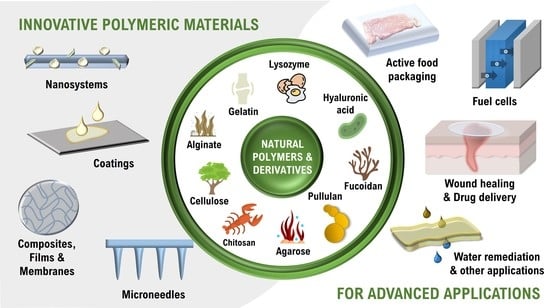
Natural Polymers-Based Materials: A Contribution to a Greener Future
Abstract
:1. Introduction
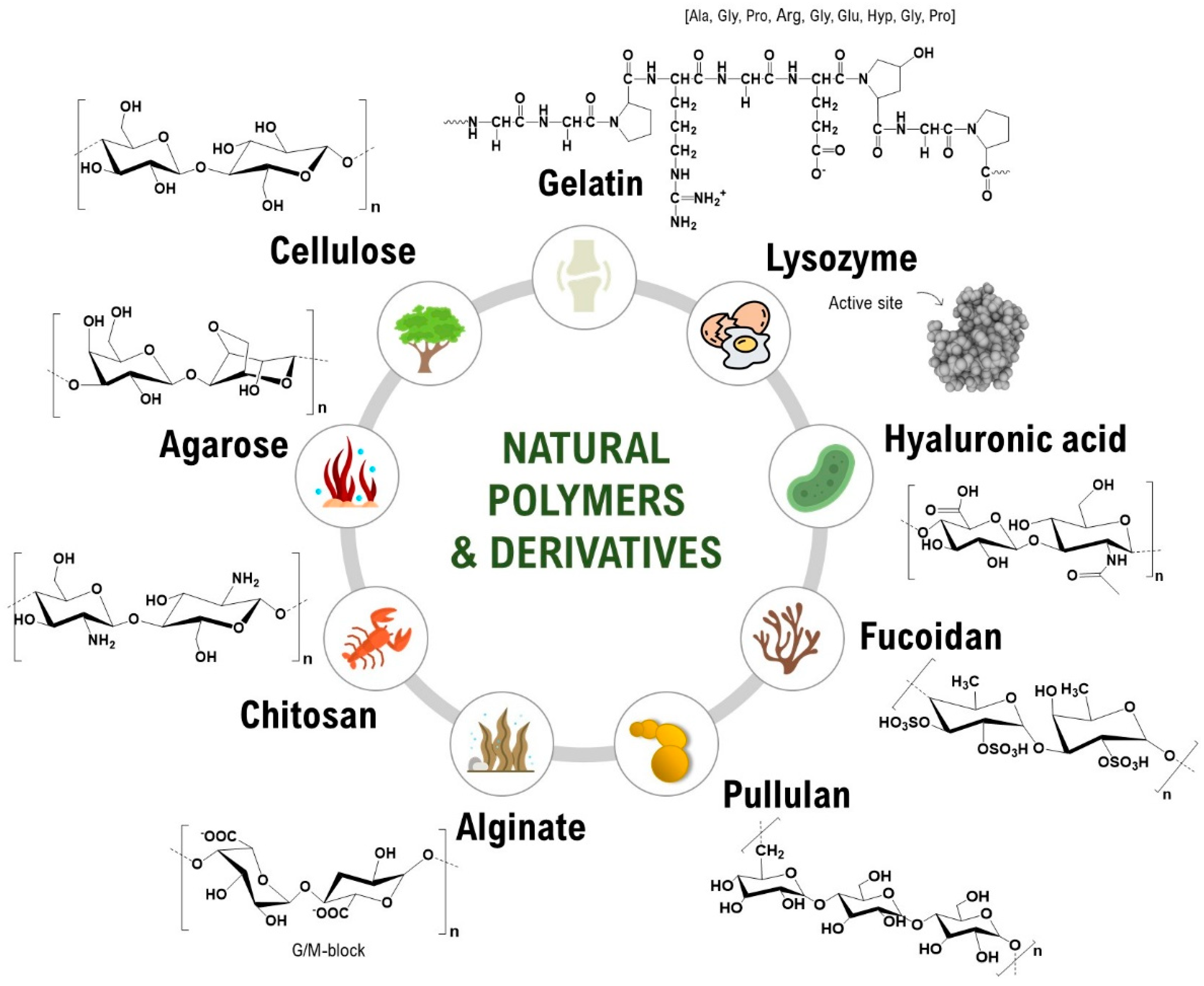
2. Natural Polymers-Based Materials at the BioPol4Fun Research Group
2.1. Cellulose-Based Materials
2.2. Other Natural Polymer-Based Materials
3. Applications of Natural Polymers-Based Materials
3.1. Natural Polymers-Based Films for Active Food Packaging
3.2. Natural Polymers-Based Ion Exchange Membranes for Fuel Cells
3.3. Natural Polymers-Based Patches for Drug Delivery and Wound Healing
3.4. Natural Polymers-Based Microneedles for Drug Delivery and Fluid Uptake
3.5. Natural Polymers-Based Materials for Other Applications
4. Perspectives and Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yu, L.; Chen, L. Polymeric materials from renewable resources. In Biodegradable Polymer Blends and Composites from Renewable Resources; Yu, L., Ed.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2009; pp. 1–15. ISBN 9780470146835. [Google Scholar]
- Klemm, D.; Heublein, B.; Fink, H.-P.; Bohn, A. Cellulose: Fascinating Biopolymer and Sustainable Raw Material. Angew. Chem. Int. Ed. 2005, 44, 3358–3393. [Google Scholar] [CrossRef]
- El Knidri, H.; Laajeb, A.; Lahsini, A. Chitin and chitosan: Chemistry, solubility, fiber formation, and their potential applications. In Handbook of Chitin and Chitosan; Gopi, S., Thomas, S., Pius, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 35–57. ISBN 978-0-12-817970-3. [Google Scholar]
- Silvetti, T.; Morandi, S.; Hintersteiner, M.; Brasca, M. Use of hen egg white lysozyme in the food industry. In Egg Innovations and Strategies for Improvements; Hester, P.Y., Ed.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 233–242. ISBN 9780128011515. [Google Scholar]
- Vilela, C.; Pinto, R.J.B.; Pinto, S.; Marques, P.A.A.P.; Silvestre, A.J.D.; Freire, C.S.R. Polysaccharide Based Hybrid Materials: Metals and Metal Oxides, Graphene and Carbon Nanotubes, 1st ed.; Springer Briefs in Molecular Science; Springer International Publishing: Cham, Switzerland, 2018; ISBN 9783030003463. [Google Scholar]
- Silva, N.H.C.S.; Vilela, C.; Marrucho, I.M.; Freire, C.S.R.; Neto, C.P.; Silvestre, A.J.D. Protein-Based materials: From sources to innovative sustainable materials for biomedical applications. J. Mater. Chem. B 2014, 2, 3715–3740. [Google Scholar] [CrossRef]
- Basu, S.; Malik, S.; Joshi, G.; Gupta, P.; Rana, V. Utilization of bio-polymeric additives for a sustainable production strategy in pulp and paper manufacturing: A comprehensive review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100050. [Google Scholar] [CrossRef]
- Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Modification of textiles for functional applications. In Fundamentals of Natural Fibres and Textiles; Mondal, M.I.H., Ed.; Woodhead Publishing, Elsevier Ltd.: Amsterdam, The Netherlands, 2021; pp. 303–365. ISBN 9780128214848. [Google Scholar]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-Induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-Based edible films for food packaging applications—A review. Int. J. Biol. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef] [PubMed]
- Alipal, J.; Pu’Ad, N.M.; Lee, T.; Nayan, N.; Sahari, N.; Basri, H.; Idris, M.; Abdullah, H. A review of gelatin: Properties, sources, process, applications, and commercialisation. Mater. Today Proc. 2021, 42, 240–250. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Wang, J.; Ma, S.; Yu, Y.; White, W.L.; Yang, S.; Yang, F.; Lu, J. Fucoidan Extracted from Undaria pinnatifida: Source for Nutraceuticals/Functional Foods. Mar. Drugs 2018, 16, 321. [Google Scholar] [CrossRef] [Green Version]
- Bukhari, S.N.A.; Roswandi, N.L.; Waqas, M.; Habib, H.; Hussain, F.; Khan, S.; Sohail, M.; Ramli, N.A.; Thu, H.E.; Hussain, Z. Hyaluronic acid, a promising skin rejuvenating biomedicine: A review of recent updates and pre-clinical and clinical investigations on cosmetic and nutricosmetic effects. Int. J. Biol. Macromol. 2018, 120, 1682–1695. [Google Scholar] [CrossRef] [PubMed]
- Gradishar, W.J.; Tjulandin, S.; Davidson, N.; Shaw, H.; Desai, N.; Bhar, P.; Hawkins, M.; O’Shaughnessy, J. Phase III Trial of Nanoparticle Albumin-Bound Paclitaxel Compared with Polyethylated Castor Oil–Based Paclitaxel in Women with Breast Cancer. J. Clin. Oncol. 2005, 23, 7794–7803. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Ibrahim, S.; Riahi, O.; Said, S.M.; Sabri, M.F.M.; Rozali, S. Biopolymers From Crop Plants. In Reference Module in Materials Science and Materials Engineering; Elsevier: Hoboken, NJ, USA, 2019; pp. 1–10. [Google Scholar] [CrossRef]
- Tongdeesoontorn, W.; Rawdkuen, S. Gelatin-Based Films and Coatings for Food Packaging Applications. In Reference Module in Food Science; Elsevier: Hoboken, NJ, USA, 2019; pp. 1–15. [Google Scholar] [CrossRef]
- Priyadarshi, R.; Rhim, J.-W. Chitosan-based biodegradable functional films for food packaging applications. Innov. Food Sci. Emerg. Technol. 2020, 62, 102346. [Google Scholar] [CrossRef]
- Mansoori, S.; Davarnejad, R.; Matsuura, T.; Ismail, A.F. Membranes based on non-synthetic (natural) polymers for wastewater treatment. Polym. Test. 2020, 84, 106381. [Google Scholar] [CrossRef]
- Vedula, S.S.; Yadav, G.D. Chitosan-based membranes preparation and applications: Challenges and opportunities. J. Indian Chem. Soc. 2021, 98, 100017. [Google Scholar] [CrossRef]
- Nechita, P.; Roman, M. Review on Polysaccharides Used in Coatings for Food Packaging Papers. Coatings 2020, 10, 566. [Google Scholar] [CrossRef]
- Song, J.; Winkeljann, B.; Lieleg, O. Biopolymer-Based Coatings: Promising Strategies to Improve the Biocompatibility and Functionality of Materials Used in Biomedical Engineering. Adv. Mater. Interfaces 2020, 7, 2000850. [Google Scholar] [CrossRef]
- Graça, M.F.P.; Miguel, S.P.; Cabral, C.S.D.; Correia, I.J. Hyaluronic acid—Based wound dressings: A review. Carbohydr. Polym. 2020, 241, 116364. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, J.P.F.; Silva, A.C.Q.; Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Spherical Cellulose Micro and Nanoparticles: A Review of Recent Developments and Applications. Nanomaterials 2021, 11, 2744. [Google Scholar] [CrossRef]
- Bennacef, C.; Desobry-Banon, S.; Probst, L.; Desobry, S. Advances on alginate use for spherification to encapsulate biomolecules. Food Hydrocoll. 2021, 118, 106782. [Google Scholar] [CrossRef]
- DeFrates, K.; Markiewicz, T.; Gallo, P.; Rack, A.; Weyhmiller, A.; Jarmusik, B.; Hu, X. Protein Polymer-Based Nanoparticles: Fabrication and Medical Applications. Int. J. Mol. Sci. 2018, 19, 1717. [Google Scholar] [CrossRef] [Green Version]
- Zhang, M.; Xie, L.; Yin, Z.; Khanal, S.K.; Zhou, Q. Biorefinery approach for cassava-based industrial wastes: Current status and opportunities. Bioresour. Technol. 2016, 215, 50–62. [Google Scholar] [CrossRef] [Green Version]
- Matharu, A.S.; de Melo, E.M.; Houghton, J.A. Opportunity for high value-added chemicals from food supply chain wastes. Bioresour. Technol. 2016, 215, 123–130. [Google Scholar] [CrossRef]
- Liguori, R.; Faraco, V. Biological processes for advancing lignocellulosic waste biorefinery by advocating circular economy. Bioresour. Technol. 2016, 215, 13–20. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: Oxford, UK, 1998; ISBN 9780198502340. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Anastas, P.; Kirchhoff, M.M. Origins, Current Status, and Future Challenges of Green Chemistry. Acc. Chem. Res. 2002, 35, 686–694. [Google Scholar] [CrossRef] [PubMed]
- United Nations Transforming Our World: The 2030 Agenda for Sustainable Development. Available online: https://sustainabledevelopment.un.org/post2015/transformingourworld (accessed on 30 November 2021).
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of Green Chemistry and Sustainability: Past, Present, and Future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef] [Green Version]
- Campalani, C.; Amadio, E.; Zanini, S.; Dall’Acqua, S.; Panozzo, M.; Ferrari, S.; De Nadai, G.; Francescato, S.; Selva, M.; Perosa, A. Supercritical CO2 as a green solvent for the circular economy: Extraction of fatty acids from fruit pomace. J. CO2 Util. 2020, 41, 101259. [Google Scholar] [CrossRef]
- Khandelwal, S.; Tailor, Y.K.; Kumar, M. Deep eutectic solvents (DESs) as eco-friendly and sustainable solvent/catalyst systems in organic transformations. J. Mol. Liq. 2016, 215, 345–386. [Google Scholar] [CrossRef]
- Gillet, S.; Aguedo, M.; Petitjean, L.; Morais, A.R.C.; Lopes, A.M.D.C.; Łukasik, R.M.; Anastas, P.T. Lignin transformations for high value applications: Towards targeted modifications using green chemistry. Green Chem. 2017, 19, 4200–4233. [Google Scholar] [CrossRef]
- Morais, E.S.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Enhanced Conversion of Xylan into Furfural using Acidic Deep Eutectic Solvents with Dual Solvent and Catalyst Behavior. ChemSusChem 2020, 13, 784–790. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Morais, E.S.; Freire, C.S.R.; Freire, M.G.; Silvestre, A.J.D. Extraction of High Value Triterpenic Acids from Eucalyptus globulus Biomass Using Hydrophobic Deep Eutectic Solvents. Molecules 2020, 25, 210. [Google Scholar] [CrossRef] [Green Version]
- Rocha, J. CICECO-Aveiro Institute of Materials: A Journey into the Future. Eur. J. Inorg. Chem. 2020, 2020, 2119–2120. [Google Scholar] [CrossRef]
- Oliveira, C.S.D.; Moreira, P.; Resende, J.; Cruz, M.T.; Pereira, C.M.F.; Silva, A.M.S.; Santos, S.A.O.; Silvestre, A.J.D. Characterization and Cytotoxicity Assessment of the Lipophilic Fractions of Different Morphological Parts of Acacia dealbata. Int. J. Mol. Sci. 2020, 21, 1814. [Google Scholar] [CrossRef] [Green Version]
- Rabah, S.; Kouachi, K.; Ramos, P.A.B.; Gomes, A.P.; Almeida, A.; Haddadi-Guemghar, H.; Madani, K.; Silvestre, A.J.D.; Santos, S.A.O. Unveiling the bioactivity of Allium triquetrum L. lipophilic fractions: Chemical characterization and in vitro antibacterial activity against methicillin-resistant Staphylococcus aureus. Food Funct. 2020, 11, 5257–5265. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial activity of pomegranate peel extracts performed by high pressure and enzymatic assisted extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef] [Green Version]
- Neto, R.T.; Santos, S.A.O.; Oliveira, J.; Silvestre, A.J.D. Tuning of Proanthocyanidin Extract’s Composition through Quaternary Eutectic Solvents Extraction. Antioxidants 2020, 9, 1124. [Google Scholar] [CrossRef] [PubMed]
- Morais, E.S.; Lopes, A.M.D.C.; Freire, M.G.; Freire, C.S.R.; Coutinho, J.A.P.; Silvestre, A.J.D. Use of Ionic Liquids and Deep Eutectic Solvents in Polysaccharides Dissolution and Extraction Processes towards Sustainable Biomass Valorization. Molecules 2020, 25, 3652. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Rua, R.; Silvestre, A.J.; Gandini, A. Polymers and copolymers from fatty acid-based monomers. Ind. Crops Prod. 2010, 32, 97–104. [Google Scholar] [CrossRef]
- Vilela, C.; Cruciani, L.; Silvestre, A.J.D.; Gandini, A. A Double Click Strategy Applied to the Reversible Polymerization of Furan/Vegetable Oil Monomers. Macromol. Rapid Commun. 2011, 32, 1319–1323. [Google Scholar] [CrossRef]
- Vilela, C.; Cruciani, L.; Silvestre, A.J.D.; Gandini, A. Reversible polymerization of novel monomers bearing furan and plant oil moieties: A double click exploitation of renewable resources. RSC Adv. 2012, 2, 2966–2974. [Google Scholar] [CrossRef]
- Vilela, C.; Silvestre, A.J.D.; Meier, M.A.R. Plant Oil-Based Long-Chain C26Monomers and Their Polymers. Macromol. Chem. Phys. 2012, 213, 2220–2227. [Google Scholar] [CrossRef]
- Vilela, C.; Silvestre, A.J.D.; Gandini, A. Thermoreversible nonlinear diels-alder polymerization of furan/plant oil monomers. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2260–2270. [Google Scholar] [CrossRef]
- Vilela, C.; Sousa, A.F.; Fonseca, A.C.; Serra, A.C.; Coelho, J.F.J.; Freire, C.S.R.; Silvestre, A.J.D. The quest for sustainable polyesters—Insights into the future. Polym. Chem. 2014, 5, 3119–3141. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Fonseca, A.C.; Matos, M.; Freire, C.S.R.; Gruter, G.-J.M.; Coelho, J.F.J.; Silvestre, A.J.D. Biobased polyesters and other polymers from 2,5-furandicarboxylic acid: A tribute to furan excellency. Polym. Chem. 2015, 6, 5961–5983. [Google Scholar] [CrossRef]
- Sousa, A.F.; Vilela, C.; Matos, M.; Freire, C.S.R.; Silvestre, A.J.D.; Coelho, J.F.J. Polyethylene terephthalate: Copolyesters, composites, and renewable alternatives. In Poly(Ethylene Terephthalate) Based Blends, Composites and Nanocomposites; Visakh, P.M., Liang, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2015; pp. 113–141. ISBN 978-0-323-31306-3. [Google Scholar]
- Soares, M.J.; Dannecker, P.-K.; Vilela, C.; Bastos, J.; Meier, M.A.R.; Sousa, A.F. Poly(1,20-eicosanediyl 2,5-furandicarboxylate), a biodegradable polyester from renewable resources. Eur. Polym. J. 2017, 90, 301–311. [Google Scholar] [CrossRef]
- Silva, A.C.; Vilela, C.; Santos, H.A.; Silvestre, A.J.; Freire, C.S. Recent trends on the development of systems for cancer diagnosis and treatment by microfluidic technology. Appl. Mater. Today 2020, 18, 100450. [Google Scholar] [CrossRef]
- Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. (Eds.) Advanced Biopolymer-Based Nanocomposites and Hybrid Materials; MDPI: Basel, Switzerland, 2021; ISBN 978-3-0365-0511-4. [Google Scholar]
- Silvestre, A.J.D.; Freire, C.S.R.; Vilela, C. Special Issue: Advanced Biopolymer-Based Nanocomposites and Hybrid Materials. Materials 2021, 14, 493. [Google Scholar] [CrossRef]
- Vilela, C.; Figueiredo, A.; Silvestre, A.; Freire, C. Multilayered materials based on biopolymers as drug delivery systems. Expert Opin. Drug Deliv. 2017, 14, 189–200. [Google Scholar] [CrossRef]
- Vilela, C.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Nanocellulose-based materials as components of polymer electrolyte fuel cells. J. Mater. Chem. A 2019, 7, 20045–20074. [Google Scholar] [CrossRef]
- Carvalho, T.; Guedes, G.; Sousa, F.L.; Freire, C.; Santos, H.A. Latest Advances on Bacterial Cellulose-Based Materials for Wound Healing, Delivery Systems, and Tissue Engineering. Biotechnol. J. 2019, 14, 1900059. [Google Scholar] [CrossRef]
- Carvalho, J.P.F.; Freire, C.S.R.; Vilela, C. Active Packaging. In Sustainable Food Processing and Engineering Challenges; Galanakis, C., Ed.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 315–341. ISBN 9780128227145. [Google Scholar]
- Vilela, C.; Freire, C.S.; Marques, P.A.; Trindade, T.; Neto, C.P.; Fardim, P. Synthesis and characterization of new CaCO3/cellulose nanocomposites prepared by controlled hydrolysis of dimethylcarbonate. Carbohydr. Polym. 2010, 79, 1150–1156. [Google Scholar] [CrossRef]
- Valente, B.F.A.; Silvestre, A.J.D.; Neto, C.P.; Vilela, C.; Freire, C.S.R. Effect of the Micronization of Pulp Fibers on the Properties of Green Composites. Molecules 2021, 26, 5594. [Google Scholar] [CrossRef] [PubMed]
- Figueiredo, A.R.P.; Vilela, C.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Bacterial Cellulose-Based Nanocomposites: Roadmap for Innovative Materials. In Nanocellulose Polymer Composites; Thakur, V.K., Ed.; Scrivener Publishing LLC: Salem, MA, USA, 2015; pp. 17–64. ISBN 9781118871904. [Google Scholar]
- Almeida, T.; Silvestre, A.; Vilela, C.; Freire, C. Bacterial Nanocellulose toward Green Cosmetics: Recent Progresses and Challenges. Int. J. Mol. Sci. 2021, 22, 2836. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Pinto, R.J.B.; Figueiredo, A.R.P.; Neto, C.P.; Silvestre, A.J.D.; Freire, C.S.R. Development and applications of cellulose nanofibres based polymer nanocomposites. In Advanced Composite Materials: Properties and Applications; Bafekrpour, E., Ed.; De Gruyter Open: Berlin, Germany, 2017; pp. 1–65. [Google Scholar] [CrossRef]
- Tomé, L.C.; Fernandes, S.C.M.; Perez, D.S.; Sadocco, P.; Silvestre, A.J.D.; Neto, C.P.; Marrucho, I.M.; Freire, C.S.R. The role of nanocellulose fibers, starch and chitosan on multipolysaccharide based films. Cellulose 2013, 20, 1807–1818. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Lameirinhas, N.S.; Guedes, G.; da Silva, G.H.R.; Oskoei, P.; Spirk, S.; Oliveira, H.; Duarte, I.F.; Vilela, C.; Freire, C.S.R. Cellulose Nanocrystals/Chitosan-Based Nanosystems: Synthesis, Characterization, and Cellular Uptake on Breast Cancer Cells. Nanomaterials 2021, 11, 2057. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.C.; Freire, C.S.; Silvestre, A.J.; Neto, C.P.; Gandini, A. Novel materials based on chitosan and cellulose. Polym. Int. 2011, 60, 875–882. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Granadeiro, C.M.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Ferreira, R.A.S.; Carlos, L.D.; Cavaleiro, A.M.V.; Trindade, T.; Nogueira, H.I.S. Luminescent Transparent Composite Films Based on Lanthanopolyoxometalates and Filmogenic Polysaccharides. Eur. J. Inorg. Chem. 2013, 2013, 1890–1896. [Google Scholar] [CrossRef]
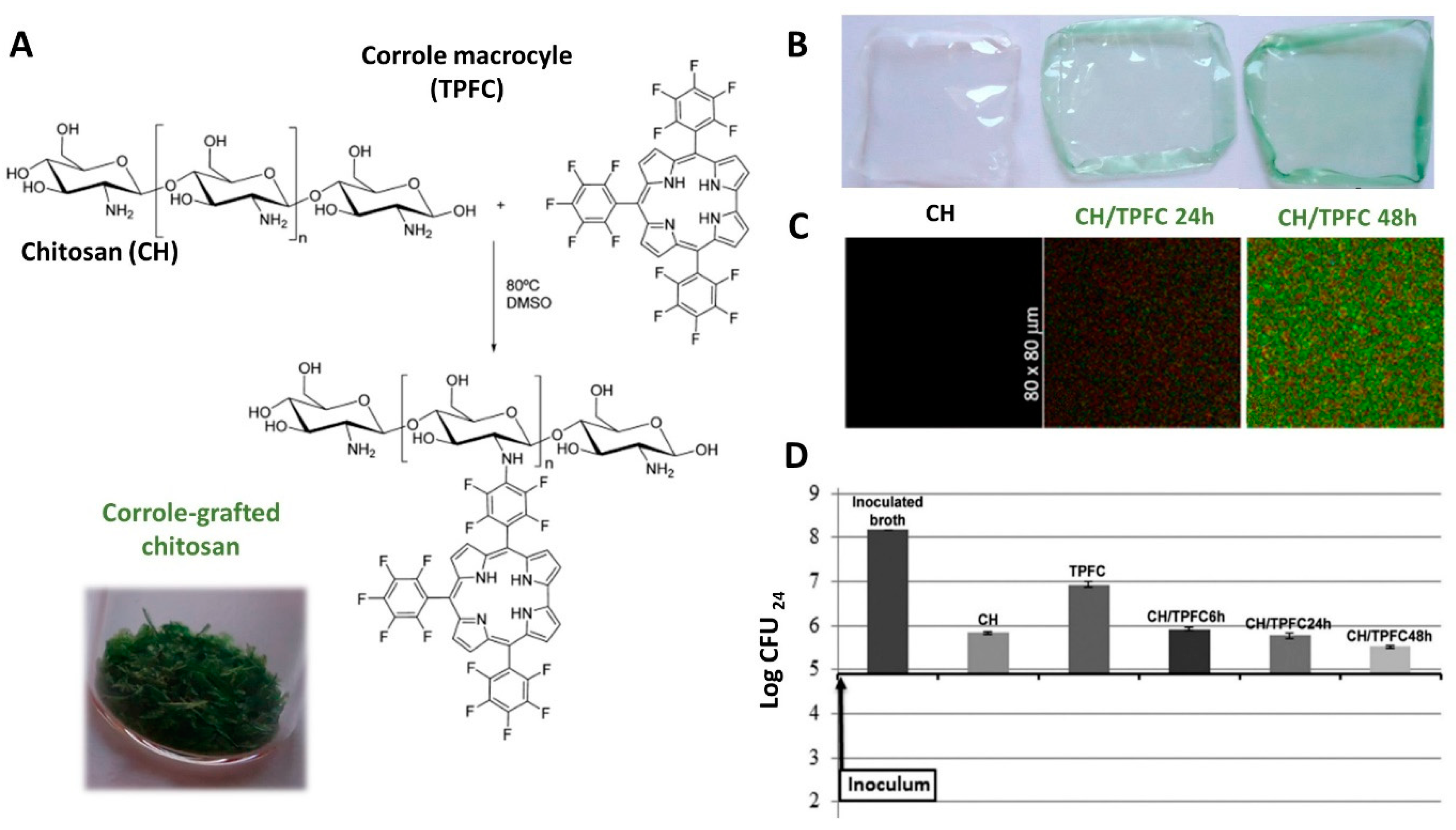
- Barata, J.F.B.; Pinto, R.J.B.; Serra, V.I.R.C.V.; Silvestre, A.J.D.; Trindade, T.; Neves, M.G.P.M.S.; Cavaleiro, J.A.S.; Daina, S.; Sadocco, P.; Freire, C.S.R. Fluorescent Bioactive Corrole Grafted-Chitosan Films. Biomacromolecules 2016, 17, 1395–1403. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.; Freire, C.; Gandini, A.; Ferreira, M.; Zheludkevich, M. Functionalized chitosan-based coatings for active corrosion protection. Surf. Coat. Technol. 2013, 226, 51–59. [Google Scholar] [CrossRef]
- Tomé, L.C.; Silva, N.H.C.S.; Soares, H.R.; Coroadinha, A.S.; Sadocco, P.; Marrucho, I.M.; Freire, C.S.R. Bioactive transparent films based on polysaccharides and cholinium carboxylate ionic liquids. Green Chem. 2015, 17, 4291–4299. [Google Scholar] [CrossRef]
- Esposito, T.; Silva, N.H.; Almeida, A.; Silvestre, A.J.; Piccinelli, A.L.; Aquino, R.P.; Sansone, F.; Mencherini, T.; Vilela, C.; Freire, C.S. Valorisation of chestnut spiny burs and roasted hazelnut skins extracts as bioactive additives for packaging films. Ind. Crops Prod. 2020, 151, 112491. [Google Scholar] [CrossRef]
- Fonseca, D.F.; Costa, P.C.; Almeida, I.F.; Dias-Pereira, P.; Correia-Sá, I.; Bastos, V.; Oliveira, H.; Duarte-Araújo, M.; Morato, M.; Vilela, C.; et al. Pullulan microneedle patches for the efficient transdermal administration of insulin envisioning diabetes treatment. Carbohydr. Polym. 2020, 241, 116314. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, D.F.; Vilela, C.; Pinto, R.J.; Bastos, V.; Oliveira, H.; Catarino, J.; Faísca, P.; Rosado, C.; Silvestre, A.J.; Freire, C.S. Bacterial nanocellulose-hyaluronic acid microneedle patches for skin applications: In vitro and in vivo evaluation. Mater. Sci. Eng. C 2021, 118, 111350. [Google Scholar] [CrossRef] [PubMed]
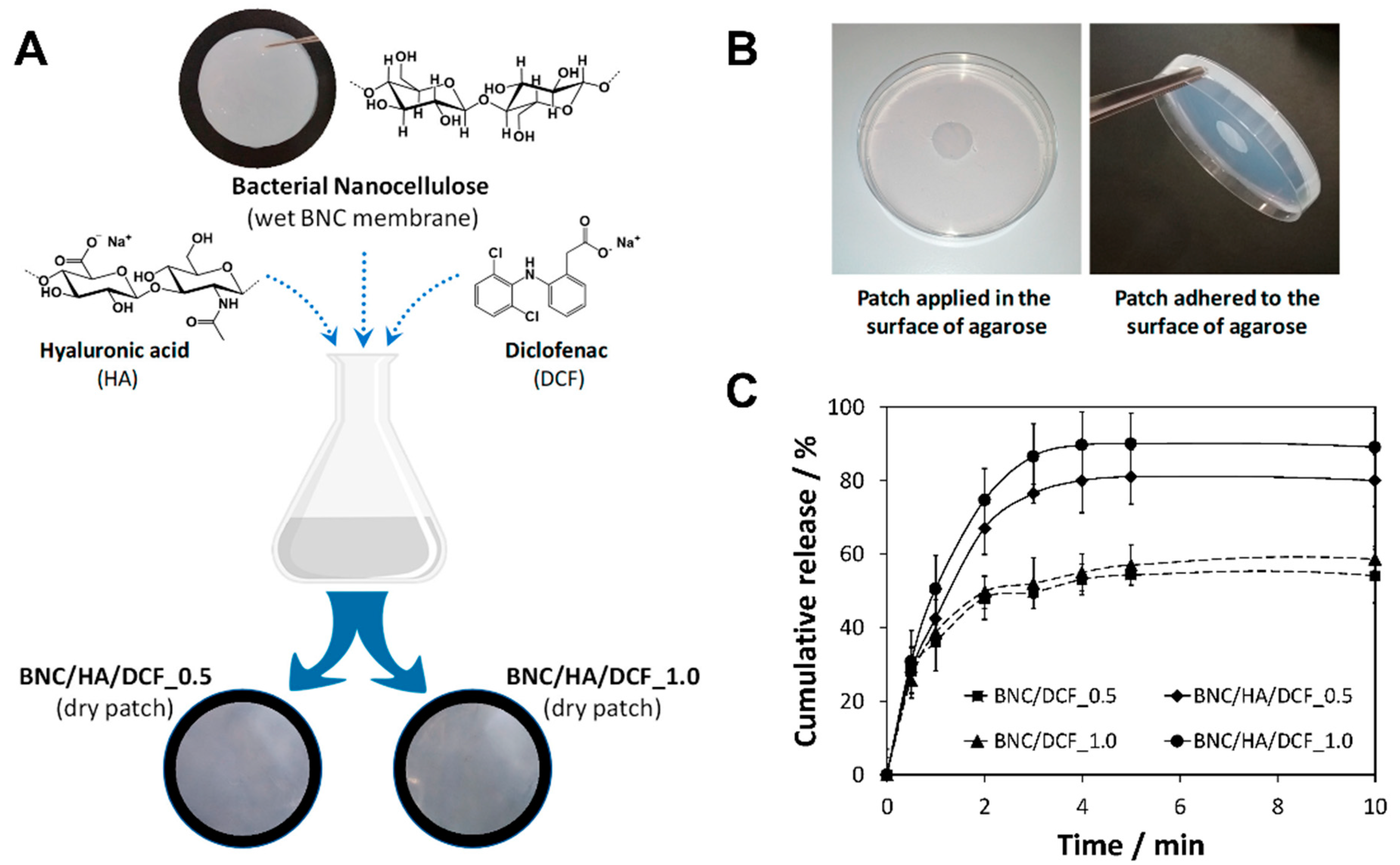
- Carvalho, J.P.F.; Silva, A.C.Q.; Bastos, V.; Oliveira, H.; Pinto, R.J.B.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Nanocellulose-Based Patches Loaded with Hyaluronic Acid and Diclofenac towards Aphthous Stomatitis Treatment. Nanomaterials 2020, 10, 628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fonseca, D.F.S.; Carvalho, J.P.F.; Bastos, V.; Oliveira, H.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Antibacterial Multi-Layered Nanocellulose-Based Patches Loaded with Dexpanthenol for Wound Healing Applications. Nanomaterials 2020, 10, 2469. [Google Scholar] [CrossRef]
- Pinto, R.J.B.; Bispo, D.; Vilela, C.; Botas, A.M.P.; Ferreira, R.A.S.; Menezes, A.C.; Campos, F.; Oliveira, H.; Abreu, M.H.; Santos, S.A.O.; et al. One-Minute Synthesis of Size-Controlled Fucoidan-Gold Nanosystems: Antitumoral Activity and Dark Field Imaging. Materials 2020, 13, 1076. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.; Silva, A.C.; Domingues, E.; Gonçalves, G.; Martins, M.A.; Figueiredo, F.M.; Santos, S.A.; Freire, C.S. Conductive polysaccharides-based proton-exchange membranes for fuel cell applications: The case of bacterial cellulose and fucoidan. Carbohydr. Polym. 2020, 230, 115604. [Google Scholar] [CrossRef]
- Makhloufi, N.; Chougui, N.; Rezgui, F.; Benramdane, E.; Freire, C.S.R.; Vilela, C.; Silvestre, A.J.D. Bio-based sustainable films from the Algerian Opuntia ficus-indica cladodes powder: Effect of plasticizer content. J. Appl. Polym. Sci. 2021, 138, 50450. [Google Scholar] [CrossRef]
- Silva, N.H.; Pinto, R.J.; Freire, C.; Marrucho, I.M. Production of lysozyme nanofibers using deep eutectic solvent aqueous solutions. Colloids Surf. B Biointerfaces 2016, 147, 36–44. [Google Scholar] [CrossRef]
- Silva, N.H.; Pinto, R.J.; Martins, M.A.; Ferreira, R.; Correia, I.; Freire, C.S.; Marrucho, I.M. Ionic liquids as promoters of fast lysozyme fibrillation. J. Mol. Liq. 2018, 272, 456–467. [Google Scholar] [CrossRef]
- Silva, N.H.; Figueira, P.; Fabre, E.; Pinto, R.J.; Pereira, M.E.; Silvestre, A.J.; Marrucho, I.M.; Vilela, C.; Freire, C.S. Dual nanofibrillar-based bio-sorbent films composed of nanocellulose and lysozyme nanofibrils for mercury removal from spring waters. Carbohydr. Polym. 2020, 238, 116210. [Google Scholar] [CrossRef]
- Silva, N.H.; Garrido-Pascual, P.; Moreirinha, C.; Almeida, A.; Palomares, T.; Alonso-Varona, A.; Vilela, C.; Freire, C.S. Multifunctional nanofibrous patches composed of nanocellulose and lysozyme nanofibers for cutaneous wound healing. Int. J. Biol. Macromol. 2020, 165, 1198–1210. [Google Scholar] [CrossRef]
- Fonseca, D.F.S.; Costa, P.C.; Almeida, I.F.; Dias-Pereira, P.; Correia-Sá, I.; Bastos, V.; Oliveira, H.; Vilela, C.; Silvestre, A.J.D.; Freire, C.S.R. Swellable Gelatin Methacryloyl Microneedles for Extraction of Interstitial Skin Fluid toward Minimally Invasive Monitoring of Urea. Macromol. Biosci. 2020, 20, e2000195. [Google Scholar] [CrossRef] [PubMed]
- Bastante, C.C.; Silva, N.H.; Cardoso, L.C.; Serrano, C.M.; de la Ossa, E.J.M.; Freire, C.S.; Vilela, C. Biobased films of nanocellulose and mango leaf extract for active food packaging: Supercritical impregnation versus solvent casting. Food Hydrocoll. 2021, 117, 106709. [Google Scholar] [CrossRef]
- Moreirinha, C.; Vilela, C.; Silva, N.H.; Pinto, R.J.; Almeida, A.; Rocha, M.A.M.; Coelho, E.; Coimbra, M.A.; Silvestre, A.J.; Freire, C.S. Antioxidant and antimicrobial films based on brewers spent grain arabinoxylans, nanocellulose and feruloylated compounds for active packaging. Food Hydrocoll. 2020, 108, 105836. [Google Scholar] [CrossRef]
- Vilela, C.; Pinto, R.J.; Coelho, J.; Domingues, M.R.; Daina, S.; Sadocco, P.; Santos, S.A.; Freire, C. Bioactive chitosan/ellagic acid films with UV-light protection for active food packaging. Food Hydrocoll. 2017, 73, 120–128. [Google Scholar] [CrossRef]
- Castro, K.A.; Moura, N.M.; Fernandes, A.; Faustino, M.A.; Simões, M.M.; Cavaleiro, J.A.; Nakagaki, S.; Almeida, A.; Cunha, Â.; Silvestre, A.J.; et al. Control of Listeria innocua biofilms by biocompatible photodynamic antifouling chitosan based materials. Dye Pigments 2017, 137, 265–276. [Google Scholar] [CrossRef]
- Vilela, C.; Moreirinha, C.; Almeida, A.; Silvestre, A.J.D.; Freire, C.S.R. Zwitterionic Nanocellulose-Based Membranes for Organic Dye Removal. Materials 2019, 12, 1404. [Google Scholar] [CrossRef] [Green Version]
- Vilela, C.; Martins, A.P.C.; Sousa, N.; Silvestre, A.J.D.; Figueiredo, F.M.L.; Freire, C.S.R. Poly(bis[2-(methacryloyloxy)ethyl] phosphate)/Bacterial Cellulose Nanocomposites: Preparation, Characterization and Application as Polymer Electrolyte Membranes. Appl. Sci. 2018, 8, 1145. [Google Scholar] [CrossRef] [Green Version]
- Gadim, T.D.O.; Figueiredo, A.G.P.R.; Navarro, N.C.R.; Vilela, C.; Gamelas, J.; Timmons, A.B.; Neto, C.P.; Silvestre, A.; Freire, C.; Figueiredo, F.M.L. Nanostructured Bacterial Cellulose–Poly(4-styrene sulfonic acid) Composite Membranes with High Storage Modulus and Protonic Conductivity. ACS Appl. Mater. Interfaces 2014, 6, 7864–7875. [Google Scholar] [CrossRef]
- Vilela, C.; Morais, J.D.; Silva, A.C.Q.; Muñoz-Gil, D.; Figueiredo, F.M.L.; Silvestre, A.J.D.; Freire, C.S.R. Flexible Nanocellulose/Lignosulfonates Ion-Conducting Separators for Polymer Electrolyte Fuel Cells. Nanomaterials 2020, 10, 1713. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro-Claro, P.; Vilela, C.; Nolasco, M.; Rudić, S.; Vaz, P.D.; Araujo, C. Structural characterization of cellulose-based nanocomposites. STFC ISIS Neutron Muon Source 2018. [Google Scholar] [CrossRef]
- Vilela, C.; Freire, C.S.R.; Araújo, C.; Rudić, S.; Silvestre, A.J.D.; Vaz, P.D.; Ribeiro-Claro, P.J.A.; Nolasco, M.M. Understanding the Structure and Dynamics of Nanocellulose-Based Composites with Neutral and Ionic Poly(methacrylate) Derivatives Using Inelastic Neutron Scattering and DFT Calculations. Molecules 2020, 25, 1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
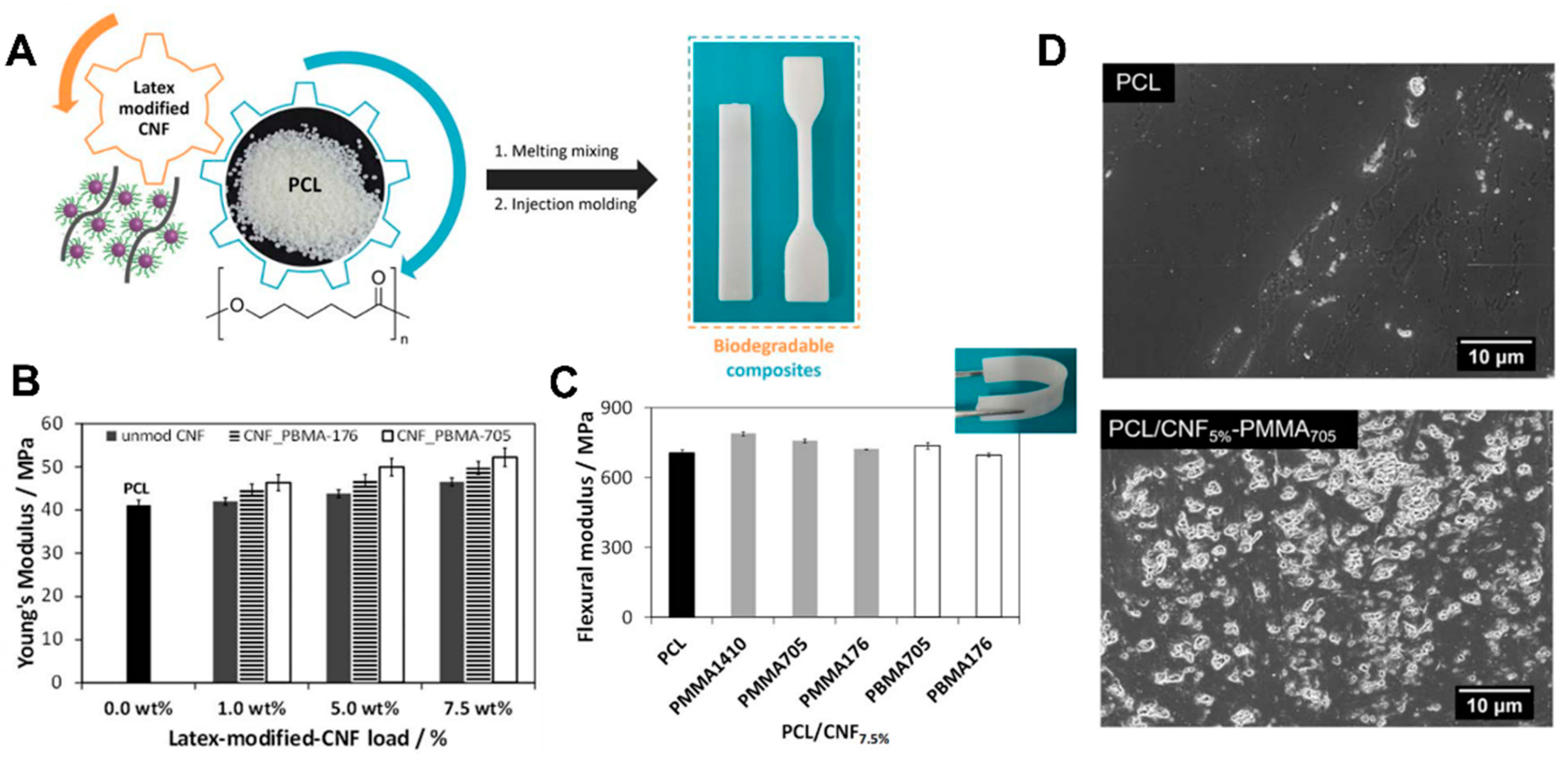
- Vilela, C.; Engström, J.; Valente, B.; Jawerth, M.; Carlmark, A.; Freire, C.S.R. Exploiting poly(ε-caprolactone) and cellulose nanofibrils modified with latex nanoparticles for the development of biodegradable nanocomposites. Polym. Compos. 2019, 40, 1342–1353. [Google Scholar] [CrossRef]
- Trovatti, E.; Fernandes, S.C.; Rubatat, L.; Perez, D.D.S.; Freire, C.S.; Silvestre, A.J.; Neto, C.P. Pullulan–nanofibrillated cellulose composite films with improved thermal and mechanical properties. Compos. Sci. Technol. 2012, 72, 1556–1561. [Google Scholar] [CrossRef]
- Martins, N.C.T.; Freire, C.; Pinto, R.; Fernandes, S.C.M.; Neto, C.; Silvestre, A.; Causio, J.; Baldi, G.; Sadocco, P.; Trindade, T. Electrostatic assembly of Ag nanoparticles onto nanofibrillated cellulose for antibacterial paper products. Cellulose 2012, 19, 1425–1436. [Google Scholar] [CrossRef]
- Martins, N.; Freire, C.; Neto, C.; Silvestre, A.; Causio, J.; Baldi, G.; Sadocco, P.; Trindade, T. Antibacterial paper based on composite coatings of nanofibrillated cellulose and ZnO. Colloids Surf. A Physicochem. Eng. Asp. 2013, 417, 111–119. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.C.M.; Freire, C.; Gandini, A.; Ferreira, M.; Zheludkevich, M. Chitosan as a Smart Coating for Controlled Release of Corrosion Inhibitor 2-Mercaptobenzothiazole. ECS Electrochem. Lett. 2013, 2, C19–C22. [Google Scholar] [CrossRef]
- Carneiro, J.; Tedim, J.; Fernandes, S.; Freire, C.; Silvestre, A.; Gandini, A.; Ferreira, M.; Zheludkevich, M. Chitosan-based self-healing protective coatings doped with cerium nitrate for corrosion protection of aluminum alloy 2024. Prog. Org. Coat. 2012, 75, 8–13. [Google Scholar] [CrossRef]
- Silva, N.H.C.S.; Mota, J.P.; De Almeida, T.S.; Carvalho, J.P.F.; Silvestre, A.J.D.; Vilela, C.; Rosado, C.; Freire, C.S.R. Topical Drug Delivery Systems Based on Bacterial Nanocellulose: Accelerated Stability Testing. Int. J. Mol. Sci. 2020, 21, 1262. [Google Scholar] [CrossRef] [Green Version]
- Trovatti, E.; Freire, C.S.; Pinto, P.C.; Almeida, I.F.; Costa, P.; Silvestre, A.J.; Neto, C.P.; Rosado, C. Bacterial cellulose membranes applied in topical and transdermal delivery of lidocaine hydrochloride and ibuprofen: In vitro diffusion studies. Int. J. Pharm. 2012, 435, 83–87. [Google Scholar] [CrossRef]
- Chantereau, G.; Sharma, M.; Abednejad, A.; Vilela, C.; Costa, E.; Veiga, M.; Antunes, F.; Pintado, M.M.; Sèbe, G.; Coma, V.; et al. Bacterial nanocellulose membranes loaded with vitamin B-based ionic liquids for dermal care applications. J. Mol. Liq. 2020, 302, 112547. [Google Scholar] [CrossRef]
- Fonseca, D.; Vilela, C.; Silvestre, A.J.; Freire, C.S. A compendium of current developments on polysaccharide and protein-based microneedles. Int. J. Biol. Macromol. 2019, 136, 704–728. [Google Scholar] [CrossRef]
- Silva, N.H.; Vilela, C.; Almeida, A.; Marrucho, I.; Freire, C. Pullulan-based nanocomposite films for functional food packaging: Exploiting lysozyme nanofibers as antibacterial and antioxidant reinforcing additives. Food Hydrocoll. 2018, 77, 921–930. [Google Scholar] [CrossRef]
- Vilela, C.; Kurek, M.; Hayouka, Z.; Röcker, B.; Yildirim, S.; Antunes, M.D.C.; Nilsen-Nygaard, J.; Pettersen, M.K.; Freire, C.S.R. A concise guide to active agents for active food packaging. Trends Food Sci. Technol. 2018, 80, 212–222. [Google Scholar] [CrossRef]
- Cacicedo, M.L.; Castro, M.C.; Servetas, I.; Bosnea, L.; Boura, K.; Tsafrakidou, P.; Dima, A.; Terpou, A.; Koutinas, A.; Castro, G.R. Progress in bacterial cellulose matrices for biotechnological applications. Bioresour. Technol. 2016, 213, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Pinto, R.J.B.; Martins, M.A.; Lucas, J.M.F.; Vilela, C.; Sales, A.; Costa, L.C.; Marques, P.; Freire, C.S.R. Highly Electroconductive Nanopapers Based on Nanocellulose and Copper Nanowires: A New Generation of Flexible and Sustainable Electrical Materials. ACS Appl. Mater. Interfaces 2020, 12, 34208–34216. [Google Scholar] [CrossRef] [PubMed]
- Tomé, L.C.; Pinto, R.J.B.; Trovatti, E.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P.; Gandini, A. Transparent bionanocomposites with improved properties prepared from acetylated bacterial cellulose and poly(lactic acid) through a simple approach. Green Chem. 2011, 13, 419–427. [Google Scholar] [CrossRef] [Green Version]
- Figueiredo, A.; Silvestre, A.; Neto, C.P.; Freire, C.S. In situ synthesis of bacterial cellulose/polycaprolactone blends for hot pressing nanocomposite films production. Carbohydr. Polym. 2015, 132, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Gadim, T.D.; Loureiro, F.J.; Vilela, C.; Rosero-Navarro, N.; Silvestre, A.J.; Freire, C.S.; Figueiredo, F.M. Protonic conductivity and fuel cell tests of nanocomposite membranes based on bacterial cellulose. Electrochim. Acta 2017, 233, 52–61. [Google Scholar] [CrossRef]
- Vilela, C.; Cordeiro, D.M.; Boas, J.V.; Barbosa, P.; Nolasco, M.; Vaz, P.D.; Rudić, S.; Ribeiro-Claro, P.; Silvestre, A.J.; Oliveira, V.B.; et al. Poly(4-styrene sulfonic acid)/bacterial cellulose membranes: Electrochemical performance in a single-chamber microbial fuel cell. Bioresour. Technol. Rep. 2019, 9, 100376. [Google Scholar] [CrossRef]
- Gadim, T.D.; Vilela, C.; Loureiro, F.; Silvestre, A.; Freire, C.S.; Figueiredo, F.M. Nafion ® and nanocellulose: A partnership for greener polymer electrolyte membranes. Ind. Crops Prod. 2016, 93, 212–218. [Google Scholar] [CrossRef]
- Vilela, C.; Gadim, T.D.O.; Silvestre, A.; Freire, C.; Figueiredo, F.M.L. Nanocellulose/poly(methacryloyloxyethyl phosphate) composites as proton separator materials. Cellulose 2016, 23, 3677–3689. [Google Scholar] [CrossRef]
- Vilela, C.; Sousa, N.; Pinto, R.; Silvestre, A.; Figueiredo, F.M.; Freire, C.S. Exploiting poly(ionic liquids) and nanocellulose for the development of bio-based anion-exchange membranes. Biomass Bioenergy 2017, 100, 116–125. [Google Scholar] [CrossRef]
- Faria, M.; Vilela, C.; Mohammadkazemi, F.; Silvestre, A.J.; Freire, C.S.; Cordeiro, N. Poly(glycidyl methacrylate)/bacterial cellulose nanocomposites: Preparation, characterization and post-modification. Int. J. Biol. Macromol. 2019, 127, 618–627. [Google Scholar] [CrossRef]
- Faria, M.; Vilela, C.; Silvestre, A.J.; Deepa, B.; Resnik, M.; Freire, C.S.; Cordeiro, N. Physicochemical surface properties of bacterial cellulose/polymethacrylate nanocomposites: An approach by inverse gas chromatography. Carbohydr. Polym. 2019, 206, 86–93. [Google Scholar] [CrossRef]
- Vilela, C.; Oliveira, H.; Almeida, A.; Silvestre, A.J.; Freire, C.S. Nanocellulose-based antifungal nanocomposites against the polymorphic fungus Candida albicans. Carbohydr. Polym. 2019, 217, 207–216. [Google Scholar] [CrossRef]
- Saïdi, L.; Vilela, C.; Oliveira, H.; Silvestre, A.; Freire, C.S. Poly(N-methacryloyl glycine)/nanocellulose composites as pH-sensitive systems for controlled release of diclofenac. Carbohydr. Polym. 2017, 169, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Vilela, C.; Moreirinha, C.; Domingues, E.M.; Figueiredo, F.M.L.; Almeida, A.; Freire, C.S.R. Antimicrobial and Conductive Nanocellulose-Based Films for Active and Intelligent Food Packaging. Nanomaterials 2019, 9, 980. [Google Scholar] [CrossRef] [Green Version]
- Trovatti, E.; Fernandes, S.C.M.; Rubatat, L.; Freire, C.S.R.; Silvestre, A.; Neto, C. Sustainable nanocomposite films based on bacterial cellulose and pullulan. Cellulose 2012, 19, 729–737. [Google Scholar] [CrossRef]
- Inácio, P.M.; Medeiros, M.D.C.; Carvalho, T.; Félix, R.C.; Mestre, A.; Hubbard, P.C.; Ferreira, Q.; Morgado, J.; Charas, A.; Freire, C.S.; et al. Ultra-low noise PEDOT:PSS electrodes on bacterial cellulose: A sensor to access bioelectrical signals in non-electrogenic cells. Org. Electron. 2020, 85, 105882. [Google Scholar] [CrossRef]
- Trovatti, E.; Silva, N.H.C.S.; Duarte, I.F.; Rosado, C.F.; Almeida, I.F.; Costa, P.; Freire, C.S.R.; Silvestre, A.J.D.; Neto, C.P. Biocellulose Membranes as Supports for Dermal Release of Lidocaine. Biomacromolecules 2011, 12, 4162–4168. [Google Scholar] [CrossRef]
- Silva, N.H.; Rodrigues, A.; Almeida, I.; da Costa, P.J.C.; Rosado, C.; Neto, C.P.; Silvestre, A.J.; Freire, C.S. Bacterial cellulose membranes as transdermal delivery systems for diclofenac: In vitro dissolution and permeation studies. Carbohydr. Polym. 2014, 106, 264–269. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.C.S.; Drumond, I.; Almeida, I.F.; Costa, P.; Rosado, C.F.; Neto, C.P.; Freire, C.S.R.; Silvestre, A.J.D. Topical caffeine delivery using biocellulose membranes: A potential innovative system for cellulite treatment. Cellulose 2014, 21, 665–674. [Google Scholar] [CrossRef] [Green Version]
- Pinto, R.J.; Fernandes, S.C.; Freire, C.S.; Sadocco, P.; Causio, J.; Neto, C.P.; Trindade, T. Antibacterial activity of optically transparent nanocomposite films based on chitosan or its derivatives and silver nanoparticles. Carbohydr. Res. 2012, 348, 77–83. [Google Scholar] [CrossRef]
- Pinto, R.; Almeida, A.; Fernandes, S.C.; Freire, C.; Silvestre, A.; Neto, C.P.; Trindade, T. Antifungal activity of transparent nanocomposite thin films of pullulan and silver against Aspergillus niger. Colloids Surf. B Biointerfaces 2013, 103, 143–148. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.H.; Vilela, C.; Pinto, R.J.; Martins, M.A.; Marrucho, I.M.; Freire, C.S. Tuning lysozyme nanofibers dimensions using deep eutectic solvents for improved reinforcement ability. Int. J. Biol. Macromol. 2018, 115, 518–527. [Google Scholar] [CrossRef]
- Sugumaran, K.R.; Ponnusami, V. Review on production, downstream processing and characterization of microbial pullulan. Carbohydr. Polym. 2017, 173, 573–591. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Mustaqeem, M.; Hassan, I.U.; Awan, T.; Arshad, F.; Salim, H.; Qurashi, A. Bioinspired and green synthesis of nanoparticles from plant extracts with antiviral and antimicrobial properties: A critical review. J. Saudi Chem. Soc. 2021, 25, 101304. [Google Scholar] [CrossRef]
- Karki, S.; Kim, H.; Na, S.-J.; Shin, D.; Jo, K.; Lee, J. Thin films as an emerging platform for drug delivery. Asian J. Pharm. Sci. 2016, 11, 559–574. [Google Scholar] [CrossRef] [Green Version]
- Mohan, C.C.; Harini, K.; Aafrin, B.V.; Priya, U.L.; Jenita, M.; Babuskin, S.; Karthikeyan, S.; Sudarshan, K.; Renuka, V.; Sukumar, M. Extraction and characterization of polysaccharides from tamarind seeds, rice mill residue, okra waste and sugarcane bagasse for its Bio-thermoplastic properties. Carbohydr. Polym. 2018, 186, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Carreira, P.; Mendes, J.A.; Trovatti, E.; Serafim, L.S.; Freire, C.S.; Silvestre, A.J.; Neto, C.P. Utilization of residues from agro-forest industries in the production of high value bacterial cellulose. Bioresour. Technol. 2011, 102, 7354–7360. [Google Scholar] [CrossRef] [PubMed]
- Horue, M.; Berti, I.R.; Cacicedo, M.L.; Castro, G.R. Microbial production and recovery of hybrid biopolymers from wastes for industrial applications- a review. Bioresour. Technol. 2021, 340, 125671. [Google Scholar] [CrossRef] [PubMed]
- Noyori, R. Synthesizing our future. Nat. Chem. 2009, 1, 5–6. [Google Scholar] [CrossRef] [PubMed]
| Cellulosic Substrate | Other Components | Type of Material | General Features | Application | Refs. |
|---|---|---|---|---|---|
| BNC | PLA | Nanocomposites | Methodology: Melt-mixing Mechanical properties: Improvement of YM up to 40% Thermal stability: Increase up to 14 °C, in the maximum degradation temperature | – | [112] |
| BNC | PCL | Nanocomposites | Methodology: Supplementation of the BNC culture medium with PCL powder and hot-pressing Thermal stability: Up to 200 °C Other characteristics: Decrease of the storage modulus with increasing amounts of PCL highlights the reinforcement role of BNC in the nanocomposites | – | [113] |
| BNC | PGMA | Nanocomposites | Methodology: in situ free radical polymerization Thermal stability: Up to 270 °C Water uptake capacity: Up to 32.8%, after 48 h Storage modulus: min. 80 MPa at 200 °C Other characteristics: Post-modification via acid-catalyzed hydrolysis improved the water-uptake capacity (up to 222%, after 48 h) | – | [119,120] |
| BNC | Pullulan | Nanocomposites | Methodology: Solvent casting Mechanical properties: Improvement of YM up to 8000% Thermal stability: Up to 40 °C, in the maximum degradation temperature | – | [124] |
| BNC | PSBMA | Films | Methodology: One-pot polymerization Water uptake capacity: Up to 559%, after 48 h Antibacterial activity: Staphylococcus aureus (4.3-log CFU reduction) and Escherichia coli (1.1-log CFU reduction) Ionic conductivity: Max. 1.5 mS cm−1, at 94 °C and 98% RH Other characteristics: UV-light barrier properties | Active and intelligent food packaging | [123] |
| BNC | PEDOT:PSS | Films | Methodology: Ink-jet printing of electrodes in BNC Other characteristics: BNC substrate lowers the PEDOT:PSS impedance and minimizes the effects of the 1/f2 noise; Ability to record low-frequency signals from non-electrogenic cells (glioma cells) | Sensors | [125] |
| BNC | PSSA | Membranes | Methodology: in situ free radical polymerization Ionic conductivity: max. 185 mS cm−1, at 94 °C and 98% RH Fuel cell tests: max. of ca. 40 mW cm−2 at 125 mA cm−2 Microbial fuel cell performance (Shewanella frigidimarina): max. power density of 2.42 mW m−2, open-circuit voltage of 0.436 V, internal resistance of 15.1 kΩ | Ion exchange membranes for fuel cells | [94,114,115] |
| BNC | NafionTM | Membranes | Methodology: Diffusion of NafionTM in the BNC matrix Ionic conductivity: 140 mS cm−1, at 94 °C and 98% RH | Ion exchange membrane for fuel cells | [116] |
| BNC | PMOEP | Membranes | Methodology: in situ free radical polymerization Water uptake capacity: Up to 206%, after 48 h Ionic conductivity: max. 100 mS cm−1, at 80 °C and 98% RH | Ion exchange membrane for fuel cells | [117] |
| BNC | PMACC | Membranes | Methodology: in situ free radical polymerization Water uptake capacity: Up to 2057%, after 48 h Ionic conductivity: max. 10 mS cm−1, at 94 °C and 98% RH | Ion exchange membrane for fuel cells | [118] |
| BNC | P(bis-MEP) | Membranes | Methodology: in situ free radical polymerization Water uptake capacity: Up to 155%, after 48 h Ionic conductivity: max. 30 mS cm−1, at 80 °C and 98% RH | Ion exchange membrane for fuel cells | [93] |
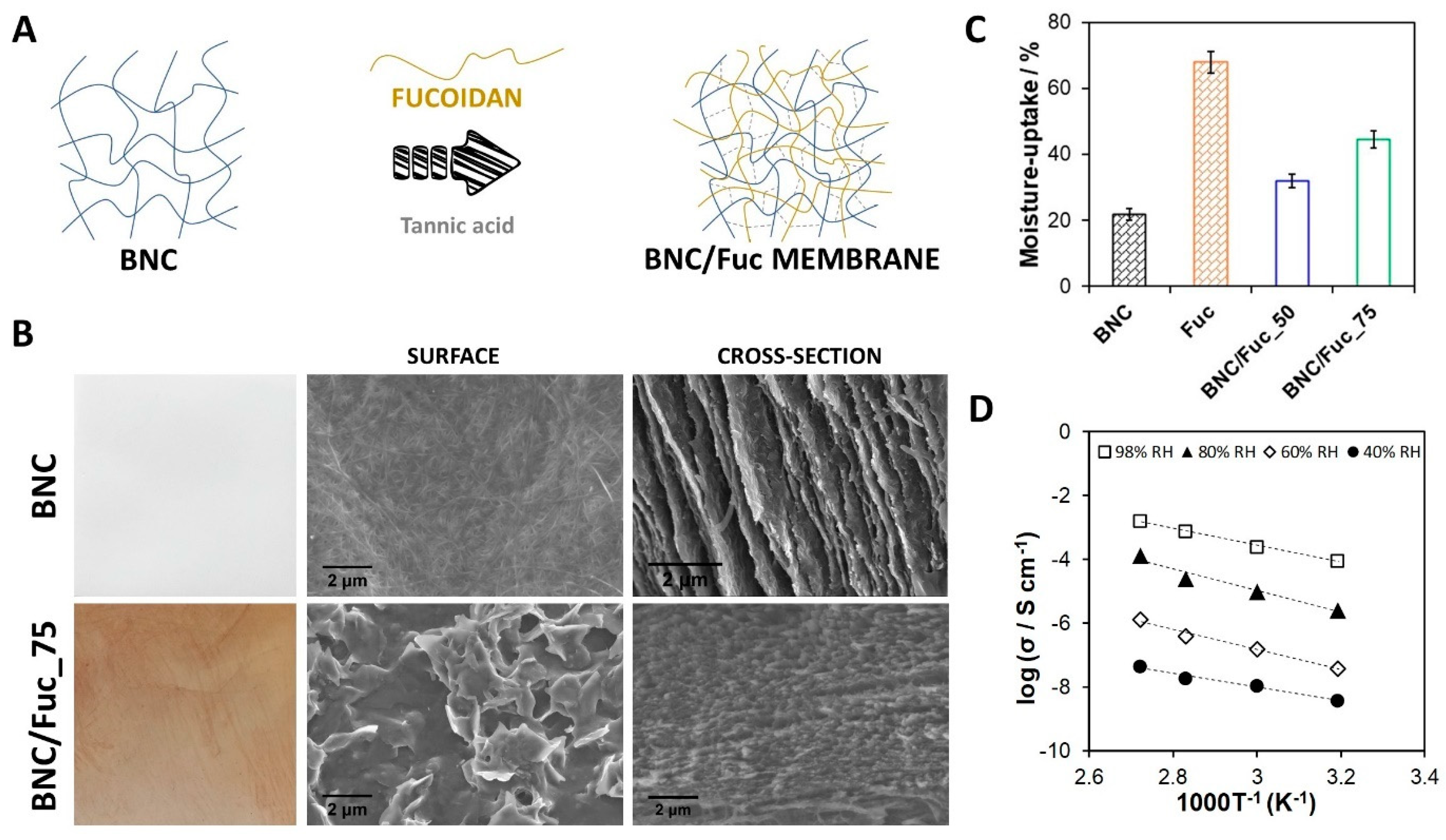
| BNC | Fucoidan | Membranes | Methodology: Diffusion of aqueous solutions in the BNC matrix Moisture uptake capacity: ca. 45%, at 98% RH after 48 h Ionic conductivity: max. 1.6 mS cm−1, at 94 °C and 98% RH | Ion exchange membrane for fuel cells | [81] |
| BNC | Lignosulfonates | Membranes | Methodology: Diffusion of aqueous solutions in the BNC matrix Moisture uptake capacity: ca. 78%, at 98% RH after 48 h Ionic conductivity: max. 23 mS cm−1, at 94 °C and 98% RH | Ion exchange membrane for fuel cells | [95] |
| BNC | PMPC | Membranes | Methodology: in situ free radical polymerization Water uptake capacity: 627–912%, for 48 h at different pH values (2.1, 7.4, 12) Antibacterial activity: S. aureus (4.3-log CFU reduction) and E. coli (1.8-log CFU reduction) Ionic dye adsorption: Up to 4.5 mg g−1 | Water remediation (removal of organic dyes) | [92] |
| BNC | Hyaluronic acid | Microneedles | Methodology: Micromolding and BNC backing layer Antioxidant activity: IC50 of 23.24 μg·mL−1 (DPPH scavenging activity) Cumulative release: ca. 94% rutin after 6.5 h (ex vivo skin) Percutaneous permeation (in vitro): 9.85%, after 12 h Other characteristics: Penetration up to 99.3 µm depth (ex vivo skin insertion); Non-cytotoxic towards HaCaT cells | Drug delivery (rutin) | [77] |
| BNC | PMETAC | Patches | Methodology: in situ free radical polymerization Water uptake capacity: Up to 873%, in distilled water after 48 h Antifungal activity: Candida albicans (4.4-log CFU reduction) Other characteristics: UV-light barrier properties; Non-cytotoxic towards HaCaT cells | Treatment of fungal infections | [121] |
| BNC | PMGly | Patches | Methodology: in situ free radical polymerization Water uptake capacity: Up to 23.5% (pH 2.1) and 77.8% (pH 7.4) after 24 h Cumulative release: pH dependent – ca. 9%, after 20 h at pH 2.1 and ca. 70%, after 1 h at pH 7.4 Other characteristics: Non-cytotoxic towards HaCaT cells | Drug delivery (diclofenac) | [122] |
| BNC | Patches | Methodology: Diffusion of aqueous solutions in the BNC matrix Moisture uptake capacity: Up to 26.0% (BNC/caffeine), 36.3% (BNC/lidocaine), 12.3% (BNC/ibuprofen) and 31.3% (BNC/diclofenac), at 75% RH, 40 °C for 3 months Cumulative release: 100% after 5 min (caffeine and lidocaine patches), 30 min (ibuprofen patch) and 15 min (diclofenac patch), in PBS Other characteristics: No significant changes in the cumulative release were observed after the accelerated stability tests; Good in vivo compatibility | Drug delivery (caffeine, lidocaine, ibuprofen, diclofenac) | [104] | |
| BNC | Patches | Methodology: Diffusion of aqueous solutions in the BNC matrix Percutaneous permeation (in vitro): 31.4 μg cm−2 h−1 (lidocaine) and 11.9 μg cm−2 h−1 (ibuprofen) | Drug delivery (lidocaine, ibuprofen) | [105] | |
| BNC | Patches | Methodology: Diffusion of aqueous solutions in the BNC matrix Cumulative release: >90% after 20 min, in PBS Percutaneous permeation (in vitro): 31.35 μg cm−2 h−1 | Drug delivery (lidocaine) | [126] | |
| BNC | Patches | Methodology: Diffusion of aqueous solutions in the BNC matrix Water uptake capacity: Up to 1400%, in PBS after 8 h Cumulative release: ca. 90% after 10 min, in PBS Percutaneous permeation (in vitro): 1.21 μg cm−2 h−1 | Drug delivery (diclofenac) | [127] | |
| BNC | Alginate + Chitosan | Patches | Methodology: LbL assembly Moisture uptake capacity: 240–250%, at 100% RH, 25 °C for 22 h Cumulative release: ca. 95% after 16 h (patch with 5 layers) up to ca. 65% after 90 h (patch with 21 layers) Antibacterial activity: S. aureus (3.2-log CFU reduction) Other characteristics: Non-cytotoxic towards HaCaT cells; Good cell migration capacity on wound healing assay | Wound healing Drug delivery (dexpanthenol) | [79] |
| BNC | Caffeine | Patches | Methodology: Diffusion of aqueous solutions in the BNC matrix Water uptake capacity: Up to 284%, in PBS solution, after 2 h Cumulative release: max. 80% after 15 min, in PBS solution Percutaneous permeation (in vitro): 2.55 μg cm−2 h−1 | Dermal care | [128] |
| BNC | ILs + vitamin B | Patches | Methodology: Diffusion of aqueous solutions in the BNC matrix Water uptake capacity: Up to 1697%, in PBS solution, after 24 h Cumulative release: At least 66% after 5 min, in PBS solution Other characteristics: Non-cytotoxic towards HaCaT cells | Dermal care | [106] |
| BNC | Hyaluronic acid | Patches | Methodology: Diffusion of aqueous solutions in the BNC matrix Water uptake capacity: Up to 484%, in agarose skin model Cumulative release: max. 90% after 4 min, in simulated salivary fluid Other characteristics: Non-cytotoxic towards HaCaT cells | Drug delivery (diclofenac) | [78] |
| Cellulose fibres | CaCO3 | Composites | Methodology: in situ synthesis Mechanical properties: Incorporation of the CaCO3/cellulose fibres improved the stiffness of polyethylene films | – | [63] |
| Cellulose fibres | PLA and PHB | Composites | Methodology: Melt-mixing Mechanical properties: Increase in YM up to 5.83 GPa, elongation at break < 3.5%, tensile strength up to 72.9 MPa and flexural modulus up to 8.1 GPa Thermal stability: Increase up to 44 °C in the maximum thermal degradation temperature Other characteristics: Decrease in water uptake, compared to neat matrices and PLA or PHB with non-micronized fibres | – | [64] |
| CNCs | Chitosan derivative with folic acid and fluorescein isothiocyanate | Nanosystems | Methodology: Physical adsorption Other characteristics: Fluorescent nanosystems; Non-cytotoxic towards MDA-MB-231 breast cancer cells; Anti-proliferative effect suggested by exometabolomics analysis | – | [69] |
| CNFs | AgNPs | Coatings | Methodology: Electrostatic assembly Air permeability: Up to 9.54 nm Pa−1 s−1 Antibacterial activity: S. aureus and Klebsiella pneumoniae (total inhibition after 24 h) | – | [100] |
| CNFs | ZnO | Coatings | Methodology: Electrostatic assembly Air permeability: Up to 10.81 nm Pa−1 s−1 Antibacterial activity: S. aureus (up to 3.4-log CFU reduction), Bacillus cereus (up to 3.5-log CFU reduction) and K. pneumoniae (total inhibition after 24 h, at [ZnO] >2%) | – | [101] |
| CNFs | PCL | Nanocomposites | Methodology: Melt-mixing Thermal stability: 335–340 °C Mechanical properties: YM of 43.6–52.3 MPa Other characteristics: Degradable under enzymatic conditions | – | [98] |
| CNFs | CuNWs | Nanocomposites | Methodology: Vacuum filtration Mechanical properties: YM of 2.62–4.72 GPa Electrical conductivity: Up to 5.43 × 104 S m−1 | – | [111] |
| CNFs | LNFs | Films | Methodology: Vacuum filtration Hg(II) removal: ca. 99% after 24 h of contact time, at pH 11 | Water remediation (mercury removal) | [85] |
| CNFs | Arabinoxylans + ferulic acid or feruloylated arabinoxylo-oligosaccharides | Films | Methodology: Solvent casting Antioxidant activity: Up to 90% (DPPH scavenging activity) Antibacterial activity: S. aureus (>3-log CFU reduction) and E. coli (up to 3-log CFU reduction) Antifungal activity: C. albicans (1.1-log CFU reduction) Other characteristics: UV-light barrier properties | Active food packaging | [89] |
| CNFs | Mango leaf extract | Films | Methodology: Supercritical solvent impregnation Antioxidant activity: ca. 84% (DPPH scavenging activity) Antibacterial activity: S. aureus (max. growth inhibition ≈ 37%) and E. coli (max. growth inhibition ≈ 91%) Other characteristics: UV-light barrier properties | Active food packaging | [88] |
| CNFs | LNFs | Patches | Methodology: Vacuum filtration Antioxidant activity: 76–79% (DPPH scavenging activity) Antibacterial activity: S. aureus (3.5-log CFU reduction) Other characteristics: Non-cytotoxic towards L929 fibroblast cells; Good cell migration capacity on wound healing assay | Wound healing | [86] |
| Natural Polymer | Other Components | Type of Material | General Features | Application | Refs. |
|---|---|---|---|---|---|
| Agar | Opuntia ficus-indica cladodes powder | Films | Methodology: Solvent casting WVTR: 12.67–14.17 g h−1 m−2 Other characteristics:UV-light barrier properties | Food packaging | [82] |
| Chitosan | MBT | Coatings | Methodology: Functionalization of the chitosan matrices Other characteristics: Corrosion protection towards Al alloy 2024; pH-dependent release | Anti-corrosion | [73,102] |
| Chitosan | Cerium (III) nitrate | Coatings | Methodology: Solvent casting Other characteristics: Corrosion protection towards Al alloy 2024; Self-healing ability | Anti-corrosion | [103] |
| Chitosan | Starch, BNC/CNFs fibres | Nanocomposites | Methodology: Solvent casting Thermal stability: Increase up to 15 °C, in the maximum degradation temperature Mechanical properties: YM up to 20 MPa Antimicrobial activity: S. aureus (up to 3-log CFU reduction) | – | [68] |
| Chitosan | AgNPs | Nanocomposites | Methodology: Solvent casting Antibacterial activity: S. aureus (up to 3-log CFU reduction), Klebsiella pneumoniae (up to 5.5-log CFU reduction) and E. coli (up to 4.5-log CFU reduction) | – | [129] |
| Chitosan | Ellagic acid | Films | Methodology: Solvent casting WVP: 2.82–3.70 g mm m−2 day−1 kPa−1 Antioxidant activity: ca. 28% (DPPH scavenging activity) Antibacterial activity: S. aureus and Pseudomonas aeruginosa (total inhibition after 24 h) Other characteristics:UV-light barrier properties | Active food packaging | [90] |
| Chitosan | Meso-tetraarylporphyrins | Films | Methodology: Solvent casting Antibacterial activity: Inhibition of Listeria innocua attachment (up to 6-log CFU reduction) and biofilm formation Other characteristics: Films are photostable and able to generate singlet oxygen under visible light irradiation | Photodynamic antifouling materials | [91] |
| Chitosan | Corrole macrocycle | Films | Methodology: Chemical grafting and solvent casting Antibacterial activity: S. aureus (2-log CFU reduction) Other characteristics: Films exhibit fluorescence | – | [72] |
| Chitosan | LnPOMs | Films | Methodology: Solvent casting Other characteristics: Films exhibit fluorescence under UV irradiation | – | [71] |
| Chitosan | Cholinium carboxylate ILs | Films | Methodology: Solvent casting Antibacterial activity: S. aureus (6.5-log CFU reduction) and K. pneumoniae (6.4-log CFU reduction) Other characteristics: Plasticizing effect of ILs | – | [74] |
| Fucoidan | AuNPs | Nanosystems | Methodology: Microwave-assisted synthesis Antitumoral activity: MNT-1, HepG2 and MG-63 tumour cell lines (reduction of cell viability up to 90%, at 72 h) Other characteristics: Cellular uptake confirmed using flow cytometry/dark-field imaging | – | [80] |
| Gelatin | Microneedles | Methodology: Photo-cross-linking and micromolding Fluid uptake: ca. 3.7 mg fluid/patch Urea recovery: >98%, in agarose skin model Other characteristics: Penetration up to 237 µm depth (ex vivo skin insertion); Non-cytotoxic towards HaCaT cells | ISF extraction for urea monitoring | [87] | |
| Pullulan | CNFs | Nanocomposites | Methodology: Solvent casting Mechanical properties: Improvement of YM up to 5500% Thermal stability: Increase up to 20 °C, in the maximum degradation temperature | – | [99] |
| Pullulan | LnPOMs | Films | Methodology: Solvent casting Other characteristics: Films exhibit fluorescence under UV irradiation | – | [71] |
| Pullulan | Cholinium carboxylate ILs | Films | Methodology: Solvent casting Antibacterial activity: S. aureus (2.6-log CFU reduction) and K. pneumoniae (3.5-log CFU reduction), only for the films with cholinium citrate Other characteristics: Plasticizing effect of ILs | – | [74] |
| Pullulan | AgNPs | Films | Methodology: Solvent casting Antifungal activity: Aspergillus niger (76% inhibition) | – | [130] |
| Pullulan | LNFs | Films | Methodology: Solvent casting Antioxidant activity: ca. 77% (DPPH scavenging activity) Antibacterial activity: S. aureus (3.2-log CFU reduction) | Active food packaging | [108,131] |
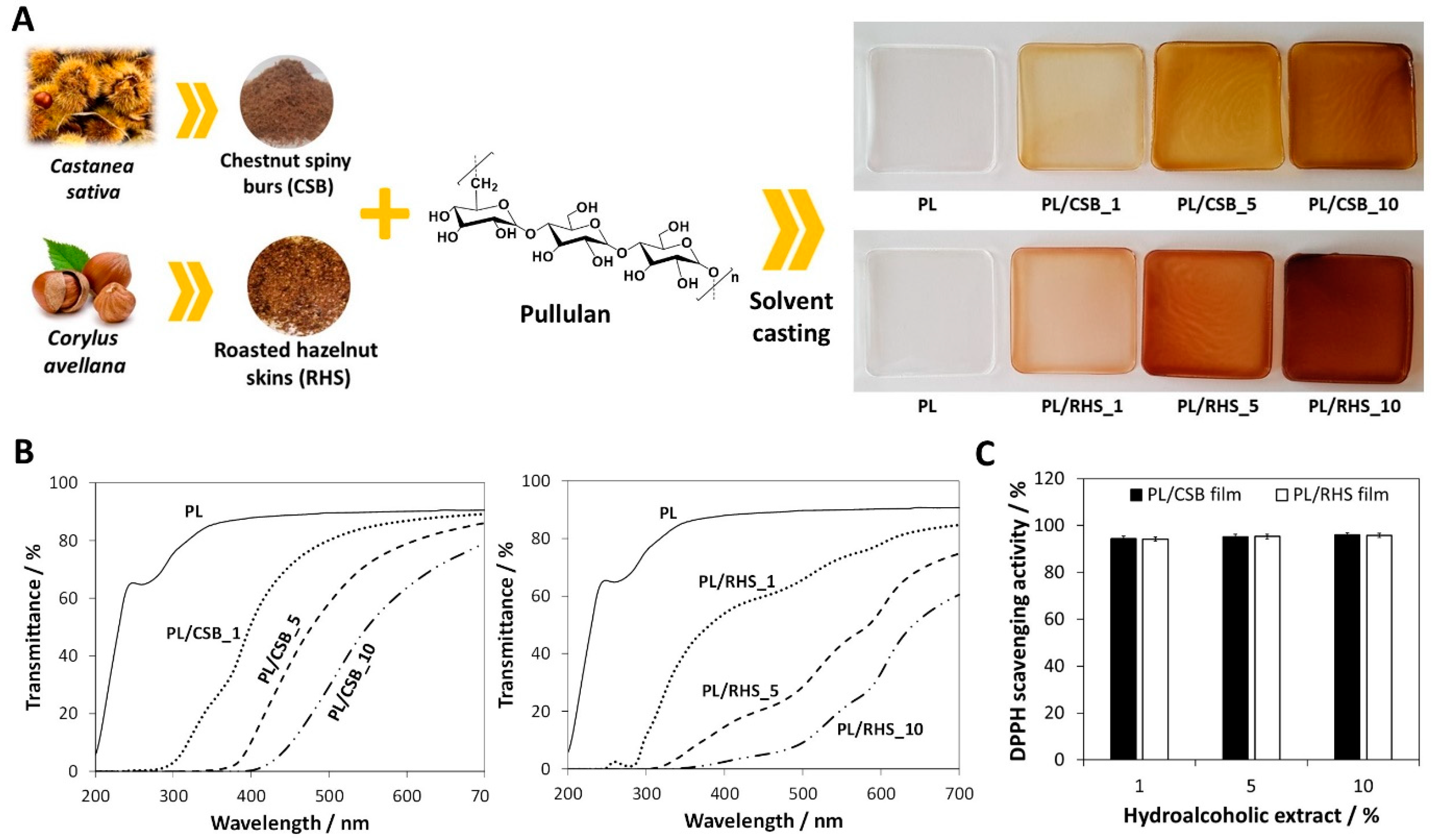
| Pullulan | Extracts from chestnut spiny burs and roasted hazelnut skins | Films | Methodology: Solvent casting Antioxidant activity: ca. 94% (DPPH scavenging activity) Antibacterial activity: S. aureus (4-log CFU reduction) Other characteristics: UV-light barrier properties | Active food packaging | [75] |
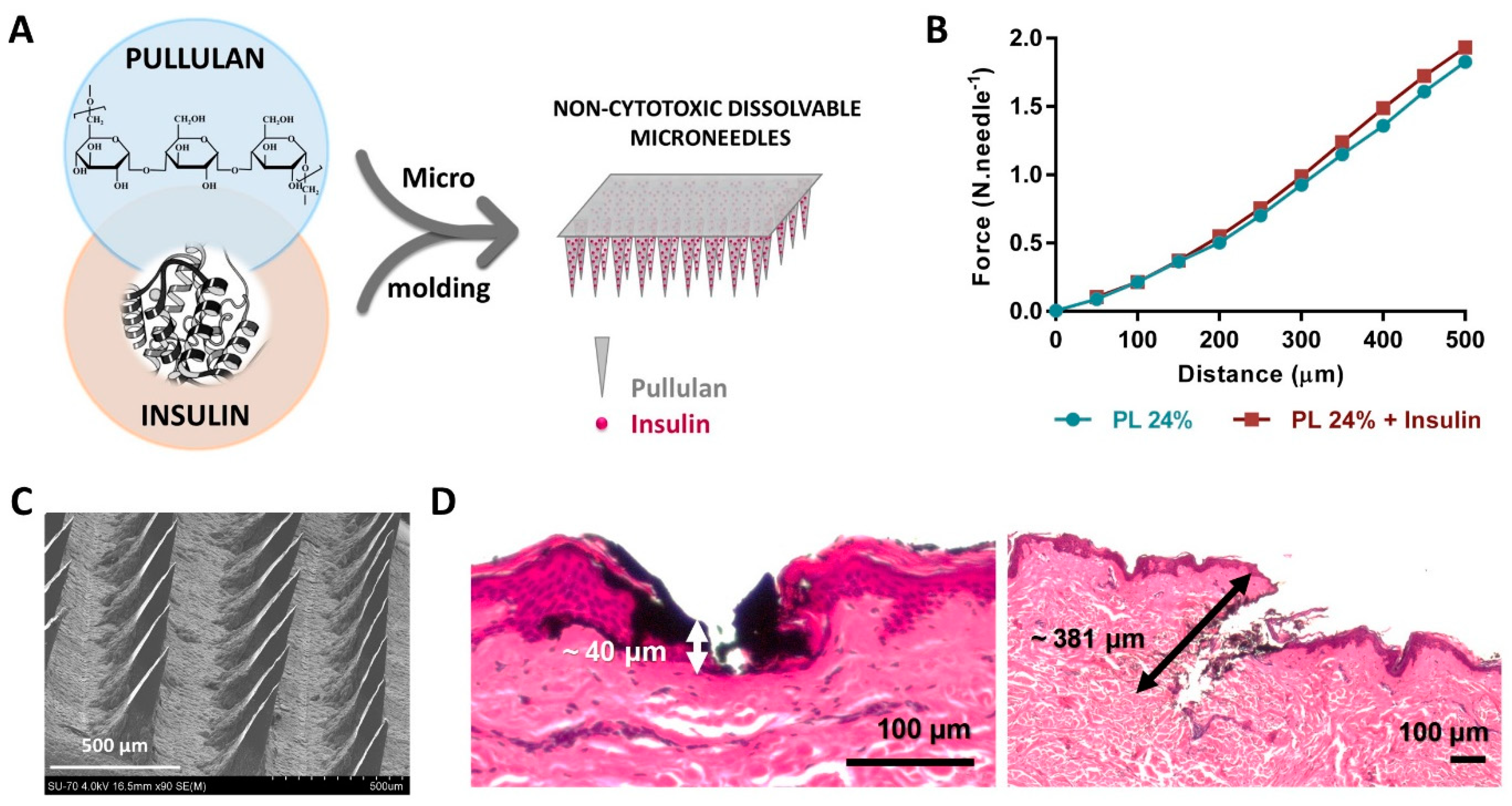
| Pullulan | Microneedles | Methodology: Micromolding Cumulative release: ca. 87% insulin after 2 h (ex vivo skin) Other characteristics: Penetration up to 381 µm depth (ex vivo skin); Non-cytotoxic towards HaCaT cells | Drug delivery (insulin) | [76] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. https://doi.org/10.3390/molecules27010094
Silva ACQ, Silvestre AJD, Vilela C, Freire CSR. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules. 2022; 27(1):94. https://doi.org/10.3390/molecules27010094
Chicago/Turabian StyleSilva, Ana C. Q., Armando J. D. Silvestre, Carla Vilela, and Carmen S. R. Freire. 2022. "Natural Polymers-Based Materials: A Contribution to a Greener Future" Molecules 27, no. 1: 94. https://doi.org/10.3390/molecules27010094
APA StyleSilva, A. C. Q., Silvestre, A. J. D., Vilela, C., & Freire, C. S. R. (2022). Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules, 27(1), 94. https://doi.org/10.3390/molecules27010094