Abstract
Increasing evidence indicates that nobiletin (NOB) is a promising neuroprotective agent. Astrocyte activation plays a key role in neurodegenerative disorders. Thus, this study aims to investigate the effects of NOB on astrocyte activation and the potential mechanisms. In this study, astrocytes were exposed to hypoxia injury for 24 h to induce activation in vitro. Glial fibrillary acidic protein (GFAP) was chosen as a marker of astrocyte activation. To evaluate the effects of NOB on the migration of activated astrocytes, we used a scratch wound healing assay and Transwell migration assay. In addition, the levels of reactive oxygen species (ROS), malondialdehyde (MDA), mitochondrial membrane potential, Nrf2 and HO-1 were measured to investigate the mechanisms of NOB in the activation of astrocytes. We found that NOB alleviated astrocyte activation and decreased GFAP expression during hypoxia. Simultaneously, NOB alleviated the migration of astrocytes induced by hypoxia. With NOB treatment, hypoxia-induced oxidative stress was partially reversed, including reducing the production of ROS and MDA. Furthermore, NOB significantly improved the mitochondrial dysfunction in activated astrocytes. Finally, NOB promoted Nrf2 nuclear translocation and HO-1 expression in response to continuous oxidative damage. Our study indicates, for the first time, that NOB alleviates the activation of astrocytes induced by hypoxia in vitro, in part by ameliorating oxidative stress and mitochondrial dysfunction. This provides new insights into the neuroprotective effects of NOB.
1. Introduction
Nerve injury is one of the most common clinical manifestations, usually observed in ischemia and trauma. It is characterized by the death of neuron and axon degeneration. Currently, there is no effective treatment to reverse the damage. After nerve injury, reactive astrogliosis occurs, including the activation and migration of astrocytes, which is associated with axon damage [1]. In addition, the glial scar, composed mainly of astrocytes, is widely recognized as the major obstacle to axon regeneration [2,3]. Recently, it has been reported that reactive astrocytes play a key role in extracellular matrix (ECM) remodeling of the nerve by influencing the synthesis and deposition of ECM [4]. When neuropathy occurs, activated astrocytes can reshape ECM, creating an environment that is not conducive to axon regeneration [5]. Therefore, alleviating astrocyte activation is a potential neuroprotective strategy that can be used to reduce axon damage and promote axon regeneration.
Nobiletin (NOB) is a polymethoxylated flavone derived from the peels of citrus fruits [6]. In recent years, NOB has attracted increasing attention for its variety of potential health benefits. Several studies in vivo and in vitro have demonstrated that NOB exerts favorable effects that are anti-inflammatory [7], anti-oxidation [8], anti-aging [9], and the improvement on endoplasmic reticulum (ER) stress [10] and mitochondrial dysfunction [11]. Increasing evidence suggests that NOB is a promising molecule that can be used to prevent neurodegenerative and neurological diseases. In animal models of Alzheimer’s disease (AD), NOB has been shown to, respectively, improve short-term memory and cognitive impairment by reducing the soluble Aβ levels and scavenging the reactive oxygen species (ROS) [12]. Besides, the treatment of nobiletin was found to ameliorate motor impairment and cognitive deficits, and protect dopaminergic neurons from neurotoxicity in animal models of Parkinson’s disease (PD) [13,14]. Furthermore, NOB also displays an outstanding antioxidative ability in ischemic injury models. NOB treatment reduced brain edema and neurological deficits, and decreased the infarct volume at 24 h after ischemic stroke [15]. Nevertheless, the effects of NOB on activated astrocytes during neuropathy remain unclear.
As is well known, oxidative stress takes an important part in the process of neuropathy [16]. To investigate the effects of NOB on activated astrocyte and further explore the related mechanisms, we used a hypoxia model to mimic the conditions for neuropathy to activate astrocytes in vitro. The results of this study provide new insights into the neuroprotective effects of NOB, involving the inhibition of astrocyte activation.
2. Materials and Methods
2.1. Drugs and Reagents
Nobiletin (LCMS purified, 99.04%; dissolved in dimethyl sulfoxide, final dose 0.0005% v/v) was obtained from MedChemExpress (Jersey City, NJ, USA). The reagents and materials for each kit are listed with each method.
2.2. Cell Culture and Hypoxia Model
Rat astrocytes were purchased from American Type Culture Collection (ATCC) and were cultured in DMEM/F12 supplemented with 10% fetal bovine serum (FBS) and 1% penicillin-streptomycin at 37 °C and 5% CO2. The medium was replaced every 48 h. Briefly, astrocytes were cultured under normal conditions for 24 h, and then placed under hypoxic conditions (0.2% O2, 94.8% N2, 5% CO2) at 37 °C for 12 h, 24 h and 48 h to induce hypoxic injury. Moreover, astrocytes were incubated with 1 μM NOB under hypoxia as NOB treatment group.
2.3. Protein Expression Analysis
Astrocytes were lysed in ice-cold RIPA buffer (Beyotime, Shanghai, China) and protease inhibitor cocktail (Roche, Mannheim, Germany). The protein concentration was quantified using a BCA protein assay kit (Beyotime, Shanghai, China). The Simple Western system (WES) (ProteinSimple, San Jose, CA, USA) was used to quantify the level of the glial fibrillary acidic protein (GFAP). Briefly, the sample was mixed with 0.1× Sample buffer and fluorescent 5× Master Mix (ProteinSimple), and then denatured at 95 °C for 5 min. The prepared samples, biotinylated ladder, blocking solution, primary antibodies, secondary antibodies, secondary streptavidin-HRP, chemiluminescent reagent and wash buffer (ProteinSimple) were loaded on the microplates according to the manufacturer’s protocol. Then, the subsequent procedures, including protein separation, washing, immunodetection and data analysis, were performed automatically by the machine. The chemiluminescent images were produced and analyzed by the Compass software (version 6.0, ProteinSimple). Primary antibodies against GFAP (1:50 dilution, Cell Signaling Technology, MA, USA), β-actin (1:100 dilution, Cell Signaling Technology), Nrf2 (1:50 dilution, Abcam, Cambridge, UK), HO-1 (1:50 dilution, Cell Signaling Technology) and Histone H3 (1:250 dilution, Cell Signaling Technology) were used in the experiment. The results are shown as the fold intensity relative to the control group.
2.4. Scratch Wound Healing Assay
Astrocytes were seeded in 6-well plates at a density of 2 × 105 cells per well. When the cell monolayer was formed, a 200 μL tip was used to introduce a linear wound gap in the center of the layer. Then, the suspended cells were washed away with PBS. The attached cells were treated with hypoxia, or both hypoxia and NOB (1 μm), and starved in a culture medium without FBS for 24 h. The wound healing at 0, 12 and 24 h was photographed using a Leica DMI 3000B microscope (×10), and the wound area at different time points was measured using ImageJ. The assay was performed in triplicate per separate experiment. The results are expressed as the ratios of the migration area to the beginning area.
2.5. Transwell Migration Assay
After hypoxia with or without NOB (1 μm), astrocytes were subsequently detached by 0.25% trypsin. Transwell chambers with an 8 µm pore size (Corning, NY, USA) were used. Approximately 1 × 104 astrocytes in serum-free medium were added into the upper chambers and the lower chambers were filled with culture medium containing 10% FBS. After migration for up to 24 h, astrocytes underneath the membrane were fixed with ice-cold absolute methyl alcohol for 15 min, and then stained with 0.5% crystal violet for another 15 min. The unmigrated astrocytes were wiped away. Five random fields of each chamber were selected to count the number of the migrated astrocytes with a Leica DMI 3000B microscope (×10), and the mean values were calculated. The assay was performed in triplicate per separate experiment.
2.6. ROS Detection
Approximately 8 × 103 astrocytes were seeded in 96-well plates. The levels of ROS in astrocytes were detected using an ROS Assay Kit (Beyotime, Shanghai, China). Briefly, the pre-treated astrocytes were incubated with 2′,7′-Dichlorodihydrofluorescein diacetate (DCFH-DA) for 20 min at 37 °C, and then gently washed three times with PBS. The images were captured using a Leica DMI 3000B microscope (×10), and the fluorescence intensities were measured using ImageJ. The assay was performed in triplicate per separate experiment.
2.7. Malondialdehyde (MDA) Detection
The pre-treated astrocytes were collected and homogenized in ice-cold RIPA buffer (Beyotime, Shanghai, China), and then centrifuged at 10,000× g for 10 min at 4 °C to obtain the supernatants. To assess the levels of MDA in astrocytes, the MDA Assay Kit (Beyotime, Shanghai, China) was performed according to the manufacturer’s protocol, and then the reactive substances were measured at 532 nm with a microplate reader (Tecan; Infnite M1000, Männedorf, Switzerland). The results were normalized with protein content using a BCA protein assay kit (Beyotime, Shanghai, China), and are presented as fold activities to the control group.
2.8. Mitochondrial Membrane Potential Detection
Astrocytes were seeded in 96-well plates at a density of 8 × 103 cells per well. Mitochondrial membrane potential was detected using a JC-1 Assay Kit (Beyotime, Shanghai, China). In brief, the pre-treated astrocytes were incubated with JC-1 for 20 min at 37 °C, and then gently rinsed twice with wash buffer. The fluorescence intensities of red aggregates and green monomers were measured by a microplate reader (Tecan; Infnite M1000, Männedorf, Switzerland), and the ratio was calculated to elucidate the mitochondrial membrane potential. The images were obtained by a Leica DMI 3000B microscope (×20). The assay was performed in triplicate per separate experiment.
2.9. Immunofluorescence
Approximately 1 × 104 astrocytes were seeded in 24-well plates. After 24 h of hypoxia, the pre-treated astrocytes were fixed with paraformaldehyde for 30 min, and then cells were permeabilized with 0.3% Triton X-100 for 15 min and blocked with 3% bovine serum albumin (BSA) for 1 h. Subsequently, cells were incubated with primary antibody against rabbit anti-Nrf2 (1:200, Abcam) at 4 °C, overnight. After washing with PBS for three times, cells were incubated with Alexa Fluor 555-conjugated donkey anti-rabbit secondary antibody (1:800, Invitrogen, CA, USA) at room temperature for 1 h. The cell nuclei were stained with DAPI. The images were obtained using a Leica microscope (×20). The assay was done in triplicate per separate experiment.
2.10. Cell Viability
Approximately 8 × 103 astrocytes were seeded in 96-well plates under normal conditions for 24 h. Then, astrocytes were treated with or without NOB (1 μm) under hypoxia for another 24 h. The cell viability was examined by the Cell Counting Kit-8 (CCK-8) (Dojindo Laboratories, Kumamoto, Japan) according to the manufacturer’s protocol. The optical density values were measured at 450 nm using a microplate reader (Tecan; Infnite M1000, Männedorf, Switzerland), and the results are shown as ratios to the control values.
2.11. Statistical Analysis
Each experiment was repeated at least three times. The data are presented as means ± standard deviation, and were analyzed statistically with a One-way analysis of variance with a Bonferroni correction using GraphPad Prism 8.0 (San Diego, CA, USA). Lastly, a p value < 0.05 was defined as statistically significant.
3. Results
3.1. NOB Alleviates the Activation of Hypoxia-Induced Astrocytes
The upregulation of GFAP is considered as a marker of astrocyte reactivity [17]. To assess whether NOB could alleviate the activation of hypoxia-induced astrocytes, the level of GFAP expression was measured. After 12 h, 24 h and 48 h of hypoxia, the protein expression significantly increased in a time-dependent manner compared with the control group (Figure 1A; p = 0.0379, 0.0021 and 0.0438, respectively; n = 3), but there was no significant difference between the 24 h and 48 h group (p = 0.9998; n = 3). Therefore, 24 h of hypoxia was used to induce the activation of astrocytes in this study.
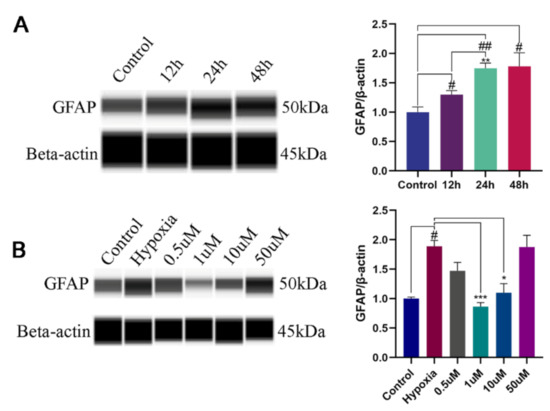
Figure 1.
The levels of GFAP expression in astrocytes by WES. (A) The levels of GFAP expression in astrocytes after hypoxia of 12 h, 24 h and 48 h, and the corresponding statistical analysis. # p < 0.05 and ## p < 0.01 vs. control, ** p < 0.01 vs. 12 h hypoxia group. (B) Effects of different NOB concentrations on GFAP expression in astrocytes after hypoxia of 24 h, and the corresponding statistical analysis. The data are shown as mean ± SD, n = 3, # p < 0.05 vs. control, * p < 0.05 and *** p ≤ 0.001 vs. 24 h hypoxia group.
To investigate the effects of NOB on activated astrocyte, cells were treated with different concentration of NOB during hypoxia. As shown in Figure 1B, the upregulation of GFAP was markedly attenuated by the administration of 1 μm and 10 μm NOB during the course of hypoxia (p = 0.0010 and 0.0309, respectively; n = 3). However, no statistical difference was found between the two groups (p = 0.4569; n = 3). The results indicate that NOB treatment can alleviate the astrocyte activation induced by hypoxia. According to the above results, we used 1 μM NOB to conduct the subsequent experiments.
3.2. NOB Inhibits the Migration of Hypoxia-Induced Astrocytes
Previous research has shown that reactive astrogliosis, including astrocyte activation and migration, occurs upon injury [18]. To explore the influence of NOB on astrocyte migration induced by hypoxia, scratch wound healing assay was performed in this study. As shown in Figure 2, astrocyte migration increased significantly after 12 h of hypoxia compared with the control (p = 0.0017, n = 3), which was reversed in the astrocytes supplemented with NOB during hypoxic conditions (p < 0.0001, n = 3). However, there was no significant difference in the area of migration after 24 h between the control group and hypoxia group (p = 0.9501, n = 3). This may be related to the severe hypoxia, and being serum-free for 24 h, which caused excessive damage to astrocytes.
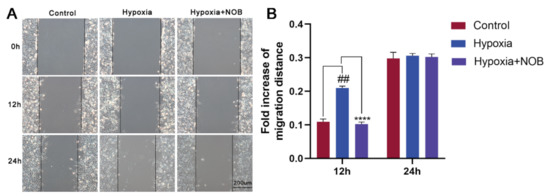
Figure 2.
NOB inhibits hypoxia-induced astrocyte migration in scratch wound healing assay. (A) The images of wound healing at 0, 12 and 24 h in different groups. Scale bar, 200 μm. (B) Statistical analysis of migration area. The data are expressed as mean ± SD, n = 3. ## p < 0.01 vs. control, **** p < 0.0001 vs. hypoxia group.
To further verify the effect of NOB on the cellular migration, we also performed a Transwell migration assay. Compared with the control group, a marked increase in the number of migrated cells was observed under hypoxia (Figure 3; p = 0.0046, n = 3). With the presence of NOB during the course of hypoxia, the change was largely attenuated (p = 0.0012, n = 3). Considering these results together, we can reach a conclusion that astrocyte activation is strongly alleviated by NOB treatment under hypoxia, which is indicated by the reduced GFAP expression and the slow cellular migration.
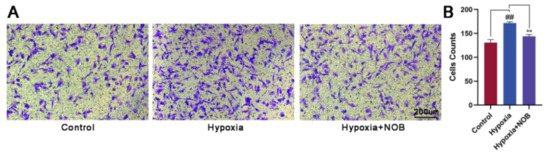
Figure 3.
NOB inhibits hypoxia-induced astrocyte migration in Transwell migration assay. (A) The images of migrated astrocytes in different groups after 24 h. Scale bar, 200 μm. (B) Statistical analysis of the number of migrated astrocytes. The values are expressed as mean ± SD, n = 3, ## p < 0.01 vs. control, ** p < 0.01 vs. hypoxia group.
3.3. NOB Reduces Oxidative Stress in Activated Astrocytes
NOB, as a kind of polymethoxylated flavone, has shown to be potentially neuroprotective in neurodegenerative diseases, such as AD and PD, which is related to its antioxidant capacity [19]. Therefore, we speculated that the inhibitory effect of NOB on activated astrocytes might also be associated with oxidative stress. To test our hypothesis, we measured two relevant biomarkers, including ROS and MDA.
As shown in Figure 4A,B, a significant increase in the mean ROS fluorescence intensity was observed in activated astrocytes by their constant exposure to hypoxic environment, relative to the control (p = 0.0084, n = 3). Nevertheless, it was largely reversed by the administration of NOB in the course of hypoxia (p = 0.0380, n = 3), which confirmed the capacity of NOB in improving hypoxia-induced oxidative stress. In addition, we found that the level of MDA was markedly higher in activated astrocytes after 24 h of hypoxia than the control level (Figure 4C; p = 0.0030, n = 3). Nevertheless, the change was attenuated by the simultaneous exposure of the astrocytes to NOB during hypoxia (p = 0.0127, n = 3). These results suggest that the inhibitory effect of NOB on astrocyte reactivity may be mediated, at least in part, by the antioxidant mechanism of ROS and MDA clearance.
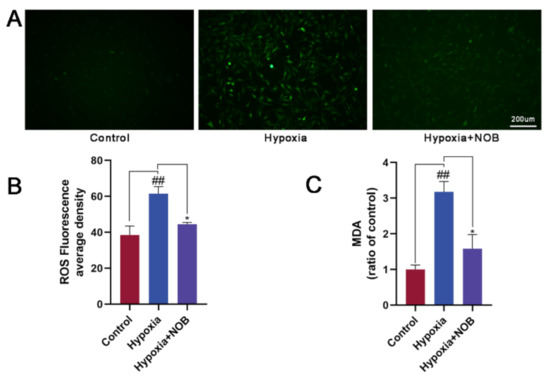
Figure 4.
NOB treatment shows strong antioxidant effects on astrocytes activated by hypoxia for 24 h, and reduces the intracellular ROS and MDA levels. (A) The ROS fluorescent images of astrocytes in different groups. Scale bar, 200 μm. (B) Quantitative analysis of ROS fluorescence intensities in different groups. (C) Statistical analysis of intracellular MDA levels in different groups. The values are shown as mean ± SD, n = 3, ## p < 0.01 vs. control, * p < 0.05 vs. hypoxia group.
3.4. NOB Ameliorates Mitochondrial Dysfunction of Activated Astrocytes
Several studies have demonstrated that NOB plays an important role in modulating mitochondrial function, including influencing the mitochondrial membrane potential [20,21,22,23]. To explore whether NOB can improve the mitochondrial function in activated astrocytes induced by hypoxia, we detected the mitochondrial membrane potential using a JC-1 Assay Kit. As shown in Figure 5B, hypoxia caused mitochondrial damage in activated astrocytes, resulting in a reduced mitochondrial membrane potential. Relative to the control, the difference was statistically significant (p = 0.0006, n = 3). Following the treatment of NOB during hypoxia, the change was largely reversed (p = 0.0206, n = 3). The fluorescent images show that the fluorescence intensities of red aggregates decreased while those of green monomers increased in activated astrocytes. Of note, NOB treatment attenuated the changes (Figure 5A). These results indicate that NOB helps to ameliorate the mitochondrial dysfunction of activated astrocytes.
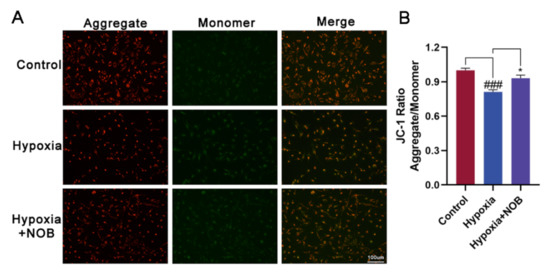
Figure 5.
NOB ameliorates mitochondrial dysfunction in activated astrocytes induced by hypoxia for 24 h. (A) The JC-1 fluorescent images of astrocytes in different groups. Scale bar, 100 μm. (B) Quantitative analysis of JC-1 fluorescence intensities in astrocytes in different groups. The data are presented as mean ± SD, n = 3, ### p < 0.001 vs. control, * p < 0.05 vs. hypoxia group.
3.5. NOB Promotes Nrf2 Nuclear Translocation and HO-1 Upregulation
As is known, nuclear factor erythroid 2-related factor 2 (Nrf2) is an important antioxidant regulator. In conditions of oxidative stress, Nrf2 is transferred into the nucleus and subsequently activates the expression of antioxidant enzymes, such as heme oxygenase 1 (HO-1) [24]. To explore the exact molecular mechanism of NOB reducing oxidative stress, we detected the level of Nrf2 and HO-1, as well as Nrf2 immunofluorescence. As shown in Figure 6A, the accumulation of Nrf2 immunofluorescence in the nuclei of astrocytes increased with NOB treatment. Furthermore, it is also supported by quantification of the nuclear Nrf2 protein (Figure 6B; p = 0.0056, n = 3). Compared with the control, the level of HO-1 increased after 24 h of hypoxia (Figure 6C; p = 0.0263, n = 3). Of note, NOB administration further upregulated the expression of HO-1 (p = 0.0135, n = 3). Above all, NOB promotes the nuclear translocation of Nrf2 and subsequently upregulates the expression of HO-1 in activated astrocytes.
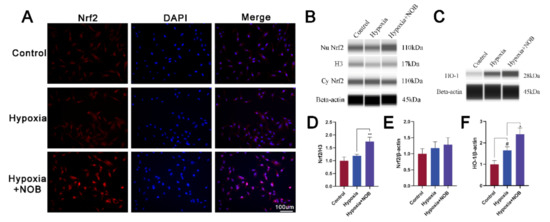
Figure 6.
NOB promotes Nrf2 nuclear translocation and HO-1 upregulation in activated astrocytes induced by hypoxia for 24 h. (A) The Nrf2 immunofluorescence images of astrocytes in different groups. Scale bar, 100 μm. (B) The levels of nuclear and cytoplasmic Nrf2 expression in astrocytes after hypoxia of 24 h. (C) The levels of HO-1 expression in astrocytes after hypoxia of 24 h. (D) Quantification of nuclear Nrf2 normalized to H3. (E) Quantification of cytoplasmic Nrf2 normalized to β-actin. (F) Quantification of HO-1 normalized to β-actin. The data are presented as mean ± SD, n = 3, # p < 0.05 vs. control, * p < 0.05 and ** p < 0.01 vs. hypoxia group.
3.6. The Effect of NOB on Cell Viability
Compared with the control level, the average viability of astrocytes was slightly decreased after 24 h of hypoxia. However, the difference was not statistically significant (Figure 7; p = 0.8730, n = 3). In addition, no apparent difference in cell viability was observed under hypoxic conditions with or without NOB treatment (p = 0.9591, n = 3). Although cell viability slightly improved under normal conditions after the treatment of NOB, there was no significant difference relative to the control group (p = 0.8238, n = 3). These results suggested that NOB alleviates hypoxia-induced astrocyte activation without influencing the cell viability and 1 μm NOB is nontoxic for astrocytes. Moreover, NOB does not affect the cell viability of astrocytes under normal conditions.
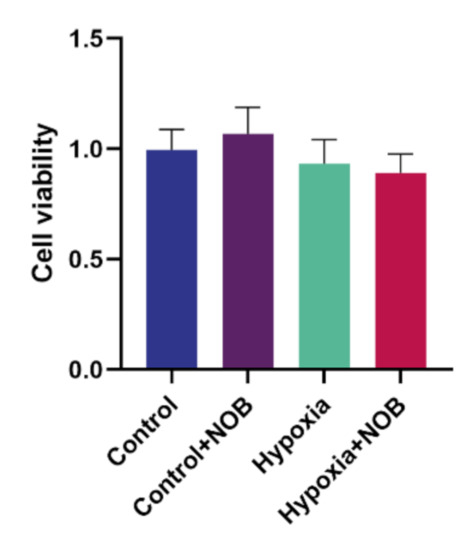
Figure 7.
Cell viability of astrocytes treated with or without 1 μm NOB under normal conditions and hypoxic conditions by the CCK-8 assay. The data are shown as mean ± SD, n = 3.
4. Discussion
Astrocytes are the major glial cells in the central nervous system (CNS), and play a critical role in the pathology of CNS. Due to their high sensitivity to changes in the microenvironment, astrocytes undergo a strong phenotypic transformation when damage occurs [18]. This process is called reactive astrogliosis, involving the activation and migration of astrocytes. In addition, previous research has indicated that it plays a key role in the secondary injury of CNS pathologies, including oxidative stress [25] and inflammation [26]. Therefore, the inhibition of astrocyte reactivity may be a potential neuroprotective target. Oxidative stress and hypoxia have been proven to play a critical role in the process of neurodegeneration in various neurological disorders, such as amyotrophic lateral sclerosis (ALS), PD and AD [27,28,29]. Oxidized free radicals at high concentrations are extremely reactive and damage neurons in CNS [30]. It also results in the dysfunction of activated astrocytes [25]. NOB is a polymethoxylated flavone and displays an excellent ability in antioxidant and anti- inflammatory in vivo and in vitro [31,32]. It is regarded as a promising therapy for alleviating neuronal degeneration. In this study, we indicate for the first time that NOB can alleviate astrocyte activation induced by hypoxia by reducing oxidative stress and improving mitochondrial dysfunction.
Consistent with previous research [33,34], hypoxia could induce the activation of astrocytes with the upregulation of GFAP expression. Notably, we found that GFAP expression significantly decreased in activated astrocytes with the administration of NOB during hypoxia. This suggests that NOB treatment alleviates astrocyte activation under hypoxic conditions. In line with a previous study [35], the results of this study indicated that NOB treatment did not affect cellular viability in either an anoxic or normal environment. Therefore, it is suggested that the inhibition of NOB on hypoxia-induced astrocyte activation is not the outcome of cytotoxic effects.
In response to damage, activated astrocytes would migrate to the site of injury and form a glial scar to surround the lesions [36]. In this study, we observed a faster migration rate of astrocytes after 24 h of hypoxia treatment than the control group using Transwell migration assay. This result is consistent with previous studies [37]. Nevertheless, the administration of NOB during hypoxia reversed the effect. Similarly, the migration of activated astrocytes was enhanced after 12 h of hypoxia treatment relative to the control in the scratch wound healing assay, and the change was attenuated by NOB treatment. Astrocyte migration is a critical step in the formation of glial scar and tissue remodeling [2]. Based on these findings, we infer that NOB treatment may help to reduce reactive astrocyte aggregation and glial scar formation at the injury site. However, the mechanisms that regulate the migration of activated astrocytes remain unclear [5]. Hence, further exploration is necessary. A better understanding of the mechanisms can lead to the development of new therapeutic strategies to reduce lesion size and promote repair in neurological diseases.
As is known, ROS has been involved as a key factor in neurodegenerative disorders, such as AD and PD [38,39]. When ROS production exceeds the cellular clear capacity, oxidative stress occurs, resulting in oxidative injury and cell death. MDA is another common biomarker of oxidative stress. It is the result of reactions between ROS and lipids, and is therefore affected by the overproduction of ROS [40]. In this study, the levels of ROS and MDA significantly increased in activated astrocytes induced by hypoxia. After NOB treatment during the course of hypoxia, the levels of ROS and MDA were remarkably decreased. It is suggested that NOB treatment can attenuate oxidative stress in activated astrocytes under hypoxic conditions. Previous research has shown that the mitochondrial respiratory chain is the main source of intracellular ROS [41]. As an organ with high energy requirements, CNS is extremely dependent on the mitochondria and their components [42]. When mitochondrial dysfunction occurs, oxidative stress is further exacerbated, followed by neuronal dysfunction and even death [43]. Mitochondrial membrane potential is a valuable indicator, reflecting the functional status of mitochondria in living cells. In this study, a decreased mitochondrial membrane potential was observed in hypoxia-induced activated astrocytes, while NOB treatment attenuated this change. Taken together, it demonstrated that NOB is a drug that targets mitochondrion, which can significantly enhance mitochondrial function and exhibits strong protective effects on astrocytes during hypoxia. Consistent with our study, Amarsanaa et al. [20] found NOB could regulate mitochondrial membrane potential through the K+ channel, and improve mitochondrial dysfunction by activating antioxidant signaling cascades. Thus, we infer that NOB alleviates astrocyte activation induced by hypoxia by ameliorating oxidative stress and mitochondrial dysfunction, to facilitate a stable transition between resting and active states of astrocytes in stress conditions.
Additionally, Nrf2 is thought to be the “master regulator” of antioxidant responses, regulating the expression of downstream antioxidant genes [44]. Recently, increasing evidence has demonstrated that Nrf2 is a potential therapeutic target for neurodegenerative disorders [44,45]. Under homeostatic conditions, Nrf2 is sequestered in the cytoplasm by binding to Kelch-like ECH associated protein 1 (Keap1). When oxidative stress occurs, Keap1 undergoes conformational changes followed by the release of Nrf2, which is then transferred into the nucleus and subsequently activates the expression of antioxidant enzymes, such as HO-1 [24]. Here, we found that NOB treatment promoted the nuclear translocation of Nrf2 in activated astrocytes, thereby reducing oxidative damage. Furthermore, HO-1 is a crucial antioxidant enzyme downstream of Nrf2. Previous research has shown that exposure to hypoxia can induce the expression of HO-1 [46]. Similarly, we observed an increased level of HO-1 in astrocytes by the constant exposure to a hypoxic environment. It may be an endogenous antioxidant response. However, this compensatory mechanism may begin to fail with the cumulative damage of hypoxia. With the treatment of NOB, the expression of HO-1 was further enhanced to resist oxidative damage.
In conclusion, our study indicates that NOB alleviates astrocyte activation induced by hypoxia in vitro by improving oxidative stress and enhancing mitochondrial function, as well as by increasing Nrf2 nuclear translocation and HO-1 upregulation. This presents a novel insight into the neuroprotective effects of NOB. NOB is a promising neuroprotective drug, so it is necessary to further investigate its exact neuroprotective mechanism and clarify its role in neurodegenerative disorders.
Author Contributions
Conceptualization, F.G. and J.W.; methodology, F.H., F.G. and J.W.; validation, D.W., F.H. and J.W.; formal analysis, D.W.; investigation, D.W.; resources, F.H., F.G. and J.W.; writing—original draft preparation, D.W.; writing—review and editing, F.G. and J.W.; visualization, D.W.; supervision, F.H. and J.W.; project administration, J.W.; funding acquisition, J.W. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants from the National Natural Science Foundation of China (No. 81770925, 81790641), Outstanding academic leaders in Shanghai (20XD1401100), Program for Outstanding Medical Academic Leader (2019LJ01) and the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences (2018PT32019).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds studied are unavailable from the authors for distribution.
References
- Lee, E.J.; Han, J.C.; Park, D.Y.; Kee, C. A neuroglia-based interpretation of glaucomatous neuroretinal rim thinning in the optic nerve head. Prog. Retin. Eye Res. 2020, 77, 100840. [Google Scholar] [CrossRef] [PubMed]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sofroniew, M.V. Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci. 2009, 32, 638–647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Munemasa, Y.; Kitaoka, Y. Molecular mechanisms of retinal ganglion cell degeneration in glaucoma and future prospects for cell body and axonal protection. Front. Cell Neurosci. 2012, 6, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yazdankhah, M.; Shang, P.; Ghosh, S.; Hose, S.; Liu, H.; Weiss, J.; Fitting, C.S.; Bhutto, I.A.; Zigler, J.S.; Qian, J.; et al. Role of glia in optic nerve. Prog. Retin. Eye Res. 2021, 81, 100886. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid composition of fruit tissues of citrus species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rong, X.; Xu, J.; Jiang, Y.; Li, F.; Chen, Y.; Dou, Q.P.; Li, D. Citrus peel flavonoid nobiletin alleviates lipopolysaccharide-induced inflammation by activating IL-6/STAT3/FOXO3a-mediated autophagy. Food Funct. 2021, 12, 1305–1317. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, D.; Zhang, P.; Wang, X.; Du, G.; Brennan, C.; Li, S.; Ho, C.-T.; Zhao, H. Antioxidant Protection of Nobiletin, 5-Demethylnobiletin, Tangeretin, and 5-Demethyltangeretin from Citrus Peel in Saccharomyces cerevisiae. J. Agric. Food Chem. 2018, 66, 3155–3160. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, H.; Li, T.; Chen, L.; Zheng, B.; Liu, R.H. Nobiletin Delays Aging and Enhances Stress Resistance of Caenorhabditis elegans. Int. J. Mol. Sci. 2020, 21, 341. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyata, Y.; Matsumoto, K.; Kusano, S.; Kusakabe, Y.; Katsura, Y.; Oshitari, T.; Kosano, H. Regulation of Endothelium-Reticulum-Stress-Mediated Apoptotic Cell Death by a Polymethoxylated Flavone, Nobiletin, Through the Inhibition of Nuclear Translocation of Glyceraldehyde 3-Phosphate Dehydrogenase in Retinal Muller Cells. Cells 2021, 10, 669. [Google Scholar] [CrossRef] [PubMed]
- Nohara, K.; Mallampalli, V.; Nemkov, T.; Wirianto, M.; Yang, J.; Ye, Y.; Sun, Y.; Han, L.; Esser, K.A.; Mileykovskaya, E.; et al. Nobiletin fortifies mitochondrial respiration in skeletal muscle to promote healthy aging against metabolic challenge. Nat. Commun. 2019, 10, 3923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, A.; Aoyama, Y.; Shin, E.J.; Nam, Y.; Kim, H.-C.; Nagai, T.; Yokosuka, A.; Mimaki, Y.; Yokoy, T.; Oshizumi, Y.; et al. Nobiletin, a citrus flavonoid, improves cognitive impairment and reduces soluble Abeta levels in a triple transgenic mouse model of Alzheimer’s disease (3XTg-AD). Behav. Brain Res. 2015, 289, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Jeong, K.H.; Jeon, M.T.; Kim, H.D.; Jung, U.J.; Jang, M.C.; Chu, J.W.; Yang, S.J.; Choi, I.Y.; Choi, M.-S.; Kim, S.R. Nobiletin protects dopaminergic neurons in the 1-methyl-4-phenylpyridinium-treated rat model of Parkinson’s disease. J. Med. Food 2015, 18, 409–414. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, Y.; Ohizumi, Y.; Yokosuka, A.; Mimaki, Y.; Fukunaga, K. Nobiletin treatment improves motor and cognitive deficits seen in MPTP-induced Parkinson model mice. Neuroscience 2014, 259, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, X.; Zhang, C.; Bai, X.; Zhang, J.; Zhao, X.; Chen, L.; Wang, L.; Zhu, C.; Cui, L.; et al. Nobiletin promotes antioxidant and anti-inflammatory responses and elicits protection against ischemic stroke in vivo. Brain Res. 2016, 1636, 130–141. [Google Scholar] [CrossRef]
- Cuenca, N.; Fernández-Sánchez, L.; Campello, L.; Maneu, V.; De la Villa, P.; Lax, P.; Pinilla, I. Cellular responses following retinal injuries and therapeutic approaches for neurodegenerative diseases. Prog. Retin. Eye Res. 2014, 43, 17–75. [Google Scholar] [CrossRef]
- Lagos-Cabre, R.; Burgos-Bravo, F.; Avalos, A.M.; Leyton, L. Connexins in Astrocyte Migration. Front. Pharmacol. 2019, 10, 1546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hart, C.G.; Karimi-Abdolrezaee, S. Recent insights on astrocyte mechanisms in CNS homeostasis, pathology, and repair. J. Neurosci. Res. 2021, 99, 2427–2462. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, A.; Ohizumi, Y. Potential Benefits of Nobiletin, A Citrus Flavonoid, against Alzheimer’s Disease and Parkinson’s Disease. Int. J. Mol. Sci. 2019, 20, 3380. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amarsanaa, K.; Kim, H.-J.; Ko, E.-A.; Jo, J.; Jung, J.J.A.S.-C. Nobiletin Exhibits Neuroprotective Effects against Mitochondrial Complex I Inhibition via Regulating Apoptotic Signaling. Exp. Neurobiol. 2021, 30, 73–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Guo, S.; Dong, Q. Nobiletin suppresses IL-21/IL-21 receptor-mediated inflammatory response in MH7A fibroblast-like synoviocytes (FLS): An implication in rheumatoid arthritis. Eur. J. Pharmacol. 2020, 875, 172939. [Google Scholar] [CrossRef] [PubMed]
- Qi, G.; Mi, Y.; Fan, R.; Li, R.; Liu, Z.; Liu, X. Nobiletin Protects against Systemic Inflammation-Stimulated Memory Impairment via MAPK and NF-kappaB Signaling Pathways. J. Agric. Food Chem. 2019, 67, 5122–5134. [Google Scholar] [CrossRef] [PubMed]
- Sharikadze, N.; Jojua, N.; Sepashvili, M.; Zhuravliova, E.; Mikeladze, D.G. Mitochondrial Target of Nobiletin’s Action. Nat. Prod. Commun. 2016, 11, 1833–1838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Q.; Liu, J.; Duan, H.; Li, R.; Peng, W.; Wu, C. Activation of Nrf2/HO-1 signaling: An important molecular mechanism of herbal medicine in the treatment of atherosclerosis via the protection of vascular endothelial cells from oxidative stress. J. Adv. Res. 2021, 34, 43–63. [Google Scholar] [CrossRef] [PubMed]
- Sheng, W.S.; Hu, S.; Feng, A.; Rock, R.B. Reactive oxygen species from human astrocytes induced functional impairment and oxidative damage. Neurochem. Res. 2013, 38, 2148–2159. [Google Scholar] [CrossRef] [Green Version]
- Goetzl, E.J.; Schwartz, J.B.; Abner, E.L.; Jicha, G.A.; Kapogiannis, D. High complement levels in astrocyte-derived exosomes of Alzheimer disease. Ann. Neurol. 2018, 83, 544–552. [Google Scholar] [CrossRef]
- Ju, Y.; Tam, K.Y. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regen. Res. 2022, 17, 543–549. [Google Scholar]
- Li, C.; Zhang, Y.; Liu, R.; Mai, Y. Ramelteon ameliorated 1-methyl-4-phenylpyridinium (MPP+)-induced neurotoxicity in neuronal cells in a mitochondrial-dependent pathway. Bioengineered 2021, 12, 4868–4877. [Google Scholar] [CrossRef] [PubMed]
- Kim, K. Glutathione in the Nervous System as a Potential Therapeutic Target to Control the Development and Progression of Amyotrophic Lateral Sclerosis. Antioxidants 2021, 10, 1011. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Zhao, Z.; Goto, S.; Koltai, E. Age-associated neurodegeneration and oxidative damage to lipids, proteins and DNA. Mol. Asp. Med. 2011, 32, 305–315. [Google Scholar] [CrossRef] [PubMed]
- Hosokawa, Y.; Hosokawa, I.; Ozaki, K.; Matsuo, T. Nobiletin Inhibits Inflammatory Reaction in Interleukin-1beta-Stimulated Human Periodontal Ligament Cells. Pharmaceutics 2021, 13, 667. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Wang, R.; Fan, H.; Ni, Z. Nobiletin ameliorates streptozotocin-cadmium-induced diabetic nephropathy via NF-kappaB signalling pathway in rats. Arch. Physiol. Biochem. 2021, 4, 34346259. [Google Scholar]
- Daverey, A.; Agrawal, S.K. Neuroprotective effects of Riluzole and Curcumin in human astrocytes and spinal cord white matter hypoxia. Neurosci. Lett. 2020, 738, 135351. [Google Scholar] [CrossRef] [PubMed]
- Mesentier-Louro, L.A.; Shariati, M.A.; Dalal, R.; Camargo, A.; Kumar, V.; Shamskhou, E.A.; Perez, V.D.J.; Liao, Y.J. Systemic hypoxia led to little retinal neuronal loss and dramatic optic nerve glial response. Exp. Eye Res. 2020, 193, 107957. [Google Scholar] [CrossRef]
- Ihara, H.; Yamamoto, H.; Ida, T.; Tsutsuki, H.; Sakamoto, T.; Fujita, T.; Okada, T.; Kozaki, S. Inhibition of nitric oxide production and inducible nitric oxide synthase expression by a polymethoxyflavone from young fruits of Citrus unshiu in rat primary astrocytes. Biosci. Biotechnol. Biochem. 2012, 76, 1843–1848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wanner, I.B.; Anderson, M.A.; Song, B.; Levine, J.; Fernandez, A.; Gray-Thompson, Z.; Ao, Y.; Sofroniew, M.V. Glial Scar Borders Are Formed by Newly Proliferated, Elongated Astrocytes That Interact to Corral Inflammatory and Fibrotic Cells via STAT3-Dependent Mechanisms after Spinal Cord Injury. J. Neurosci. 2013, 33, 12870–12886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, W.; Liu, X.; Gao, L.; Tian, C.; Shi, Q. αA-Crystallin inhibits optic nerve astrocyte activation induced by oxygen-glucose deprivation in vitro. Life Sci. 2021, 278, 119533. [Google Scholar] [CrossRef] [PubMed]
- Oli, V.; Gupta, R.; Kumar, P. FOXO and related transcription factors binding elements in the regulation of neurodegenerative disorders. J. Chem. Neuroanat. 2021, 116, 102012. [Google Scholar] [CrossRef]
- Savitt, J.M.; Dawson, V.L.; Dawson, T.M. Diagnosis and treatment of Parkinson disease: Molecules to medicine. J. Clin. Investig. 2006, 116, 1744–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.; Behl, T.; Sehgal, A.; Singh, S.; Sharma, N.; Aleya, L.; Bungau, S. Connecting the dots between mitochondrial dysfunction and Parkinson’s disorder: Focus mitochondria-targeting therapeutic paradigm in mitigating the disease severity. Environ. Sci. Pollut. Res. Int. 2021, 28, 37060–37081. [Google Scholar] [CrossRef]
- Panes, J.D.; Wendt, A.; Ramirez-Molina, O.; Castro, P.A.; Fuentealba, J. Deciphering the role of PGC-1alpha in neurological disorders: From mitochondrial dysfunction to synaptic failure. Neural Regen. Res. 2022, 17, 237–245. [Google Scholar]
- Hybertson, B.M.; Gao, B.F.; Bose, S.K.; McCord, J.M. Oxidative stress in health and disease: The therapeutic potential of Nrf2 activation. Mol. Asp. Med. 2011, 32, 234–246. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Li, J.; Zheng, Y. The Potential Role of Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2) in Glaucoma: A Review. Med. Sci. Monit. 2020, 26, e921514. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.J.; Jiang, B.-H.; Chin, B.Y.; Iyer, N.V.; Alam, J.; Semenza, G.L.; Choi, A. Hypoxia-inducible Factor-1 Mediates Transcriptional Activation of the Heme Oxygenase-1 Gene in Response to Hypoxia. J. Biol. Chem. 1997, 272, 5375–5381. [Google Scholar] [CrossRef] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).