A Double-Edged Sword: Thioxanthenes Act on Both the Mind and the Microbiome
Abstract
1. Introduction
2. Therapeutic Usage of Thioxanthenes
3. Pharmacological Properties of Thioxanthenes
4. Bacterial Inhibitory Action of Thioxanthenes
5. Bacteriostatic Action of Thioxanthenes
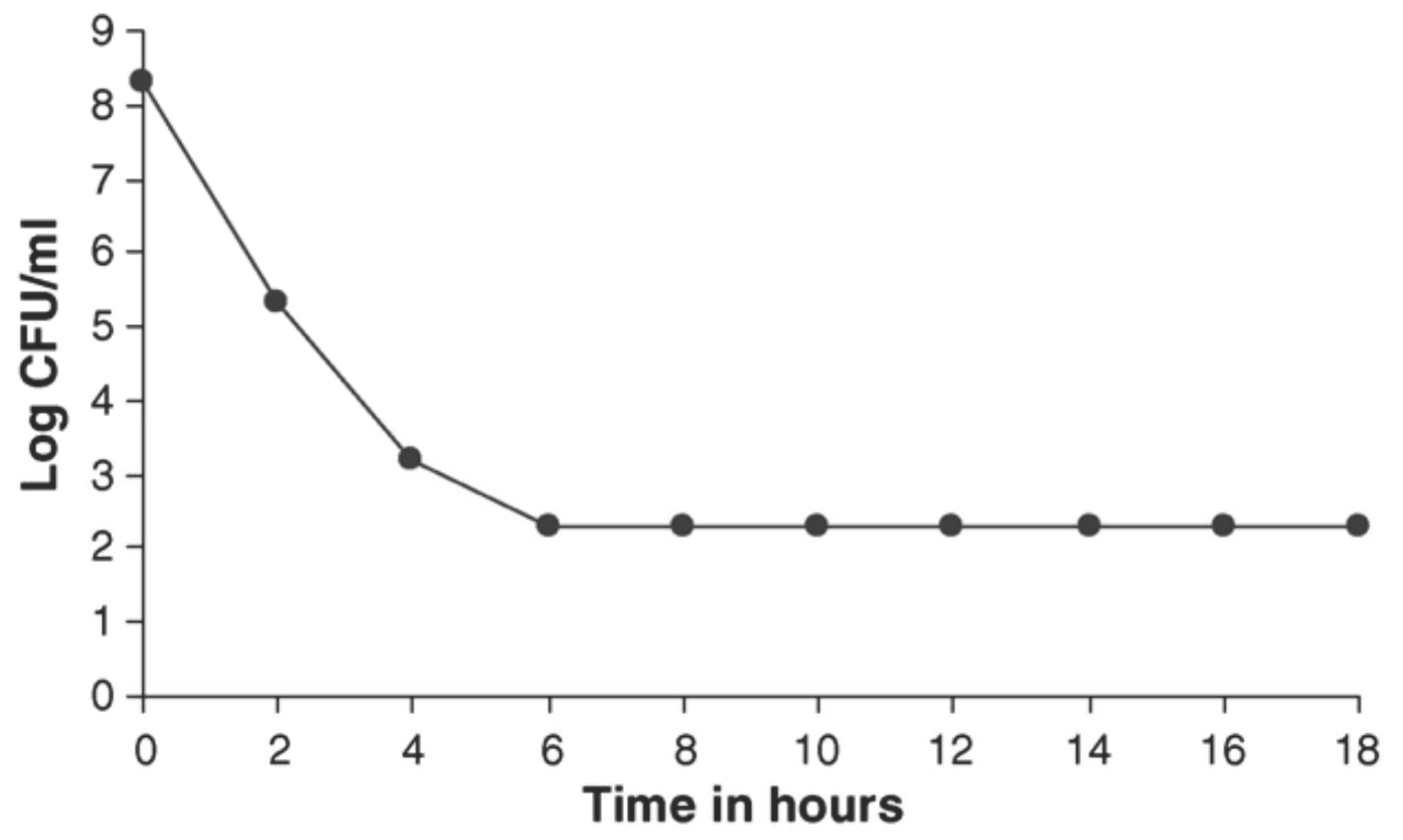
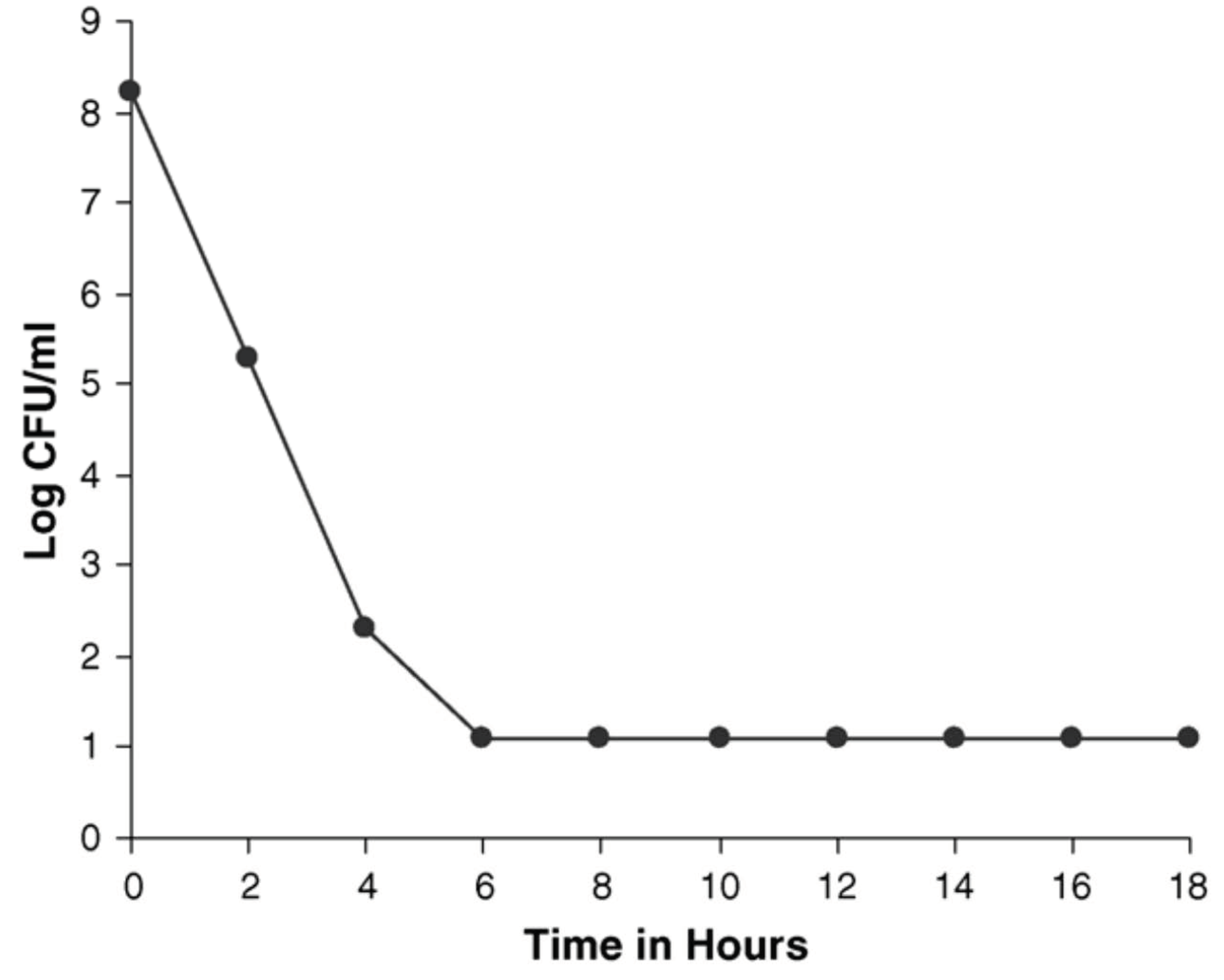
6. In Vivo Observation with Flupenthixol
7. Effect of Thioxanthenes on Slow Growing Mycobacteria
8. Effect of Thioxanthenes on Viruses and Eukaryotic Cells
9. In Vitro Modulation of Human Neutrophil by E-Clopenthixol
10. Inhibition of HIV Replication by Thioxanthenes
11. Antiparasitic Action of Thioxanthenes
12. Efflux Pump Inhibition by Thioxanthenes
13. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ahmed, U.; Jones, H.; Adams, C.E. Chlorpromazine for psychosis induced aggression or agitation. Cochrane Database Syst. Rev. 2010, 4. [Google Scholar] [CrossRef]
- Baumeister, A.A. The Chlorpromazine Enigma. J. Hist. Neurosci. 2013, 22, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Tomida, K.; Takahashi, N.; Saito, S.; Maeno, N.; Iwamoto, K.; Yoshida, K.; Kimura, H.; Iidaka, T.; Ozaki, N. Relationship of psychopathological symptoms and cognitive function to subjective quality of life in patients with chronic schizophrenia. Psychiatry Clin. Neurosci. 2010, 64, 62–69. [Google Scholar] [CrossRef]
- Renard, J.; Norris, C.; Rushlow, W.; Laviolette, S.R. Neuronal and molecular effects of cannabidiol on the mesolimbic dopamine system: Implications for novel schizophrenia treatments. Neurosci. Biobehav. Rev. 2017, 75, 157–165. [Google Scholar] [CrossRef]
- Newman, W.J.; Newman, B.M. Rediscovering clozapine: After a turbulent history, current guidance on initiating and monitoring. Curr. Psychiatr. 2016, 15, 42–49. [Google Scholar]
- Meltzer, H.Y. Update on typical and atypical antipsychotic drugs. Annu. Rev. Med. 2013, 64, 393–406. [Google Scholar] [CrossRef]
- Baumann, P.; Kirchherr, H.; Berney, P.; Hiemke, C. Flupentixol: Relevance of stereoselective therapeutic drug monitoring. Psychopharmacology 2012, 221, 719–720. [Google Scholar] [CrossRef][Green Version]
- Behere, P.B.; Das, A.; Behere, A.P. Antipsychotics. In Clinical Psychopharmacology; Springer: Singapore, 2019; pp. 39–87. [Google Scholar]
- Nasrallah, H.; Tandon, R. Classic Antipsychotic Medications. In Essentials of Clinical Psychopharmacology; Schatzberg, A.F., Nemeroff, C.B., Eds.; American Psychiatric Publishing Inc.: Arlington, TX, USA, 2013; pp. 219–236. [Google Scholar]
- Kendall, T. The rise and fall of the atypical antipsychotics. Br. J. Psychiatry 2011, 199, 266–268. [Google Scholar] [CrossRef]
- Remvig, J.; Sonne, L.M. Chlorprothixene (“Truxal”) compared to chlorpromazine. Psychopharmacologia 1961, 2, 203–208. [Google Scholar] [CrossRef]
- Madhusoodanan, S.; Alexeenko, L.; Sanders, R.; Brenner, R. Extrapyramidal symptoms associated with antidepressants—A review of the literature and an analysis of spontaneous reports. Ann. Clin. Psychiatry 2010, 22, 148–156. [Google Scholar] [PubMed]
- Correll, C.U. Mechanism of Action of Antipsychotic Medications. J. Clin. Psychiatry 2014, 75, e23. [Google Scholar] [CrossRef]
- Taylor, D. Psychopharmacology and adverse effects of antipsychotic long-acting injections: A review. Br. J. Psychiatry 2009, 195, s13–s19. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, H.; Gen, K.; Inoue, Y. Comparison of the anti-dopamine D2 and anti-serotonin 5-HT 2A activities of chlorpromazine, bromperidol, haloperidol and second-generation antipsychotics parent compounds and metabolites thereof. J. Psychopharmacol. 2013, 27, 396–400. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekaran, S.; Kar, S.; Yadav, S. Chlorpromazine-induced severe exfoliative photoallergic reaction. Int. J. Nutr. Pharm. Neurol. Dis. 2015, 5, 34–36. [Google Scholar]
- Edler, K.; Gottfries, C.G.; Haslund, J.; Ravn, J. Eye changes in connection with neuroleptic treatment especially concerning phenothiazines and thioxanthenes. Acta Psychiatr. Scand. 1971, 47, 377–384. [Google Scholar] [CrossRef]
- Ayano, G. First Generation Antipsychotics: Pharmacokinetics, Pharmacodynamics, Therapeutic Effects and Side effects: A review. RRJChem 2016, 4, 90–94. [Google Scholar]
- Lambert, T.; Taylor, D. Pharmacology of antipsychotic long-acting injections. In Antipsychotic Long-Acting Injections; Haddad, P., Lambert, T., Lauriello, J., Eds.; Oxford University Press Inc.: New York, NY, USA, 2011; pp. 23–47. ISBN 978-0-19-958604-2. [Google Scholar]
- Li, P.L.; Snyder, G.E.; Vanover, K. Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future. Curr. Top. Med. Chem. 2016, 16, 3385–3403. [Google Scholar] [CrossRef]
- Novir, S.B. A theoretical study of the structural and electronic properties of trans and cis structures of chlorprothixene as a nano-drug. Curr. Appl. Phys. 2017, 17, 1754–1764. [Google Scholar] [CrossRef]
- de Wit, H. Flupenthixol. In Encyclopedia of Psychopharmacology; Stolerman, I.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 538–539. [Google Scholar] [CrossRef]
- Bostwick, J.R.; Guthrie, S.K.; Ellingrod, V.L. Antipsychotic-induced hyperprolactinemia. Pharmacotherapy 2009, 29, 64–73. [Google Scholar] [CrossRef]
- Tardy, M.; Dold, M.; Engel, R.R.; Leucht, S. Flupenthixol versus low-potency first-generation antipsychotic drugs for schizophrenia. Cochrane Database Syst. Rev. 2014, 2014, 1–26. [Google Scholar] [CrossRef]
- Van Coller, P.E. Flupenthixol (fluanxol) in the treatment of psychosomatic disorders in medicine. Psychosomatics 1971, 12, 256–259. [Google Scholar] [CrossRef]
- Trueman, H.R.; Valentine, M.G. Flupenthixol decanoate in schizophrenia. Br. J. Psychiatry 1974, 124, 58–59. [Google Scholar] [CrossRef]
- Leucht, S.; Cipriani, A.; Spineli, L.; Mavridis, D.; Örey, D.; Richter, F.; Samara, M.; Barbui, C.; Engel, R.R.; Geddes, J.R.; et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: A multiple-treatments meta-analysis. Lancet 2013, 382, 951–962. [Google Scholar] [CrossRef]
- Ravn, J.; Rud, C.; Wendelboe, J. 252 mit dem neuen Psykofarmakon Clopenthixol (Sordinol, Ciatyl) behandelte psychiatrische Patienten. In Neuro-Psychopharmacology; Bradley, P.B., Flugel, F., Hoch, P., Eds.; Elsevier Publishing Co.: Amsterdam, The Netherlands, 1964; pp. 285–289. [Google Scholar]
- Gordon, M. (Ed.) Psychopharmacological Agents; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 1483274462/9781483274461. [Google Scholar]
- Schatzberg, A.F.; DeBattista, C. Manual of Clinical Psychopharmacology; American Psychiatric Publishing: Washington, DC, USA, 2015; ISBN 1-58562-481-0. [Google Scholar]
- Iversen, L.L.; Miller, R.J.; Horn, A.S. Effects of dopaminergic agonist and antagonist drugs on cyclic 3′,5′ –adenosine Monophosphate (cyclic AMP) production in rat brain homogenates. J. Pharmacol. 1974, 5, 117–118. [Google Scholar]
- Moller Nielsen, I.; Fjalland, B.; Pedersen, V.; Nymark, M. Pharmacology of neuroleptics upon repeated administration. Psychopharmacologia 1974, 34, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Casey, D.; Christensen, A. (Eds.) Psychopharmacology: Current Trends: Current Trends; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 3642732801/9783642732805. [Google Scholar]
- Moloney, R.D.; Desbonnet, L.; Clarke, G.; Dinan, T.G.; Cryan, J.F. The microbiome: Stress, health and disease. Mamm. Genome 2014, 25, 49–74. [Google Scholar] [CrossRef]
- Parashar, A.; Udayabanu, M. Gut microbiota regulates key modulators of social behavior. Eur. Neuropsychopharmacol. 2016, 26, 78–91. [Google Scholar] [CrossRef] [PubMed]
- Valles-Colomer, M.; Falony, G.; Darzi, Y.; Tigchelaar, E.F.; Wang, J.; Tito, R.Y.; Schiweck, C.; Kurilshikov, A.; Joossens, M.; Wijmenga, C.; et al. The neuroactive potential of the human gut microbiota in quality of life and depression. Nat. Microbiol. 2019, 4, 623–632. [Google Scholar] [CrossRef] [PubMed]
- Matarazzo, I.; Toniato, E.; Robuffo, I. Psychobiome Feeding Mind: Polyphenolics in Depression and Anxiety. Curr. Top. Med. Chem. 2018, 18, 2108–2115. [Google Scholar] [CrossRef]
- Zhu, X.; Li, B.; Lou, P.; Dai, T.; Chen, Y.; Zhuge, A.; Yuan, Y.; Li, L. The Relationship Between the Gut Microbiome and Neurodegenerative Diseases. Neurosci. Bull. 2021, 37, 1510–1522. [Google Scholar] [CrossRef]
- Hu, X.; Wang, T.; Jin, F. Alzheimer’s disease and gut microbiota. Sci. China Life Sci. 2016, 59, 1006–1023. [Google Scholar] [CrossRef]
- Sampson, T.R.; Debelius, J.W.; Thron, T.; Janssen, S.; Shastri, G.G.; Ilhan, Z.E.; Challis, C.; Schretter, C.E.; Rocha, S.; Gradinaru, V.; et al. Gut Microbiota Regulate Motor Deficits and Neuroinflammation in a Model of Parkinson’s Disease. Cell 2016, 167, 1469–1480.e12. [Google Scholar] [CrossRef]
- Socała, K.; Doboszewska, U.; Szopa, A.; Serefko, A.; Włodarczyk, M.; Zielińska, A.; Poleszak, E.; Fichna, J.; Wlaź, P. The role of microbiota-gut-brain axis in neuropsychiatric and neurological disorders. Pharmacol. Res. 2021, 172, 105840. [Google Scholar] [CrossRef]
- Geiger, H.; Finkeistein, B.A. Largactil in the treatment of tuberculosis. Schweiz. Med. Wochenschr. 1954, 84, 1063–1064. [Google Scholar]
- Kristiansen, J.E. The antimicrobial activity of non-antibiotics. Report from a congress on the antimicrobial effect of drugs other than antibiotics on bacteria, viruses, protozoa, and other organisms. Acta Pathol. Microbiol. Immunol. Scand. Suppl. 1992, 100, 7–14. [Google Scholar]
- Chakrabarty, A.N.; Bhattacharya, C.P.; Dastidar, S.G. Antimycobacterial activity of methdilazine (Md), an antimicrobic phenothiazine. APMIS 1993, 101, 449–454. [Google Scholar] [CrossRef] [PubMed]
- Annadurai, S.; Guha-Thakurta, A.; Sa, B.; Dastidar, S.G.; Ray, R.; Chakrabarty, A.N. Experimental studies on synergism between aminoglycosides and the antimicrobial antiinflammatory agent diclofenac sodium. J. Chemother. 2002, 14, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, A.; Kumar, K.A.; Dutta, N.K.; Chakraborty, P.; Dastidar, S.G. Evaluation of in vitro and in vivo antibacterial activity of dobutamine hydrochloride. Indian J. Med. Microbiol. 2003, 21, 172–178. [Google Scholar] [CrossRef]
- Kumar, K.A.; Ganguly, K.; Mazumdar, K.; Dutta, N.K.; Dastidar, S.G.; Chakrabarty, A.N. Amlodipine: A cardiovascular drug with powerful antimicrobial property. Acta Microbiol. Pol. 2003, 52, 285–292. [Google Scholar]
- Mazumdar, K.; Ganguly, K.; Kumar, K.A.; Dutta, N.K.; Chakrabarty, A.N.; Dastidar, S.G. Antimicrobial potentiality of a new non-antibiotic: The cardiovascular drug oxyfedrine hydrochloride. Microbiol. Res. 2003, 158, 259–264. [Google Scholar] [CrossRef]
- Karak, P.; Kumar, K.A.; Mazumdar, K.; Mookerjee, M.; Dastidar, S.G. Antibacterial potential of an antispasmodic drug dicyclomine hydrochloride. Indian J. Med. Res. 2003, 118, 192–196. [Google Scholar] [PubMed]
- Pal, T.; Dutta, N.K.; Mazumdar, K.; DasGupta, A.; Jeyaseeli, L.; Dastidar, S.G. Assessment of antibacterial activity of the cardiovascular drug nifedipine. Orient. Pharm. Exp. Med. 2006, 6, 126–133. [Google Scholar]
- Dasgupta, A.; Jeyaseeli, L.; Dutta, N.K.; Mazumdar, K.; Karak, P.; Dastidar, S.G.; Motohashi, N.; Shirataki, Y. Studies on the antimicrobial potential of the cardiovascular drug lacidipine. In Vivo 2007, 21, 847–850. [Google Scholar] [PubMed]
- Mortensen, I.; Kristiansen, J.E. The antibacterial activity of the psychopharmacological agent clopenthixol and its two main metabolites. Acta Pathol. Microbiol. Immunol. Scand. B 1987, 95, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Jeyaseeli, L.; DasGupta, A.; Asok Kumar, K.; Mazumdar, K.; Dutta, N.K.; Dastidar, S.G. Antimicrobial potentiality of the thioxanthene flupenthixol through extensive in vitro and in vivo experiments. Int. J. Antimicrob. Agents 2006, 27, 58–62. [Google Scholar] [CrossRef]
- Poulsen, M.Ø.; Jacobsen, K.; Thorsing, M.; Kristensen, N.R.D.; Clasen, J.; Lillebæk, E.M.S.; Skov, M.N.; Kallipolitis, B.H.; Kolmos, H.J.; Klitgaard, J.K. Thioridazine potentiates the effect of a beta-lactam antibiotic against Staphylococcus aureus independently of mecA expression. Res. Microbiol. 2013, 164, 181–188. [Google Scholar] [CrossRef]
- Poulsen, M.Ø.; Klitgaard, J.K.; Christensen, J.B.; Kallipolitis, B.H.; Kaatz, G.W.; Plenge, P.; Fey, S.J.; Kristiansen, J.E. Comparison of Antibacterial Activity of (–) Thioridazine and Racemic Thioridazine in Staphylococcus aureus. Am. J. Bioavailab. Bioequivalence 2018, 1, 1–9. [Google Scholar]
- Poulsen, M.Ø.; Schøler, L.; Nielsen, A.; Skov, M.N.; Kolmos, H.J.; Kallipolitis, B.H.; Olsen, A.; Klitgaard, J.K. Combination therapy with thioridazine and dicloxacillin combats meticillin-resistant Staphylococcus aureus infection in Caenorhabditis elegans. J. Med. Microbiol. 2014, 63, 1174–1180. [Google Scholar] [CrossRef]
- Jeyaseeli, L.; Dasgupta, A.; Dastidar, S.G.; Molnar, J.; Amaral, L. Evidence of significant synergism between antibiotics and the antipsychotic, antimicrobial drug flupenthixol. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 1243–1250. [Google Scholar] [CrossRef]
- Laborit, H.; Huguenard, P.; Alluaume, R. A new vegetative stabilizer; 4560 RP. Presse Med. 1952, 60, 206–208. [Google Scholar]
- Sigwald, J.; Bouttier, D. 3-Chloro-10-(3′-dimethylaminopropyl)-phenothiazine hydrochloride in current neuro-psychiatry. Bull. Mem. Soc. Med. Hop. Paris 1953, 54, 150–182. [Google Scholar] [PubMed]
- Delay, J.; Deniker, P. Réactions biologiques observées au cours du traitement par le chlorhydrate de diméthyl-amino-propyl-N-chlorophénothiazine (4560 R.P.). CR Congrés Médicine Alién Neurol. 1952, 50, 514–518. [Google Scholar]
- Popper, M.; Lorian, V. Effects of chlorpromazine on bacteria and bacteriostatic complex in vitro. Presse Med. 1959, 67, 212. [Google Scholar] [PubMed]
- Bourdon, J.L. Contribution to the study of the antibiotic properties of chlorpromazine or 4560 RP. Ann. Inst. Pasteur 1961, 101, 876–886. [Google Scholar]
- Crowle, A.J.; Douvas, G.S.; May, M.H. Chlorpromazine: A drug potentially useful for treating mycobacterial infections. Chemotherapy 1992, 38, 410–419. [Google Scholar] [CrossRef]
- Kristiansen, J.E.; Vergmann, B. The antibacterial effect of selected phenothiazines and thioxanthenes on slow-growing mycobacteria. Acta Pathol. Microbiol. Immunol. Scand. B 1986, 94, 393–398. [Google Scholar] [CrossRef]
- Molnár, J.; Béládi, I.; Földes, I. Studies on antituberculotic action of some phenothiazine derivatives in vitro. Zent. Bakteriol. Orig. A 1977, 239, 521–526. [Google Scholar]
- Ordway, D.; Viveiros, M.; Leandro, C.; Bettencourt, R.; Almeida, J.; Martins, M.; Kristiansen, J.E.; Molnar, J.; Amaral, L. Clinical concentrations of thioridazine kill intracellular multidrug-resistant Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2003, 47, 917–922. [Google Scholar] [CrossRef]
- Amaral, L.; Kristiansen, J.E.; Abebe, L.S.; Millett, W. Inhibition of the respiration of multi-drug resistant clinical isolates of Mycobacterium tuberculosis by thioridazine: Potential use for initial therapy of freshly diagnosed tuberculosis. J. Antimicrob. Chemother. 1996, 38, 1049–1053. [Google Scholar] [CrossRef]
- Ratnakar, P.; Murthy, P.S. Trifluoperazine inhibits the incorporation of labelled precursors into lipids, proteins and DNA of Mycobacterium tuberculosis H37Rv. Fems Microbiol. Lett. 1993, 110, 291–294. [Google Scholar] [CrossRef]
- Simons, S.O.; Kristiansen, J.E.; Hajos, G.; van der Laan, T.; Molnár, J.; Boeree, M.J.; van Ingen, J.; Christensen, J.B.; Viveiros, M.; Riedl, Z.; et al. Activity of the efflux pump inhibitor SILA 421 against drug-resistant tuberculosis. Int. J. Antimicrob. Agents 2013, 41, 488–489. [Google Scholar] [CrossRef][Green Version]
- van Ingen, J.; van der Laan, T.; Amaral, L.; Dekhuijzen, R.; Boeree, M.J.; van Soolingen, D. In vitro activity of thioridazine against mycobacteria. Int. J. Antimicrob. Agents 2009, 34, 190–191. [Google Scholar] [CrossRef]
- Kristiansen, J.E.; Dastidar, S.G.; Palchoudhuri, S.; Roy, D.S.; Das, S.; Hendricks, O.; Christensen, J.B. Phenothiazines as a solution for multidrug resistant tuberculosis: From the origin to present. Int. Microbiol. 2015, 18, 1–12. [Google Scholar]
- Rajšner, M.; Metyšová, J.; Svátek, E.; Mikšík, F.; Protiva, M. 2- And 3-fluoro derivatives of clorotepin and related compounds; 6- And 7-fluoro derivative of chlorprothixene. Collect. Czechoslov. Chem. Commun. 1975, 40, 719–737. [Google Scholar] [CrossRef]
- Kristiansen, J.E.; Mortensen, I. Stereo-isomeric dissociation of the antibacterial and the neuroleptic effect of clopenthixol. Acta Pathol. Microbiol. Scand. B 1981, 89, 437–438. [Google Scholar]
- Hyttel, J.; Arnt, J.; Bogoso, K.B. Antipsychotic Drugs: Configurational Stereoisomers. In Handbook of Stereoisomers: Drugs in Psychopharmacology; Smith, D.F., Ed.; CRC Press Inc.: Boca Raton, FL, USA, 1984; pp. 143–214. ISBN 084932940X. [Google Scholar]
- Kristiansen, J.E.; Andersen, L.P.; Vestergaard, B.F.; Hvidberg, E.F. Effect of Selected Neuroleptic Agents and Stereo-Isomeric Analogues on Virus and Eukaryotic Cells. Pharmacol. Toxicol. 1991, 69, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Elferink, J.G.; Deierkauf, M.; Riemersma, J.C. Involvement of calmodulin in granulocyte chemotaxis: The effect of calmodulin inhibitors. Res. Commun. Chem. Pathol. Pharmacol. 1982, 38, 77–84. [Google Scholar] [PubMed]
- Ochs, D.L.; Reed, P.W. Inhibition of the neutrophil oxidative burst and degranulation by phenothiazines. Biochem. Biophys. Res. Commun. 1981, 102, 958–962. [Google Scholar] [CrossRef]
- Smith, R.J.; Bowman, B.J.; Iden, S.S. Effects of trifluoperazine on human neutrophil function. Immunology 1981, 44, 677–684. [Google Scholar]
- Elferink, J.G. Chlorpromazine inhibits phagocytosis and exocytosis in rabbit polymorphonuclear leukocytes. Biochem. Pharmacol. 1979, 28, 965–968. [Google Scholar] [CrossRef]
- Suda, T.; Shimizu, D.; Maeda, N.; Shiga, T. Decreased viscosity of human erythrocyte suspension induced by chlorpromazine and isoxsuprine. Biochem. Pharmacol. 1981, 30, 2057–2064. [Google Scholar] [CrossRef]
- Ogiso, T.; Iwaki, M.; Mori, K. Fluidity of human erythrocyte membrane and effect of chlorpromazine on fluidity and phase separation of membrane. Biochim. Biophys. Acta 1981, 649, 325–335. [Google Scholar] [CrossRef]
- Rechnitzer, C.; Kristiansen, J.E.; Kharazmi, A. In vitro modulation of human neutrophil chemotaxis by cis(Z)- and trans(E)-clopenthixol, and chlorpromazine. Acta Pathol. Microbiol. Immunol. Scand. C 1985, 93, 199–203. [Google Scholar] [CrossRef] [PubMed]
- Keyzer, H.; Fey, S.J.; Thornton, B.; Kristiansen, J.E. Molar ratios of therapeutic water-soluble phenothiazine·water-insoluble phospholipid adducts reveal a Fibonacci correlation and a putative link for structure–activity relationships. RSC Adv. 2015, 5, 20865–20877. [Google Scholar] [CrossRef]
- Pike, M.C.; Snyderman, R. Lipid requirements for leukocyte chemotaxis and phagocytosis: Effects of inhibitors of phospholipid and cholesterol synthesis. J. Immunol. 1980, 124, 1963–1969. [Google Scholar] [PubMed]
- Petersen, P.V.; Møller-Nielsen, I.; Pedersen, V.; Jørgensen, A.; Lassen, N. The Thioxanthenes. In Psychotherapeutic Drugs, Part II; Usdin, E., Forrest, I., Marcel Dekker, Eds.; Marcel Dekker Inc.: New York, NY, USA; Basel, Switzerland, 1977; pp. 827–867. [Google Scholar]
- Larsen, J.J. A study on inhibition of cholera toxin-induced intestinal hypersecretion by neuroleptics. Acta Pharmacol. Toxicol. 1982, 50, 294–299. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, I.; Kristiansen, J.E.; Christensen, A.V.; Hvidberg, E.F. The antibacterial effect of some neuroleptics on strains isolated from patients with meningitis. Pharmacol. Toxicol. 1992, 71, 449–451. [Google Scholar] [CrossRef]
- Kristiansen, J.E.; Hansen, J.B. Inhibition of HIV replication by neuroleptic agents and their potential use in HIV infected patients with AIDS related dementia. Int. J. Antimicrob. Agents 2000, 14, 209–213. [Google Scholar] [CrossRef]
- Sarin, P.S.; Agrawal, S.; Civeira, M.P.; Goodchild, J.; Ikeuchi, T.; Zamecnik, P.C. Inhibition of acquired immunodeficiency syndrome virus by oligodeoxynucleoside methylphosphonates. Proc. Natl. Acad. Sci. USA 1988, 85, 7448–7451. [Google Scholar] [CrossRef]
- Ross, R.; MacGregor, W. The Fight Against Malaria: An Industrial Necessity for Our African Colonies. J. R. Afr. Soc. 1903, 2, 149–160. [Google Scholar]
- Schapira, A. Concomitant resistance to pyrimethamine and cycloguanil of chloroquine-resistant falciparum malaria from East Africa: An in vitro study of 12 isolates. Trans. R. Soc. Trop. Med. Hyg. 1984, 78, 359–362. [Google Scholar] [CrossRef]
- Jepsen, S.; Fogh, S.; Peterslund, N.; Black, F. RII-RIII chloroquine resistant Plasmodium falciparum malaria from East Africa: Studies of the in vivo and in vitro response to chloroquine. Ann. Trop. Med. Parasitol. 1983, 77, 349–354. [Google Scholar] [CrossRef] [PubMed]
- Guttmann, P.; Erlich, P. Über die wirkung des MethylenBlau bei Malaria. Berl. Klin. Wochenschr. 1891, 28, 953–956. [Google Scholar]
- Kristiansen, J.E.; Jepsen, S. The susceptibility of Plasmodium falciparum in vitro to chlorpromazine and the stereo-isomeric compounds cis(Z)- and trans(E)-clopenthixol. Acta Pathol. Microbiol. Immunol. Scand. B 1985, 93, 249–251. [Google Scholar] [CrossRef]
- Desjardins, R.E.; Canfield, C.J.; Haynes, J.D.; Chulay, J.D. Quantitative assessment of antimalarial activity in vitro by a semiautomated microdilution technique. Antimicrob. Agents Chemother. 1979, 16, 710–718. [Google Scholar] [CrossRef] [PubMed]
- Molnár, J.; Gálfi, M.; Lózsa, A.; Nakamura, M.J. Inhibition of bacterial plasmid replication by stereoselective binding by tricyclic psychopharmacons. Res. Commun. Chem. Pathol. Pharmacol. 1984, 43, 235–249. [Google Scholar]
- Poole, K.; Srikumar, R. Multidrug efflux in Pseudomonas aeruginosa: Components, mechanisms and clinical significance. Curr. Top. Med. Chem. 2001, 1, 59–71. [Google Scholar] [CrossRef]
- Van Bambeke, F.; Balzi, E.; Tulkens, P.M. Antibiotic efflux pumps. Biochem. Pharmacol. 2000, 60, 457–470. [Google Scholar] [CrossRef]
- Kuroda, M.; Ohta, T.; Uchiyama, I.; Baba, T.; Yuzawa, H.; Kobayashi, I.; Cui, L.; Oguchi, A.; Aoki, K.; Nagai, Y.; et al. Whole genome sequencing of meticillin-resistant Staphylococcus aureus. Lancet 2001, 357, 1225–1240. [Google Scholar] [CrossRef]
- Markham, P.N.; Westhaus, E.; Klyachko, K.; Johnson, M.E.; Neyfakh, A.A. Multiple novel inhibitors of the NorA multidrug transporter of Staphylococcus aureus. Antimicrob. Agents Chemother. 1999, 43, 2404–2408. [Google Scholar] [CrossRef] [PubMed]
- Dey, S.; Hafkemeyer, P.; Pastan, I.; Gottesman, M.M. A single amino acid residue contributes to distinct mechanisms of inhibition of the human multidrug transporter by stereoisomers of the dopamine receptor antagonist flupentixol. Biochemistry 1999, 38, 6630–6639. [Google Scholar] [CrossRef]
- Kristiansen, J.E.; Sebbesen, O.; Frimodt-Møller, N.; Aaes-Jørgensen, T.; Hvidberg, E.F. Synergy between a non-neuroleptic thioxanthene stereo-isomer and penicillin in vivo. APMIS 1988, 96, 1079–1084. [Google Scholar] [CrossRef]
- Ford, J.M.; Prozialeck, W.C.; Hait, W.N. Structural features determining activity of phenothiazines and related drugs for inhibition of cell growth and reversal of multidrug resistance. Mol. Pharmacol. 1989, 35, 105–115. [Google Scholar] [PubMed]
- Zilberstein, D.; Liveanu, V.; Gepstein, A. Tricyclic drugs reduce proton motive force in Leishmania donovani promastigotes. Biochem. Pharmacol. 1990, 39, 935–940. [Google Scholar] [CrossRef]
- Kaatz, G.W.; Moudgal, V.V.; Seo, S.M.; Kristiansen, J.E. Phenothiazines and thioxanthenes inhibit multidrug efflux pump activity in Staphylococcus aureus. Antimicrob. Agents Chemother. 2003, 47, 719–726. [Google Scholar] [CrossRef] [PubMed]
- Kristiansen, J.E.; Fey, S.J. The Accepted “Clinical Interaction Model”: A Special Case of Reality. J. Bioequiv. Availab. 2017, 9, 418–423. [Google Scholar]
- Nehme, H.; Saulnier, P.; Ramadan, A.A.; Cassisa, V.; Guillet, C.; Eveillard, M.; Umerska, A. Antibacterial activity of antipsychotic agents, their association with lipid nanocapsules and its impact on the properties of the nanocarriers and on antibacterial activity. PLoS ONE 2018, 13, e0189950. [Google Scholar] [CrossRef]
- Palmeira, A.; Sousa, E.; Fernandes, M.X.; Pinto, M.M.; Helena Vasconcelos, M. Multidrug resistance reversal effects of aminated thioxanthones and interaction with cytochrome P450 3A4. J. Pharm. Pharm. Sci. 2012, 15, 31–45. [Google Scholar] [CrossRef]
- Torregrosa-Chinillach, A.; Chinchilla, R. Synthesis of xanthones, thioxanthones and Acridones by a metal-free photocatalytic oxidation using visible light and molecular oxygen. Molecules 2021, 26, 974. [Google Scholar] [CrossRef]
- Noori Tahneh, A.; Bagheri Novir, S.; Balali, E. Density functional theory study of structural and electronic properties of trans and cis structures of thiothixene as a nano-drug. J. Mol. Model. 2017, 23, 1–5. [Google Scholar] [CrossRef]
- Wrzesinski, K.; Alnøe, S.; Jochumsen, H.H.; Mikkelsen, K.; Bryld, T.D.; Vistisen, J.S.; Alnøe, P.W.; Fey, S.J. A Purpose-Built System for Culturing Cells as In Vivo Mimetic 3D Structures. In BioMechanics and Functional Tissue Engineering [Working Title]; IntechOpen: London, UK, 2021; ISBN 978-1-83880-286-8. [Google Scholar]
- Möhle, L.; Mattei, D.; Heimesaat, M.M.; Bereswill, S.; Fischer, A.; Alutis, M.; French, T.; Hambardzumyan, D.; Matzinger, P.; Dunay, I.R.; et al. Ly6Chi Monocytes Provide a Link between Antibiotic-Induced Changes in Gut Microbiota and Adult Hippocampal Neurogenesis. Cell Rep. 2016, 15, 1945–1956. [Google Scholar] [CrossRef] [PubMed]
- Sundarakrishnan, A.; Chen, Y.; Black, L.D.; Aldridge, B.B.; Kaplan, D.L. Engineered cell and tissue models of pulmonary fibrosis. Adv. Drug Deliv. Rev. 2018, 129, 78–94. [Google Scholar] [CrossRef] [PubMed]
- Co, J.Y.; Margalef-Català, M.; Li, X.; Mah, A.T.; Kuo, C.J.; Monack, D.M.; Amieva, M.R. Controlling Epithelial Polarity: A Human Enteroid Model for Host-Pathogen Interactions. Cell Rep. 2019, 26, 2509–2520. [Google Scholar] [CrossRef] [PubMed]
- Kester, J.C.; Brubaker, D.K.; Velazquez, J.; Wright, C.; Lauffenburger, D.A.; Griffith, L.G. Clostridioides difficile-associated antibiotics alter human mucosal barrier functions by microbiome-independent mechanisms. Antimicrob. Agents Chemother. 2020, 64, e01404–e01419. [Google Scholar] [CrossRef] [PubMed]
- Jensen, A.S. Cardiac Action Potential Prolongation Induced by Isolated Thioridazine Enantiomers; Aalborg University: Aalborg, Denmark, 2014; pp. 1–86. [Google Scholar]
- Jensen, A.S.; Pennisi, C.P.; Sevcencu, C.; Christensen, J.B.; Kristiansen, J.E.; Struijk, J.J. Differential effects of thioridazine enantiomers on action potential duration in rabbit papillary muscle. Eur. J. Pharmacol. 2014, 747C, 7–12. [Google Scholar] [CrossRef]
- Antonsen, S.; Monsen, E.B.; Ovchinnikov, K.; Nolsoe, J.M.J.; Ekeberg, D.; Kristiansen, J.E.; Diep, D.B.; Stenstrom, Y. Synthesis of the Enantiomers of Thioridazine. SynOpen 2020, 4, 12–16. [Google Scholar] [CrossRef]
| Class of Antipsychotics | Drugs and Their Chemical Structures |
|---|---|
1. Phenothiazines Phenothiazine basic ring structure | |
| a. Amino alkyl compounds: (Low/medium potency agents that can antagonize α1-adenoreceptors, histamine H1 receptors and muscarinic cholinergic receptors) | Chlorpromazine: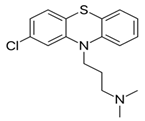 |
| b. Piperidine compounds: (Low/medium potency agents and also muscarinic antagonist) | Thioridazine: |
| c. Piperazine compounds: (Medium/high potency agents) | Trifluoperazine: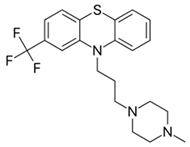 |
2. Butyrophenones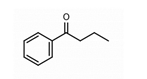 Butyrophenone basic ring structure (High potency agents) | Haloperidol: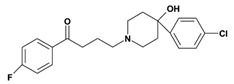 |
Droperidol: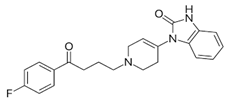 | |
3. Thioxanthenes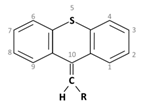 Thioxanthene basic ring structure (Medium potency agents) | Chlorprothixene: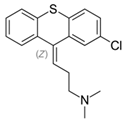 |
Flupenthixol: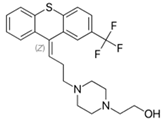 | |
Clopenthixol: |
| Adverse Effects | Drugs | ||||||
|---|---|---|---|---|---|---|---|
| (1) Phenothiazines | (2) Butyro- Phenones | (3) Thioxanthenes | |||||
| Chlorpromazine | Thioridazine | Trifluoperazine | Haloperidol | Chlorprothixene | Flupenthixol | Clopenthixol | |
| Extra Pyramidal Side Effects: The muscle related side effects observed with antipsychotic medications are termed as ‘Extra -Pyramidal Side Effects’ or EPS [10] | Low | Low | High | Very high | In a comparative study it was observed that Parkinsonian symptoms were more often found with chlorpromazine than chlorprothixene [11] | Develops in high dosages, can be controlled by anti-parkinsonian drugs [12,13] | High |
| Anti-cholinergic Effects: This includes symptoms like urinary difficulties, constipation, dry mouth, blurred visions and may lead to cognitive impairments. | High | High | Low | Very low | Moderate | Low | Both clopenthixol and flupenthixol were found to have lower effect in comparison to chlorprothixene [14] |
| Sedation: This is common with antipsychotic medications and is dose dependent. | High | High | Low | Produces much lesser sleepiness and calming effect than chlorpromazine [15] | High | Low | Low |
| Hypotension: Antipsychotics commonly cause orthostatic hypotension, depending on the degree of α1 adrenoreceptor antagonism. | High | High | Low | Very low | High | Moderate | Treatment with clopenthixol is often associated with orthostatic hypotension [19] |
| Other Effects: | Photosensitivity: Chlorpromazine is known to induce photosensitivity and skin pigmentation [16]. An intensive study with phenothiazines and thioxanthenes on schizophrenic patients [17] reported that patients receiving chlorpromazine showed statistically significant changes in the lens and cornea while patients treated with thioxanthenes did not. | Hyperprolactinemia: Thioxanthenes cause high prolactin levels due to the blockade of prolactin inhibitory factors (PIF), that inhibits release of prolactin from the pituitary gland [18]. | |||||
| Bacteria | No. Strains Tested | Range of MIC (µg/mL) Observed | |||
|---|---|---|---|---|---|
| Z-Clopenthixol | E-Clopenthixol | N-Dealkyl-Clopenthixol | Clopenthixol Sulfoxide | ||
| Staphylococci/Micrococci | 4 | 12.5–25 | 12.5 | 3.1–6.2 | >100 |
| Streptococci | 13 | 6.2–50 | 3.1–25 | 1.6–12.5 | >100 |
| Corynebacteria | 3 | 12.5 | 3.1–12.5 | 1.6–3.1 | >100 |
| Listeria/Erisipelothrix | 2 | 12.5–25 | 6.2–12.5 | 6.2 | >100 |
| Clostridium/Propionibacterium | 2 | 50 | 12.5 | 3.1–12.5 | >100 |
| Enterobacteriaceae | 19 | 25–>100 | 6.2–>100 | 6.2–>100 | 50–100 |
| Aeromonas/Pseudomonas | 7 | 50–>100 | 25–>100 | 12.5–>100 | >100 |
| Other Gram-negative bacteria | 11 | 12.5–>100 | 1.6–>100 | 3.1–>100 | 100–>100 |
| Bacteria | No. of Strains Tested | No. of Strains Inhibited by Flupenthixol (µg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| 5 | 10 | 25 | 50 | 100 | 200 | >200 | ||
| Bacillus spp | 6 | 1 | 5 | |||||
| Staphylococcus aureus | 84 | 9 | 30 | 31 | 12 | 2 | ||
| Streptococcus spp | 4 | 1 | 2 | 1 | ||||
| Escherichia coli | 47 | 1 | 5 | 4 | 5 | 3 | 29 | |
| Salmonella spp. | 15 | 1 | 5 | 1 | 8 | |||
| Arizona spp. | 1 | 1 | ||||||
| Providencia spp. | 1 | 1 | ||||||
| Proteus spp | 4 | 1 | 3 | |||||
| Shigella spp | 26 | 2 | 11 | 1 | 1 | 11 | ||
| Pseudomonas spp. | 12 | 1 | 2 | 9 | ||||
| Pasteurella septica | 1 | 1 | ||||||
| Bordetella bronchiseptica | 1 | 1 | ||||||
| Hafnia spp. | 1 | 1 | ||||||
| Klebsiella spp. | 5 | 5 | ||||||
| Vibrio cholerae | 111 | 5 | 9 | 23 | 26 | 5 | 4 | 39 |
| Vibrio parahaemolyticus | 33 | 1 | 1 | 9 | 1 | 5 | 16 | |
| Total | 352 | 17 | 56 | 80 | 51 | 13 | 15 | 120 |
| Group | Drug Injected Per Mouse | Mice Died |
|---|---|---|
| Control (N = 60) | 0.1 mL sterile saline | 48 |
| Group I (N = 20) | 15 µg flupenthixol | 3 * |
| Group II (N = 20) | 30 µg flupenthixol | 10 ** |
| Group | No. Mice Tested | Drug (µg/Mouse) | CFU/mL Count a | ||
|---|---|---|---|---|---|
| Heart Blood | Liver | Spleen | |||
| I | 5 | Flupenthixol 15 µg | 1.2–44 × 103 | 6.5–73 × 103 | 3.2–75 × 103 |
| Control | 5 | Saline | 5.3–74 × 108 | 8.5–50 × 108 | 1.8–80 × 108 |
| Strains | Highest Drug Concentration Permitting Growth Quantitatively Similar to the Vehicle-Treated Control | |||||
|---|---|---|---|---|---|---|
| E-Flupenthixol | Z-Flupenthixol | E-Clopenthixol | Z-Clopenthixol | E-Chlorprothixene | Z-Chlorprothixene | |
| M. tuberculosis St. 5 | 6.25 | 6.25 | 12.5 | 6.25 | 6.25 | 12.5 |
| M. bovis I 264 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 |
| B.C.G T 1443 | 6.25 | 6.25 | 12.5 | 6.25 | 6.25 | 6.25 |
| M. marinum T 2401 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 | 6.25 |
| M. scrofulaceum T14447 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| M. szulgai 908 | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 |
| M. xenopi E 1613 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 |
| M. avium T 10350 | 25 | 25 | 25 | 25 | 25 | 25 |
| M. intracellulare ATCC 23432 | 6.25 | 6.25 | 12.5 | 12.5 | 12.5 | 12.5 |
| M. intracellulare E 48067 | 12.5 | 12.5 | 12.5 | 12.5 | 12.5 | 25 |
| Inhibitory Agents | Number of Strains with Growth Similar to Vehicle Treated Controls | |||||
|---|---|---|---|---|---|---|
| Drug Concentration (µg/mL) | ||||||
| <6.25 | 6.25 | 12.5 | 25 | 50 | 100 | |
| E-flupenthixol | 10 | 9 | 3 | 1 | 0 | 0 |
| Z-flupenthixol | 10 | 9 | 4 | 1 | 0 | 0 |
| E-clopenthixol | 10 | 10 | 8 | 1 | 0 | 0 |
| Z-clopenthixol | 10 | 10 | 6 | 1 | 0 | 0 |
| E-chlorprothixen | 10 | 9 | 6 | 1 | 0 | 0 |
| Z-chlorprothixen | 10 | 9 | 7 | 2 | 0 | 0 |
| Concentration (mg/mL) | Chlorprothixene HCI (mol.wt. 352) | E-Flupentixol (mol.wt. 508) | |||
|---|---|---|---|---|---|
| µmol/L | CTE | µmol/L | CTE | ||
| Z- | E- | ||||
| 0.3 | 1.1 | * | * | 0.8 | * |
| 0.7 | 2.2 | * | * | 1.5 | * |
| 1.56 | 4.4 | 0 | 0 | 3.1 | 0 |
| 3.13 | 8.9 | 0 | ++ | 6.2 | + |
| 6.25 | 17.6 | 0 | ++++ | 12.3 | ++++ |
| 12.5 | 35.5 | 0 | ++++ | 24.6 | ++++ |
| 25.0 | 71.0 | 0 | ++++ | 49.2 | ++++ |
| Z-Clopenthixol Concentration in µm (µg/mL) | Percentage of Viable Cells | E-Clopenthixol Concentration in µm (µg/mL) | Percentage of Viable Cells | ||||
|---|---|---|---|---|---|---|---|
| ½ h | 1 h | 2 h | ½ h | 1 h | 2 h | ||
| 105 (50) | 86 | 77 | 67 | 105 (50) | 89 | 88 | 78 |
| 53 (25) | 94 | 91 | 90 | 53 (25) | 99 | 99 | 98 |
| 26 (12.5) | 98 | 97 | 99 | 26 (12.5) | 96 | 97 | 97 |
| Compound a | Concentration b (mg/L) | % Inhibition of HIV Expression c | |||
|---|---|---|---|---|---|
| Syncytia | P17 | P24 | RT | ||
| Z-flupenthixol | 0.08 | 0 | 0 | 0 | 0 |
| 0.4 | 0 | 0 | 0 | 0 | |
| 2.0 | 34 | 49 | 43 | 57 | |
| E-flupenthixol | 0.1 | 0 | 0 | 0 | 0 |
| 1.0 | 0 | 0 | 0 | 0 | |
| 10.0 | 43 | 66 | 67 | 67 | |
| Compound | Percent Inhibition of Plasmodium falciparum In Vitro | |||||||
|---|---|---|---|---|---|---|---|---|
| Drug Concentration (µg/mL) | ||||||||
| 0 | 0.039 | 0.078 | 0.156 | 0.312 | 0.625 | 1.25 | 2.5 | |
| Z-clopenthixol | 0 | 0 | 0 | 17 | 30 | 76 | 99 | 100 |
| E-clopenthixol | 0 | 0 | 0 | 9 | 12 | 36 | 83 | 95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poulsen, M.Ø.; Dastidar, S.G.; Roy, D.S.; Palchoudhuri, S.; Kristiansen, J.E.H.; Fey, S.J. A Double-Edged Sword: Thioxanthenes Act on Both the Mind and the Microbiome. Molecules 2022, 27, 196. https://doi.org/10.3390/molecules27010196
Poulsen MØ, Dastidar SG, Roy DS, Palchoudhuri S, Kristiansen JEH, Fey SJ. A Double-Edged Sword: Thioxanthenes Act on Both the Mind and the Microbiome. Molecules. 2022; 27(1):196. https://doi.org/10.3390/molecules27010196
Chicago/Turabian StylePoulsen, Marianne Ø., Sujata G. Dastidar, Debalina Sinha Roy, Shauroseni Palchoudhuri, Jette Elisabeth H. Kristiansen, and Stephen J. Fey. 2022. "A Double-Edged Sword: Thioxanthenes Act on Both the Mind and the Microbiome" Molecules 27, no. 1: 196. https://doi.org/10.3390/molecules27010196
APA StylePoulsen, M. Ø., Dastidar, S. G., Roy, D. S., Palchoudhuri, S., Kristiansen, J. E. H., & Fey, S. J. (2022). A Double-Edged Sword: Thioxanthenes Act on Both the Mind and the Microbiome. Molecules, 27(1), 196. https://doi.org/10.3390/molecules27010196





