Abstract
Three isopimarane diterpenes [fladins B (1), C (2), and D (3)] were isolated from the twigs and leaves of Chinese folk medicine, Isodon flavidus. The chemical structures were determined by the analysis of the comprehensive spectroscopic data, and the absolute configuration was confirmed by X-ray crystallographic analysis. The structures of 1–3 were formed from isopimaranes through the rearrangement of ring A by the bond break at C-3 and C-4 to form a new δ-lactone ring system between C-3 and C-9. This structure type represents the first discovery of a natural isopimarane diterpene with an unusual lactone moiety at C-9 and C-10. In the crystal of 1, molecules are linked to each other by intermolecular O-H···O bonds, forming chains along the b axis. Compounds 1–3 were evaluated for their bioactivities against different diseases. None of these compounds displayed cytotoxic activities against HCT116 and A549 cancer cell lines, antifungal activities against Trichophyton rubrum and T. mentagrophytes, or antiviral activities against HIV entry at 20 µg/mL (62.9–66.7) µM. Compounds 1 and 3 did not show antiviral activities against Ebola entry at 20 µg/mL either; only 2 was found to show an 81% inhibitory effect against Ebola entry activity at 20 µg/mL (66.7 µM). The bioactivity evidence suggested that this type of compound could be a valuable antiviral lead for further structure modification to improve the antiviral potential.
1. Introduction
Natural products, especially plant-derived natural products, have long been an important source of molecules for drug discovery [1]. Diterpenes are one class of plant compounds that have been constantly investigated for their valuable potential in drug discovery due to their diversified structures and pronounced biological activities [2,3]. Isopimaranes are a subtype of diterpenes featuring carbotricyclic rings. Their rich stereochemistry features and broad biological activities render them the interesting molecules applicable to the pharmaceutical industry. They have been reported to own remarkable biological activities, including cytotoxic, antimicrobial, and anti-inflammatory activities [4,5,6,7,8]. The 3,4-Seco-isopimarane diterpenes belong to isopimaranes with the rearrangement of ring A by the bond break between C-3 and C-4. To date, there have been few reports on natural 3,4-seco-isopimaranes [9,10], and no antiviral activity has been reported for this type of compound.
The genus Isodon (formally Rabdosia) contains approximately 150 species of wild plants, and about 30 species have a long tradition of use as Chinese popular folk medicines [11,12]. Isodon species are rich in producing diterpenes with a diversity of carbon skeletons and a variety of biological activities [11,13,14]. Diterpenoids with a tricyclic core system and their seco-derivatives were also reported from this genus, including I. rubescens, I. lophanthoides, and I. flavidus [15,16,17,18]. I. flavidus is a commonly used herb to defend against tinea pedis by the Miao minority in China [10]. Phytochemical investigation of this plant revealed the presence of isopirmarane [19,20] and ent-kaurane diterpenoids [15], along with other types of compounds such as flavonoids and steroids [19,20]. However, no biological evaluation of this medicinal plant was reported in these articles. In our previous study, two antifungal constituents, fladin A and lophanic acid, were isolated and identified from this plant. Fladin A was a 3,4-seco-isopimarane diterpenoid, and lophanic acid was an abietane diterpenoid [10]. In a search for novel bioactive compounds from I. flavidus, we further obtained three new diterpenes, namely fladins B (1), C (2), and D (3). They also belong to 3,4-seco-isopimarane diterpenes but with a δ-lactone group formed between C-3 and C-9 (Figure 1). This article reports the isolation, structural identification, as well as biological evaluation (cytotoxicity against cancer cells, antifungal activity against Trichophyton fungi, and anti-Ebola entry activity) of these novel 3,4-seco-isopimarane diterpenes from the plant I. flavidus.
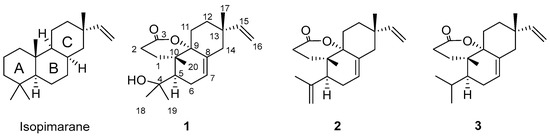
Figure 1.
Chemical structures of isopimarane skeleton and compounds 1–3.
2. Results
Plants from the genus Isodon are claimed to be a rich source of bioactive diterpenes with a diversity of carbon skeletons. The folk medicinal uses of I. flavidus [21] and our previous discovery of antifungal diterpenes from I. flavidus [10] gave rise to our great interest in this medicinal plant. Our ongoing research of the methanolic extract of the twigs and leaves of I. flavidus led to the identification of three new 3,4-seco-isopimarane diterpenes.
2.1. Chemistry and Structure Elucidation
Compound 1, [α −8.59° (c 0.33, CHCl3), was obtained as colorless cubic crystals, and the molecular formula was determined to be C20H30O3 by positive HR-ESI-MS ([M + H]+ m/z 319.2269, calcd. 319.2273). The IR spectrum of 1 displayed a hydroxyl group (νmax 450 cm−1) and a δ-lactone group (νmax 1694 cm−1). Evidenced from the 1H and 13C NMR (Table 1), DEPT spectral, and the HSQC correlation data, the structure of 1 showed signals of four tertiary methyls [δH 0.88, 1.14, 1.28, and 1.33 (each 3H, s); δC 19.9, 21.2, 26.4, and 34.4], a vinyl [δH 5.80 (1H, dd, J = 17.5, 10.7 Hz), 4.89 (1H, dd, J = 10.7, 1.2 Hz), 4.94 (1H, dd, J = 17.5, 1.2 Hz); δC 149.2 and 110.2], one trisubstituted carbon–carbon double bond [δH 5.57 (1H, dt, J = 5.6, 2.1); δC 127.5 and 132.8], six methylenes, one methine [δH 2.07 (1H, m); δC 43.8], two oxygenated quaternary carbons (δC 74.6 and 87.0), two sp3 aliphatic carbons (δC 37.8 and 21.2), and one carbonyl carbon (δC 172.7). These data indicated 1 to be a diterpenoid.

Table 1.
1H and 13C NMR spectral data (J in Hz) of 1–3 in CDCl3.
According to the HMBC spectral data (Figure 2) of 1, the presence of correlations from H-15 to C-12, -13, -14, and -17, from H-16 to C-13 and -15, and from H-17 to C-12, -13, -14, and -15 indicated the vinyl group at C-13. The presence of correlations from H-7 to C-6 and -8 assigned the trisubstituted carbon–carbon double bond formed between C-7 and C-8. The correlations observed from H-5 to C-4, -6, -10, -18, -19, and -20, and from H-20 to C-1, -5, -9, and -10 assigned the two oxyquaternary carbons at C-4 and -9, respectively. The correlations from H-2 to C-1 and -3, from H-1 to C-3, -9, and -10, and from the methyl signal H-20 to C-1, -5, -9, and -10 revealed a δ-lactone ring present in the structure of 1.
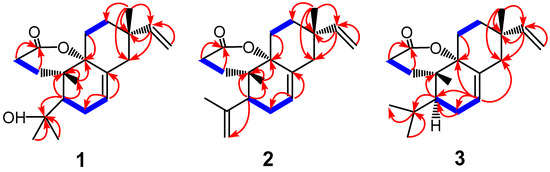
Figure 2.
Key HMBC (in red) and COSY (in blue) correlations for compounds 1–3.
From the molecular formula of C20H30O3 of compound 1, six double bond equivalents were calculated, which were assigned to the three rings (the lactone ring, ring B, ring C, and the three double bonds (the carbonyl group and the two carbon–carbon double bonds)). As a result, no additional ring existed in the structure of 1, which determined that the original ring A in an isopimarane skeleton was opened in 1. No HMBC correlation signal was observed from H-5 to the carbonyl carbon (C-3), revealing that the ring A opening occurred at the bond between C-3 and C-4, which was further confirmed by the presence of the δ-lactone ring present in the structure of 1.
In order to further determine the absolute configuration, 1 was recrystallized in methanol to afford colorless crystals and was analyzed by X-ray crystallography. The crystallographic data of 1 (CCDC 1033449) are given in the supporting information. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre [22]. The structural refinement of the Cu Kα data of the crystal of 1 resulted in a Flack parameter of −0.1 (4) [23,24], allowing an explicit assignment of the absolute structure of 1 (Figure 3). The four chiral centers, C-5, -9, -10, and -13, were thus determined as R, S, S, and S, respectively. Accordingly, compound 1 was thus identified as 4α-hydroxy-3,4-seco-isopimara-7,15-diene-3,9α-olide, and it was given the trivial name of fladin B.
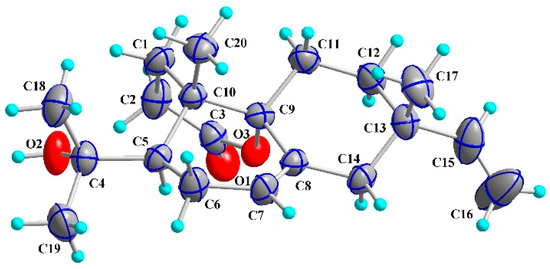
Figure 3.
X-ray crystallographic structure of 1.
Compound 2, [α −2.14° (c 0.19, CHCl3), was obtained as colorless cubic crystals, and the molecular formula was determined to be C20H28O2 by positive HR-ESI-MS ([M + Na]+ m/z 323.1978 (calcd. 323.1987). The IR spectrum of 2 displayed a carbonyl group (νmax 1718 cm−1). A comparison of the 1H and 13C NMR data suggested that the structure resembled compound 1. When comparing the 13C NMR data, we observed that the signals at δC 74.2 and 34.1 in 1 disappeared. Instead, new signals appeared at δC 145.4 and 115.9, which were assigned to the double bond of Δ4,19 through the analysis of the 2D spectral correlation data of 2 (Figure 2). Accordingly, 2 was elucidated as 3,4-seco-isopimara-4 (18),7,15-triene-3,9α-olide, and it was given the trivial name of fladin C.
Compound 3, [α −12.9° (c 1.09, MeOH), was also obtained as colorless cubic crystals, and the molecular formula was determined to be C20H30O2 by positive HR-ESI-MS ([M + Na]+ m/z 325.2045, calcd. 325.2144). Compound 3 was found to be another closely related congener of 1. In comparison with 1, the signal at δC 74.2 disappeared, but a new signal was observed at δC 25.5 in the 13C NMR spectrum. Analysis of the HSQC and HMBC spectral data (Figure 2) of 3 assigned the signal of δC 25.5 belonging to the isopropyl group attached at C-5. Compound 3 was, thus, determined as 3,4-seco-isopimara-7,15-diene-3,9α-olide, and it was given a trivial name of fladin D.
2.2. X-ray Crystallographic Analysis
The molecular structures of the title compounds were built up from a bicycle [4.4.0] decene ring bearing δ-lactone, methyl, and vinyl substituents. The structural differences arose at C-5, with 1 having a 2-hydroxypropyl group, 2 having an isopropenyl group, and 3 having an isopropyl group. The X-ray analysis provided solid evidence to reveal the stereochemistry of this type of compound. In the molecule of 1, the 2-hydroxypropyl group was β-oriented, as were the methyls of C-10 and C-13, whereas the δ-lactone ring and the C-13 vinyl group were α-oriented. In the skeleton, the δ-lactone ring (atoms C-1 to C-3/C-9/C-10/O-3) was confirmed to be a chair conformation, with C-3 and C-10 deviating from the best plane through atoms C-1/C-2/C-9/O-3 by -0.191 Å and 0.680 Å, respectively. The two trans-fused six-membered rings B (atoms C-5 to C-10) and C (atoms C-8 to C-9/C-11 to C-14) adopted half-chair and chair conformations, respectively. In the cyclohexene ring B, the deviations of atoms C-5 and C-6 from the best plane through atoms C-7/C-8/C-9/C-10 were 0.361 Å and 0.932 Å, respectively. In cyclohexane ring C, atoms C-9 and C-13 deviated by 0.585 Å and −0.674 Å from the best plane through atoms C-8/C-11/C-12/C-14.
The packing of compound 1 was characterized by a network of hydrogen bonds. A strong O—H···O hydrogen bond, namely, O1—H1···O2i (Table 2), was formed via the hydroxyl group and the lactone oxo group running along the b axis direction (Figure 4).

Table 2.
Hydrogen bond geometry of 1 (Å, °).
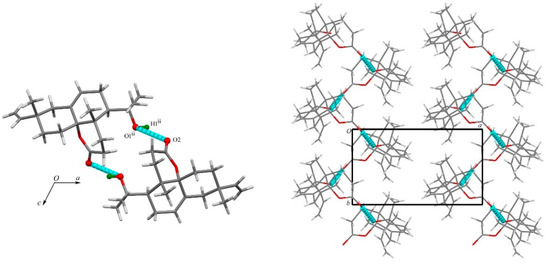
Figure 4.
The crystal structure of 1 (hydrogen bonds shown as blue dashed lines). The view is along the b axis (left) and c axis (right). [Symmetry code: (ii) −x, 1/2 + y, −z.].
2.3. Plausible Biogenetic Pathway of Fladins B–D (1–3)
Fladins B–D (1–3) possess a rare 3,4-seco-isopimarane skeleton with an unusual lactone moiety formed at C-9 and C-10. Hypothetically, they might be derived from isopimarane diterpenes, which are found abundantly in Isodon plants [11,12]. The original precursor of the diversified isopimarane-type diterpenes in Isodon plants could well be 3β-hydroxy-8,15-isopimaradiene (4), a rich isopimarane diterpenoid observed in several Isodon plants [10]. Given that background, a plausible biogenetic pathway of 1–3 originating from the natural precursor (4) is proposed (Scheme 1) [10,25,26]. Compound 4 was initially converted to 5 through the oxidation of 3-OH and the epoxidation of the Δ8,9 double bond. By insertion of an oxygen atom between C-3 and C-4 of 5 (an enzymatic Baeyer–Villager reaction), compound 6 with a seven-membered lactone ring was formed. Ester hydrolysis of 6 by esterase produced diterpene carboxylic acid 7, which underwent protonation in the epoxide group, followed by an SN2 reaction between the carboxylic acid group and the protonated epoxide group to provide 9. Elimination of a hydroxy group in 9 yielded fladin B (1), which eliminated another hydroxy group to generate fladin C (2). Compound 2 underwent a hydrogenation reduction on the Δ4,19 double bond to produce fladin D (3).
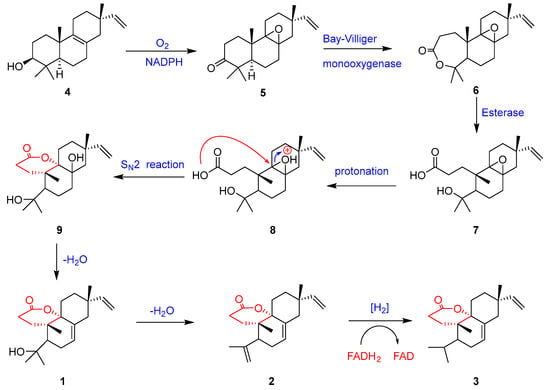
Scheme 1.
Proposed biogenetic pathway of 1–3.
2.4. Bioactivity Evaluation of Fladins B–D (1–3)
Previously, two diterpenoids isolated from I. flavidus were reported to have inhibition activity against Trichophyton rubrum with MIC values around 25.5–197 μM [10]. In order to find out whether our isolates were the active ingredients of this medicinal plant, we evaluated the antifungal potential against two athlete’s foot fungal strains (T. rubrum and T. mentagrophytes) of compounds 1–3. However, no inhibitory activities against the two fungi were observed at concentrations below 20 µg/mL (62.9–66.7 µM). Cell viability was also measured for each pure compound to determine the cytotoxic activities of the compounds, but no compound was found to show inhibitory effects against HCT116 and A549 cancer cells at concentrations below 20 µg/mL (62.9–66.7 µM) as well. The compounds were then tested for their antiviral potential using our established pseudovirus screening assay system. No compounds displayed antiviral activities against HIV entry below the concentration of 20 µg/mL (62.9–66.7 µM), and compounds 1 and 3 also showed no antiviral activities against Ebola entry below the concentration of 20 µg/mL (62.9–66.7 µM). Interestingly, only fladin C (2) showed 81% inhibition against the Ebola virus at a concentration of 20 µg/mL (66.7 μM). To the best of our knowledge, there have been no reports of seco-isopimarane-type diterpenes having antiviral activity.
3. Discussion
Plants from the genus Isodon are claimed to be a rich source of bioactive diterpenes with a diversity of carbon skeletons. The folk medicinal uses of I. flavidus [22] and our previous discovery of antifungal diterpenes from I. flavidus [10] gave rise to our great interest in this medicinal plant. Our ongoing research of the methanolic extract of the twigs and leaves of I. flavidus led to the identification of three new 3,4-seco-isopimarane diterpenes [fladins B–D (1–3)]. Their structures were determined based on the analysis of the comprehensive spectroscopic data, and the absolute configuration of fladin B (1) was determined by X-ray crystallographic data. The structures of 1–3 with a new δ-lactone ring system between C-3 and C-9 were formed through the rearrangement at ring A of an isopimarane skeleton by the bond break of C-3 and C-4. In the crystal of 1, molecules were linked to each other by intermolecular O-H···O bonds, forming chains along the b axis. The differences among the structures of 1–3 were only in the isopropyl group presented with a hydroxy group at C-4 for fladin B (1), a Δ4,19 double bond for fladin C (2), and no substituent at C-4 for fladin D (3). Accordingly, compounds 1–3 were identified as 3,4-seco-isopimarane-type diterpenes. The 3,4-seco-isopimaranes could be produced from the naturally occurring precursor 3β-hydroxy-8,15-isopimaradiene (4) in the I. flavidus plant through the biogenetic pathway outlined in Scheme 1.
Natural isopimaranes were demonstrated with broad biological activities, including antimicrobial activities. For example, our previous isolated 3,4-seco-isopimarane diterpene fladin A, which has a cyclic ether group formed between C-4 and C-9, showed inhibition activity against T. rubrum with a MIC value of 62.5 μg/mL [10]. In the present study, the cytotoxic, antifungal, and antiviral activities of 1–3 were investigated. Although the three compounds possessed similar structures, only compound 2 was observed to inhibit Ebola entry at the concentration of 20 µg/mL (66.7 µM). The results revealed that the antiviral potential of 3,4-seco-isopimarane-type diterpenes could be improved by further structural modification on some specific functional groups.
4. Materials and Methods
4.1. General Experimental Procedures
One-dimensional and two-dimensional NMR spectra of fladins B and D were recorded on a JEOL JNM-ECS400 (400 MHz) spectrometer (JEOL, Tokyo, Japan), and those of fladin C were recorded on a JEOL ECX 500M (500 MHz) spectrometer (JEOL, Tokyo, Japan). Chemical shifts (δ) were expressed in ppm, and coupling constants (J) were reported in Hz. All NMR experiments were obtained by using standard pulse sequences supplied by the vendor. Optical rotation was measured with a Perkin-Elmer model 241 polarimeter (Perkin Elmer, Waltham, MA, USA). IR spectra were recorded on a Bruker VECTOR22 spectrophotometer (Bruker, Rheinstetten, Germany) with KBr pellets. High-resolution electrospray ionization mass spectroscopy (HR-ESI-MS) was recorded on a VG Autospec-3000 spectrometer (VG, Manchester, England). Column chromatography was performed with silica gel (200–300 mesh; Qingdao Marine Chemical, Inc., Qingdao, China). Thin-layer chromatography (TLC) was performed on glass plates coated with silica gel GF254 (Qingdao Marine Chemical Inc.). All solvents, including petroleum ether (60–90 °C), were distilled prior to use. X-ray crystallographic data were obtained on a Bruker APEX-II CCD instrument (Bruker, Rheinstetten, Germany) using Cu Kα radiation.
4.2. Plant Materials
The collection of the twigs and leaves samples of I. flavidus was made in September 2012 in Leishan county, Guizhou, China. This plant species was authenticated by Professor De-Yuan Chen of the Guizhou University of Traditional Chinese Medicine and deposited in the same university with the accession number No. 20120903.
4.3. Extraction and Isolation
The air-dried milled plant material of I. flavdus (5.5 kg) was extracted with 95% methanol (3 × 10 L) at room temperature and concentrated in vacuo to give 556 g of extract. The methanol extract was processed as previously described [10] to provide six fractions (A–F). Fraction C was chromatographed over an additional silica gel column, which was developed by gradient elution (CHCl3/MeOH gradient, from 5:1 to 1:1) to provide fladin B (1, 5 g) and sub-fractions (C1–C4). Fladin B (1) was then recrystallized from methanol at room temperature to provide colorless crystals suitable for X-ray crystallographic analysis. Sub-fraction C3 was separated over a Sephadex LH-20 column (CHCl3/MeOH gradient, 1:1) to afford fladins C (2, 32 mg) and D (3, 5 mg).
4.3.1. Fladin B (1)
Colorless cubic crystals (MeOH); m.p. 155–157 °C; [α −8.59° (c 0.33, CHCl3); UV (CHCl3) λmax (log ε) 244 (0.94) nm; IR (KBr) νmax 3450, 2970, 2928, 1693, 1462, 1424, 1383, 1367, 1153, 842 cm−1; 1H and 13C NMR, see Table 1; HR-ESI-MS ([M + H]+ m/z 319.2269, calcd. 319.2273 for C20H31O3).
4.3.2. Fladin C (2)
Colorless cubic crystals (MeOH); m.p. 146–148 °C; [α −2.14° (c 0.19, CHCl3); UV (CHCl3) λmax (log ε) 243 (0.93) nm; IR (KBr) νmax 3454, 2960, 2922, 1718, 1638, 1434, 1386, 1328, 1033, 853 cm−1; 1H and 13C NMR, see Table 1; HR-ESI-MS ([M + Na]+ m/z 323.1978, calcd. 323.1987 for C20H28O2Na).
4.3.3. Fladin D (3)
Colorless cubic crystals (MeOH); m.p. 149–151 °C; [α −12.9° (c 1.09, MeOH); UV (CHCl3) λmax (log ε) 223 (0.93) nm; IR (KBr) νmax 3432, 2955, 1728, 1719, 1712, 1470, 1346, 1065, 539 cm−1; 1H and 13C NMR, see Table 1; HR-ESI-MS ([M + Na]+ m/z 325.2045, calcd. 325.2144 for C20H30O2Na).
4.4. Single Crystal X-ray Data of Fladin B (1)
Crystal data of 1 (from MeOH): space group P21, C20H30O3, M = 318.44, a = 11.4575 (12) Å, b = 6.3205 (6) Å, c = 12.5407 (13) Å, α = 90.00°, β = 108.004 (4)°, γ = 90.00°, V = 863.69 (15) Å3, T = 100 (2) K, Z = 2. μ (CuKα) = 0.632 mm−1, 4892 reflections measured, 2556 independent reflections (Rint = 0.0574). The final R1 values were 0.0936 (I > 2σ (I)). The final wR (F2) values were 0.2355 (I > 2σ(I)). The final R1 values were 0.0948 (all data). The final wR (F2) values were 0.2382 (all data). The goodness of fit on F2 was 1.108. The Flack parameter was −0.1 (4). The Hooft parameter was 0.10 (15) for 917 Bijvoet pairs. Data were deposited in the Cambridge Crystallographic Data Centre with No. CCDC 1033449.
4.5. Bioactivity Evaluation
Cytotoxicity assays were performed against human colon (HCT116) and lung (A549) cancer cell lines by using Sulforhodamine B (SRB, Fluka, Cat. No. 86183, Buchs, Switzerland), as previously reported [27,28].
The antifungal activity was evaluated against Trichophyton rubrum (ATCC MYA-4438) and T. mentagrophytes (ATCC 28185) (obtained from the Institute of Dermatology, Chinese Academy of Medical Science, Nanjing, China). The antifungal assays were performed using the broth microdilution method, as described in M38-A2, with modifications [29,30,31].
Compounds 1–3 were then evaluated for their anti-HIV and anti-Ebola activities using our previously established “One-Stone-Two-Birds” assay evaluation system as previously described with a modified procedure [32,33].
5. Conclusions
In conclusion, we isolated three novel compounds [fladins B-D (1-3)] from I. flavidus belonging to 3,4-seco-isopimarane diterpenes characterized by a unique δ-lactone ring formed between C-3 and C-9. The biological evaluation revealed that only 2 could inhibit viral replication against Ebola entry. The differences among the structures of 1–3 occurred only in the C-5 isopropyl group. The presence of the carbon–carbon double bond at the isopropyl group could be a key contributor to the antiviral activity of a 3,4-seco-isopimarane. Further structural modification is thus needed to reveal the structure–activity relationship of 3,4-seco-isopimaranes in order to improve their biological activities.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules27103098/s1, X-ray analysis of fladin B (1); Figure S1: The UV spectrum of fladin B (1) in CHCl3; Figure S2: The IR (KBr disc) spectrum of fladin B (1); Figure S3: The HR-ESI-MS spectrum of fladin B (1); Figure S4: 1H NMR spectrum of fladin B (1) in CDCl3 (400 MHz); Figure S5: 13C NMR spectrum of fladin B (1) in CDCl3 (100 MHz); Figure S6: DEPT spectra of fladin B (1) in CDCl3; Figure S7: HSQC spectrum of fladin B (1) in CDCl3; Figure S8: HMBC spectrum of fladin B (1) in CDCl3; Figure S9: 1H-1H COSY spectrum of fladin B (1) in CDCl3; Figure S10: NOESY spectrum of fladin B (1) in CDCl3; Figure S11: The UV spectrum of fladin C (2) in CHCl3; Figure S12: The IR (KBr disc) spectrum of fladin C (2); Figure S13: The HR-ESI-MS spectrum of fladin C (2); Figure S14: 1H NMR spectrum of fladin C (2) in CDCl3 (500 MHz); Figure S15: 13C NMR spectrum of fladin C (2) in CDCl3 (125 MHz); Figure S16: DEPT spectra of fladin C (2) in CDCl3; Figure S17: HSQC spectrum of fladin C (2) in CDCl3; Figure S18: HMBC spectrum of fladin C (2) in CDCl3; Figure S19: 1H-1H COSY spectrum of fladin C (2) in CDCl3; Figure S20: NOESY spectrum of fladin C (2) in CDCl3; Figure S21: The UV spectrum of fladin D (3) in CHCl3; Figure S22: The IR (KBr disc) spectrum of fladin D (3); Figure S23: The HR-ESI-MS spectrum of fladin D (3); Figure S24: 1H NMR spectrum of fladin D (3) in CDCl3 (400 MHz); Figure S25: 13C NMR spectrum of fladin D (3) in CDCl3 (100 MHz); Figure S26: DEPT spectra of fladin D (3) in CDCl3; Figure S27: HSQC spectrum of fladin D (3) in CDCl3; Figure S28: HMBC spectrum of fladin D (3) in CDCl3; Figure S29: 1H-1H COSY spectrum of fladin D (3) in CDCl3; Figure S30: NOESY spectrum of fladin D (3) in CDCl3. Refs [34,35] are cited in Supplementary Materials.
Author Contributions
J.Z. and H.-J.Z. conceived and designed the experiments with the assistance of L.-T.P., W.-F.L. and Z.-M.L. carried out procurement, extraction, isolation, structure elucidation, and drafting work. J.-X.L. performed the antifungal evaluation and the SRB assay. C.-L.Z. and K.H. performed X-ray crystallographic analysis. N.Y.T. and Y.-H.L. performed the “One-Stone-Two-Birds” assay with the guidance of L.R. All authors have read and agreed to the published version of the manuscript.
Funding
This project was funded by the National Key R&D Program of China (2019YFC1712500), the National Natural Science Foundation of China (No. 81760772), the Tip-top Talent Foundation of the Department of Education [grant number KY (2021)034], the Research Grants Council of the Hong Kong Special Administrative Region, China (Project No. 13103917), Hong Kong Baptist University, Research Committee, and University Grants Committee of the Hong Kong Special Administrative Region, China (UGC Research Matching Grant Scheme RMGS2019_1_19).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data associated with this study are included in this published article. Supplementary Data with original NMR, IR, and MS spectra for compounds 1–3 are included in the article. Additional files are available from the corresponding authors upon reasonable request.
Acknowledgments
The authors are grateful to the Department of Chemistry, Hong Kong Baptist University, for access to the Bruker Ascend 400 MHz instrument and to the School of Chinese Medicine, Hong Kong Baptist University, for the acquisition of MS data. We thank the Key Laboratory of Chemistry for Natural Products of Guizhou Province and the Chinese Academy of Sciences for providing JEOL JNM-ECS400 and JEOL ECX 500M (500 MHz) spectrometers. We also wish to thank the State Key Laboratory of Phytochemistry and Plant Resources in West China, Kunming Institute of Botany, Chinese Academy of Sciences for measuring X-ray crystallographic data.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from the authors.
References
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.; Zhang, H.; Liu, J. Structural Diversity and Biological Activities of Diterpenoids Derived from Euphorbia fischeriana Steud. Molecules 2018, 23, 935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanson, J.R. Diterpenoids. Nat. Prod. Rep. 2007, 24, 1332–1341. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.H.; Li, Z.H.; Feng, T.; Liu, J.K. A New Isopimarane diterpenoid from cultures of Inonotus sinensis. Chem. Nat. Compd. 2020, 56, 458–460. [Google Scholar] [CrossRef]
- Rozimamat, R.; Hu, R.; Aisa, H.A. New isopimarane diterpenes and nortriterpene with cytotoxic activity from Ephorbia alatavica Boiss. Fitoterapia 2018, 127, 328–333. [Google Scholar] [CrossRef]
- Win, N.N.; Ito, T.; Aimaiti, S.; Kodama, T.; Imagawa, H.; Ngwe, H.; Abe, I.; Morita, H. Kaempulchraols I-O: New isopimarane diterpenoids from Kaempferia pulchra rhizomes collected in Myanmar and their antiproliferative activity. Tetrahedron 2015, 71, 4707–4713. [Google Scholar] [CrossRef] [Green Version]
- Isca, V.M.S.; Andrade, J.; Fernandes, A.S.; Paixão, P.; Uriel, C.; Gómez, A.M.; Duarte, N.; Rijo, P. In vitro antimicrobial activity of isopimarane-type diterpenoids. Molecules 2020, 25, 4250. [Google Scholar] [CrossRef]
- Chokchaisiri, R.; Chaichompoo, W.; Chunglok, W.; Cheenpracha, S.; Ganranoo, L.; Phutthawong, N.; Bureekaew, S.; Suksamrarn, A. Isopimarane diterpenoids from the rhizomes of Kaempferia marginata and their potential anti-inflammatory activities. J. Nat. Prod. 2020, 83, 14–19. [Google Scholar] [CrossRef]
- Huang, H.; Tang, C.P.; Ke, C.Q.; Shu, R.G.; Ye, Y. 3,4-seco-Isopimarane and 3, 4-seco-pimarane diterpenoids from Callicarpa nudiflora. Chin. J. Nat. Med. 2021, 19, 632–640. [Google Scholar] [CrossRef]
- Li, J.X.; Li, Q.J.; Guan, Y.F.; Song, X.; Liu, Y.H.; Zhang, J.J.; Li, W.F.; Du, J.; Zhu, M.; Banas, J.A.; et al. Discovery of antifungal constituents from the Miao medicinal plant Isodon flavidus. J. Ethnopharmacol. 2016, 191, 372–378. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.D.; Xu, Y.L.; Jiang, B. Diterpenoids from Isodon Species; Science Press: Beijing, China, 2001. [Google Scholar]
- Sun, H.D.; Huang, S.X.; Han, Q.B. Diterpenoids from Isodon species and their biological activities. Nat. Prod. Rep. 2006, 23, 673–698. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.P.; Li, C.; Yang, H.Z.; Lu, Y.Q.; Yu, H.Y.; Gao, H.M.; Wang, Z.M. Three new cytotoxic ent-kaurane diterpenes from Isodon excisoides. Molecules 2015, 20, 17544–17556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.X.; Xiao, W.L.; Li, L.M.; Li, S.H.; Zhou, Y.; Ding, L.S.; Lou, L.G.; Sun, H.D. Bisrubescensins A-C: Three new dimeric ent-kauranoids isolated from Isodon rubescens. Org. Lett. 2006, 8, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.S.; Tian, J.; Yue, J.M.; Chen, S.N.; Lin, Z.W.; Sun, H.D. Diterpenoids from Isodon flavidus. Phytochemistry 1998, 48, 1025–1029. [Google Scholar] [CrossRef]
- Zou, J.; Pan, L.T.; Li, Q.J.; Zhao, J.H.; Pu, J.X.; Yao, P.; Gong, N.; Lu, Y.; Kondratyuk, T.P.; Pezzuto, J.M.; et al. Rubesanolides A and B: Diterpenoids from Isodon rubescens. Org. Lett. 2011, 13, 1406–1409. [Google Scholar] [CrossRef] [Green Version]
- Zou, J.; Pan, L.T.; Li, Q.J.; Pu, J.X.; Yao, P.; Zhu, M.; Banas, J.A.; Zhang, H.J.; Sun, H.D. Rubesanolides C-E: Abietane diterpenoids isolated from Isodon rubescens and evaluation of their anti-biofilm activity. Org. Biomol. Chem. 2012, 10, 5039–5044. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Xu, F.; Dong, H.; Node, M.; Fuji, K. The chemical structure of lophanic acid. Nat. Prod. Res. Dev. 1995, 4, 24–28. [Google Scholar]
- Zhao, M.Z.; Li, J.Q.; Zhang, Y.; Zhang, X.J.; Jiang, B. Study on chemical constituents from rhizome of Rabdosia flavida. J. Chin. Med. Mater. 2014, 37, 1193–1196. [Google Scholar]
- Li, J.Q.; Zhao, M.Z.; Zhang, Y.; Zhang, X.J.; Cheng, X.; Jiang, B. Chemical constituents from underground part of Isodon flavida. Chin. J. Exp. Tradit. Med. Formulae 2014, 24, 114–117. [Google Scholar]
- CCDC1033449 (1) Contains the Supplementary Crystallographic Data for This Paper. These Data Can Be Obtained Free of Charge from the Cambridge Crystallographic Data Centre. Available online: www.ccdc.cam.ac.uk/data_request/cif (accessed on 10 November 2014).
- The Editorial Committee of Flora of Guizhou. Flora of Guizhou (Guizhou Zhiwu Zhi); Guizhou People Press: Guiyang, China, 1986. [Google Scholar]
- Flack, H.D. On enantiomorph--polarity estimation. Acta Crystallogr. Sect. A 1983, A39, 876–881. [Google Scholar] [CrossRef]
- Flack, H.D.; Bernardinelli, G. The use of X-ray crystallography to determine absolute configuration. Chirality 2008, 20, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.S.; Choi, S.U.; Lee, K.R. Three new diterpenoids from the leaves of Thuja orientalis. Planta Med. 2012, 78, 485–487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Zhu, H.J.; Zhou, X.J.; Yang, T.H.; Wang, Y.Y.; Su, J.; Li, Y.; Cheng, Y.X. Diterpenoids from the feces of Trogopterus xanthipes. J. Nat. Prod. 2010, 73, 865–869. [Google Scholar] [CrossRef] [PubMed]
- Jutiviboonsuk, A.; Zhang, H.J.; Tan, G.T.; Ma, C.; Hung, N.V.; Cuong, N.M.; Bunyapraphatsara, N.; Soejarto, D.D.; Fong, H.H.S. Bioactive constituents from roots of Bursera tonkinensis. Phytochemistry 2005, 66, 2745–2751. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.Y.; Tsang, S.W.; Guan, Y.F.; Liu, K.L.; Pan, W.H.; Lam, C.S.; Lee, K.M.; Xia, Y.X.; Xie, W.J.; Wong, W.Y.; et al. The in vitro and in vivo antitumor effects of plant-derived miliusanes and their induction of cellular senescence. J. Med. Chem. 2019, 62, 1541–1561. [Google Scholar] [CrossRef]
- Wong-Deyrup, S.W.; Song, X.; Ng, T.W.; Liu, X.B.; Zeng, J.G.; Qing, Z.X.; Deyrup, S.T.; He, Z.D.; Zhang, H.J. Plant-derived isoquinoline alkaloids that target ergosterol biosynthesis discovered by using a novel antifungal screening tool. Biomed. Pharmacother. 2021, 137, 111348. [Google Scholar] [CrossRef]
- Yang, H.C.; Mikami, Y.; Yazawa, K.; Taguchi, H.; Nishimura, K.; Miyaji, M.; Branchini, M.L.M.; Aoki, F.H.; Yamamoto, K. Colorimetric MTT assessment of antifungal activity of D0870 against fluconazole-resistant Candida albicans: Kolorimetrischer MTT-Test zum Nachweis antimyzetischer Aktivität von D0870 gegen Fluconazol-resistente Candida albicans-Stämme. Mycoses 2008, 41, 477–480. [Google Scholar] [CrossRef]
- M38-A2; Reference Method For Broth Dilution Antifungal Susceptibility Testing of Filamentous Fungi. Approved Standard-Second Edition; Clinical and Laboratory Standards Institute: Malvern, PA, USA, 2008.
- Zhang, H.J.; Rumschlag-Booms, E.; Guan, Y.F.; Wang, D.Y.; Liu, K.L.; Li, W.F.; Nguyen, V.H.; Cuong, N.M.; Soejarto, D.D.; Fong, H.H.S.; et al. Potent inhibitor to drug-resistant HIV-1 strains identified from the medicinal plant Justicia gendarussa. J. Nat. Prod. 2017, 80, 1798–1807. [Google Scholar] [CrossRef] [Green Version]
- Rumschlag-Booms, E.; Zhang, H.J.; Soejarto, D.D.; Fong, H.H.S.; Rong, L.J. Development of an antiviral screening protocol: One-Stone-Two-birds. J. Antivir. Antiretrovir. 2011, 3, 8–10. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. A short history of SHELX. Acta Cryst. A. 2008, 64, 112–122. [Google Scholar] [CrossRef] [Green Version]
- Sheldrick, G.M. SHELXTL-PC (Version 5.1); Siemens Analytical Instruments, Inc: Madison, WI, USA, 1997. [Google Scholar]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).