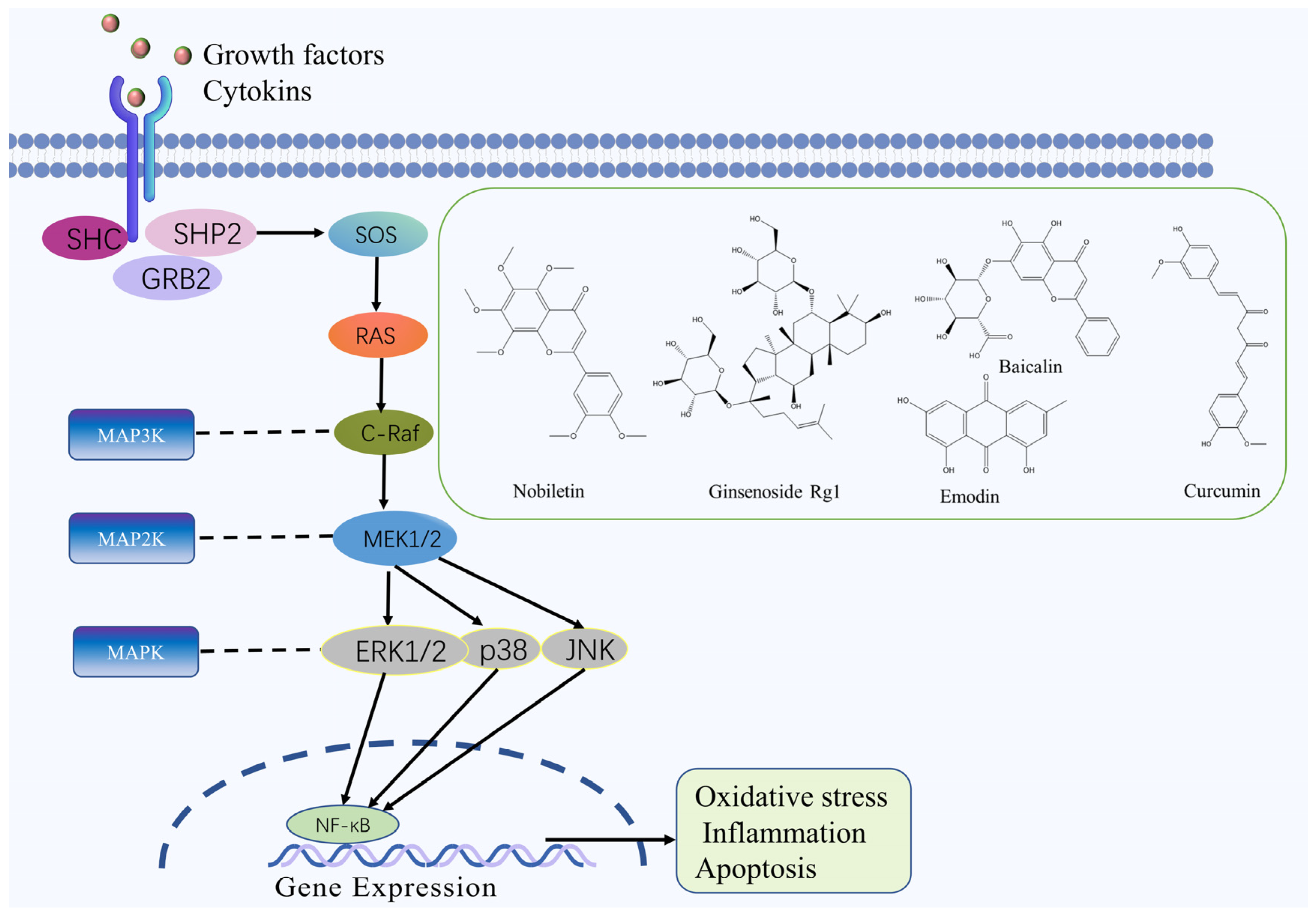
2.3. MAPK Signaling Pathway
2.4. Notch Signaling Pathway
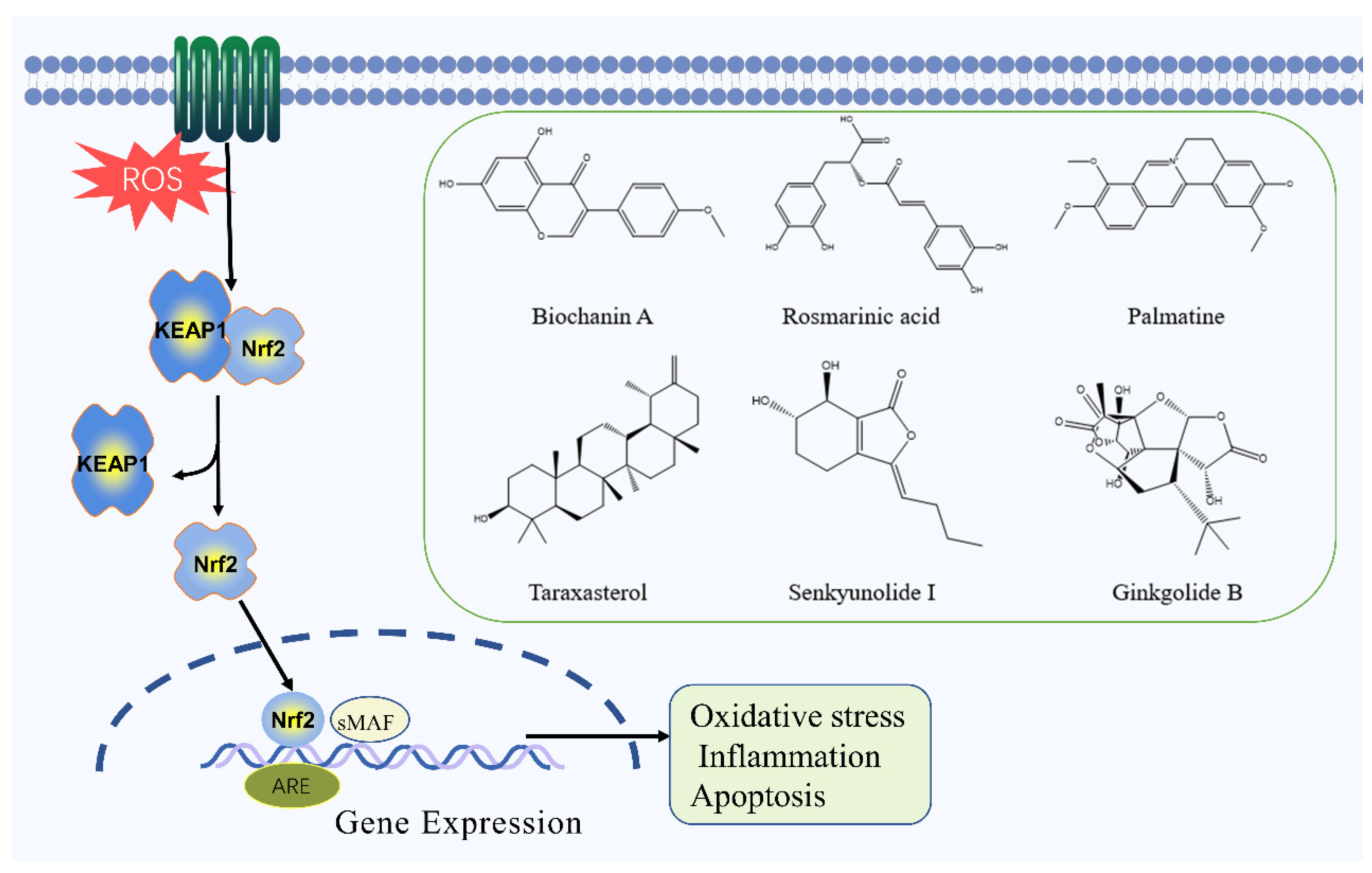
2.5. Nrf2 Signaling Pathway
2.6. PI3K/Akt Signaling Pathway
3. The Target Protein of Natural Compounds in the Treatment of IS
3.1. SIRT1
3.2. MMP9
3.3. TLR4
3.4. HIF-α
4. Conclusions and Future Aspects
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| IS | Ischemic stroke |
| r-tPA | Recombinant tissue plasminogen activator |
| FDA | Food and drug administration |
| US | United States |
| BBB | Blood–brain barrier |
| TCM | Traditional chinese medicine |
| CIRI | Cerebral ischemia/reperfusion injury |
| ROS | Reactive oxygen species |
| JAK | Janus Kinase |
| STAT | Signal Transducer And Activator Of Transcription |
| IL-1β | Interleukin-1β |
| TNF-α | Tumor necrosis factor-α |
| IL-6 | Interleukin-6 |
| ICAM-1α | Intercellular cell adhesion molecule-1 α |
| EPO | Erythropoietin |
| ECF | Epidermal Growth Factor |
| PDGF | Platelet-derivedgrowthfactorreceptor |
| MCAO | Middle Cerebral Artery Occlusion |
| NF-κB | Nuclear Factor Kappa-B |
| TLR4 | Toll Like Receptor 4 |
| FOXO3a | Forkhead box O3 |
| MPO | Myeloperoxidase |
| NLRP3 | NOD-like receptor protein 3 |
| HMGB1 | High Mobility Group Box 1 |
| MyD88 | Myeloiddifferentiationfactor88 |
| MAPK | Mitogen-Activated Protein Kinase |
| CytC | Cytochrome c |
| Bcl-2 | B-cell lymphoma-2 |
| Bax | Bcl2-associated x protein |
| JNK | Jun N-Terminal Kinase |
| ERK | Extracellular Signal-Regulated Kinase |
| CSL | CBF1/suppressor of hairless/Lag |
| NICD | Notch intracellular domain |
| HIF | Hypoxia-inducible factor |
| VEGF | Vascular endothelial growth factor |
| Nrf2 | Nuclear Factor E2-Related Factor 2 |
| NQO1 | Nad(p)h quinone oxidoreductase |
| HO-1 | Heme oxygenase-1 |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| GPX-Px | Glutathione peroxidase |
| GSH | Reduced glutathione |
| AMPK | Adenosine monophosphate |
| OGD/R | Oxygen-Glucose Deprivation/Re-Oxygenation |
| MDA | Malondialdehyde |
| PI3K | Phosphoinositide 3-kinase |
| Akt | Protein kinase B |
| mTOR | mammalian target of rapamycin |
| SIRT1 | Silent mating type information regulation 2 homolog-1. |
| MMP9 | Matrix Metalloproteinase 9 |
References
- Hankey, G.J. Stroke. Lancet 2017, 389, 641–654. [Google Scholar] [CrossRef]
- Benjamin, E.J.; Muntner, P.; Alonso, A. Heart Disease and Stroke Statistics-2019 Update: A Report from the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef] [PubMed]
- Johnson, W.; Onuma, O.; Owolabi, M.; Sachdev, S. Stroke: A global response is needed. Bull. WHO 2016, 94, 634. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Huang, Z. Effect of Comprehensive Cerebral Protection Program on Cerebral Oxygen Metabolism and Vascular Endothelial Function in Elderly Patients with Acute Cerebral Infarction. Iran. J. Public Health 2019, 48, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Hong, P.; Li, F.X.; Gu, R.N.; Fang, Y.Y.; Lai, L.Y.; Wang, Y.W.; Tao, T.; Xu, S.Y.; You, Z.J.; Zhang, H.F. Inhibition of NLRP3 Inflammasome Ameliorates Cerebral Ischemia-Reperfusion Injury in Diabetic Mice. Neural Plast. 2018, 2018, 9163521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seners, P.; Turc, G.; Maïer, B.; Mas, J.L.; Oppenheim, C.; Baron, J.C. Incidence and Predictors of Early Recanalization After Intravenous Thrombolysis: A Systematic Review and Meta-Analysis. Stroke 2016, 47, 2409–2412. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.H.; Yeh, J.C.; Tsai, C.H.; Yang, J.T.; Lou, S.L.; Seak, C.J.; Wang, C.Y.; Wei, K.C.; Liu, H.L. Improved thrombolytic effect with focused ultrasound and neuroprotective agent against acute carotid artery thrombosis in rat. Sci. Rep. 2017, 7, 1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, L.; Xiong, X.; Wu, X.; Ye, Y.; Jian, Z.; Zhi, Z.; Gu, L. Targeting Oxidative Stress and Inflammation to Prevent Ischemia-Reperfusion Injury. Front. Mol. Neurosci. 2020, 13, 28. [Google Scholar] [CrossRef] [Green Version]
- Huang, P.; Wan, H.; Shao, C.; Li, C.; Zhang, L.; He, Y. Recent Advances in Chinese Herbal Medicine for Cerebral Ischemic Reperfusion Injury. Front. Pharmacol. 2021, 12, 688596. [Google Scholar] [CrossRef] [PubMed]
- Skała, E.; Sitarek, P.; Różalski, M.; Krajewska, U.; Szemraj, J.; Wysokińska, H.; Śliwiński, T. Antioxidant and DNA Repair Stimulating Effect of Extracts from Transformed and Normal Roots of Rhaponticum carthamoides against Induced Oxidative Stress and DNA Damage in CHO Cells. Oxid. Med. Cell. Longev. 2016, 2016, 5753139. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Li, H.; Fang, F.; Deng, X.; Ma, S. Astragaloside IV attenuates cognitive impairments induced by transient cerebral ischemia and reperfusion in mice via anti-inflammatory mechanisms. Neurosci. Lett. 2017, 639, 114–119. [Google Scholar] [CrossRef]
- Chamorro, Á.; Dirnagl, U.; Urra, X.; Planas, A.M. Neuroprotection in acute stroke: Targeting excitotoxicity, oxidative and nitrosative stress, and inflammation. Lancet Neurol. 2016, 15, 869–881. [Google Scholar] [CrossRef]
- Li, J.; Zhao, T.; Qiao, H.; Li, Y.; Xia, M.; Wang, X.; Liu, C.; Zheng, T.; Chen, R.; Xie, Y.; et al. Research progress of natural products for the treatment of ischemic stroke. J. Integr. Neurosci. 2022, 21, 14. [Google Scholar] [CrossRef]
- Zhang, A.-H.; Yu, J.-B.; Sun, H.; Kong, L.; Wang, X.-Q.; Zhang, Q.-Y.; Wang, X.-J. Identifying quality-markers from Shengmai San protects against transgenic mouse model of Alzheimer’s disease using chinmedomics approach. Phytomed. Int. J. Phytother. Phytopharm. 2018, 45, 84–92. [Google Scholar] [CrossRef]
- Cao, G.Q.; Hu, Z.P.; Ma, F.G.; Zhang, Y.P. Research progress of commonly used traditional Chinese medicines for the treatment of ischemic stroke. Liaoning J. Tradit. Chin. Med. 2019, 46, 2666–2671. [Google Scholar]
- Satriotomo, I.; Bowen, K.K.; Vemuganti, R. JAK2 and STAT3 activation contributes to neuronal damage following transient focal cerebral ischemia. J. Neurochem. 2006, 98, 1353–1368. [Google Scholar] [CrossRef]
- Xu, G.; Wang, X.; Xiong, Y.; Ma, X.; Qu, L. Effect of sevoflurane pretreatment in relieving liver ischemia/reperfusion-induced pulmonary and hepatic injury. Acta Cir. Bras. 2019, 34, e201900805. [Google Scholar] [CrossRef]
- Zhong, Y.; Yin, B.; Ye, Y.; Dekhel, O.; Xiong, X.; Jian, Z.; Gu, L. The bidirectional role of the JAK2/STAT3 signaling pathway and related mechanisms in cerebral ischemia-reperfusion injury. Exp. Neurol. 2021, 341, 113690. [Google Scholar] [CrossRef]
- Wu, Y.; Xu, J.; Xu, J.; Zheng, W.; Chen, Q.; Jiao, D. Study on the mechanism of JAK2/STAT3 signaling pathway-mediated inflammatory reaction after cerebral ischemia. Mol. Med. Rep. 2018, 17, 5007–5012. [Google Scholar] [CrossRef]
- Gong, P.; Zhang, Z.; Zou, Y.; Tian, Q.; Han, S.; Xu, Z.; Liao, J.; Gao, L.; Chen, Q.; Li, M. Tetramethylpyrazine attenuates blood-brain barrier disruption in ischemia/reperfusion injury through the JAK/STAT signaling pathway. Eur. J. Pharmacol. 2019, 854, 289–297. [Google Scholar] [CrossRef]
- Chen, J.; Huang, C.; Ye, L.; Yao, B.; Yang, M.; Cai, Q. Effect of matrine on JAK2/STAT3 signaling pathway and brain protection in rats with cerebral ischemia-reperfusion. Adv. Clin. Exp. Med. 2020, 29, 959–966. [Google Scholar] [CrossRef]
- Yu, L.; Liu, Z.; He, W.; Chen, H.; Lai, Z.; Duan, Y.; Cao, X.; Tao, J.; Xu, C.; Zhang, Q.; et al. Hydroxysafflor Yellow A Confers Neuroprotection from Focal Cerebral Ischemia by Modulating the Crosstalk Between JAK2/STAT3 and SOCS3 Signaling Pathways. Cell. Mol. Neurobiol. 2020, 40, 1271–1281. [Google Scholar] [CrossRef]
- Dong, W.; Xian, Y.; Yuan, W.; Huifeng, Z.; Tao, W.; Zhiqiang, L.; Shan, F.; Ya, F.; Hongli, W.; Jinghuan, W.; et al. Catalpol stimulates VEGF production via the JAK2/STAT3 pathway to improve angiogenesis in rats’ stroke model. J. Ethnopharmacol. 2016, 191, 169–179. [Google Scholar] [CrossRef]
- Hu, G.Q.; Du, X.; Li, Y.J.; Gao, X.Q.; Chen, B.Q.; Yu, L. Inhibition of cerebral ischemia/reperfusion injury-induced apoptosis: Nicotiflorin and JAK2/STAT3 pathway. Neural Regen. Res. 2017, 12, 96–102. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Chen, J.; Wu, J.; Wu, Q.; Jia, C.; Xu, Y.X.Z.; Chen, L.; Tu, W.; Yang, G.; Kong, J.; et al. Atractylenolide III ameliorates cerebral ischemic injury and neuroinflammation associated with inhibiting JAK2/STAT3/Drp1-dependent mitochondrial fission in microglia. Phytomedicine 2019, 59, 152922. [Google Scholar] [CrossRef]
- Li, L.; Sun, L.; Qiu, Y.; Zhu, W.; Hu, K.; Mao, J. Protective Effect of Stachydrine Against Cerebral Ischemia-Reperfusion Injury by Reducing Inflammation and Apoptosis Through P65 and JAK2/STAT3 Signaling Pathway. Front. Pharmacol. 2020, 11, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gasparini, C.; Feldmann, M. NF-κB as a target for modulating inflammatory responses. Curr. Pharm. Des. 2012, 18, 5735–5745. [Google Scholar] [CrossRef]
- Gordon, J.W.; Shaw, J.A.; Kirshenbaum, L.A. Multiple facets of NF-kappaB in the heart: To be or not to NF-kappaB. Circul. Res. 2011, 108, 1122–1132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, L.; Yang, X.P.; Janic, B.; Rhaleb, N.E.; Harding, P.; Nakagawa, P.; Peterson, E.L.; Carretero, O.A. Ac-SDKP suppresses TNF-alpha-induced ICAM-1 expression in endothelial cells via inhibition of IkappaB kinase and NF-kappaB activation. Am. J. Physiol. Heart Circ. Physiol. 2016, 310, H1176–H1183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Delft, M.A.; Huitema, L.F.; Tas, S.W. The contribution of NF-kappaB signalling to immune regulation and tolerance. Eur. J. Clin. Investig. 2015, 45, 529–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Dang, W.; Zhang, S.; Wang, L.; Zhang, X. Artesunate attenuates inflammatory injury and inhibits the NF-κB pathway in a mouse model of cerebral ischemia. J. Int. Med. Res. 2021, 49, 3000605211053549. [Google Scholar] [CrossRef]
- Zhao, D.; Ji, J.; Li, S.; Wu, A. Skullcapflavone II protects neuronal damage in cerebral ischemic rats via inhibiting NF-ĸB and promoting angiogenesis. Microvasc. Res. 2022, 141, 104318. [Google Scholar] [CrossRef]
- Tan, J.; Luo, J.; Meng, C.; Jiang, N.; Cao, J.; Zhao, J. Syringin exerts neuroprotective effects in a rat model of cerebral ischemia through the FOXO3a/NF-κB pathway. Int. Immunopharmacol. 2021, 90, 107268. [Google Scholar] [CrossRef]
- Fan, X.; Elkin, K.; Shi, Y.; Zhang, Z.; Cheng, Y.; Gu, J.; Liang, J.; Wang, C.; Ji, X. Schisandrin B improves cerebral ischemia and reduces reperfusion injury in rats through TLR4/NF-κB signaling pathway inhibition. Neurol. Res. 2020, 42, 693–702. [Google Scholar] [CrossRef]
- Shi, C.; Li, J.; Li, J. Ephedrine attenuates cerebral ischemia/reperfusion injury in rats through NF-κB signaling pathway. Hum. Exp. Toxicol. 2021, 40, 994–1002. [Google Scholar] [CrossRef]
- Zhang, W.; Song, J.; Li, W.; Kong, D.; Liang, Y.; Zhao, X.; Du, G. Salvianolic Acid D Alleviates Cerebral Ischemia-Reperfusion Injury by Suppressing the Cytoplasmic Translocation and Release of HMGB1-Triggered NF-κB Activation to Inhibit Inflammatory Response. Mediat. Inflamm. 2020, 2020, 9049614. [Google Scholar] [CrossRef] [Green Version]
- Zhang, B.; Song, C.; Feng, B.; Fan, W. Neuroprotection by triptolide against cerebral ischemia/reperfusion injury through the inhibition of NF-κB/PUMA signal in rats. Ther. Clin. Risk Manag. 2016, 12, 817–824. [Google Scholar] [CrossRef] [Green Version]
- Zhang, F.B.; Wang, J.P.; Zhang, H.X.; Fan, G.M.; Cui, X. Effect of β-patchoulene on cerebral ischemia-reperfusion injury and the TLR4/NF-κB signaling pathway. Exp. Ther. Med. 2019, 17, 3335–3342. [Google Scholar] [CrossRef] [Green Version]
- Pan, J.; Li, X.; Guo, F.; Yang, Z.; Zhang, L.; Yang, C. Ginkgetin attenuates cerebral ischemia-reperfusion induced autophagy and cell death via modulation of the NF-κB/p53 signaling pathway. Biosci. Rep. 2019, 39, BSR20191452. [Google Scholar] [CrossRef] [Green Version]
- Song, Z.; Feng, J.; Zhang, Q.; Deng, S.; Yu, D.; Zhang, Y.; Li, T. Tanshinone IIA Protects against Cerebral Ischemia Reperfusion Injury by Regulating Microglial Activation and Polarization via NF-κB Pathway. Front. Pharmacol. 2021, 12, 641848. [Google Scholar] [CrossRef]
- Li, Y.; Li, S.; Li, D. Breviscapine Alleviates Cognitive Impairments Induced by Transient Cerebral Ischemia/Reperfusion through Its Anti-Inflammatory and Anti-Oxidant Properties in a Rat Model. ACS Chem. Neurosci. 2020, 11, 4489–4498. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, Z.; Zhu, S.; Guo, Y.; Zhang, Y.; Dou, J.; Zhang, J.; Ito, Y.; Guo, Z. Diosgenin revealed potential effect against cerebral ischemia reperfusion through HIKESHI/HSP70/NF-κB anti-inflammatory axis. Phytomedicine 2022, 99, 153991. [Google Scholar] [CrossRef]
- Xiong, D.; Deng, Y.; Huang, B.; Yin, C.; Liu, B.; Shi, J.; Gong, Q. Icariin attenuates cerebral ischemia-reperfusion injury through inhibition of inflammatory response mediated by NF-κB, PPARα and PPARγ in rats. Int. Immunopharmacol. 2016, 30, 157–162. [Google Scholar] [CrossRef]
- Sun, K.; Luo, Z.L.; Hu, C.; Gong, T.L.; Tang, G.H.; Wu, S.P. Protective effect and immune mechanism of berberine on cerebral ischemia/reperfusion injury in rats. Zhongguo Ying Yong Sheng Li Xue Za Zhi 2020, 36, 656–661. [Google Scholar]
- Zheng, Y.; Han, Z.; Zhao, H.; Luo, Y. MAPK: A Key Player in the Development and Progression of Stroke. CNS Neurol. Disord. Drug Targets 2020, 19, 248–256. [Google Scholar] [CrossRef]
- Arthur, J.S.; Ley, S.C. Mitogen-activated protein kinases in innate immunity. Nat. Rev. Immunol. 2013, 13, 679–692. [Google Scholar] [CrossRef]
- Peti, W.; Page, R. Molecular basis of MAP kinase regulation. Protein Sci. 2013, 22, 1698–1710. [Google Scholar] [CrossRef]
- Turjanski, A.G.; Vaqué, J.P.; Gutkind, J.S. MAP kinases and the control of nuclear events. Oncogene 2007, 26, 3240–3253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.J.; Pan, W.W.; Liu, S.B.; Shen, Z.F.; Xu, Y.; Hu, L.L. ERK/MAPK signalling pathway and tumorigenesis. Exp. Ther. Med. 2020, 19, 1997–2007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oh, C.C.; Lee, J.; D’Souza, K.; Zhang, W.; Migrino, R.Q.; Thornburg, K.; Reaven, P. Activator protein-1 and caspase 8 mediate p38alpha MAPK-dependent cardiomyocyte apoptosis induced by palmitic acid. Apoptosis 2019, 24, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.R.; Liu, C.L.; Park, D.J. Alteration of MAP kinase pathways after transient forebrain ischemia. J. Cereb. Blood Flow Metab. 2000, 20, 1089–1095. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Wang, F.; Yu, L.; Li, Z. Nobiletin alleviates cerebral ischemic-reperfusion injury via MAPK signaling pathway. Am. J. Transl. Res. 2019, 11, 5967–5977. [Google Scholar]
- Li, L.; Li, Y.; Miao, C.; Liu, Y.; Liu, R. Coriolus versicolor polysaccharides (CVP) regulates neuronal apoptosis in cerebral ischemia-reperfusion injury via the p38MAPK signaling pathway. Ann. Transl. Med. 2020, 8, 1168. [Google Scholar] [CrossRef]
- Ma, S.; Liu, X.; Cheng, B.; Jia, Z.; Hua, H.; Xin, Y. Chemical characterization of polysaccharides isolated from scrophularia ningpoensis and its protective effect on the cerebral ischemia/reperfusin injury in rat model. Int. J. Biol. Macromol. 2019, 139, 955–966. [Google Scholar] [CrossRef]
- Leung, S.W.; Lai, J.H.; Wu, J.C.; Tsai, Y.R.; Chen, Y.H.; Kang, S.J.; Chiang, Y.H.; Chang, C.F.; Chen, K.Y. Neuroprotective Effects of Emodin against Ischemia/Reperfusion Injury through Activating ERK-1/2 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 2899. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Suo, L.; Liu, Y.; Li, H.; Xue, W. Protective effects of ginsenoside Rg1 against oxygen-glucose-deprivation-induced apoptosis in neural stem cells. J. Neurol. Sci. 2017, 373, 107–112. [Google Scholar] [CrossRef]
- Li, C.; Sui, C.; Wang, W.; Yan, J.; Deng, N.; Du, X.; Cheng, F.; Ma, X.; Wang, X.; Wang, Q. Baicalin Attenuates Oxygen-Glucose Deprivation/Reoxygenation-Induced Injury by Modulating the BDNF-TrkB/PI3K/Akt and MAPK/Erk1/2 Signaling Axes in Neuron-Astrocyte Cocultures. Front. Pharmacol. 2021, 12, 599543. [Google Scholar] [CrossRef]
- Huang, L.; Chen, C.; Zhang, X.; Li, X.; Chen, Z.; Yang, C.; Liang, X.; Zhu, G.; Xu, Z. Neuroprotective Effect of Curcumin against Cerebral Ischemia-Reperfusion via Mediating Autophagy and Inflammation. J. Mol. Neurosci. 2018, 64, 129–139. [Google Scholar] [CrossRef]
- Pancewicz-Wojtkiewicz, J. Epidermal growth factor receptor and notch signaling in non-small-cell lung cancer. Cancer Med. 2016, 5, 3572–3578. [Google Scholar] [CrossRef]
- Oya, S.; Yoshikawa, G.; Takai, K.; Tanaka, J.I.; Higashiyama, S.; Saito, N.; Kirino, T.; Kawahara, N. Attenuation of Notch signaling promotes the differentiation of neural progenitors into neurons in the hippocampal CA1 region after ischemic injury. Neuroscience 2009, 158, 683–692. [Google Scholar] [CrossRef]
- Li, Y.Z.; Sun, Z.; Xu, H.R.; Zhang, Q.G.; Zeng, C.Q. Osthole inhibits proliferation of kainic acid-activated BV-2 cells by modulating the Notch signaling pathway. Mol. Med. Rep. 2020, 22, 3759–3766. [Google Scholar] [CrossRef]
- Sjöqvist, M.; Antfolk, D.; Ferraris, S.; Rraklli, V.; Haga, C.; Antila, C.; Mutvei, A.; Imanishi, S.Y.; Holmberg, J.; Jin, S.; et al. PKCζ regulates Notch receptor routing and activity in a Notch signaling-dependent manner. Cell Res. 2014, 24, 433–450. [Google Scholar] [CrossRef] [Green Version]
- Liang, C.; Ni, G.X.; Shi, X.L.; Jia, L.; Wang, Y.L. Astragaloside IV regulates the HIF/VEGF/Notch signaling pathway through miRNA-210 to promote angiogenesis after ischemic stroke. Restor. Neurol. Neurosci. 2020, 38, 271–282. [Google Scholar] [CrossRef]
- Guan, J.; Wei, X.; Qu, S.; Lv, T.; Fu, Q.; Yuan, Y. Osthole prevents cerebral ischemia-reperfusion injury via the Notch signaling pathway. Biochem. Cell Biol. 2017, 95, 459–467. [Google Scholar] [CrossRef] [Green Version]
- Liu, L.; Locascio, L.M.; Doré, S. Critical Role of Nrf2 in Experimental Ischemic Stroke. Front. Pharmacol. 2019, 10, 153. [Google Scholar] [CrossRef] [Green Version]
- Canning, P.; Sorrell, F.J.; Bullock, A.N. Structural basis of Keap1 interactions with Nrf2. Free Radic. Biol. Med. 2015, 88 Pt B, 101–107. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.M.; Maltagliati, A.J. Nrf2 at the heart of oxidative stress and cardiac protection. Physiol. Genom. 2018, 50, 77–97. [Google Scholar] [CrossRef]
- Li, W.; Kong, A.N. Molecular mechanisms of Nrf2-mediated antioxidant response. Mol. Carcinog. 2009, 48, 91–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruns, D.R.; Drake, J.C.; Biela, L.M.; Peelor, F.F., 3rd; Miller, B.F.; Hamilton, K.L. Nrf2 Signaling and the Slowed Aging Phenotype: Evidence from Long-Lived Models. Oxid. Med. Cell. Longev. 2015, 2015, 732596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Yang, J.; Wu, S.; Jin, C.; Lu, X.; Hu, X.; Sun, Y.; Gao, X.; Cai, Y. Activation of Nrf2/ARE signaling pathway attenuates lanthanum chloride induced injuries in primary rat astrocytes. Met. Integr. Biometal Sci. 2017, 9, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Lu, H.; Qin, J.; Qu, S.; Wang, W.; Guo, Y.; Liao, W.; Song, M.; Chen, J.; Wang, Y. Biochanin A Provides Neuroprotection Against Cerebral Ischemia/Reperfusion Injury by Nrf2-Mediated Inhibition of Oxidative Stress and Inflammation Signaling Pathway in Rats. Med. Sci. Monit. 2019, 25, 8975–8983. [Google Scholar] [CrossRef]
- Sahraroo, A.; Babalar, M.; Mirjalili, M.H.; Fattahi Moghaddam, M.R.; Nejad Ebrahimi, S. In-vitro Callus Induction and Rosmarinic Acid Quantification in Callus Culture of Satureja khuzistanica Jamzad (Lamiaceae). Iran. J. Pharm. Res. 2014, 13, 1447–1456. [Google Scholar]
- Cui, H.Y.; Zhang, X.J.; Yang, Y.; Zhang, C.; Zhu, C.H.; Miao, J.Y.; Chen, R. Rosmarinic acid elicits neuroprotection in ischemic stroke via Nrf2 and heme oxygenase 1 signaling. Neural Regen. Res. 2018, 13, 2119–2128. [Google Scholar]
- Tang, C.; Hong, J.; Hu, C.; Huang, C.; Gao, J.; Huang, J.; Wang, D.; Geng, Q.; Dong, Y. Palmatine Protects against Cerebral Ischemia/Reperfusion Injury by Activation of the AMPK/Nrf2 Pathway. Oxid. Med. Cell. Longev. 2021, 2021, 6660193. [Google Scholar] [CrossRef]
- He, Y.; Jiang, K.; Zhao, X. Taraxasterol protects hippocampal neurons from oxygen-glucose deprivation-induced injury through activation of Nrf2 signalling pathway. Artif. Cells Nanomed. Biotechnol. 2020, 48, 252–258. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Duan, M.; Liang, S.; Wang, Y.; Feng, Y. Senkyunolide I protects rat brain against focal cerebral ischemia-reperfusion injury by up-regulating p-Erk1/2, Nrf2/HO-1 and inhibiting caspase 3. Brain Res. 2015, 1605, 39–48. [Google Scholar] [CrossRef]
- Liu, Q.; Jin, Z.; Xu, Z.; Yang, H.; Li, L.; Li, G.; Li, F.; Gu, S.; Zong, S.; Zhou, J.; et al. Antioxidant effects of ginkgolides and bilobalide against cerebral ischemia injury by activating the Akt/Nrf2 pathway in vitro and in vivo. Cell Stress Chaperones 2019, 24, 441–452. [Google Scholar] [CrossRef]
- Lv, H.; Li, J.; Che, Y.Q. CXCL8 gene silencing promotes neuroglial cells activation while inhibiting neuroinflammation through the PI3K/Akt/NF-κB-signaling pathway in mice with ischemic stroke. J. Cell. Physiol. 2019, 234, 7341–7355. [Google Scholar] [CrossRef]
- Wang, C.; Wei, Z.; Jiang, G.; Liu, H. Neuroprotective mechanisms of miR-124 activating PI3K/Akt signaling pathway in ischemic stroke. Exp. Ther. Med. 2017, 13, 3315–3318. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Li, H.; Mo, Z.; Lei, J.; Zhu, L.; Huang, Y.; Fu, R.; Li, C.; Huang, Y.; Liu, K.; et al. Connectivity map identifies luteolin as a treatment option of ischemic stroke by inhibiting MMP9 and activation of the PI3K/Akt signaling pathway. Exp. Mol. Med. 2019, 51, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; An, R.; Tian, X.; Yang, M.; Li, M.; Lou, J.; Xu, L.; Dong, Z. β-Caryophyllene Pretreatment Alleviates Focal Cerebral Ischemia-Reperfusion Injury by Activating PI3K/Akt Signaling Pathway. Neurochem. Res. 2017, 42, 1459–1469. [Google Scholar] [CrossRef] [PubMed]
- Whitman, M.; Downes, C.P.; Keeler, M.; Keller, T.; Cantley, L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 1988, 332, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Burke, J.E. Structural Basis for Regulation of Phosphoinositide Kinases and Their Involvement in Human Disease. Mol. Cell. 2018, 71, 653–673. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugiyama, M.G.; Fairn, G.D.; Antonescu, C.N. Akt-ing Up Just About Everywhere: Compartment-Specific Akt Activation and Function in Receptor Tyrosine Kinase Signaling. Front. Cell Dev. Biol. 2019, 7, 70. [Google Scholar] [CrossRef]
- Mayer, I.A.; Arteaga, C.L. The PI3K/AKT Pathway as a Target for Cancer Treatment. Annu. Rev. Med. 2016, 67, 11–28. [Google Scholar] [CrossRef]
- Zhou, Z.; Dun, L.; Wei, B.; Gan, Y.; Liao, Z.; Lin, X.; Lu, J.; Liu, G.; Xu, H.; Lu, C.; et al. Musk Ketone Induces Neural Stem Cell Proliferation and Differentiation in Cerebral Ischemia via Activation of the PI3K/Akt Signaling Pathway. Neuroscience 2020, 435, 1–9. [Google Scholar] [CrossRef]
- Lei, J.; Chen, Q. Resveratrol attenuates brain damage in permanent focal cerebral ischemia via activation of PI3K/Akt signaling pathway in rats. Neurol. Res. 2018, 40, 1014–1020. [Google Scholar] [CrossRef]
- Ding, Y.; Du, J.; Cui, F.; Chen, L.; Li, K. The protective effect of ligustrazine on rats with cerebral ischemia-reperfusion injury via activating PI3K/Akt pathway. Hum. Exp. Toxicol. 2019, 38, 1168–1177. [Google Scholar] [CrossRef]
- Xie, W.; Wulin, H.; Shao, G.; Wei, L.; Qi, R.; Ma, B.; Chen, N.; Shi, R. Polygalasaponin F inhibits neuronal apoptosis induced by oxygen-glucose deprivation and reoxygenation through the PI3K/Akt pathway. Basic Clin. Pharmacol. Toxicol. 2020, 127, 196–204. [Google Scholar] [CrossRef]
- Tao, J.; Cui, Y.; Duan, Y.; Zhang, N.; Wang, C.; Zhang, F. Puerarin attenuates locomotor and cognitive deficits as well as hippocampal neuronal injury through the PI3K/Akt1/GSK-3β signaling pathway in an in vivo model of cerebral ischemia. Oncotarget 2017, 8, 106283–106295. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Wu, Y.; Zhao, B.; Hu, H.; Zhu, B.; Sun, Z.; Li, P.; Du, S. Panax notoginseng Saponins Protect Cerebral Microvascular Endothelial Cells against Oxygen-Glucose Deprivation/Reperfusion-Induced Barrier Dysfunction via Activation of PI3K/Akt/Nrf2 Antioxidant Signaling Pathway. Molecules 2018, 23, 2781. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.; Hong, H.; Zhang, X.; Lai, W.; Wang, Y.; Chu, K.; Brown, J.; Hong, G.; Chen, L. Salidroside Inhibits Inflammation Through PI3K/Akt/HIF Signaling After Focal Cerebral Ischemia in Rats. Inflammation 2017, 40, 1297–1309. [Google Scholar] [CrossRef]
- D’Onofrio, N.; Servillo, L.; Balestrieri, M.L. SIRT1 and SIRT6 Signaling Pathways in Cardiovascular Disease Protection. Antioxid. Redox Signal. 2018, 28, 711–732. [Google Scholar] [CrossRef]
- Fujita, Y.; Fujiwara, K.; Zenitani, S.; Yamashita, T. Acetylation of NDPK-D Regulates Its Subcellular Localization and Cell Survival. PLoS ONE 2015, 10, e0139616. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Yao, J.; Li, Z.; Zu, G.; Feng, D.; Shan, W.; Li, Y.; Hu, Y.; Zhao, Y.; Tian, X. miR-34a-5p Inhibition Alleviates Intestinal Ischemia/Reperfusion-Induced Reactive Oxygen Species Accumulation and Apoptosis via Activation of SIRT1 Signaling. Antioxid. Redox Signal. 2016, 24, 961–973. [Google Scholar] [CrossRef]
- Cheng, Z.; Zhang, M.; Ling, C.; Zhu, Y.; Ren, H.; Hong, C.; Qin, J.; Liu, T.; Wang, J. Neuroprotective Effects of Ginsenosides against Cerebral Ischemia. Molecules 2019, 24, 1102. [Google Scholar] [CrossRef] [Green Version]
- Kou, D.Q.; Jiang, Y.L.; Qin, J.H.; Huang, Y.H. Magnolol attenuates the inflammation and apoptosis through the activation of SIRT1 in experimental stroke rats. Pharmacol. Rep. 2017, 69, 642–647. [Google Scholar] [CrossRef]
- Lv, H.; Wang, L.; Shen, J.; Hao, S.; Ming, A.; Wang, X.; Su, F.; Zhang, Z. Salvianolic acid B attenuates apoptosis and inflammation via SIRT1 activation in experimental stroke rats. Brain Res. Bull. 2015, 115, 30–36. [Google Scholar] [CrossRef]
- Yan, X.; Yu, A.; Zheng, H.; Wang, S.; He, Y.; Wang, L. Calycosin-7-O-β-D-glucoside Attenuates OGD/R-Induced Damage by Preventing Oxidative Stress and Neuronal Apoptosis via the SIRT1/FOXO1/PGC-1α Pathway in HT22 Cells. Neural Plast. 2019, 2019, 8798069. [Google Scholar] [CrossRef] [Green Version]
- Turner, R.J.; Sharp, F.R. Implications of MMP9 for Blood Brain Barrier Disruption and Hemorrhagic Transformation Following Ischemic Stroke. Front. Cell. Neurosci. 2016, 10, 56. [Google Scholar] [CrossRef] [Green Version]
- Asahi, M.; Wang, X.; Mori, T.; Sumii, T.; Jung, J.C.; Moskowitz, M.A.; Fini, M.E.; Lo, E.H. Effects of matrix metalloproteinase-9 gene knock-out on the proteolysis of blood-brain barrier and white matter components after cerebral ischemia. J. Neurosci. 2001, 21, 7724–7732. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Zou, H.; Wang, G.; Yang, H.; Xie, Z.; Bi, J. Matrix metalloproteinase-9 and tissue inhibitor of metalloproteinase-1 expression in early focal cerebral infarction following urokinase thrombolysis in rats. Neural Regen. Res. 2012, 7, 1325–1330. [Google Scholar]
- Wu, H.; Zhang, Z.; Li, Y.; Zhao, R.; Li, H.; Song, Y.; Qi, J.; Wang, J. Time course of upregulation of inflammatory mediators in the hemorrhagic brain in rats: Correlation with brain edema. Neurochem. Int. 2010, 57, 248–253. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.B.; Wang, W.; Gao, J.M.; Li, F.; Shi, J.S.; Gong, Q.H. Icariside II attenuates cerebral ischemia/reperfusion-induced blood-brain barrier dysfunction in rats via regulating the balance of MMP9/TIMP1. Acta Pharmacol. Sin. 2020, 41, 1547–1556. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; He, Z.; Deng, S. Ursolic acid reduces the metalloprotease/anti-metalloprotease imbalance in cerebral ischemia and reperfusion injury. Drug Des. Devel. Ther. 2016, 10, 1663–1674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, Y.; Qing, L.; Xi, L.; Mei, Z.; Ting, S.; Na, H.; Wei, J.; Tao, Z.; Peng, Y.; Qi, Y. Oxymatrine improves blood-brain barrier integrity after cerebral ischemia-reperfusion injury by downregulating CAV1 and MMP9 expression. Phytomedicine 2021, 84, 153505. [Google Scholar]
- Fu, S.; Gu, Y.; Jiang, J.Q.; Chen, X.; Xu, M.; Chen, X.; Shen, J. Calycosin-7-O-β-D-glucoside regulates nitric oxide/caveolin-1/matrix metalloproteinases pathway and protects blood-brain barrier integrity in experimental cerebral ischemia-reperfusion injury. J. Ethnopharmacol. 2014, 155, 692–701. [Google Scholar] [CrossRef]
- Song, Y.; Hou, M.; Li, Z.; Luo, C.; Ou, J.S.; Yu, H.; Yan, J.; Lu, L. TLR4/NF-κB/Ceramide signaling contributes to Ox-LDL-induced calcification of human vascular smooth muscle cells. Eur. J. Pharmacol. 2017, 794, 45–51. [Google Scholar] [CrossRef]
- Wang, P.F.; Xiong, X.Y.; Chen, J.; Wang, Y.C.; Duan, W.; Yang, Q.W. Function and mechanism of toll-like receptors in cerebral ischemic tolerance: From preconditioning to treatment. J. Neuroinflamm. 2015, 12, 80. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Jin, M.; Lv, T.; Guan, J. Mechanism of focal cerebral ischemic tolerance in rats with ischemic preconditioning involves MyD88- and TRIF-dependent pathways. Exp. Ther. Med. 2013, 6, 1375–1379. [Google Scholar] [CrossRef] [Green Version]
- Hyakkoku, K.; Hamanaka, J.; Tsuruma, K.; Shimazawa, M.; Tanaka, H.; Uematsu, S.; Akira, S.; Inagaki, N.; Nagai, H.; Hara, H. Toll-like receptor 4 (TLR4), but not TLR3 or TLR9, knock-out mice have neuroprotective effects against focal cerebral ischemia. Neuroscience 2010, 171, 258–267. [Google Scholar] [CrossRef]
- Wang, N.; Liu, Y.; Jia, C.; Gao, C.; Zheng, T.; Wu, M.; Zhang, Q.; Zhao, X.; Li, Z.; Chen, J.; et al. Machine learning enables discovery of Gentianine targeting TLR4/NF-κB pathway to repair ischemic stroke injury. Pharmacol. Res. 2021, 173, 105913. [Google Scholar] [CrossRef]
- Yang, B.; Sun, Y.; Lv, C.; Zhang, W.; Chen, Y. Procyanidins exhibits neuroprotective activities against cerebral ischemia reperfusion injury by inhibiting TLR4-NLRP3 inflammasome signal pathway. Psychopharmacology 2020, 237, 3283–3293. [Google Scholar] [CrossRef]
- Koyasu, S.; Kobayashi, M.; Goto, Y.; Hiraoka, M.; Harada, H. Regulatory mechanisms of hypoxia-inducible factor 1 activity: Two decades of knowledge. Cancer Sci. 2018, 109, 560–571. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Tao, T.; Xu, J.; Liu, Z.; Zou, Z.; Jin, M. HIF-1α attenuates neuronal apoptosis by upregulating EPO expression following cerebral ischemia-reperfusion injury in a rat MCAO model. Int. J. Mol. Med. 2020, 45, 1027–1036. [Google Scholar] [CrossRef] [Green Version]
- Du, M.; Huang, K.; Huang, D.; Yang, L.; Gao, L.; Wang, X.; Huang, D.; Li, X.; Wang, C.; Zhang, F.; et al. Renalase is a novel target gene of hypoxia-inducible factor-1 in protection against cardiac ischaemia-reperfusion injury. Cardiovasc. Res. 2015, 105, 182–191. [Google Scholar] [CrossRef]
- Fuhrmann, D.C.; Brüne, B. Mitochondrial composition and function under the control of hypoxia. Redox Biol. 2017, 12, 208–215. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Yin, Y.; Yu, S.; Ren, H.; Xue, Q.; Xu, X. Catalpol protects vascular structure and promotes angiogenesis in cerebral ischemic rats by targeting HIF-1α/VEGF. Phytomedicine 2020, 78, 153300. [Google Scholar] [CrossRef]
- Ni, H.; Li, J.; Zheng, J.; Zhou, B. Cardamonin attenuates cerebral ischemia/reperfusion injury by activating the HIF-1α/VEGFA pathway. Phytother. Res. 2022, 36, 1736–1747. [Google Scholar] [CrossRef]
- Zheng, Y.; Zhang, R.; Shi, W.; Li, L.; Liu, H.; Chen, Z.; Wu, L. Metabolism and pharmacological activities of the natural health-benefiting compound diosmin. Food Funct. 2020, 11, 8472–8492. [Google Scholar] [CrossRef]
- Sadeghipour, M.; Terreux, R.; Phipps, J. Flavonoids and tyrosine nitration: Structure-activity relationship correlation with enthalpy of formation. Toxicol. In Vitro 2005, 19, 155–165. [Google Scholar] [CrossRef] [PubMed]
- Chemler, S.R. Phenanthroindolizidines and Phenanthroquinolizidines: Promising Alkaloids for Anti-Cancer Therapy. Curr. Bioact. Compd. 2009, 5, 2–19. [Google Scholar] [CrossRef]
- Chen, L.; Huang, G. Antitumor Activity of Polysaccharides: An Overview. Curr. Drug Targets 2018, 19, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Chen, T.; Yan, M.; Zhao, W.; Li, F.; Cheng, W.; Yuan, L. Synthesis, characterization, antioxidant activity and neuroprotective effects of selenium polysaccharide from Radix hedysari. Carbohydr. Polym. 2015, 125, 161–168. [Google Scholar] [CrossRef]
- Chen, Y.; Song, M.; Wang, Y.; Xiong, W.; Zeng, L.; Zhang, S.; Xu, M.; Du, H.; Liu, J.; Wang, D.; et al. The anti-DHAV activities of Astragalus polysaccharide and its sulfate compared with those of BSRPS and its sulfate. Carbohydr. Polym. 2015, 117, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Wang, Y.; Shen, H.-l.; Shen, X.-d.; Nie, Y.; Wang, Y.; Han, T.; Yin, M.; Zhang, Q.-y. Structural characterization and radioprotection of bone marrow hematopoiesis of two novel polysaccharides from the root of Angelica sinensis (Oliv.) Diels. Fitoterapia 2012, 83, 1712–1720. [Google Scholar] [CrossRef]
- Cai, W.; Xie, L.; Chen, Y.; Zhang, H. Purification, characterization and anticoagulant activity of the polysaccharides from green tea. Carbohydr. Polym. 2013, 92, 1086–1090. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Shan, B.; Liao, C.-H.; Xie, J.-H.; Wen, P.-W.; Shi, J.-Y. Anti-diabetic properties of Momordica charantia L. polysaccharide in alloxan-induced diabetic mice. Int. J. Biol. Macromol. 2015, 81, 538–543. [Google Scholar] [CrossRef]
- Rushdi, M.I.; Abdel-Rahman, I.A.M.; Saber, H.; Attia, E.Z.; Abdelraheem, W.M.; Madkour, H.A.; Abdelmohsen, U.R. The genus: Chemical and pharmacological diversity. Nat. Prod. Res. 2021, 35, 4560–4578. [Google Scholar] [CrossRef]
- Qu, J.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef]
- He, Y.; Hu, Z.; Li, A.; Zhu, Z.; Yang, N.; Ying, Z.; He, J.; Wang, C.; Yin, S.; Cheng, S. Recent Advances in Biotransformation of Saponins. Molecules 2019, 24, 2365. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Zhang, S.; Peng, D.; Wang, C.; Zhao, D.; Ma, K.; Wu, J.; Huang, L. Transcriptome Analysis of (Benth.) O. Kuntze and Identification of Genes Involved in Triterpenoid Saponin Biosynthesis. Int. J. Mol. Sci. 2019, 20, 2643. [Google Scholar] [CrossRef] [Green Version]
- Segovia, F.J.; Hidalgo, G.I.; Villasante, J.; Ramis, X.; Almajano, M.P. Avocado Seed: A Comparative Study of Antioxidant Content and Capacity in Protecting Oil Models from Oxidation. Molecules 2018, 23, 2421. [Google Scholar] [CrossRef] [Green Version]
- El-Demerdash, A.; Kumla, D.; Kijjoa, A. Chemical Diversity and Biological Activities of Meroterpenoids from Marine Derived-Fungi: A Comprehensive Update. Mar. Drugs 2020, 18, 317. [Google Scholar] [CrossRef]
- Menezes, L.R.; Costa, C.O.; Rodrigues, A.C.; Santo, F.R.; Nepel, A.; Dutra, L.M.; Silva, F.M.; Soares, M.B.; Barison, A.; Costa, E.V.; et al. Cytotoxic Alkaloids from the Stem of Xylopia laevigata. Molecules 2016, 21, 890. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.H.; Kim, Y.H.; Chi, S.W.; Choo, S.J.; Ryoo, I.J.; Ahn, J.S.; Yoo, I.D. Evaluation of human neutrophil elastase inhibitory effect of iridoid glycosides from Hedyotis diffusa. Bioorg. Med. Chem. Lett. 2010, 20, 513–515. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, H.L.; Zhang, A.H.; Zhou, X.H.; Wang, X.Q.; Han, Y.; Yan, G.L.; Liu, L.; Wang, X.J. Network pharmacology combined with functional metabolomics discover bile acid metabolism as a promising target for mirabilite against colorectal cancer. RSC Adv. 2018, 8, 30061–30070. [Google Scholar] [CrossRef] [Green Version]
- Gao, H.-L.; Zhang, A.-H.; Yu, J.-B.; Sun, H.; Kong, L.; Wang, X.-Q.; Yan, G.-L.; Liu, L.; Wang, X.-J. High-throughput lipidomics characterize key lipid molecules as potential therapeutic targets of Kaixinsan protects against Alzheimer’s disease in APP/PS1 transgenic mice. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2018, 1092, 286–295. [Google Scholar] [CrossRef]
- Zhang, A.; Sun, H.; Wang, X. Mass spectrometry-driven drug discovery for development of herbal medicine. Mass Spectrom. Rev. 2018, 37, 307–320. [Google Scholar] [CrossRef]



| Natural Compounds | Categories | Plants | Experiments Model | Mechanisms | Signaling Pathways | Ref. | |
|---|---|---|---|---|---|---|---|
| In Vivo | In Vitro | ||||||
| Matrine | Alkaloid | Sophora flavescens Aiton. | MCAO rats | -- | ↑: SOD, ↓: MDA, p-JAK2, p-STAT3 | JAK2/STAT3 | [21] |
| Hydroxy saffron yellow A | Flavonoid | Carthamus tinctorius L. | MCAO rats | -- | ↑: SOCS3 ↓: p-JAK2, p-STAT3 | JAK2/STAT3 | [22] |
| Catalpol | Terpenoid | Rehmannia glutinosa (Gaertn.) Libosch. ex Fisch. & C. A. Mey. | MCAO rats | -- | ↑: VEGF, EPO, EPOR ↓: p-JAK2, p-STAT3 | JAK2/STAT3 | [23] |
| Nicotiflorin | Flavonoid | Carthamus tinctorius L. | MCAO rats | -- | ↑: Bcl-2 ↓: p-JAK2, p-STAT3, caspase-3, Bax | JAK2/STAT3 | [24] |
| Atractylenolide III | Terpenoid | Atractylodes macrocephala Koidz. | MCAO rats | OGD/R cells | ↓: IL-1β, TNF-α, IL-6, Drp1, p-JAK2, p-STAT3 | JAK2/STAT3 | [25] |
| Stachydrine | Alkaloid | Leonurus japonicus Houtt. | MCAO rats | OGD/R cells | ↑: SOD ↓: p-65, p-iκB, p-JAK2, p-STAT3, MDA, IL-1β, TNF-α | JAK2/STAT3 | [26] |
| Artesunate | Terpenoid | Artemisia annua L. | MCAO mice | -- | ↑: IκB ↓: IL-1β, TNF-α, NF-κB | NF-κB | [31] |
| Skullcapflavone II | Flavonoid | Scutellaria baicalensis Georgi | MCAO rats | -- | ↑: SOD, GSH, VEGF, Ang-1,Tie-2, ↓: MDA, IL-1β, TNF-α, IL-6, caspase-3 and -9, NF-ĸb, TLR4 | NF-κB | [32] |
| Syringin | Saponin | Eleutherococcus senticosus (Rupr. & Maxim.) Maxim. | MCAO rats | -- | ↑: p-FOXO3a ↓: NF-κB, IL-1β, IL-6, TNF-α, MPO | NF-κB | [33] |
| Schisandrin B | Lignan | Schisandra chinensis (Turcz.) Baill. | MCAO rats | -- | ↓: NF-κB, TLR4, IL-1β, IL-6, TNF-α | NF-κB | [34] |
| Ephedrine | Alkaloid | Ephedra sinica Stapf Ephedra sinica Stapf | MCAO rats | -- | ↑: Bcl-2 ↓: IL-1β, TNF-α, IL-6, Bax, NO, p-NF-κB | NF-κB | [35] |
| Berberine | Alkaloid | Coptis chinensis Franch. | MCAO rats | -- | ↑: SOD, GSH-Px, CD4+, CD8 ↓: NO, TNF-α, IFN-β, IL-6, NF-κB p65, NLRP3, ASC, caspase-3 | NF-κB | [44] |
| Salvianolic acid D | Polyphenol | Salvia miltiorrhiza Bunge | MCAO rats | OGD/R cells | ↑: Bcl-2 ↓: Bax, Cyt c, caspase-3 and -9, TLR4, MyD88, TRAF6, NF-κB, HMGB1 | NF-κB | [36] |
| Triptolide | Terpenoid | Tripterygium wilfordii Hook. f. | MCAO rats | -- | ↓: NF-κBp65, PUMA, caspase-3 | NF-κB | [37] |
| β-patchoulene | Terpenoid | Pogostemon cablin (Blanco) Benth. | MCAO rats | -- | ↑: IκBα,SOD, GSH-Px, Bcl-2 ↓: NF-κBp65, TLR4, caspase-3, Bax, TNF-α, IFN-β, IL-6 | NF-κB | [38] |
| Ginkgetin | Flavonoid | Ginkgo biloba L. | MCAO rats | -- | ↑: Bcl-2 ↓: LC3-II/LC3-I, DRAM, Beclin 1, cathepsin B, cathepsin D, DRAM, PUMA, Beclin 1, p53, Bax | NF-κB | [39] |
| Tanshinone IIA | Terpenoid | Salvia miltiorrhiza Bunge | MCAO rats | OGD/R cells | ↑: SOD ↓: MDA, TNF-α, IL-1β, IL-6, p-iκB, p-p65 | NF-κB | [40] |
| Breviscapine | Flavonoid | Erigeronbreviscapus (Vant.) Hand.-Mazz. | MCAO rats | -- | ↑: SOD, GSH-Px ↓: MDA, IL-6, IL-1β, TNF-α, PARP-1, COX2, iNOS, p65 | NF-κB | [41] |
| Diosgenin | Saponin | Dioscorea zingiberensis C. H. Wright | MCAO rats | OGD/R cells | ↑: HIKESHI, HSP70, IκBα ↓: TNF-α, IL-1β, IL-6, NF-κB | NF-κB | [42] |
| Icariin | Flavonoid | Epimedium brevicornum Maxim. | MCAO rats | -- | ↑: PPARα,PPARγ, IκBα ↓: TNF-α, IL-1β, IL-6, NF-κB | NF-κB | [43] |
| Berberine | Alkaloid | Coptis chinensis Franch. | MCAO rats | -- | ↑: SOD, GSH-Px, CD4+, CD8 ↓: NO, TNF-α, IFN-β, IL-6, NF-κB p65, NLRP3, ASC, caspase-3 | NF-κB | [44] |
| Nobiletin | Flavonoid | Citrus reticulata Blanco | MCAO rats | -- | ↑: Bcl-2, IL-10, ↓: TNF-α, IL-6, caspase-3, Bax, p-p38, MAPKAP-2 | MAPK | [52] |
| Coriolus versicolor polysaccharides | Polysaccharide | Coriolus versicolor (L. ex Fr.) Quel | MCAO rats | -- | ↑: Bcl-2, IL-10, ↓: Bax, TNF-α, IL-1β, caspase-3, p38 MAPK | MAPK | [53] |
| Scrophularia ningpoensis polysaccharides | Polysaccharide | Scrophularia ningpoensis Hemsl. | MCAO rats | -- | ↑: p-ERK, SOD ↓: p-JNK, p-p38, TNF-α, IL-1β, MDA, NO, NOS | MAPK | [54] |
| Emodin | Quinone | Rheum palmatum L. | -- | OGD/R cells | ↑: p-ERK-1/2, GLT-1, Bcl-2 ↓: caspase-3 | MAPK | [55] |
| Ginsenoside Rg1 | Terpenoid | Panax ginseng C. A. Mey. | MCAO rats | -- | ↑: Bcl-2 ↓: p-JNK, p-p38, caspase-3, Bax | MAPK | [56] |
| Baicalin | Flavonoid | Scutellaria baicalensis Georgi | -- | OGD/R cells | ↑: MAPK, ERK, MAP2, Bcl ↓: Bax, caspase-3 and -9 | MAPK | [57] |
| Curcumin | Polyphenol | Curcuma longa L. | MCAO rats | -- | ↓: LC3-II/LC3-I, IL-1, TLR4, p-38, p-p38 | MAPK | [58] |
| Astragaloside IV | Saponin | Astragalus penduliflorus subsp. mongholicus var. dahuricus (Fisch. ex DC.) X. Y. Zhu | MCAO rats | -- | ↑: HIF-1α, VEGF, Notch, DLL4 | Notch | [63] |
| Osthole | Coumarin | Cnidium monnieri (L.) Cusson | MCAO rats | -- | ↑: Bcl-2, Notch, NICD, Hes 1 ↓: Bax, caspase-3, | Notch | [64] |
| Biochanin A | Flavonoid | Trifolium pratense L. | MCAO rats | -- | ↑: SOD, GSH-Px, HO-1, Nrf2 ↓: MDA | Nrf2 | [71] |
| Rosmarinic acid | Polyphenol | Rosmarinus officinalis L. | MCAO rats | -- | ↑: Bcl-2, HO-1, Nrf2, SOD ↓: MDA, Bax | Nrf2 | [73] |
| Palmatine | Alkaloid | Coptis chinensis Franch | MCAO rats | OGD/R cells | ↑: Bcl-2, HO-1, Nrf2, SOD, CAT, p-AMPK ↓: MDA, Bax, TNF-α, IL-1β, IL-6 | Nrf2 | [74] |
| Taraxasterol | Terpenoid | Taraxacum mongolicum Hand.-Mazz. | OGD/R cells | ↑: HO-1, NQO-1, GPx-3, Nrf2, Bcl-2 ↓: ROS, MDA, Bax | Nrf2 | [75] | |
| Senkyunolide I | Terpenoid | Ligusticum chuanxiong Hort. | MCAO rats | -- | ↑: SOD, Erk1/2, Nrf2, NQO1, Bcl-2 ↓: MDA, caspase-3, caspase-9, Bax | Nrf2 | [76] |
| Ginkgolide B | Terpenoid | Ginkgo biloba L. | MCAO rats | OGD/R cells | ↑: SOD, p-Akt, HO-1, Nqo1p-Nrf2 ↓: ROS | Nrf2 | [77] |
| Resveratrol | Polyphenol | Reynoutria japonica Houtt. | MCAO rats | -- | ↑: p-AKT ↓: IL-1β, TNFα, COX2, MPO | PI3K/Akt | [87] |
| Ligustrazine | Alkaloid | Ligusticum chuanxiong Hort. | MCAO rats | OGD/R cells | ↑: p-eNOS, p-AKT | PI3K/Akt | [88] |
| Polygalasaponin F | Terpenoid | Polygala tenuifolia Willd. | OGD/R cells | ↑: p-AKT, Nrf2, HO-1 ↓: Bcl-2/Bax caspase-3 | PI3K/Akt | [89] | |
| Puerarin | Flavonoid | Puerariae Lobata (Willd.) Ohwi | 4-vessel occlusion rats | -- | ↑: p-GSK-3β, MCL-1, p-AKT ↓: caspase-3 | PI3K/Akt | [90] |
| Panax notoginseng Saponins | Saponin | Panax notoginseng (Burkill) F. H. Chen ex C. H. Chow | -- | OGD/R cells | ↑: p-AKT, Nrf2, HO-1 ↓: ROS | PI3K/Akt | [91] |
| Salidroside | Polyphenol | Rhodiola rosea L. | MCAO rats | -- | ↑: p-Akt ↓: IL-6, IL-1β, TNF-α, CD14, CD44, iNOs | PI3K/Akt | [92] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, X.-H.; Yin, F.-T.; Zhou, X.-H.; Zhang, A.-H.; Sun, H.; Yan, G.-L.; Wang, X.-J. The Signaling Pathways and Targets of Natural Compounds from Traditional Chinese Medicine in Treating Ischemic Stroke. Molecules 2022, 27, 3099. https://doi.org/10.3390/molecules27103099
Li X-H, Yin F-T, Zhou X-H, Zhang A-H, Sun H, Yan G-L, Wang X-J. The Signaling Pathways and Targets of Natural Compounds from Traditional Chinese Medicine in Treating Ischemic Stroke. Molecules. 2022; 27(10):3099. https://doi.org/10.3390/molecules27103099
Chicago/Turabian StyleLi, Xing-Hua, Feng-Ting Yin, Xiao-Hang Zhou, Ai-Hua Zhang, Hui Sun, Guang-Li Yan, and Xi-Jun Wang. 2022. "The Signaling Pathways and Targets of Natural Compounds from Traditional Chinese Medicine in Treating Ischemic Stroke" Molecules 27, no. 10: 3099. https://doi.org/10.3390/molecules27103099
APA StyleLi, X.-H., Yin, F.-T., Zhou, X.-H., Zhang, A.-H., Sun, H., Yan, G.-L., & Wang, X.-J. (2022). The Signaling Pathways and Targets of Natural Compounds from Traditional Chinese Medicine in Treating Ischemic Stroke. Molecules, 27(10), 3099. https://doi.org/10.3390/molecules27103099






