Plants as a Source of Anticancer Agents: From Bench to Bedside
Abstract
1. Introduction
2. Cancer Hallmarks as Targets for Natural Products
2.1. Genomic Instability
2.2. Sustained Proliferative Signaling
2.3. Evasion of Anti-Growth Signaling
2.4. Resistance to Apoptosis
2.5. Replicative Immortality
2.6. Dysregulated Metabolism
2.7. Tumor-Promoting Inflammation
2.8. Angiogenesis
2.9. Tissue Invasion and Metastasis
2.10. Immune Evasion
3. Anticancer Drug Discovery
4. Chemoprevention Mechanisms of Plant-Derived Products
5. Plant-Derived Natural Products with Potential Anticancer Effects
5.1. Curcumin
5.2. Resveratrol
5.3. Quercetin
5.4. EGCG (Epigallocatechin Gallate)
5.5. Allicin
5.6. Thymoquinone
5.7. Emodin
5.8. Genistein
5.9. Parthenolide
5.10. Luteolin
6. Clinical Studies
6.1. Curcumin
6.2. Resveratrol
6.3. Quercetin
6.4. EGCG (Epigallocatechin Gallate)
6.5. Allicin
6.6. Thymoquinone
6.7. Emodin
6.8. Genistein
6.9. Parthenolide
6.10. Luteolin
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kocarnik, J.M.; Compton, K.; Dean, F.E.; Fu, W.; Gaw, B.L.; Harvey, J.D.; Henrikson, H.J.; Lu, D.; Pennini, A.; Xu, R. Cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life years for 29 cancer groups from 2010 to 2019: A systematic analysis for the Global Burden of Disease Study 2019. JAMA Oncol. 2022, 8, 420–444. [Google Scholar] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Zou, H.; Li, Y.; Liu, X.; Wu, Z.; Li, J.; Ma, Z. Roles of plant-derived bioactive compounds and related microRNAs in cancer therapy. Phytother. Res. 2021, 35, 1176–1186. [Google Scholar] [CrossRef]
- Iqbal, J.; Abbasi, B.A.; Mahmood, T.; Kanwal, S.; Ali, B.; Shah, S.A.; Khalil, A.T. Plant-derived anticancer agents: A green anticancer approach. Asian Pac. J. Trop. Biomed. 2017, 7, 1129–1150. [Google Scholar] [CrossRef]
- Leitzmann, C. Characteristics and health benefits of phytochemicals. Complementary Med. Res. 2016, 23, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Avato, P.; Migoni, D.; Argentieri, M.; Fanizzi, F.P.; Tava, A. Activity of saponins from Medicago species against HeLa and MCF-7 cell lines and their capacity to potentiate cisplatin effect. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2017, 17, 1508–1518. [Google Scholar] [CrossRef]
- Joshi, P.; Vishwakarma, R.A.; Bharate, S.B. Natural alkaloids as P-gp inhibitors for multidrug resistance reversal in cancer. Eur. J. Med. Chem. 2017, 138, 273–292. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H. Anticancer and antimicrobial potential of plant-derived natural products. In Phytochemicals—Bioactivities and Impact on Health; Rasooli, I., Ed.; InTech: Rijeka, Croatia, 2011; pp. 141–158. [Google Scholar]
- Talib, W.H.; Alsalahat, I.; Daoud, S.; Abutayeh, R.F.; Mahmod, A.I. Plant-Derived Natural Products in Cancer Research: Extraction, Mechanism of Action, and Drug Formulation. Molecules 2020, 25, 5319. [Google Scholar] [CrossRef] [PubMed]
- Rayan, A.; Raiyn, J.; Falah, M. Nature is the best source of anticancer drugs: Indexing natural products for their anticancer bioactivity. PLoS ONE 2017, 12, e0187925. [Google Scholar] [CrossRef] [PubMed]
- Thakore, P.; Mani, R.K.; Kavitha, S.J. A brief review of plants having anti-cancer property. Int. J. Pharm. Res. Dev. 2012, 3, 129–136. [Google Scholar]
- Tariq, A.; Sadia, S.; Pan, K.; Ullah, I.; Mussarat, S.; Sun, F.; Abiodun, O.O.; Batbaatar, A.; Li, Z.; Song, D. A systematic review on ethnomedicines of anti-cancer plants. Phytother. Res. 2017, 31, 202–264. [Google Scholar] [CrossRef] [PubMed]
- Rana, P.; Shrama, A.; Mandal, C.C. Molecular insights into phytochemicals-driven break function in tumor microenvironment. J. Food Biochem. 2021, 45, e13824. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, L.R.; Chen, H.; Collins, A.R.; Connell, M.; Damia, G.; Dasgupta, S.; Malhotra, M.; Meeker, A.K.; Amedei, A.; Amin, A.; et al. Genomic instability in human cancer: Molecular insights and opportunities for therapeutic attack and prevention through diet and nutrition. Semin. Cancer Biol. 2015, 35, S5–S24. [Google Scholar] [CrossRef] [PubMed]
- Rusin, M.; Zajkowicz, A.; Butkiewicz, D. Resveratrol induces senescence-like growth inhibition of U-2 OS cells associated with the instability of telomeric DNA and upregulation of BRCA1. Mech. Ageing Dev. 2009, 130, 528–537. [Google Scholar] [CrossRef]
- Talib, W.H. Melatonin and cancer hallmarks. Molecules 2018, 23, 518. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Yaswen, P.; MacKenzie, K.L.; Keith, W.N.; Hentosh, P.; Rodier, F.; Zhu, J.; Firestone, G.L.; Matheu, A.; Carnero, A.; Bilsland, A.; et al. Therapeutic targeting of replicative immortality. Semin. Cancer Biol. 2015, 35, S104–S128. [Google Scholar] [CrossRef]
- Bodnar, A.G.; Ouellette, M.; Frolkis, M.; Holt, S.E.; Chiu, C.-P.; Morin, G.B.; Harley, C.B.; Shay, J.W.; Lichtsteiner, S.; Wright, W.E. Extension of life-span by introduction of telomerase into normal human cells. Science 1998, 279, 349–352. [Google Scholar] [CrossRef]
- Bensinger, S.J.; Christofk, H.R. New aspects of the Warburg effect in cancer cell biology. Semin. Cell Dev. Biol. 2012, 23, 352–361. [Google Scholar] [CrossRef] [PubMed]
- Dang, C.V.; Kim, J.-w.; Gao, P.; Yustein, J. The interplay between MYC and HIF in cancer. Nat. Rev. Cancer 2008, 8, 51–56. [Google Scholar] [CrossRef]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.-S. The Interplay among miRNAs, Major Cytokines, and Cancer-Related Inflammation. Mol. Ther. Nucleic Acids 2020, 20, 606–620. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Karin, M.; Sun, B. Targeting cancer-promoting inflammation—Have anti-inflammatory therapies come of age? Nat. Rev. Clin. Oncol. 2021, 18, 261–279. [Google Scholar] [CrossRef]
- Chakraborty, S.; Njah, K.; Hong, W. Agrin Mediates Angiogenesis in the Tumor Microenvironment. Trends Cancer 2020, 6, 81–85. [Google Scholar] [CrossRef] [PubMed]
- Bergers, G.; Benjamin, L.E. Tumorigenesis and the angiogenic switch. Nat. Rev. Cancer 2003, 3, 401–410. [Google Scholar] [CrossRef]
- Martin, T.A.; Ye, L.; Sanders, A.J.; Lane, J.; Jiang, W.G. Cancer invasion and metastasis: Molecular and cellular perspective. In Madame Curie Bioscience Database [Internet]; Landes Bioscience: Georgetown, TX, USA, 2013. [Google Scholar]
- Habli, Z.; Toumieh, G.; Fatfat, M.; Rahal, O.N.; Gali-Muhtasib, H. Emerging cytotoxic alkaloids in the battle against cancer: Overview of molecular mechanisms. Molecules 2017, 22, 250. [Google Scholar] [CrossRef] [PubMed]
- N Nwodo, J.; Ibezim, A.; Simoben, C.V.; Ntie-Kang, F. Exploring cancer therapeutics with natural products from African medicinal plants, part II: Alkaloids, terpenoids and flavonoids. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2016, 16, 108–127. [Google Scholar] [CrossRef] [PubMed]
- Khan, H.; Saeedi, M.; Nabavi, S.M.; Mubarak, M.S.; Bishayee, A. Glycosides from medicinal plants as potential anticancer agents: Emerging trends towards future drugs. Curr. Med. Chem. 2019, 26, 2389–2406. [Google Scholar]
- Moraes, D.F.C.; Mesquita, L.S.S.d.; Amaral, F.M.M.d.; Sousa Ribeiro, M.N.d.; Malik, S. Anticancer drugs from plants. In Biotechnology and Production of Anti-Cancer Compounds; Springer: Berlin/Heidelberg, Germany, 2017; pp. 121–142. [Google Scholar]
- Habtemariam, S.; Lentini, G. Plant-derived anticancer agents: Lessons from the pharmacology of geniposide and its aglycone, genipin. Biomedicines 2018, 6, 39. [Google Scholar] [CrossRef] [PubMed]
- Dey, P.; Kundu, A.; Kumar, A.; Gupta, M.; Lee, B.M.; Bhakta, T.; Dash, S.; Kim, H.S. Analysis of alkaloids (indole alkaloids, isoquinoline alkaloids, tropane alkaloids). In Recent Advances in Natural Products Analysis; Elsevier: Amsterdam, The Netherlands, 2020; pp. 505–567. [Google Scholar]
- da Costa, R.; Passos, G.F.; Quintao, N.L.; Fernandes, E.S.; Maia, J.R.L.; Campos, M.M.; Calixto, J.B. Taxane-induced neurotoxicity: Pathophysiology and therapeutic perspectives. Br. J. Pharmacol. 2020, 177, 3127–3146. [Google Scholar] [CrossRef]
- Zhang, X.; Rakesh, K.; Shantharam, C.; Manukumar, H.; Asiri, A.; Marwani, H.; Qin, H.-L. Podophyllotoxin derivatives as an excellent anticancer aspirant for future chemotherapy: A key current imminent needs. Bioorganic Med. Chem. 2018, 26, 340–355. [Google Scholar] [CrossRef]
- Ramos, A.C.; Peláez, R.; López, J.L.; Caballero, E.; Medarde, M.; San Feliciano, A. Heterolignanolides. Furo-and thieno-analogues of podophyllotoxin and thuriferic acid. Tetrahedron 2001, 57, 3963–3977. [Google Scholar] [CrossRef]
- Montecucco, A.; Zanetta, F.; Biamonti, G. Molecular mechanisms of etoposide. EXCLI J. 2015, 14, 95. [Google Scholar] [PubMed]
- George, B.P.; Chandran, R.; Abrahamse, H. Role of Phytochemicals in Cancer Chemoprevention: Insights. Antioxidants 2021, 10, 1455. [Google Scholar] [CrossRef]
- Bishayee, A.; Sethi, G. Bioactive natural products in cancer prevention and therapy: Progress and promise. In Seminars in Cancer Biology; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1–3. [Google Scholar]
- Aggarwal, B.B.; Kumar, A.; Bharti, A.C. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003, 23, 363–398. [Google Scholar] [PubMed]
- Kaltschmidt, B.; Greiner, J.F.W.; Kadhim, H.M.; Kaltschmidt, C. Subunit-Specific Role of NF-κB in Cancer. Biomedicines 2018, 6, 44. [Google Scholar] [CrossRef] [PubMed]
- Sanlier, N.; Kocabas, Ş.; Erdogan, K.; Sanlier, N.T. Effects of curcumin, its analogues, and metabolites on various cancers: Focusing on potential mechanisms. Food Rev. Int. 2022, 1–21. [Google Scholar] [CrossRef]
- Shen, J.; Zhang, Y.; Yu, H.; Shen, B.; Liang, Y.; Jin, R.; Liu, X.; Shi, L.; Cai, X. Role of DUSP1/MKP1 in tumorigenesis, tumor progression and therapy. Cancer Med. 2016, 5, 2061–2068. [Google Scholar] [CrossRef] [PubMed]
- Truong, V.L.; Jun, M.; Jeong, W.S. Role of resveratrol in regulation of cellular defense systems against oxidative stress. Biofactors 2018, 44, 36–49. [Google Scholar] [CrossRef]
- Shao, B.-Z.; Xu, Z.-Q.; Han, B.-Z.; Su, D.-F.; Liu, C. NLRP3 inflammasome and its inhibitors: A review. Front. Pharmacol. 2015, 6, 262. [Google Scholar] [CrossRef]
- Manju, S.; Ethiraj, K.; Elias, G. Safer anti-inflammatory therapy through dual COX-2/5-LOX inhibitors: A structure-based approach. Eur. J. Pharm. Sci. 2018, 121, 356–381. [Google Scholar]
- Elshaer, M.; Chen, Y.; Wang, X.J.; Tang, X. Resveratrol: An overview of its anti-cancer mechanisms. Life Sci. 2018, 207, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Rather, R.A.; Bhagat, M. Quercetin as an innovative therapeutic tool for cancer chemoprevention: Molecular mechanisms and implications in human health. Cancer Med. 2020, 9, 9181–9192. [Google Scholar] [CrossRef]
- Rafiq, R.A.; Quadri, A.; Nazir, L.A.; Peerzada, K.; Ganai, B.A.; Tasduq, S.A. A potent inhibitor of phosphoinositide 3-kinase (PI3K) and mitogen activated protein (MAP) kinase signalling, quercetin (3, 3′, 4′, 5, 7-pentahydroxyflavone) promotes cell death in ultraviolet (UV)-B-irradiated B16F10 melanoma cells. PLoS ONE 2015, 10, e0131253. [Google Scholar] [CrossRef] [PubMed]
- Granado-Serrano, A.B.; Martín, M.A.; Bravo, L.; Goya, L.; Ramos, S. Quercetin induces apoptosis via caspase activation, regulation of Bcl-2, and inhibition of PI-3-kinase/Akt and ERK pathways in a human hepatoma cell line (HepG2). J. Nutr. 2006, 136, 2715–2721. [Google Scholar] [CrossRef]
- Rabiya, A.; Paithankar, V.; Kochar, A.C.; Bobade, N. Review on Chemoprevention of Cancer by Dietry Phytochemicals. World J. Pharm. Res. 2020, 9, 743–755. [Google Scholar]
- Almatroodi, S.A.; Almatroudi, A.; Khan, A.A.; Alhumaydhi, F.A.; Alsahli, M.A.; Rahmani, A.H. Potential therapeutic targets of epigallocatechin gallate (EGCG), the most abundant catechin in green tea, and its role in the therapy of various types of cancer. Molecules 2020, 25, 3146. [Google Scholar] [CrossRef]
- Tedeschi, E.; Suzuki, H.; Menegazzi, M. Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann. N. Y. Acad. Sci. 2002, 973, 435–437. [Google Scholar] [CrossRef] [PubMed]
- Sen, T.; Dutta, A.; Chatterjee, A. Epigallocatechin-3-gallate (EGCG) downregulates gelatinase-B (MMP-9) by involvement of FAK/ERK/NFκB and AP-1 in the human breast cancer cell line MDA-MB-231. Anti-Cancer Drugs 2010, 21, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Masuda, M.; Wakasaki, T.; Toh, S.; Shimizu, M.; Adachi, S. Chemoprevention of head and neck cancer by green tea extract: EGCG—the role of EGFR signaling and “lipid raft”. J. Oncol. 2011, 2011, 540148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Tighiouart, M.; Lee, J.E.; Shin, H.J.; Khuri, F.R.; Yang, C.S.; Chen, Z.; Shin, D.M. Synergistic inhibition of head and neck tumor growth by green tea (−)-epigallocatechin-3-gallate and EGFR tyrosine kinase inhibitor. Int. J. Cancer 2008, 123, 1005–1014. [Google Scholar] [CrossRef]
- Pahlke, G.; Ngiewih, Y.; Kern, M.; Jakobs, S.; Marko, D.; Eisenbrand, G. Impact of quercetin and EGCG on key elements of the Wnt pathway in human colon carcinoma cells. J. Agric. Food Chem. 2006, 54, 7075–7082. [Google Scholar] [CrossRef] [PubMed]
- Hossain, M.M.; Banik, N.L.; Ray, S.K. Survivin knockdown increased anti-cancer effects of (−)-epigallocatechin-3-gallate in human malignant neuroblastoma SK-N-BE2 and SH-SY5Y cells. Exp. Cell Res. 2012, 318, 1597–1610. [Google Scholar] [CrossRef] [PubMed]
- Vézina, A.; Chokor, R.; Annabi, B. EGCG targeting efficacy of NF-κB downstream gene products is dictated by the monocytic/macrophagic differentiation status of promyelocytic leukemia cells. Cancer Immunol. Immunother. 2012, 61, 2321–2331. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.S.; Kim, M.H.; Jeong, M.; Hwang, Y.S.; Lim, S.H.; Shin, B.A.; Ahn, B.W.; Jung, Y.D. EGCG blocks tumor promoter-induced MMP-9 expression via suppression of MAPK and AP-1 activation in human gastric AGS cells. Anticancer Res. 2004, 24, 747–754. [Google Scholar] [PubMed]
- Lee, Y.-H.; Kwak, J.; Choi, H.-K.; Choi, K.-C.; Kim, S.; Lee, J.; Jun, W.; Park, H.-J.; Yoon, H.-G. EGCG suppresses prostate cancer cell growth modulating acetylation of androgen receptor by anti-histone acetyltransferase activity. Int. J. Mol. Med. 2012, 30, 69–74. [Google Scholar] [PubMed]
- Li, Y.; Yuan, Y.-Y.; Meeran, S.M.; Tollefsbol, T.O. Synergistic epigenetic reactivation of estrogen receptor-α (ERα) by combined green tea polyphenol and histone deacetylase inhibitor in ERα-negative breast cancer cells. Mol. Cancer 2010, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Song, Y.; Lian, J.; Wang, Z. Allicin inhibits the invasion of lung adenocarcinoma cells by altering tissue inhibitor of metalloproteinase/matrix metalloproteinase balance via reducing the activity of phosphoinositide 3-kinase/AKT signaling. Oncol. Lett. 2017, 14, 468–474. [Google Scholar] [CrossRef][Green Version]
- Patra, S.; Nayak, R.; Patro, S.; Pradhan, B.; Sahu, B.; Behera, C.; Bhutia, S.K.; Jena, M. Chemical diversity of dietary phytochemicals and their mode of chemoprevention. Biotechnol. Rep. 2021, 30, e00633. [Google Scholar] [CrossRef]
- Bat-Chen, W.; Golan, T.; Peri, I.; Ludmer, Z.; Schwartz, B. Allicin purified from fresh garlic cloves induces apoptosis in colon cancer cells via Nrf2. Nutr. Cancer 2010, 62, 947–957. [Google Scholar] [CrossRef] [PubMed]
- Gomathinayagam, R.; Ha, J.H.; Jayaraman, M.; Song, Y.S.; Isidoro, C.; Dhanasekaran, D.N. Chemopreventive and anticancer effects of thymoquinone: Cellular and molecular targets. J. Cancer Prev. 2020, 25, 136. [Google Scholar] [CrossRef] [PubMed]
- Mercan, T.; Yamasan, B.; Erkan, O.; Özdemir, S. Thymoquinone alters ionic currents and decreases β adrenergic response in rat ventricle myocytes. J. Mol. Cell. Cardiol. 2018, 120, 22. [Google Scholar] [CrossRef]
- Shao, Y.-y.; Li, B.; Huang, Y.-m.; Luo, Q.; Xie, Y.-m.; Chen, Y.-h. Thymoquinone attenuates brain injury via an antioxidative pathway in a status epilepticus rat model. Transl. Neurosci. 2017, 8, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.H.; Lee, M.S.; Cha, E.Y.; Sul, J.Y.; Lee, J.S.; Kim, J.S.; Park, J.B.; Kim, J.Y. Inhibitory effect of emodin on fatty acid synthase, colon cancer proliferation and apoptosis. Mol. Med. Rep. 2017, 15, 2163–2173. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Cui, C.-f.; Yang, L.; Wang, L.; Jiang, X.-h. Emodin inhibits colon cancer cell invasion and migration by suppressing epithelial–mesenchymal transition via the Wnt/β-Catenin pathway. Oncol. Res. 2019, 27, 193. [Google Scholar] [CrossRef] [PubMed]
- Saunders, I.T.; Mir, H.; Kapur, N.; Singh, S. Emodin inhibits colon cancer by altering BCL-2 family proteins and cell survival pathways. Cancer Cell Int. 2019, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, X.; Zhou, Q.; Lu, Y.; Zhang, H.; Chen, Q.; Zhao, M.; Su, S. Inhibitory effect of emodin on migration, invasion and metastasis of human breast cancer MDA-MB-231 cells in vitro and in vivo. Oncol. Rep. 2015, 33, 338–346. [Google Scholar] [CrossRef] [PubMed]
- Kaneshiro, T.; Morioka, T.; Inamine, M.; Kinjo, T.; Arakaki, J.; Chiba, I.; Sunagawa, N.; Suzui, M.; Yoshimi, N. Anthraquinone derivative emodin inhibits tumor-associated angiogenesis through inhibition of extracellular signal-regulated kinase 1/2 phosphorylation. Eur. J. Pharmacol. 2006, 553, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, G.; Babykutty, S.; Sathiadevan, P.P.; Srinivas, P. Molecular mechanism of emodin action: Transition from laxative ingredient to an antitumor agent. Med. Res. Rev. 2007, 27, 591–608. [Google Scholar] [CrossRef] [PubMed]
- El-Far, Y.M.; Khodir, A.E.; Emarah, Z.A.; Ebrahim, M.A.; Al-Gayyar, M.M. Chemopreventive and hepatoprotective effects of genistein via inhibition of oxidative stress and the versican/PDGF/PKC signaling pathway in experimentally induced hepatocellular carcinoma in rats by thioacetamide. Redox Rep. 2022, 27, 9–20. [Google Scholar] [CrossRef] [PubMed]
- Sahin, K.; Yenice, E.; Bilir, B.; Orhan, C.; Tuzcu, M.; Sahin, N.; Ozercan, I.H.; Kabil, N.; Ozpolat, B.; Kucuk, O. Genistein Prevents Development of Spontaneous Ovarian Cancer and Inhibits Tumor Growth in Hen ModelGenistein Prevents Development of Ovarian Cancer. Cancer Prev. Res. 2019, 12, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Ullrich, A.; Schlessinger, J. Signal transduction by receptors with tyrosine kinase activity. Cell 1990, 61, 203–212. [Google Scholar] [CrossRef]
- Tian, B.; Xiao, Y.; Ma, J.; Ou, W.; Wang, H.; Wu, J.; Tang, J.; Zhang, B.; Liao, X.; Yang, D. Parthenolide inhibits angiogenesis in esophageal squamous cell carcinoma through suppression of VEGF. OncoTargets Ther. 2020, 13, 7447. [Google Scholar] [CrossRef]
- Carlisi, D.; Lauricella, M.; D’Anneo, A.; De Blasio, A.; Celesia, A.; Pratelli, G.; Notaro, A.; Calvaruso, G.; Giuliano, M.; Emanuele, S. Parthenolide and its soluble analogues: Multitasking compounds with antitumor properties. Biomedicines 2022, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- D’anneo, A.; Carlisi, D.; Lauricella, M.; Puleio, R.; Martinez, R.; Di Bella, S.; Di Marco, P.; Emanuele, S.; Di Fiore, R.; Guercio, A. Parthenolide generates reactive oxygen species and autophagy in MDA-MB231 cells. A soluble parthenolide analogue inhibits tumour growth and metastasis in a xenograft model of breast cancer. Cell Death Dis. 2013, 4, e891. [Google Scholar] [CrossRef]
- Kato, H.; Naiki-Ito, A.; Suzuki, S.; Inaguma, S.; Komura, M.; Nakao, K.; Naiki, T.; Kachi, K.; Kato, A.; Matsuo, Y. DPYD, down-regulated by the potentially chemopreventive agent luteolin, interacts with STAT3 in pancreatic cancer. Carcinogenesis 2021, 42, 940–950. [Google Scholar] [CrossRef] [PubMed]
- Raina, R.; Pramodh, S.; Rais, N.; Haque, S.; Shafarin, J.; Bajbouj, K.; Hamad, M.; Hussain, A. Luteolin inhibits proliferation, triggers apoptosis and modulates Akt/mTOR and MAP kinase pathways in HeLa cells. Oncol. Lett. 2021, 21, 192. [Google Scholar] [CrossRef] [PubMed]
- Lal, J. Turmeric, curcumin and our life: A review. Bull. Environ. Pharm. Life Sci. 2012, 1, 11–17. [Google Scholar]
- Talib, W.H.; Al-Hadid, S.A.; Ali, M.B.W.; Al-Yasari, I.H.; Abd Ali, M.R. Role of curcumin in regulating p53 in breast cancer: An overview of the mechanism of action. Breast Cancer Targets Ther. 2018, 10, 207. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.-Y.; Meng, X.; Li, S.; Gan, R.-Y.; Li, Y.; Li, H.-B. Bioactivity, health benefits, and related molecular mechanisms of curcumin: Current progress, challenges, and perspectives. Nutrients 2018, 10, 1553. [Google Scholar] [CrossRef]
- Hewlings, S.J.; Kalman, D.S. Curcumin: A review of its effects on human health. Foods 2017, 6, 92. [Google Scholar] [CrossRef] [PubMed]
- Rathore, S.; Mukim, M.; Sharma, P.; Devi, S.; Nagar, J.C.; Khalid, M. Curcumin: A review for health benefits. Int. J. Res. Rev. 2020, 7, 273–290. [Google Scholar]
- Ismail, N.I.; Othman, I.; Abas, F.; H Lajis, N.; Naidu, R. Mechanism of apoptosis induced by curcumin in colorectal cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef] [PubMed]
- Sahebkar, A.; Serban, M.-C.; Ursoniu, S.; Banach, M. Effect of curcuminoids on oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Funct. Foods 2015, 18, 898–909. [Google Scholar] [CrossRef]
- Menon, V.P.; Sudheer, A.R.J. Antioxidant and anti-inflammatory properties of curcumin. In The Molecular Targets Therapeutic Uses of Curcumin in Health Disease; Springer: Berlin/Heidelberg, Germany, 2007; pp. 105–125. [Google Scholar]
- Panahi, Y.; Alishiri, G.H.; Parvin, S.; Sahebkar, A. Mitigation of systemic oxidative stress by curcuminoids in osteoarthritis: Results of a randomized controlled trial. J. Diet. Suppl. 2016, 13, 209–220. [Google Scholar] [CrossRef] [PubMed]
- Notarbartolo, M.; Poma, P.; Perri, D.; Dusonchet, L.; Cervello, M.; D’Alessandro, N. Antitumor effects of curcumin, alone or in combination with cisplatin or doxorubicin, on human hepatic cancer cells. Analysis of their possible relationship to changes in NF-kB activation levels and in IAP gene expression. Cancer Lett. 2005, 224, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-W.; Chang, C.-L.; Lee, H.-C.; Chi, C.-W.; Pan, J.-P.; Yang, W.-C.J. Curcumin induces the apoptosis of human monocytic leukemia THP-1 cells via the activation of JNK/ERK pathways. BMC Complementary Altern. Med. 2012, 12, 1–8. [Google Scholar] [CrossRef]
- Kabir, M.; Rahman, M.; Akter, R.; Behl, T.; Kaushik, D.; Mittal, V.; Pandey, P.; Akhtar, M.F.; Saleem, A.; Albadrani, G.M. Potential role of curcumin and its nanoformulations to treat various types of cancers. Biomolecules 2021, 11, 392. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xu, Y.; Meng, L.; Huang, L.; Sun, H. Curcumin inhibits proliferation and promotes apoptosis of breast cancer cells. Exp. Ther. Med. 2018, 16, 1266–1272. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, J.; Li, X.; Jia, Y.; Huai, L.; He, K.; Yu, P.; Wang, M.; Xing, H.; Rao, Q. Role of the Wilms’ tumor 1 gene in the aberrant biological behavior of leukemic cells and the related mechanisms. Oncol. Rep. 2014, 32, 2680–2686. [Google Scholar] [CrossRef]
- Lu, Y.; Wei, C.; Xi, Z. Curcumin suppresses proliferation and invasion in non-small cell lung cancer by modulation of MTA1-mediated Wnt/β-catenin pathway. In Vitro Cell. Dev. Biol. Anim. 2014, 50, 840–850. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, H.; Masoudifar, A.; Sahebkar, A.; Zare, N.; Sadri Nahand, J.; Rashidi, B.; Mehrabian, E.; Mohammadi, M.; Mirzaei, H.R.; Jaafari, M.R. MicroRNA: A novel target of curcumin in cancer therapy. J. Cell. Physiol. 2018, 233, 3004–3015. [Google Scholar] [CrossRef] [PubMed]
- Goradel, N.H.; Hour, F.G.; Negahdari, B.; Malekshahi, Z.V.; Hashemzehi, M.; Masoudifar, A.; Mirzaei, H. Stem cell therapy: A new therapeutic option for cardiovascular diseases. J. Cell. Biochem. 2018, 119, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Tan, B.L.; Norhaizan, M.E. Curcumin combination chemotherapy: The implication and efficacy in cancer. Molecules 2019, 24, 2527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Lai, Z.-L.; Chen, H.-F.; Zhang, M.; Wang, A.; Jia, T.; Sun, W.-Q.; Zhu, X.-M.; Chen, X.-F.; Zhao, Z.J. Curcumin synergizes with 5-fluorouracil by impairing AMPK/ULK1-dependent autophagy, AKT activity and enhancing apoptosis in colon cancer cells with tumor growth inhibition in xenograft mice. J. Exp. Clin. Cancer Res. 2017, 36, 1–12. [Google Scholar]
- Guorgui, J.; Wang, R.; Mattheolabakis, G.; Mackenzie, G.G. Curcumin formulated in solid lipid nanoparticles has enhanced efficacy in Hodgkin’s lymphoma in mice. Arch. Biochem. Biophys. 2018, 648, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Singh, S.K.; Chowdhury, I.; Lillard Jr, J.W.; Singh, R. Combinatorial effect of curcumin with docetaxel modulates apoptotic and cell survival molecules in prostate cancer. Front. Biosci. 2017, 9, 235. [Google Scholar]
- Park, B.H.; Lim, J.E.; Jeon, H.G.; Seo, S.I.; Lee, H.M.; Choi, H.Y.; Jeon, S.S.; Jeong, B.C. Curcumin potentiates antitumor activity of cisplatin in bladder cancer cell lines via ROS-mediated activation of ERK1/2. Oncotarget 2016, 7, 63870. [Google Scholar] [CrossRef] [PubMed]
- Abadi, A.J.; Mirzaei, S.; Mahabady, M.K.; Hashemi, F.; Zabolian, A.; Hashemi, F.; Raee, P.; Aghamiri, S.; Ashrafizadeh, M.; Aref, A.R. Curcumin and its derivatives in cancer therapy: Potentiating antitumor activity of cisplatin and reducing side effects. Phytother. Res. 2022, 36, 189–213. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Zabolian, A.; Saleki, H.; Bagherian, M.; Azami, N.; Bejandi, A.K.; Hushmandi, K.; Ang, H.L. Polychemotherapy with curcumin and doxorubicin via biological nanoplatforms: Enhancing antitumor activity. Pharmaceutics 2020, 12, 1084. [Google Scholar] [CrossRef]
- Hassanalilou, T.; Ghavamzadeh, S.; Khalili, L. Curcumin and gastric cancer: A review on mechanisms of action. J. Gastrointest. Cancer 2019, 50, 185–192. [Google Scholar] [CrossRef]
- Tang, X.-Q.; Bi, H.; Feng, J.-Q.; Cao, J.-G. Effect of curcumin on multidrug resistance in resistant human gastric carcinoma cell line SGC7901/VCR. Acta Pharmacol. Sin. 2005, 26, 1009–1016. [Google Scholar] [CrossRef]
- Zhang, N.; Gao, M.; Wang, Z.; Zhang, J.; Cui, W.; Li, J.; Zhu, X.; Zhang, H.; Yang, D.-H.; Xu, X.J. Curcumin reverses doxorubicin resistance in colon cancer cells at the metabolic level. J. Pharm. Biomed. Anal. 2021, 201, 114129. [Google Scholar] [CrossRef]
- Alsamydai, A.; Jaber, N. Pharmacological aspects of curcumin. Int. J. Pharm. 2018, 5, 313–326. [Google Scholar]
- Meng, T.; Xiao, D.; Muhammed, A.; Deng, J.; Chen, L.; He, J. Anti-inflammatory action and mechanisms of resveratrol. Molecules 2021, 26, 229. [Google Scholar] [CrossRef] [PubMed]
- Kukreja, A.; Wadhwa, N.; Tiwari, A. Therapeutic role of resveratrol and piceatannol in disease prevention. Blood Disord. Transfus. 2014, 5, 9. [Google Scholar] [CrossRef]
- Gülçin, İ. Antioxidant properties of resveratrol: A structure–activity insight. Innov. Food Sci. Emerg. Technol. 2010, 11, 210–218. [Google Scholar] [CrossRef]
- de Sá Coutinho, D.; Pacheco, M.T.; Frozza, R.L.; Bernardi, A. Anti-inflammatory effects of resveratrol: Mechanistic insights. Int. J. Mol. Sci. 2018, 19, 1812. [Google Scholar] [CrossRef]
- Cho, S.; Namkoong, K.; Shin, M.; Park, J.; Yang, E.; Ihm, J.; Thu, V.T.; Kim, H.K.; Han, J. Cardiovascular protective effects and clinical applications of resveratrol. J. Med. Food 2017, 20, 323–334. [Google Scholar] [CrossRef]
- Kasiotis, K.M.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and related stilbenes: Their anti-aging and anti-angiogenic properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef]
- Ko, J.-H.; Sethi, G.; Um, J.-Y.; Shanmugam, M.K.; Arfuso, F.; Kumar, A.P.; Bishayee, A.; Ahn, K.S. The role of resveratrol in cancer therapy. Int. J. Mol. Sci. 2017, 18, 2589. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H.; Alsayed, A.R.; Farhan, F.; Al Kury, L.T. Resveratrol and tumor microenvironment: Mechanistic basis and therapeutic targets. Molecules 2020, 25, 4282. [Google Scholar] [CrossRef]
- Xia, N.; Daiber, A.; Förstermann, U.; Li, H. Antioxidant effects of resveratrol in the cardiovascular system. Br. J. Pharmacol. 2017, 174, 1633–1646. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.; Chen, C.; Jiang, X.; Zhang, Z. Resveratrol synergistically triggers apoptotic cell death with arsenic trioxide via oxidative stress in human lung adenocarcinoma A549 cells. Biol. Trace Elem. Res. 2015, 163, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Hogg, S.J.; Chitcholtan, K.; Hassan, W.; Sykes, P.H.; Garrill, A.J. Resveratrol, acetyl-resveratrol, and polydatin exhibit antigrowth activity against 3D cell aggregates of the SKOV-3 and OVCAR-8 ovarian cancer cell lines. Obstet. Gynecol. Int. 2015, 2015, 279591. [Google Scholar] [CrossRef] [PubMed]
- Varoni, E.M.; Lo Faro, A.F.; Sharifi-Rad, J.; Iriti, M. Anticancer molecular mechanisms of resveratrol. Front. Nutr. 2016, 3, 8. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Wang, L.; Cui, J.; Huoc, Z.; Xue, J.; Cui, H.; Mao, Q.; Yang, R. Resveratrol inhibits NF-κB signaling through suppression of p65 and IB kinase activities. Die Pharm.-Int. J. Pharm. Sci. 2013, 68, 689–694. [Google Scholar]
- Li, D.; Wang, G.; Jin, G.; Yao, K.; Zhao, Z.; Bie, L.; Guo, Y.; Li, N.; Deng, W.; Chen, X. Resveratrol suppresses colon cancer growth by targeting the AKT/STAT3 signaling pathway. Int. J. Mol. Med. 2019, 43, 630–640. [Google Scholar] [CrossRef]
- Heo, J.R.; Kim, S.M.; Hwang, K.A.; Kang, J.H.; Choi, K.C. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int. J. Mol. Med. 2018, 42, 1427–1435. [Google Scholar] [CrossRef]
- Li, L.; Qiu, R.L.; Lin, Y.; Cai, Y.; Bian, Y.; Fan, Y.; Gao, X.J. Resveratrol suppresses human cervical carcinoma cell proliferation and elevates apoptosis via the mitochondrial and p53 signaling pathways. Oncol. Lett. 2018, 15, 9845–9851. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Tong, L.; Luo, Y.; Li, X.; Chen, G.; Wang, Y. Resveratrol inhibits the proliferation and induces the apoptosis in ovarian cancer cells via inhibiting glycolysis and targeting AMPK/mTOR signaling pathway. J. Cell. Biochem. 2018, 119, 6162–6172. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Ma, J.; Ma, Q.; Li, B.; Han, L.; Liu, J.; Xu, Q.; Duan, W.; Yu, S.; Wang, F. Resveratrol inhibits the epithelial-mesenchymal transition of pancreatic cancer cells via suppression of the PI-3K/Akt/NF-κB pathway. Curr. Med. Chem. 2013, 20, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Chong, T.; Wang, Z.; Chen, H.; Li, H.; Cao, J.; Zhang, P.; Li, H. A novel anti-cancer effect of resveratrol: Reversal of epithelial-mesenchymal transition in prostate cancer cells. Mol. Med. Rep. 2014, 10, 1717–1724. [Google Scholar] [CrossRef]
- Chhabra, G.; Singh, C.K.; Amiri, D.; Akula, N.; Ahmad, N. Recent advancements on immunomodulatory mechanisms of resveratrol in tumor microenvironment. Molecules 2021, 26, 1343. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.; Shin, H.; Kim, J. In vivo anti-cancer effects of resveratrol mediated by NK cell activation. J. Innate Immun. 2021, 13, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Cocetta, V.; Quagliariello, V.; Fiorica, F.; Berretta, M.; Montopoli, M. Resveratrol as chemosensitizer agent: State of art and future perspectives. Int. J. Mol. Sci. 2021, 22, 2049. [Google Scholar] [CrossRef] [PubMed]
- Robertson, I.; Hau, T.W.; Sami, F.; Ali, M.S.; Badgujar, V.; Murtuja, S.; Hasnain, M.S.; Khan, A.; Majeed, S.; Ansari, M.T. The science of resveratrol, formulation, pharmacokinetic barriers and its chemotherapeutic potential. Int. J. Pharm. 2022, 121605. [Google Scholar] [CrossRef]
- Harborne, J.B.; Williams, C.A. Advances in flavonoid research since 1992. Phytochemistry 2000, 55, 481–504. [Google Scholar] [CrossRef]
- Williams, C.A.; Grayer, R.J. Anthocyanins and other flavonoids. Nat. Prod. Rep. 2004, 21, 539–573. [Google Scholar] [CrossRef]
- Ross, J.A.; Kasum, C.M. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002, 22, 19–34. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.; Bijsman, M.N.; Van Gameren, Y.; Cnossen, E.P.; De Vries, J.H.; Katan, M.B. The sugar moiety is a major determinant of the absorption of dietary flavonoid glycosides in man. Free. Radic. Res. 1999, 31, 569–573. [Google Scholar] [CrossRef] [PubMed]
- Chang, Q.; Wong, Y.-S. Identification of flavonoids in Hakmeitau beans (Vigna sinensis) by high-performance liquid chromatography− electrospray mass spectrometry (LC-ESI/MS). J. Agric. Food Chem. 2004, 52, 6694–6699. [Google Scholar] [CrossRef]
- Middleton, E.; Kandaswami, C. The Flavonoids: Advances in Research Since 1986; Harborne, J.B., Ed.; Chapman & Hall: London, UK, 1994. [Google Scholar]
- Häkkinen, S.H.; Kärenlampi, S.O.; Heinonen, I.M.; Mykkänen, H.M.; Törrönen, A.R. Content of the flavonols quercetin, myricetin, and kaempferol in 25 edible berries. J. Agric. Food Chem. 1999, 47, 2274–2279. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G.; Manach, C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005, 81, 243S–255S. [Google Scholar] [CrossRef] [PubMed]
- Wiczkowski, W.; Romaszko, J.; Bucinski, A.; Szawara-Nowak, D.; Honke, J.; Zielinski, H.; Piskula, M.K. Quercetin from shallots (Allium cepa L. var. aggregatum) is more bioavailable than its glucosides. J. Nutr. 2008, 138, 885–888. [Google Scholar] [CrossRef] [PubMed]
- Varsha, K.; Sharma, A.; Kaur, A.; Madan, J.; Pandey, R.S.; Jain, U.K.; Chandra, R. Chapter 28—Natural plant-derived anticancer drugs nanotherapeutics: A review on preclinical to clinical success. In Nanostructures for Cancer Therapy; Ficai, A., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 775–809. [Google Scholar] [CrossRef]
- Li, Y.; Yao, J.; Han, C.; Yang, J.; Chaudhry, M.T.; Wang, S.; Liu, H.; Yin, Y. Quercetin, inflammation and immunity. Nutrients 2016, 8, 167. [Google Scholar] [CrossRef] [PubMed]
- Bule, M.; Abdurahman, A.; Nikfar, S.; Abdollahi, M.; Amini, M. Antidiabetic effect of quercetin: A systematic review and meta-analysis of animal studies. Food Chem. Toxicol. 2019, 125, 494–502. [Google Scholar] [CrossRef]
- Wang, H.; Yuan, Z.; Chen, Z.; Yao, F.; Hu, Z.; Wu, B. Effect of quercetin on glioma cell U87 apoptosis and feedback regulation of MDM2-p53. Nan Fang Yi Ke Da Xue Xue Bao J. South. Med. Univ. 2014, 34, 686–689. [Google Scholar]
- Xiang, T.; Fang, Y.; Wang, S.-X. Quercetin suppresses HeLa cells by blocking PI3K/Akt pathway. J. Huazhong Univ. Sci. Technol. 2014, 34, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Russo, M.; Palumbo, R.; Tedesco, I.; Mazzarella, G.; Russo, P.; Iacomino, G.; Russo, G.L. Quercetin and anti-CD95 (Fas/Apo1) enhance apoptosis in HPB-ALL cell line. FEBS Lett. 1999, 462, 322–328. [Google Scholar] [CrossRef]
- Aalinkeel, R.; Bindukumar, B.; Reynolds, J.L.; Sykes, D.E.; Mahajan, S.D.; Chadha, K.C.; Schwartz, S.A. The dietary bioflavonoid, quercetin, selectively induces apoptosis of prostate cancer cells by down-regulating the expression of heat shock protein 90. Prostate 2008, 68, 1773–1789. [Google Scholar] [CrossRef] [PubMed]
- Kim, G.N.; Jang, H.D. Protective Mechanism of Quercetin and Rutin Using Glutathione Metabolism on H2O2-induced Oxidative Stress in HepG2 Cells. Ann. N. Y. Acad. Sci. 2009, 1171, 530–537. [Google Scholar] [CrossRef]
- Fernald, K.; Kurokawa, M. Evading apoptosis in cancer. Trends Cell Biol. 2013, 23, 620–633. [Google Scholar] [CrossRef] [PubMed]
- Granato, M.; Rizzello, C.; Montani, M.S.G.; Cuomo, L.; Vitillo, M.; Santarelli, R.; Gonnella, R.; D’Orazi, G.; Faggioni, A.; Cirone, M. Quercetin induces apoptosis and autophagy in primary effusion lymphoma cells by inhibiting PI3K/AKT/mTOR and STAT3 signaling pathways. J. Nutr. Biochem. 2017, 41, 124–136. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Ma, J.; Li, X.Y.; Wu, Y.; Shi, H.; Chen, Y.; Lu, G.; Shen, H.M.; Lu, G.D.; Zhou, J. Quercetin induces p53-independent cancer cell death through lysosome activation by the transcription factor EB and Reactive Oxygen Species-dependent ferroptosis. Br. J. Pharmacol. 2021, 178, 1133–1148. [Google Scholar] [CrossRef] [PubMed]
- Iriti, M.; Kubina, R.; Cochis, A.; Sorrentino, R.; Varoni, E.M.; Kabała-Dzik, A.; Azzimonti, B.; Dziedzic, A.; Rimondini, L.; Wojtyczka, R.D. Rutin, a quercetin glycoside, restores chemosensitivity in human breast cancer cells. Phytother. Res. 2017, 31, 1529–1538. [Google Scholar] [CrossRef] [PubMed]
- Bulavin, D.V.; Phillips, C.; Nannenga, B.; Timofeev, O.; Donehower, L.A.; Anderson, C.W.; Appella, E.; Fornace, A.J. Inactivation of the Wip1 phosphatase inhibits mammary tumorigenesis through p38 MAPK–mediated activation of the p16Ink4a-p19Arf pathway. Nat. Genet. 2004, 36, 343–350. [Google Scholar] [CrossRef]
- Hong, Y.; Lee, J.; Moon, H.; Ryu, C.H.; Seok, J.; Jung, Y.-S.; Ryu, J.; Baek, S.J. Quercetin induces anticancer activity by upregulating pro-NAG-1/GDF15 in differentiated thyroid cancer cells. Cancers 2021, 13, 3022. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.L.; Prawira, A.; Kaye, A.H.; Hovens, C.M. Tumour angiogenesis: Its mechanism and therapeutic implications in malignant gliomas. J. Clin. Neurosci. 2009, 16, 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.H.; Alitalo, K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat. Rev. Mol. Cell Biol. 2007, 8, 464–478. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Pei, Y.; Wang, W.; Liu, F.; Zheng, K.; Zhang, X. Quercetin suppresses the proliferation and metastasis of metastatic osteosarcoma cells by inhibiting parathyroid hormone receptor 1. Biomed. Pharmacother. 2019, 114, 108839. [Google Scholar] [CrossRef]
- Ryu, S.; Park, S.; Lim, W.; Song, G. Quercetin augments apoptosis of canine osteosarcoma cells by disrupting mitochondria membrane potential and regulating PKB and MAPK signal transduction. J. Cell. Biochem. 2019, 120, 17449–17458. [Google Scholar] [CrossRef] [PubMed]
- Mutlu Altundağ, E.; Kasacı, T.; Yılmaz, A.M.; Karademir, B.; Koçtürk, S.; Taga, Y.; Yalçın, A.S. Quercetin-induced cell death in human papillary thyroid cancer (B-CPAP) cells. J. Thyroid. Res. 2016, 2016, 9843675. [Google Scholar] [CrossRef] [PubMed]
- Quagliariello, V.; Armenia, E.; Aurilio, C.; Rosso, F.; Clemente, O.; de Sena, G.; Barbarisi, M.; Barbarisi, A. New treatment of medullary and papillary human thyroid cancer: Biological effects of hyaluronic acid hydrogel loaded with quercetin alone or in combination to an inhibitor of aurora kinase. J. Cell. Physiol. 2016, 231, 1784–1795. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Wang, B.; Wei, X.; Men, K.; Zheng, F.; Zhou, Y.; Zheng, Y.; Gou, M.; Huang, M.; Guo, G. Anticancer effect and mechanism of polymer micelle-encapsulated quercetin on ovarian cancer. Nanoscale 2012, 4, 7021–7030. [Google Scholar] [CrossRef] [PubMed]
- Dhanaraj, T.; Mohan, M.; Arunakaran, J. Quercetin attenuates metastatic ability of human metastatic ovarian cancer cells via modulating multiple signaling molecules involved in cell survival, proliferation, migration and adhesion. Arch. Biochem. Biophys. 2021, 701, 108795. [Google Scholar] [CrossRef]
- Kee, J.-Y.; Han, Y.-H.; Kim, D.-S.; Mun, J.-G.; Park, J.; Jeong, M.-Y.; Um, J.-Y.; Hong, S.-H. Inhibitory effect of quercetin on colorectal lung metastasis through inducing apoptosis, and suppression of metastatic ability. Phytomedicine 2016, 23, 1680–1690. [Google Scholar] [CrossRef] [PubMed]
- Rotimi, D.E.; Olaolu, T.D.; Adeyemi, O.S. Pharmacological action of quercetin against testicular dysfunction: A mini review. J. Integr. Med. 2022, S2095–S4964. [Google Scholar] [CrossRef] [PubMed]
- Botten, D.; Fugallo, G.; Fraternali, F.; Molteni, C. Structural properties of green tea catechins. J. Phys. Chem. B 2015, 119, 12860–12867. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrasco, Y.; Gaspari, A.; Graziani, G.; Santini, A.; Ritieni, A. Fast analysis of polyphenols and alkaloids in cocoa-based products by ultra-high performance liquid chromatography and Orbitrap high resolution mass spectrometry (UHPLC-Q-Orbitrap-MS/MS). Food Res. Int. 2018, 111, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Lambert, J.D.; Elias, R. The antioxidant and pro-oxidant activities of green tea polyphenols: A role in cancer prevention. Arch. Biochem. Biophys. 2010, 501, 65–72. [Google Scholar] [CrossRef]
- Mukai, K.; Mitani, S.; Ohara, K.; Nagaoka, S.-I. Structure–activity relationship of the tocopherol-regeneration reaction by catechins. Free. Radic. Biol. Med. 2005, 38, 1243–1256. [Google Scholar] [CrossRef] [PubMed]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free. Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Rodier, M.; Fillon, L.; Faure, S.; Clere, N. Epigallocatechin gallate: A review of its beneficial properties to prevent metabolic syndrome. Nutrients 2015, 7, 5443–5468. [Google Scholar] [CrossRef] [PubMed]
- Xicota, L.; Rodriguez-Morato, J.; Dierssen, M.; de la Torre, R. Potential role of (-)-epigallocatechin-3-gallate (EGCG) in the secondary prevention of Alzheimer disease. Curr. Drug Targets 2017, 18, 174–195. [Google Scholar] [CrossRef] [PubMed]
- H Farzaei, M.; Rahimi, R.; Abdollahi, M. The role of dietary polyphenols in the management of inflammatory bowel disease. Curr. Pharm. Biotechnol. 2015, 16, 196–210. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, Y.; Gaynor, R.B. Therapeutic potential of inhibition of the NF-κB pathway in the treatment of inflammation and cancer. J. Clin. Investig. 2001, 107, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Thongboonkerd, V. Molecular mechanisms of epigallocatechin-3-gallate for prevention of chronic kidney disease and renal fibrosis: Preclinical evidence. Curr. Dev. Nutr. 2019, 3, nzz101. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, B.; Du, F.; Su, X.; Sun, G.; Zhou, G.; Bian, X.; Liu, N. Epigallocatechin-3-Gallate Attenuates Oxidative Stress and Inflammation in Obstructive Nephropathy via NF-κB and Nrf2/HO-1 Signalling Pathway Regulation. Basic Clin. Pharmacol. Toxicol. 2015, 117, 164–172. [Google Scholar] [CrossRef] [PubMed]
- Riegsecker, S.; Wiczynski, D.; Kaplan, M.J.; Ahmed, S. Potential benefits of green tea polyphenol EGCG in the prevention and treatment of vascular inflammation in rheumatoid arthritis. Life Sci. 2013, 93, 307–312. [Google Scholar] [CrossRef]
- Zan, L.; Chen, Q.; Zhang, L.; Li, X. Epigallocatechin gallate (EGCG) suppresses growth and tumorigenicity in breast cancer cells by downregulation of miR-25. Bioengineered 2019, 10, 374–382. [Google Scholar] [CrossRef]
- Kwak, T.W.; Park, S.B.; Kim, H.-J.; Jeong, Y.-I.; Kang, D.H. Anticancer activities of epigallocatechin-3-gallate against cholangiocarcinoma cells. OncoTargets Ther. 2017, 10, 137. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Ju, Y.; Wang, J.; Zhou, R. Epigallocatechin-3-gallate promotes apoptosis and reversal of multidrug resistance in esophageal cancer cells. Pathol.-Res. Pract. 2017, 213, 1242–1250. [Google Scholar] [CrossRef]
- Wu, D.; Liu, Z.; Li, J.; Zhang, Q.; Zhong, P.; Teng, T.; Chen, M.; Xie, Z.; Ji, A.; Li, Y. Epigallocatechin-3-gallate inhibits the growth and increases the apoptosis of human thyroid carcinoma cells through suppression of EGFR/RAS/RAF/MEK/ERK signaling pathway. Cancer Cell Int. 2019, 19, 1–17. [Google Scholar] [CrossRef]
- Gu, J.; Qiao, K.; Sun, P.; Chen, P.; Li, Q. Study of EGCG induced apoptosis in lung cancer cells by inhibiting PI3K/Akt signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 4557–4563. [Google Scholar] [PubMed]
- Zhu, F.; Xu, Y.; Pan, J.; Li, M.; Chen, F.; Xie, G. Epigallocatechin gallate protects against MNNG-induced precancerous lesions of gastric carcinoma in rats via PI3K/Akt/mTOR pathway. Evid.-Based Complementary Altern. Med. 2021, 2021, 8846813. [Google Scholar] [CrossRef]
- Amin, A.; Wang, D.; Nannapaneni, S.; Lamichhane, R.; Chen, Z.G.; Shin, D.M. Combination of resveratrol and green tea epigallocatechin gallate induces synergistic apoptosis and inhibits tumor growth in vivo in head and neck cancer models. Oncol. Rep. 2021, 45, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Khiewkamrop, P.; Phunsomboon, P.; Richert, L.; Pekthong, D.; Srisawang, P. Epistructured catechins, EGCG and EC facilitate apoptosis induction through targeting de novo lipogenesis pathway in HepG2 cells. Cancer Cell Int. 2018, 18, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Jankun, J.; Selman, S.H.; Swiercz, R.; Skrzypczak-Jankun, E. Why drinking green tea could prevent cancer. Nature 1997, 387, 561. [Google Scholar] [CrossRef] [PubMed]
- Garbisa, S.; Sartor, L.; Biggin, S.; Salvato, B.; Benelli, R.; Albini, A. Tumor gelatinases and invasion inhibited by the green tea flavanol epigallocatechin-3-gallate. Cancer 2001, 91, 822–832. [Google Scholar] [CrossRef]
- Rao, S.D.; Pagidas, K. Epigallocatechin-3-gallate, a natural polyphenol, inhibits cell proliferation and induces apoptosis in human ovarian cancer cells. Anticancer Res. 2010, 30, 2519–2523. [Google Scholar] [PubMed]
- Liotta, L.A.; Tryggvason, K.; Garbisa, S.; Hart, I.; Foltz, C.; Shafie, S. Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 1980, 284, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Jin, G.; Yang, Y.; Liu, K.; Zhao, J.; Chen, X.; Liu, H.; Bai, R.; Li, X.; Jiang, Y.; Zhang, X. Combination curcumin and (−)-epigallocatechin-3-gallate inhibits colorectal carcinoma microenvironment-induced angiogenesis by JAK/STAT3/IL-8 pathway. Oncogenesis 2017, 6, e384. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Ma, W.-y.; Huang, C.; Yang, C.S. Inhibition of tumor promoter-induced activator protein 1 activation and cell transformation by tea polyphenols,(-)-epigallocatechin gallate, and theaflavins. Cancer Res. 1997, 57, 4414–4419. [Google Scholar] [PubMed]
- Shimizu, M.; Shirakami, Y.; Sakai, H.; Yasuda, Y.; Kubota, M.; Adachi, S.; Tsurumi, H.; Hara, Y.; Moriwaki, H. (−)-Epigallocatechin gallate inhibits growth and activation of the VEGF/VEGFR axis in human colorectal cancer cells. Chem.-Biol. Interact. 2010, 185, 247–252. [Google Scholar] [CrossRef]
- Mahadevan, D.; Thanki, N.; Aroca, P.; McPhie, P.; Yu, J.-C.; Beeler, J.; Santos, E.; Wlodawer, A.; Heidaran, M. A Divalent Metal Ion Binding Site in the Kinase Insert Domain of the. alpha.-Platelet-Derived Growth Factor Receptor Regulates Its Association with SH2 Domains. Biochemistry 1995, 34, 2095–2106. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Blayney, A.; Liu, X.; Gandy, L.; Jin, W.; Yan, L.; Ha, J.-H.; Canning, A.J.; Connelly, M.; Yang, C. EGCG binds intrinsically disordered N-terminal domain of p53 and disrupts p53-MDM2 interaction. Nat. Commun. 2021, 12, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Tsuneoka, M. Gallic Acid Derivatives Propyl Gallate and Epigallocatechin Gallate Reduce rRNA Transcription via Induction of KDM2A Activation. Biomolecules 2022, 12, 30. [Google Scholar] [CrossRef]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Cavallito, C.J.; Bailey, J.H. Allicin, the antibacterial principle of Allium sativum. I. Isolation, physical properties and antibacterial action. J. Am. Chem. Soc. 1944, 66, 1950–1951. [Google Scholar] [CrossRef]
- Izigov, N.; Farzam, N.; Savion, N. S-allylmercapto-N-acetylcysteine up-regulates cellular glutathione and protects vascular endothelial cells from oxidative stress. Free. Radic. Biol. Med. 2011, 50, 1131–1139. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Foerster, J.; Kappler, U.; Antelmann, H.; Noll, U.; Gruhlke, M.C.H.; Slusarenko, A.J. Allicin, the odor of freshly crushed garlic: A review of recent progress in understanding allicin’s effects on cells. Molecules 2021, 26, 1505. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.Y.Y.; Yuen, A.C.Y.; Chan, R.Y.K.; Chan, S.W.J.P.R. A review of the cardiovascular benefits and antioxidant properties of allicin. Phytother. Res. 2013, 27, 637–646. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Y.; Zhao, J.; Zhao, M.; Wang, K. Allicin activates autophagic cell death to alleviate the malignant development of thyroid cancer. Exp. Ther. Med. 2018, 15, 3537–3543. [Google Scholar] [CrossRef] [PubMed]
- Padilla-Camberos, E.; Zaitseva, G.; Padilla, C.; Puebla, A.M. Antitumoral activity of allicin in murine lymphoma L5178Y. Asian Pac. J. Cancer Prev. 2010, 11, 1241–1244. [Google Scholar] [PubMed]
- Hussain, Z.V.M.; Sivanandhan, S.K. Antiproliferative and Apoptotic Induction of Allicin in Human Lung Cancer Cell Lines. Indian J. Pharm. Educ. Res. 2021, 55, 566–573. [Google Scholar] [CrossRef]
- Maitisha, G.; Aimaiti, M.; An, Z.; Li, X. Allicin induces cell cycle arrest and apoptosis of breast cancer cells in vitro via modulating the p53 pathway. Mol. Biol. Rep. 2021, 48, 7261–7272. [Google Scholar] [CrossRef]
- Lee, C.G.; Lee, H.-W.; Kim, B.-O.; Rhee, D.-K.; Pyo, S. Allicin inhibits invasion and migration of breast cancer cells through the suppression of VCAM-1: Regulation of association between p65 and ER-α. J. Funct. Foods 2015, 15, 172–185. [Google Scholar] [CrossRef]
- Sun, L.; Wang, X. Effects of allicin on both telomerase activity and apoptosis in gastric cancer SGC-7901 cells. World J. Gastroenterol. 2003, 9, 1930. [Google Scholar] [CrossRef]
- Cha, J.H.; Choi, Y.J.; Cha, S.H.; Choi, C.H.; Cho, W.H. Allicin inhibits cell growth and induces apoptosis in U87MG human glioblastoma cells through an ERK-dependent pathway. Oncol. Rep. 2012, 28, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Weeranantanapan, O.; Satsantitham, K.; Sritangos, P.; Chudapongse, N. Allicin suppresses human glioblastoma cell growth by inducing cell cycle arrest and apoptosis, and by promoting autophagy. Arch. Biol. Sci. 2020, 72, 313–319. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, D. Allicin suppresses the migration and invasion in cervical cancer cells mainly by inhibiting NRF2. Exp. Ther. Med. 2019, 17, 1523–1528. [Google Scholar] [CrossRef]
- Khakbaz, P.; Panahizadeh, R.; Vatankhah, M.A.; Najafzadeh, N. Allicin Reduces 5-fluorouracil-resistance in Gastric Cancer Cells through Modulating MDR1, DKK1, and WNT5A Expression. Drug Res. 2021, 71, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Huang, Y.; Wang, J.-P.; Yu, X.-Y.; Zhang, L.-Y. The synergistic anticancer effect of artesunate combined with allicin in osteosarcoma cell line in vitro and in vivo. Asian Pac. J. Cancer Prev. 2013, 14, 4615–4619. [Google Scholar] [CrossRef] [PubMed]
- Jobani, B.M.; Najafzadeh, N.; Mazani, M.; Arzanlou, M.; Vardin, M.M. Molecular mechanism and cytotoxicity of allicin and all-trans retinoic acid against CD44+ versus CD117+ melanoma cells. Phytomedicine 2018, 48, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.F.; Anwer, M. Effect of Electromagnetic Field and Allicin as Natural Extract on Hepatocellular Carcinoma (HepG2). Med. J. Cairo Univ. 2020, 88, 815–825. [Google Scholar]
- Ahmad, A.; Mishra, R.K.; Vyawahare, A.; Kumar, A.; Rehman, M.U.; Qamar, W.; Khan, A.Q.; Khan, R. Thymoquinone (2-Isopropyl-5-methyl-1, 4-benzoquinone) as a chemopreventive/anticancer agent: Chemistry and biological effects. Saudi Pharm. J. 2019, 27, 1113–1126. [Google Scholar] [CrossRef] [PubMed]
- Dajani, E.Z.; Shahwan, T.G.; Dajani, N.E. Overview of the preclinical pharmacological properties of Nigella sativa (black seeds): A complementary drug with historical and clinical significance. J. Physiol. Pharm. 2016, 67, 801–817. [Google Scholar]
- Jain, A.; Dhruw, L.; Sinha, P.; Pradhan, A.; Sharma, R.; Gupta, B. Thymoquinone. In Nutraceuticals; Elsevier: Amsterdam, The Netherlands, 2021; pp. 891–901. [Google Scholar]
- Younus, H.; Sawhney. Molecular and Therapeutic: Actions of Thymoquinone; Springer: Berlin/Heidelberg, Germany, 2018. [Google Scholar]
- Alobaedi, O.H.; Talib, W.H.; Basheti, I.A. Antitumor effect of thymoquinone combined with resveratrol on mice transplanted with breast cancer. Asian Pac. J. Trop. Med. 2017, 10, 400–408. [Google Scholar] [CrossRef] [PubMed]
- Darakhshan, S.; Pour, A.B.; Colagar, A.H.; Sisakhtnezhad, S. Thymoquinone and its therapeutic potentials. Pharmacol. Res. 2015, 95, 138–158. [Google Scholar] [CrossRef] [PubMed]
- Phua, C.Y.H.; Teoh, Z.L.; Goh, B.-H.; Yap, W.H.; Tang, Y.-Q. Triangulating the pharmacological properties of thymoquinone in regulating reactive oxygen species, inflammation, and cancer: Therapeutic applications and mechanistic pathways. Life Sci. 2021, 287, 120120. [Google Scholar] [CrossRef] [PubMed]
- Homayoonfal, M.; Asemi, Z.; Yousefi, B. Potential anticancer properties and mechanisms of thymoquinone in osteosarcoma and bone metastasis. Cell. Mol. Biol. Lett. 2022, 27, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Alhmied, F.; Alammar, A.; Alsultan, B.; Alshehri, M.; Pottoo, F.H. Molecular mechanisms of thymoquinone as anticancer agent. Comb. Chem. High Throughput Screen. 2021, 24, 1644–1653. [Google Scholar] [CrossRef]
- Wirries, A.; Breyer, S.; Quint, K.; Schobert, R.; Ocker, M. Thymoquinone hydrazone derivatives cause cell cycle arrest in p53-competent colorectal cancer cells. Exp. Ther. Med. 2010, 1, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Gali-Muhtasib, H.; Kuester, D.; Mawrin, C.; Bajbouj, K.; Diestel, A.; Ocker, M.; Habold, C.; Foltzer-Jourdainne, C.; Schoenfeld, P.; Peters, B. Thymoquinone triggers inactivation of the stress response pathway sensor CHEK1 and contributes to apoptosis in colorectal cancer cells. Cancer Res. 2008, 68, 5609–5618. [Google Scholar] [CrossRef]
- Gali-Muhtasib, H.U.; Abou Kheir, W.G.; Kheir, L.A.; Darwiche, N.; Crooks, P.A. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anti-Cancer Drugs 2004, 15, 389–399. [Google Scholar] [CrossRef] [PubMed]
- Motaghed, M.; Al-Hassan, F.M.; Hamid, S.S. Cellular responses with thymoquinone treatment in human breast cancer cell line MCF-7. Pharmacogn. Res. 2013, 5, 200. [Google Scholar]
- Ma, J.; Zhang, Y.; Deng, H.; Liu, Y.; Lei, X.; He, P.; Dong, W. Thymoquinone inhibits the proliferation and invasion of esophageal cancer cells by disrupting the AKT/GSK-3β/Wnt signaling pathway via PTEN upregulation. Phytother. Res. 2020, 34, 3388–3399. [Google Scholar] [CrossRef] [PubMed]
- Guler, E.M.; Sisman, B.H.; Kocyigit, A.; Hatiboglu, M.A. Investigation of cellular effects of thymoquinone on glioma cell. Toxicol. Rep. 2021, 8, 162–170. [Google Scholar] [CrossRef] [PubMed]
- El-Sheikh, A.A.K.; Morsy, M.A.; Abdalla, A.M.; Hamouda, A.H.; Alhaider, I.A. Mechanisms of thymoquinone hepatorenal protection in methotrexate-induced toxicity in rats. Mediat. Inflamm. 2015, 2015, 859383. [Google Scholar] [CrossRef] [PubMed]
- Chehl, N.; Chipitsyna, G.; Gong, Q.; Yeo, C.J.; Arafat, H.A. Anti-inflammatory effects of the Nigella sativa seed extract, thymoquinone, in pancreatic cancer cells. Hpb 2009, 11, 373–381. [Google Scholar] [CrossRef] [PubMed]
- El Mezayen, R.; El Gazzar, M.; Nicolls, M.R.; Marecki, J.C.; Dreskin, S.C.; Nomiyama, H. Effect of thymoquinone on cyclooxygenase expression and prostaglandin production in a mouse model of allergic airway inflammation. Immunol. Lett. 2006, 106, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Choi, B.Y.; Jeong, C.-H.; Kundu, J.K.; Chun, K.-S. Thymoquinone induces apoptosis in human colon cancer HCT116 cells through inactivation of STAT3 by blocking JAK2-and Src-mediated phosphorylation of EGF receptor tyrosine kinase. Oncol. Rep. 2014, 32, 821–828. [Google Scholar] [CrossRef]
- Raut, P.K.; Lee, H.S.; Joo, S.H.; Chun, K.-S. Thymoquinone induces oxidative stress-mediated apoptosis through downregulation of Jak2/STAT3 signaling pathway in human melanoma cells. Food Chem. Toxicol. 2021, 157, 112604. [Google Scholar] [CrossRef] [PubMed]
- Almajali, B.; Al-Jamal, H.A.N.; Taib, W.R.W.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N.; Tajudin, S.A. Thymoquinone suppresses cell proliferation and enhances apoptosis of HL60 leukemia cells through re-expression of JAK/STAT negative regulators. Asian Pac. J. Cancer Prev. 2021, 22, 879. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.C.; Loo, S.Y.; Gee, V.; Yap, C.W.; Sethi, G.; Kumar, A.P.; Tan, K.H.B. Anticancer activity of thymoquinone in breast cancer cells: Possible involvement of PPAR-γ pathway. Biochem. Pharmacol. 2011, 82, 464–475. [Google Scholar] [CrossRef]
- Zhu, W.-Q.; Wang, J.; Guo, X.-F.; Liu, Z.; Dong, W.-G. Thymoquinone inhibits proliferation in gastric cancer via the STAT3 pathway in vivo and in vitro. World J. Gastroenterol. 2016, 22, 4149. [Google Scholar] [CrossRef] [PubMed]
- Talib, W.H. Regressions of breast carcinoma syngraft following treatment with piperine in combination with thymoquinone. Sci. Pharm. 2017, 85, 27. [Google Scholar] [CrossRef]
- Ayan, M.; Tas, U.; Sogut, E.; Caylı, S.; Kaya, H.; Esen, M.; Erdemir, F.; Uysal, M. Protective effect of thymoquinone against testicular torsion induced oxidative injury. Andrologia 2016, 48, 143–151. [Google Scholar] [CrossRef]
- Fouad, A.A.; Jresat, I. Thymoquinone therapy abrogates toxic effect of cadmium on rat testes. Andrologia 2015, 47, 417–426. [Google Scholar] [CrossRef]
- Mabrouk, A.; Ben Cheikh, H. Thymoquinone supplementation ameliorates lead-induced testis function impairment in adult rats. Toxicol. Ind. Health 2016, 32, 1114–1121. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Muneer, K.M.; Tamimi, I.A.; Chang, M.E.; Ata, M.O.; Yusuf, N. Thymoquinone suppresses metastasis of melanoma cells by inhibition of NLRP3 inflammasome. Toxicol. Appl. Pharmacol. 2013, 270, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Badary, O.A.; Taha, R.A.; Gamal El-Din, A.M.; Abdel-Wahab, M.H. Thymoquinone is a potent superoxide anion scavenger. Drug Chem. Toxicol. 2003, 26, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Nagi, M.N.; Mansour, M.A. Protective effect of thymoquinone against doxorubicin–induced cardiotoxicity in rats: A possible mechanism of protection. Pharmacol. Res. 2000, 41, 283–289. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, R.M.A.; Alkharfy, K.M.; Raish, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Effects of thymoquinone on the pharmacokinetics and pharmacodynamics of glibenclamide in a rat model. Nat. Prod. Commun. 2015, 10, 1934578X1501000821. [Google Scholar] [CrossRef]
- Elbarbry, F.; Ragheb, A.; Marfleet, T.; Shoker, A. Modulation of hepatic drug metabolizing enzymes by dietary doses of thymoquinone in female New Zealand White rabbits. Phytother. Res. 2012, 26, 1726–1730. [Google Scholar] [CrossRef] [PubMed]
- Arafa, E.-S.A.; Zhu, Q.; Shah, Z.I.; Wani, G.; Barakat, B.M.; Racoma, I.; El-Mahdy, M.A.; Wani, A.A. Thymoquinone up-regulates PTEN expression and induces apoptosis in doxorubicin-resistant human breast cancer cells. Mutat. Res. Fundam. Mol. Mech. Mutagenesis 2011, 706, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Homayoonfal, M.; Asemi, Z.; Yousefi, B. Targeting microRNAs with thymoquinone: A new approach for cancer therapy. Cell. Mol. Biol. Lett. 2021, 26, 1–22. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, F.; Wu, H.; Chen, X.; Zhang, Y.; Lin, J.; Cai, Y.; Xiang, J.; He, N.; Hu, Z. Thymoquinone Suppresses the Proliferation, Migration and Invasiveness through Regulating ROS, Autophagic Flux and miR-877-5p in Human Bladder Carcinoma Cells. Int. J. Biol. Sci. 2021, 17, 3456. [Google Scholar] [CrossRef]
- Ünal, T.D.; Hamurcu, Z.; Delibaşı, N.; Çınar, V.; Güler, A.; Gökçe, S.; Nurdinov, N.; Ozpolat, B. Thymoquinone inhibits proliferation and migration of MDA-MB-231 triple negative breast cancer cells by suppressing autophagy, Beclin-1 and LC3. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem.-Anti-Cancer Agents) 2021, 21, 355–364. [Google Scholar] [CrossRef]
- Atteia, H.H.; Arafa, M.H.; Mohammad, N.S.; Amin, D.M.; Sakr, A.T. Thymoquinone upregulates miR-125a-5p, attenuates STAT3 activation, and potentiates doxorubicin antitumor activity in murine solid Ehrlich carcinoma. J. Biochem. Mol. Toxicol. 2021, 35, e22924. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Zhao, X.; Cui, T. Full Length Research Paper Production of emodin from Aspergillus ochraceus at preparative scale. Afr. J. Biotechnol. 2010, 9, 512–517. [Google Scholar]
- Tang, T.; Yin, L.; Yang, J.; Shan, G. Emodin, an anthraquinone derivative from Rheum officinale Baill, enhances cutaneous wound healing in rats. Eur. J. Pharmacol. 2007, 567, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Fu, J.; Yin, X.; Cao, S.; Li, X.; Lin, L.; Huyiligeqi; Ni, J. Emodin: A review of its pharmacology, toxicity and pharmacokinetics. Phytother. Res. 2016, 30, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Gao, J.; Pang, X.; Chen, A.; Wang, Y. Molecular mechanisms of action of emodin: As an anti-cardiovascular disease drug. Front. Pharmacol. 2020, 11, 559607. [Google Scholar] [CrossRef]
- Huang, H.-C.; Chang, J.-H.; Tung, S.-F.; Wu, R.-T.; Foegh, M.L.; Chu, S.-H. Immunosuppressive effect of emodin, a free radical generator. Eur. J. Pharmacol. 1992, 211, 359–364. [Google Scholar] [CrossRef]
- Hsu, S.-C.; Chung, J.-G. Anticancer potential of emodin. BioMedicine 2012, 2, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Jin, S.; Cao, Y.; Xu, J.; Zhu, S.; Li, Z. Emodin regulates cell cycle of non-small lung cancer (NSCLC) cells through hyaluronan synthase 2 (HA2)-HA-CD44/receptor for hyaluronic acid-mediated motility (RHAMM) interaction-dependent signaling pathway. Cancer Cell Int. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Qin, B.; Zeng, Z.; Xu, J.; Shangwen, J.; Ye, Z.J.; Wang, S.; Wu, Y.; Peng, G.; Wang, Q.; Gu, W. Emodin inhibits invasion and migration of hepatocellular carcinoma cells via regulating autophagy-mediated degradation of snail and β-catenin. BMC Cancer 2022, 22, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Li, G.; Han, G.; Feng, S.; Liu, Y.; Chen, J.; Liu, C.; Zhao, L.; Jin, F. Emodin induced necroptosis in the glioma cell line U251 via the TNF-α/RIP1/RIP3 pathway. Investig. New Drugs 2020, 38, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Oei, A.L.M.; Sweep, F.C.G.J.; Thomas, C.M.G.; Boerman, O.C.; Massuger, L.F.A.G. The use of monoclonal antibodies for the treatment of epithelial ovarian cancer. Int. J. Oncol. 2008, 32, 1145–1157. [Google Scholar] [CrossRef] [PubMed]
- Zou, G.; Zhang, X.; Wang, L.; Li, X.; Xie, T.; Zhao, J.; Yan, J.; Wang, L.; Ye, H.; Jiao, S. Herb-sourced emodin inhibits angiogenesis of breast cancer by targeting VEGFA transcription. Theranostics 2020, 10, 6839. [Google Scholar] [CrossRef]
- Wu, F.L.; Chu, P.Y.; Chen, G.Y.; Wang, K.; Hsu, W.Y.; Ahmed, A.; Ma, W.L.; Cheng, W.C.; Wu, Y.C.; Yang, J.C. Natural anthraquinone compound emodin as a novel inhibitor of aurora A kinase: A pilot study. Chem. Biol. Drug Des. 2022, 99, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.; Lee, Y.H.; Lee, C.H.; Lee, S.K. Emodin, an anthraquinone derivative isolated from the rhizomes of Rheum palmatum, selectively inhibits the activity of casein kinase II as a competitive inhibitor. Planta Med. 1999, 65, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-j.; Meng, X.-y.; Chen, J.-f.; Wang, K.-y.; Zhou, C.; Yu, R.; Ma, Q. Emodin induced necroptosis and inhibited glycolysis in the renal cancer cells by enhancing ROS. Oxidative Med. Cell. Longev. 2021, 2021, 8840590. [Google Scholar] [CrossRef] [PubMed]
- Yon, J.-M.; Baek, I.-J.; Lee, B.J.; Yun, Y.W.; Nam, S.-Y. Emodin and [6]-gingerol lessen hypoxia-induced embryotoxicities in cultured mouse whole embryos via upregulation of hypoxia-inducible factor 1α and intracellular superoxide dismutases. Reprod. Toxicol. 2011, 31, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Yan, J.; Chang, Y.; ShiDu Yan, S.; Shi, H. Hypoxia inducible factor-1 as a target for neurodegenerative diseases. Curr. Med. Chem. 2011, 18, 4335–4343. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.-L.; Bu, H.; Li, H.; Chen, H.; Guo, H.-C.; Wang, Z.-H.; Tong, H.-F.; Ni, Z.-L.; Liu, H.-B.; Lin, S.-Z. Emodin reverses gemcitabine resistance in pancreatic cancer cells via the mitochondrial apoptosis pathway in vitro. Int. J. Oncol. 2012, 40, 1049–1057. [Google Scholar] [CrossRef]
- Wang, Z.-H.; Chen, H.; Guo, H.-C.; Tong, H.-F.; Liu, J.-X.; Wei, W.-T.; Tan, W.; Ni, Z.-L.; Liu, H.-B.; Lin, S.-Z. Enhanced antitumor efficacy by the combination of emodin and gemcitabine against human pancreatic cancer cells via downregulation of the expression of XIAP in vitro and in vivo. Int. J. Oncol. 2011, 39, 1123–1131. [Google Scholar]
- Wang, W.; Sun, Y.-p.; Huang, X.-z.; He, M.; Chen, Y.-y.; Shi, G.-y.; Li, H.; Yi, J.; Wang, J. Emodin enhances sensitivity of gallbladder cancer cells to platinum drugs via glutathion depletion and MRP1 downregulation. Biochem. Pharmacol. 2010, 79, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-z.; Wang, J.; Huang, C.; Chen, Y.-y.; Shi, G.-y.; Hu, Q.-s.; Yi, J. Emodin enhances cytotoxicity of chemotherapeutic drugs in prostate cancer cells: The mechanisms involve ROS-mediated suppression of multidrug resistance and hypoxia inducible factor-1. Cancer Biol. Ther. 2008, 7, 468–475. [Google Scholar] [CrossRef]
- Spagnuolo, C.; Russo, G.L.; Orhan, I.E.; Habtemariam, S.; Daglia, M.; Sureda, A.; Nabavi, S.F.; Devi, K.P.; Loizzo, M.R.; Tundis, R. Genistein and cancer: Current status, challenges, and future directions. Adv. Nutr. 2015, 6, 408–419. [Google Scholar] [CrossRef] [PubMed]
- Mukund, V.; Mukund, D.; Sharma, V.; Mannarapu, M.; Alam, A. Genistein: Its role in metabolic diseases and cancer. Crit. Rev. Oncol. Hematol. 2017, 119, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhu, C.-F.; Iwamoto, H.; Chen, J.-S. Genistein inhibits invasive potential of human hepatocellular carcinoma by altering cell cycle, apoptosis, and angiogenesis. World J. Gastroenterol. 2005, 11, 6512. [Google Scholar] [CrossRef]
- Chodon, D.; Banu, S.M.; Padmavathi, R.; Sakthisekaran, D. Inhibition of cell proliferation and induction of apoptosis by genistein in experimental hepatocellular carcinoma. Mol. Cell. Biochem. 2007, 297, 73–80. [Google Scholar] [CrossRef]
- Tatsuta, M.; Iishi, H.; Baba, M.; Yano, H.; Uehara, H.; Nakaizumi, A. Attenuation by genistein of sodium-chloride-enhanced gastric carcinogenesis induced by N-methyl-N′-nitro-N-nitrosoguanidine in Wistar rats. Int. J. Cancer 1999, 80, 396–399. [Google Scholar] [CrossRef]
- Mentor-Marcel, R.; Lamartiniere, C.A.; Eltoum, I.-E.; Greenberg, N.M.; Elgavish, A. Genistein in the diet reduces the incidence of poorly differentiated prostatic adenocarcinoma in transgenic mice (TRAMP). Cancer Res. 2001, 61, 6777–6782. [Google Scholar] [PubMed]
- Perabo, F.G.E.; Von Löw, E.C.; Ellinger, J.; Von Rücker, A.; Müller, S.C.; Bastian, P.J. Soy isoflavone genistein in prevention and treatment of prostate cancer. Prostate Cancer Prostatic Dis. 2008, 11, 6–12. [Google Scholar] [CrossRef]
- Pollard, M.; Wolter, W. Prevention of spontaneous prostate-related cancer in Lobund-Wistar rats by a soy protein isolate/isoflavone diet. Prostate 2000, 45, 101–105. [Google Scholar] [CrossRef]
- Sohel, M.; Biswas, P.; Al Amin, M.; Dey, D.; Sultana, H.; Aktar, S.; Khan, M.S.; Hossain, M.A.; Paul, P.; Nurul, I.M. Genistein Mediated Molecular Pharmacology, Cell-Specific Anti-Breast Cancer Mechanism with Synergistic Effect and in silico Safety Measurement. Preprints 2021, 2021090123. [Google Scholar] [CrossRef]
- Banerjee, S.; Li, Y.; Wang, Z.; Sarkar, F.H. Multi-targeted therapy of cancer by genistein. Cancer Lett. 2008, 269, 226–242. [Google Scholar] [CrossRef] [PubMed]
- King-Batoon, A.; Leszczynska, J.M.; Klein, C.B. Modulation of gene methylation by genistein or lycopene in breast cancer cells. Environ. Mol. Mutagenesis 2008, 49, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Basak, S.; Pookot, D.; Noonan, E.J.; Dahiya, R. Genistein down-regulates androgen receptor by modulating HDAC6-Hsp90 chaperone function. Mol. Cancer Ther. 2008, 7, 3195–3202. [Google Scholar] [CrossRef] [PubMed]
- Estrela, J.M.; Mena, S.; Obrador, E.; Benlloch, M.; Castellano, G.; Salvador, R.; Dellinger, R.W. Polyphenolic phytochemicals in cancer prevention and therapy: Bioavailability versus bioefficacy. J. Med. Chem. 2017, 60, 9413–9436. [Google Scholar] [CrossRef]
- Meeran, S.M.; Katiyar, S.K. Cell cycle control as a basis for cancer chemoprevention through dietary agents. Front. Biosci. J. Virtual Libr. 2008, 13, 2191. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, F.H.; Adsule, S.; Padhye, S.; Kulkarni, S.; Li, Y. The role of genistein and synthetic derivatives of isoflavone in cancer prevention and therapy. Mini Rev. Med. Chem. 2006, 6, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Bi, Y.-l.; Min, M.; Shen, W.; Liu, Y. Genistein induced anticancer effects on pancreatic cancer cell lines involves mitochondrial apoptosis, G0/G1cell cycle arrest and regulation of STAT3 signalling pathway. Phytomedicine 2018, 39, 10–16. [Google Scholar] [CrossRef] [PubMed]
- Farina, H.G.; Pomies, M.; Alonso, D.F.; Gomez, D.E. Antitumor and antiangiogenic activity of soy isoflavone genistein in mouse models of melanoma and breast cancer. Oncol. Rep. 2006, 16, 885–891. [Google Scholar] [CrossRef]
- Shafiee, G.; Saidijam, M.; Tayebinia, H.; Khodadadi, I. Beneficial effects of genistein in suppression of proliferation, inhibition of metastasis, and induction of apoptosis in PC3 prostate cancer cells. Arch. Physiol. Biochem. 2020, 128, 694–702. [Google Scholar] [CrossRef]
- Sharma, M.; Arora, I.; Chen, M.; Wu, H.; Crowley, M.R.; Tollefsbol, T.O.; Li, Y. Therapeutic Effects of Dietary Soybean Genistein on Triple-Negative Breast Cancer via Regulation of Epigenetic Mechanisms. Nutrients 2021, 13, 3944. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Li, Y.; Nedeljkovic-Kurepa, A.; Sarkar, F.H. Inactivation of NF-κB by genistein is mediated via Akt signaling pathway in breast cancer cells. Oncogene 2003, 22, 4702–4709. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Malhotra, L.; Yadav, P.; Mishra, V.; Sharma, R.S.; Samath, E.A. Genistein: A novel inhibitor of IL-6/IL-6R interface of the Interleukin-6–mediated STAT3 dependent pathway of carcinogenesis. J. Mol. Struct. 2022, 1258, 132668. [Google Scholar] [CrossRef]
- Nakamura, Y.; Yogosawa, S.; Izutani, Y.; Watanabe, H.; Otsuji, E.; Sakai, T. A combination of indole-3-carbinol and genistein synergistically induces apoptosis in human colon cancer HT-29 cells by inhibiting Akt phosphorylation and progression of autophagy. Mol. Cancer 2009, 8, 1–15. [Google Scholar] [CrossRef]
- Oh, H.Y.; Leem, J.; Yoon, S.J.; Yoon, S.; Hong, S.J. Lipid raft cholesterol and genistein inhibit the cell viability of prostate cancer cells via the partial contribution of EGFR-Akt/p70S6k pathway and down-regulation of androgen receptor. Biochem. Biophys. Res. Commun. 2010, 393, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Kim, S.H.; Kim, Y.B.; Jeon, Y.T.; Lee, S.C.; Song, Y.S. Genistein inhibits cell growth by modulating various mitogen-activated protein kinases and AKT in cervical cancer cells. Ann. N. Y. Acad. Sci. 2009, 1171, 495–500. [Google Scholar] [CrossRef] [PubMed]
- Javed, Z.; Khan, K.; Herrera-Bravo, J.; Naeem, S.; Iqbal, M.J.; Sadia, H.; Qadri, Q.R.; Raza, S.; Irshad, A.; Akbar, A. Genistein as a regulator of signaling pathways and microRNAs in different types of cancers. Cancer Cell Int. 2021, 21, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Q.; Liu, B.; Zheng, X.; Li, P.; Zhao, T.; Jin, X.; Ye, F.; Zhang, P.; Chen, W. Genistein inhibits radiation-induced invasion and migration of glioblastoma cells by blocking the DNA-PKcs/Akt2/Rac1 signaling pathway. Radiother. Oncol. 2021, 155, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Sohel, M.; Biswas, P.; Al Amin, M.; Hossain, M.A.; Sultana, H.; Dey, D.; Aktar, S.; Setu, A.; Khan, M.S.; Paul, P.; et al. Genistein, a Potential Phytochemical against Breast Cancer Treatment-Insight into the Molecular Mechanisms. Processes 2022, 10, 415. [Google Scholar] [CrossRef]
- Mahood, H.E.; Abbas, M.K.; Zahid, N.A. Micropropagation of Feverfew (Tanacetum parthenium) and Quantification of Parthenolide Content in Its Micropropagated and Conventionally Grown Plants. Horticulturae 2022, 8, 50. [Google Scholar] [CrossRef]
- Freund, R.R.; Gobrecht, P.; Fischer, D.; Arndt, H.-D. Advances in chemistry and bioactivity of parthenolide. Nat. Prod. Rep. 2020, 37, 541–565. [Google Scholar] [CrossRef] [PubMed]
- Sztiller-Sikorska, M.; Czyz, M. Parthenolide as cooperating agent for anti-cancer treatment of various malignancies. Pharmaceuticals 2020, 13, 194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Tang, B.; Wang, F.-C.; Tang, L.; Lei, Y.-Y.; Luo, Y.; Huang, S.-J.; Yang, M.; Wu, L.-Y.; Wang, W. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics 2020, 10, 5225. [Google Scholar] [CrossRef]
- Siddiqui, N.A.; Alam, P.; Alrehaily, A.J.; Alqahtani, A.S.; Akhtar, A.; Alhowiriny, T.A.; Almarfadi, O.M.; Mothana, R.A. Optimization of ultrasound-assisted parthenolide extraction from Tarchonanthus camphoratus leaves using response surface methodology: HPTLC and cytotoxicity analysis. Arab. J. Chem. 2021, 14, 103194. [Google Scholar] [CrossRef]
- Sun, L.; Yuan, W.; Wen, G.; Yu, B.; Xu, F.; Gan, X.; Tang, J.; Zeng, Q.; Zhu, L.; Chen, C. Parthenolide inhibits human lung cancer cell growth by modulating the IGF-1R/PI3K/Akt signaling pathway. Oncol. Rep. 2020, 44, 1184–1193. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Banerjee, S.; Mondal, A.; Chakraborty, U.; Pumarol, J.; Croley, C.R.; Bishayee, A. Targeting the JAK/STAT signaling pathway using phytocompounds for cancer prevention and therapy. Cells 2020, 9, 1451. [Google Scholar] [CrossRef]
- Lu, L.; Feng, Q.; Su, T.; Cheng, Y.; Huang, Z.; Huang, Q.; Liu, Z. Pharmacoepigenetics of Chinese Herbal Components in Cancer. In Pharmacoepigenetics; Elsevier: Amsterdam, The Netherlands, 2019; pp. 859–869. [Google Scholar]
- Jafari, N.; Nazeri, S.; Enferadi, S.T. Parthenolide reduces metastasis by inhibition of vimentin expression and induces apoptosis by suppression elongation factor α− 1 expression. Phytomedicine 2018, 41, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, T.G. Role of nuclear factor kappa B (NF-κB) signalling in neurodegenerative diseases: An mechanistic approach. Curr. Neuropharmacol. 2020, 18, 918–935. [Google Scholar] [CrossRef] [PubMed]
- Zanelli, M.; Sanguedolce, F.; Zizzo, M.; Palicelli, A.; Bassi, M.C.; Santandrea, G.; Martino, G.; Soriano, A.; Caprera, C.; Corsi, M. Primary effusion lymphoma occurring in the setting of transplanted patients: A systematic review of a rare, life-threatening post-transplantation occurrence. BMC Cancer 2021, 21, 1–13. [Google Scholar] [CrossRef]
- Narkhede, M.; Arora, S.; Ujjani, C. Primary effusion lymphoma: Current perspectives. OncoTargets Ther. 2018, 11, 3747. [Google Scholar] [CrossRef] [PubMed]
- Karam, L.; Abou Staiteieh, S.; Chaaban, R.; Hayar, B.; Ismail, B.; Neipel, F.; Darwiche, N.; Abou Merhi, R. Anticancer activities of parthenolide in primary effusion lymphoma preclinical models. Mol. Carcinog. 2021, 60, 567–581. [Google Scholar] [CrossRef] [PubMed]
- Moorthy, R.K.; Jayamohan, S.; Kannan, M.K.; Arockiam, A.J.V. Parthenolide Induces Apoptosis and Cell Cycle Arrest by the Suppression of miR-375 Through Nucleolin in Prostate Cancer. J. Pharm. Res. Int. 2021, 215–227. [Google Scholar] [CrossRef]
- Sun, T.; Liu, Z.; Yang, Q. The role of ubiquitination and deubiquitination in cancer metabolism. Mol. Cancer 2020, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Ju, X.; Yang, Q.; Zhu, Y.; Fan, D.; Su, G.; Kong, L.; Li, Y. USP47 maintains the stemness of colorectal cancer cells and is inhibited by parthenolide. Biochem. Biophys. Res. Commun. 2021, 562, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lin, M.; Bi, H.; Yan, Y.; Huang, W.; Zhang, G.; Zhang, G.; Tang, S.; Liu, Y.; Zhang, L.; Ma, J. Parthenolide suppresses non-small cell lung cancer GLC-82 cells growth via B-Raf/MAPK/Erk pathway. Oncotarget 2017, 8, 23436. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Han, Y.; Liu, L.; Ren, X.; Zhang, H.; Fan, S.; Qin, T.; Li, L. Parthenolide inhibits the tumor characteristics of renal cell carcinoma. Int. J. Oncol. 2021, 58, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yang, J.; Sun, Y.; Fan, S.; Lu, Y.; Li, L. Parthenolide induces autophagy and apoptosis of breast cancer cells associated with the PI3K/AKT/mTOR pathway. Res. Sq. 2021. preprint. [Google Scholar]
- Berdan, C.A.; Ho, R.; Lehtola, H.S.; To, M.; Hu, X.; Huffman, T.R.; Petri, Y.; Altobelli, C.R.; Demeulenaere, S.G.; Olzmann, J.A. Parthenolide covalently targets and inhibits focal adhesion kinase in breast cancer cells. Cell Chem. Biol. 2019, 26, 1027–1035.e22. [Google Scholar] [CrossRef]
- Liu, W.; Wang, X.; Sun, J.; Yang, Y.; Li, W.; Song, J. Parthenolide suppresses pancreatic cell growth by autophagy-mediated apoptosis. OncoTargets Ther. 2017, 10, 453. [Google Scholar] [CrossRef] [PubMed]
- Sufian, H.B.; Santos, J.M.; Khan, Z.S.; Munir, M.T.; Zahid, M.K.; Al-Harrasi, A.; Gollahon, L.S.; Hussain, F.; Rahman, S.M. Parthenolide Inhibits Migration and Reverses the EMT Process in Breast Cancer Cells by Suppressing TGFβ and TWIST1. Res. Sq. 2021. preprint. [Google Scholar]
- Zhu, S.M.; Park, Y.R.; Seo, S.Y.; Kim, I.H.; Lee, S.T.; Kim, S.W. Parthenolide inhibits transforming growth factor β1-induced epithelial-mesenchymal transition in colorectal cancer cells. Intest. Res. 2019, 17, 527. [Google Scholar] [CrossRef]
- Che, S.-T.; Bie, L.; Li, X.; Qi, H.; Yu, P.; Zuo, L. Parthenolide inhibits the proliferation and induces the apoptosis of human uveal melanoma cells. Int. J. Ophthalmol. 2019, 12, 1531. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xiao, C.; Sun, M.; Tan, M.; Hu, L.; Yu, Q. Parthenolide inhibits STAT3 signaling by covalently targeting janus kinases. Molecules 2018, 23, 1478. [Google Scholar] [CrossRef] [PubMed]
- Jeyamohan, S.; Moorthy, R.K.; Kannan, M.K.; Arockiam, A.J.V. Parthenolide induces apoptosis and autophagy through the suppression of PI3K/Akt signaling pathway in cervical cancer. Biotechnol. Lett. 2016, 38, 1251–1260. [Google Scholar] [CrossRef] [PubMed]
- Aziz, N.; Kim, M.-Y.; Cho, J.Y. Anti-inflammatory effects of luteolin: A review of in vitro, in vivo, and in silico studies. J. Ethnopharmacol. 2018, 225, 342–358. [Google Scholar] [CrossRef]
- Gendrisch, F.; Esser, P.R.; Schempp, C.M.; Wölfle, U. Luteolin as a modulator of skin aging and inflammation. Biofactors 2021, 47, 170–180. [Google Scholar] [CrossRef] [PubMed]
- Gupta, G.; Tiwari, J.; Dahiya, R.; Sharma, R.K.; Mishra, A.; Dua, K. Recent updates on neuropharmacological effects of luteolin. EXCLI J. 2018, 17, 211–214. [Google Scholar] [PubMed]
- Luo, Y.; Shang, P.; Li, D. Luteolin: A flavonoid that has multiple cardio-protective effects and its molecular mechanisms. Front. Pharmacol. 2017, 8, 692. [Google Scholar] [CrossRef] [PubMed]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial mechanism of luteolin against Staphylococcus aureus and Listeria monocytogenes and its antibiofilm properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef]
- Ahmadi, S.M.; Farhoosh, R.; Sharif, A.; Rezaie, M. Structure-antioxidant activity relationships of luteolin and catechin. J. Food Sci. 2020, 85, 298–305. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Chang, Y.; Wang, R.; Lv, T.; Cui, C.; Liu, M. Investigation of the anti-inflammatory and antioxidant activities of luteolin, kaempferol, apigenin and quercetin. S. Afr. J. Bot. 2021, 137, 257–264. [Google Scholar] [CrossRef]
- Ganai, S.A.; Sheikh, F.A.; Baba, Z.A.; Mir, M.A.; Mantoo, M.A.; Yatoo, M.A. Anticancer activity of the plant flavonoid luteolin against preclinical models of various cancers and insights on different signalling mechanisms modulated. Phytother. Res. 2021, 35, 3509–3532. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Wei, P.; Song, J.; Jia, X.; Zhang, Z. Enhanced anticancer activity in vitro and in vivo of luteolin incorporated into long-circulating micelles based on DSPE-PEG2000 and TPGS. J. Pharm. Pharmacol. 2016, 68, 1290–1298. [Google Scholar] [CrossRef] [PubMed]
- Palko-Labuz, A.; Sroda-Pomianek, K.; Uryga, A.; Kostrzewa-Suslow, E.; Michalak, K. Anticancer activity of baicalein and luteolin studied in colorectal adenocarcinoma LoVo cells and in drug-resistant LoVo/Dx cells. Biomed. Pharmacother. 2017, 88, 232–241. [Google Scholar] [CrossRef]
- Mishan, M.A.; Khazeei Tabari, M.A.; Mahrooz, A.; Bagheri, A. Role of microRNAs in the anticancer effects of the flavonoid luteolin: A systematic review. Eur. J. Cancer Prev. 2021, 30, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Franco, Y.E.; de Lima, C.A.; Rosa, M.N.; Silva, V.A.; Reis, R.M.; Priolli, D.G.; Carvalho, P.O.; do Nascimento, J.R.; da Rocha, C.Q.; Longato, G.B. Investigation of U-251 cell death triggered by flavonoid luteolin: Towards a better understanding on its anticancer property against glioblastomas. Nat. Prod. Res. 2021, 35, 4807–4813. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-T.; Liu, Y.-E.; Hsu, K.-W.; Wang, Y.-F.; Chan, Y.-C.; Chen, Y.; Chen, D.-R. MLL3 induced by luteolin causes apoptosis in tamoxifen-resistant breast cancer cells through H3K4 monomethylation and suppression of the PI3K/AKT/mTOR pathway. Am. J. Chin. Med. 2020, 48, 1221–1241. [Google Scholar] [CrossRef]
- Yang, P.-W.; Lu, Z.-Y.; Pan, Q.; Chen, T.-T.; Feng, X.-J.; Wang, S.-M.; Pan, Y.-C.; Zhu, M.-H.; Zhang, S.-H. MicroRNA-6809-5p mediates luteolin-induced anticancer effects against hepatoma by targeting flotillin 1. Phytomedicine 2019, 57, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Khodayari, N.; Oshins, R.; Holliday, L.S.; Clark, V.; Xiao, Q.; Marek, G.; Mehrad, B.; Brantly, M. Alpha-1 antitrypsin deficient individuals have circulating extracellular vesicles with profibrogenic cargo. Cell Commun. Signal. 2020, 18, 1–18. [Google Scholar] [CrossRef]
- Cao, Z.; Zhang, H.; Cai, X.; Fang, W.; Chai, D.; Wen, Y.; Chen, H.; Chu, F.; Zhang, Y. Luteolin promotes cell apoptosis by inducing autophagy in hepatocellular carcinoma. Cell. Physiol. Biochem. 2017, 43, 1803–1812. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.; Wang, J. Modulatory effect of luteolin on redox homeostasis and inflammatory cytokines in a mouse model of liver cancer. Oncol. Lett. 2016, 12, 4767–4772. [Google Scholar] [CrossRef]
- You, Y.; Wang, R.; Shao, N.; Zhi, F.; Yang, Y. Luteolin suppresses tumor proliferation through inducing apoptosis and autophagy via MAPK activation in glioma. OncoTargets Ther. 2019, 12, 2383. [Google Scholar] [CrossRef]
- Imran, M.; Rauf, A.; Abu-Izneid, T.; Nadeem, M.; Shariati, M.A.; Khan, I.A.; Imran, A.; Orhan, I.E.; Rizwan, M.; Atif, M. Luteolin, a flavonoid, as an anticancer agent: A review. Biomed. Pharmacother. 2019, 112, 108612. [Google Scholar] [CrossRef]
- Potočnjak, I.; Šimić, L.; Gobin, I.; Vukelić, I.; Domitrović, R. Antitumor activity of luteolin in human colon cancer SW620 cells is mediated by the ERK/FOXO3a signaling pathway. Toxicol. Vitr. 2020, 66, 104852. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S.; Won, S.B.; Kwon, Y.H. Luteolin induces apoptosis and autophagy in HCT116 colon cancer cells via p53-dependent pathway. Nutr. Cancer 2021, 74, 677–686. [Google Scholar] [CrossRef]
- Huang, L.; Jin, K.; Lan, H. Luteolin inhibits cell cycle progression and induces apoptosis of breast cancer cells through downregulation of human telomerase reverse transcriptase. Oncol. Lett. 2019, 17, 3842–3850. [Google Scholar] [CrossRef] [PubMed]
- Xiang, C.; Wu, X.; Zhao, Z.; Feng, X.; Bai, X.; Liu, X.; Zhao, J.; Takeda, S.; Qing, Y. Nonhomologous end joining and homologous recombination involved in luteolin-induced DNA damage in DT40 cells. Toxicol. Vitr. 2020, 65, 104825. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.-B.; Wang, W.-J.; Xu, C.; Xie, Y.-J.; Wang, X.-R.; Zhang, Y.-Z.; Huang, J.-M.; Huang, M.; Xie, C.; Liu, P. Luteolin and its derivative apigenin suppress the inducible PD-L1 expression to improve anti-tumor immunity in KRAS-mutant lung cancer. Cancer Lett. 2021, 515, 36–48. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Lim, T.; Han, M.S.; Lee, S.-H.; Baek, S.H.; Nan, H.-Y.; Lee, C. Anticancer effect of luteolin is mediated by downregulation of TAM receptor tyrosine kinases, but not interleukin-8, in non-small cell lung cancer cells. Oncol. Rep. 2017, 37, 1219–1226. [Google Scholar] [CrossRef] [PubMed]
- Pu, Y.; Zhang, T.; Wang, J.; Mao, Z.; Duan, B.; Long, Y.; Xue, F.; Liu, D.; Liu, S.; Gao, Z. Luteolin exerts an anticancer effect on gastric cancer cells through multiple signaling pathways and regulating miRNAs. J. Cancer 2018, 9, 3669. [Google Scholar] [CrossRef]
- Seo, Y.; Ryu, K.; Park, J.; Jeon, D.-k.; Jo, S.; Lee, H.K.; Namkung, W. Inhibition of ANO1 by luteolin and its cytotoxicity in human prostate cancer PC-3 cells. PLoS ONE 2017, 12, e0174935. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.; Gao, J.; Guan, L.; Chen, X.; Gao, J.; Wang, K. Inhibition of ANO1/TMEM16A induces apoptosis in human prostate carcinoma cells by activating TNF-α signaling. Cell Death Dis. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.-C.; Hsu, W.-H.; Ho, J.-Y.; Lin, C.-W.; Chu, C.-Y.; Kandaswami, C.C.; Lee, M.-T.; Cheng, C.-H. Flavonoids Luteolin and Quercetin Inhibit RPS19 and contributes to metastasis of cancer cells through c-Myc reduction. J. Food Drug Anal. 2018, 26, 1180–1191. [Google Scholar] [CrossRef]
- Liu, Q.; Hodge, J.; Wang, J.; Wang, Y.; Wang, L.; Singh, U.P.; Li, Y.; Yao, Y.; Wang, D.; Ai, W. Emodin reduces breast cancer lung metastasis by suppressing macrophage-induced breast cancer cell epithelial-mesenchymal transition and cancer stem cell formation. Theranostics 2020, 10, 8365. [Google Scholar] [CrossRef] [PubMed]
- Belcaro, G.; Hosoi, M.; Pellegrini, L.; Appendino, G.; Ippolito, E.; Ricci, A.; Ledda, A.; Dugall, M.; Cesarone, M.R.; Maione, C. A controlled study of a lecithinized delivery system of curcumin (Meriva®) to alleviate the adverse effects of cancer treatment. Phytother. Res. 2014, 28, 444–450. [Google Scholar] [CrossRef] [PubMed]
- Shehzad, A.; Wahid, F.; Lee, Y.S. Curcumin in cancer chemoprevention: Molecular targets, pharmacokinetics, bioavailability, and clinical trials. Arch. Der. Pharm. 2010, 343, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.J.; Mukhtar, H. Curcumin for chemoprevention of colon cancer. Cancer Lett. 2007, 255, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Ledda, A.; Belcaro, G.; Dugall, M.; Luzzi, R.; Scoccianti, M.; Togni, S.; Appendino, G.; Ciammaichella, G. Meriva®, a lecithinized curcumin delivery system, in the control of benign prostatic hyperplasia: A pilot, product evaluation registry study. Panminerva Med. 2012, 54, 17. [Google Scholar] [PubMed]
- Bayet-Robert, M.; Kwiatowski, F.; Leheurteur, M.; Gachon, F.; Planchat, E.; Abrial, C.; Mouret-Reynier, M.-A.; Durando, X.; Barthomeuf, C.; Chollet, P. Phase I dose escalation trial of docetaxel plus curcumin in patients with advanced and metastatic breast cancer. Cancer Biol. Ther. 2010, 9, 8–14. [Google Scholar] [CrossRef]
- Cruz–Correa, M.; Shoskes, D.A.; Sanchez, P.; Zhao, R.; Hylind, L.M.; Wexner, S.D.; Giardiello, F.M. Combination treatment with curcumin and quercetin of adenomas in familial adenomatous polyposis. Clin. Gastroenterol. Hepatol. 2006, 4, 1035–1038. [Google Scholar] [CrossRef]
- Dhillon, N.; Aggarwal, B.B.; Newman, R.A.; Wolff, R.A.; Kunnumakkara, A.B.; Abbruzzese, J.L.; Ng, C.S.; Badmaev, V.; Kurzrock, R. Phase II trial of curcumin in patients with advanced pancreatic cancer. Clin. Cancer Res. 2008, 14, 4491–4499. [Google Scholar] [CrossRef]
- Saghatelyan, T.; Tananyan, A.; Janoyan, N.; Tadevosyan, A.; Petrosyan, H.; Hovhannisyan, A.; Hayrapetyan, L.; Arustamyan, M.; Arnhold, J.; Rotmann, A.-R. Efficacy and safety of curcumin in combination with paclitaxel in patients with advanced, metastatic breast cancer: A comparative, randomized, double-blind, placebo-controlled clinical trial. Phytomedicine 2020, 70, 153218. [Google Scholar] [CrossRef] [PubMed]
- Bachmeier, B.E.; Blaheta, R. Curcumin 3.0—Therapeutic and Diagnostic Potential in Cancer and Beyond. Int. J. Mol. Sci. 2022, 23, 5398. [Google Scholar] [CrossRef] [PubMed]
- Thambamroong, T.; Seetalarom, K.; Saichaemchan, S.; Pumsutas, Y.; Prasongsook, N. Efficacy of Curcumin on Treating Cancer Anorexia-Cachexia Syndrome in Locally or Advanced Head and Neck Cancer: A Double-Blind, Placebo-Controlled Randomised Phase IIa Trial (CurChexia). J. Nutr. Metab. 2022, 2022, 5425619. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.P.; Singh, R.; Verma, S.S.; Rai, V.; Kaschula, C.H.; Maiti, P.; Gupta, S.C. Health benefits of resveratrol: Evidence from clinical studies. Med. Res. Rev. 2019, 39, 1851–1891. [Google Scholar] [CrossRef] [PubMed]
- Brown, V.A.; Patel, K.R.; Viskaduraki, M.; Crowell, J.A.; Perloff, M.; Booth, T.D.; Vasilinin, G.; Sen, A.; Schinas, A.M.; Piccirilli, G. Repeat dose study of the cancer chemopreventive agent resveratrol in healthy volunteers: Safety, pharmacokinetics, and effect on the insulin-like growth factor axis. Cancer Res. 2010, 70, 9003–9011. [Google Scholar] [CrossRef]
- Zhu, W.; Qin, W.; Zhang, K.; Rottinghaus, G.E.; Chen, Y.-C.; Kliethermes, B.; Sauter, E.R. Trans-resveratrol alters mammary promoter hypermethylation in women at increased risk for breast cancer. Nutr. Cancer 2012, 64, 393–400. [Google Scholar] [CrossRef]
- Nguyen, A.V.; Martinez, M.; Stamos, M.J.; Moyer, M.P.; Planutis, K.; Hope, C.; Holcombe, R.F. Results of a phase I pilot clinical trial examining the effect of plant-derived resveratrol and grape powder on Wnt pathway target gene expression in colonic mucosa and colon cancer. Cancer Manag. Res. 2009, 1, 25. [Google Scholar]
- Shen, W.-W.; Zeng, Z.; Zhu, W.-X.; Fu, G.-H. MiR-142-3p functions as a tumor suppressor by targeting CD133, ABCG2, and Lgr5 in colon cancer cells. J. Mol. Med. 2013, 91, 989–1000. [Google Scholar] [CrossRef]
- Howells, L.M.; Berry, D.P.; Elliott, P.J.; Jacobson, E.W.; Hoffmann, E.; Hegarty, B.; Brown, K.; Steward, W.; Gescher, A.J. Phase I randomized, double-blind pilot study of micronized resveratrol (SRT501) in patients with hepatic metastases—safety, pharmacokinetics, and pharmacodynamics. Cancer Prev. Res. 2011, 4, 1419–1425. [Google Scholar] [CrossRef]
- Moutabian, H.; Majdaeen, M.; Ghahramani-Asl, R.; Yadollahi, M.; Gharepapagh, E.; Ataei, G.; Falahatpour, Z.; Bagheri, H.; Farhood, B. A systematic review of the therapeutic effects of resveratrol in combination with 5-fluorouracil during colorectal cancer treatment: With a special focus on the oxidant, apoptotic, and anti-inflammatory activities. Cancer Cell Int. 2022, 22, 1–14. [Google Scholar] [CrossRef]
- Jurczyk, M.; Kasperczyk, J.; Wrześniok, D.; Beberok, A.; Jelonek, K. Nanoparticles Loaded with Docetaxel and Resveratrol as an Advanced Tool for Cancer Therapy. Biomedicines 2022, 10, 1187. [Google Scholar] [CrossRef]
- Ferry, D.R.; Smith, A.; Malkhandi, J.; Fyfe, D.W.; deTakats, P.G.; Anderson, D.; Baker, J.; Kerr, D.J. Phase I clinical trial of the flavonoid quercetin: Pharmacokinetics and evidence for in vivo tyrosine kinase inhibition. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 1996, 2, 659–668. [Google Scholar]
- Sun, C.-L.; Yuan, J.-M.; Lee, M.-J.; Yang, C.S.; Gao, Y.-T.; Ross, R.K.; Yu, M.C. Urinary tea polyphenols in relation to gastric and esophageal cancers: A prospective study of men in Shanghai, China. Carcinogenesis 2002, 23, 1497–1503. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Freedman, N.; Kamangar, F.; Dawsey, S.; Hollenbeck, A.; Schatzkin, A.; Abnet, C. Tea, coffee, carbonated soft drinks and upper gastrointestinal tract cancer risk in a large United States prospective cohort study. Eur. J. Cancer 2010, 46, 1873–1881. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Sun, Z.; Han, C.; Chen, J. The chemopreventive effects of tea on human oral precancerous mucosa lesions. Proc. Soc. Exp. Biol. Med. 1999, 220, 218–224. [Google Scholar] [PubMed]
- Inoue, M.; Sasazuki, S.; Wakai, K.; Suzuki, T.; Matsuo, K.; Shimazu, T.; Tsuji, I.; Tanaka, K.; Mizoue, T.; Nagata, C. Green tea consumption and gastric cancer in Japanese: A pooled analysis of six cohort studies. Gut 2009, 58, 1323–1332. [Google Scholar] [CrossRef]
- Lee, K.J.; Inoue, M.; Otani, T.; Iwasaki, M.; Sasazuki, S.; Tsugane, S.; Group, J.S. Coffee consumption and risk of colorectal cancer in a population-based prospective cohort of Japanese men and women. Int. J. Cancer 2007, 121, 1312–1318. [Google Scholar] [CrossRef]
- Zhang, G.; Wang, Y.; Zhang, Y.; Wan, X.; Li, J.; Liu, K.; Wang, F.; Liu, Q.; Yang, C.; Yu, P. Anti-cancer activities of tea epigallocatechin-3-gallate in breast cancer patients under radiotherapy. Curr. Mol. Med. 2012, 12, 163–176. [Google Scholar] [CrossRef]
- Ogunleye, A.A.; Xue, F.; Michels, K.B. Green tea consumption and breast cancer risk or recurrence: A meta-analysis. Breast Cancer Res. Treat. 2010, 119, 477–484. [Google Scholar] [CrossRef]
- Jatoi, A.; Ellison, N.; Burch, P.A.; Sloan, J.A.; Dakhil, S.R.; Novotny, P.; Tan, W.; Fitch, T.R.; Rowland, K.M.; Young, C.Y. A phase II trial of green tea in the treatment of patients with androgen independent metastatic prostate carcinoma. Cancer Interdiscip. Int. J. Am. Cancer Soc. 2003, 97, 1442–1446. [Google Scholar] [CrossRef]
- Choan, E.; Segal, R.; Jonker, D.; Malone, S.; Reaume, N.; Eapen, L.; Gallant, V. A prospective clinical trial of green tea for hormone refractory prostate cancer: An evaluation of the complementary/alternative therapy approach. In Urologic Oncology: Seminars and Original Investigations; Elsevier: Amsterdam, The Netherlands, 2005; pp. 108–113. [Google Scholar]
- McGlynn, K.A.; Petrick, J.L.; El-Serag, H.B. Epidemiology of hepatocellular carcinoma. Hepatology 2021, 73, 4–13. [Google Scholar] [CrossRef]
- Ji, B.T.; Chow, W.H.; Hsing, A.W.; McLaughlin, J.K.; Dai, Q.; Gao, Y.T.; Blot, W.J.; Fraumeni Jr, J.F. Green tea consumption and the risk of pancreatic and colorectal cancers. Int. J. Cancer 1997, 70, 255–258. [Google Scholar] [CrossRef]
- Alam, M.; Ali, S.; Ashraf, G.M.; Bilgrami, A.L.; Yadav, D.K.; Hassan, M.I. Epigallocatechin 3-gallate: From green tea to cancer therapeutics. Food Chem. 2022, 132135. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Wu, Y.; Zhou, B.; Wang, B.; Yu, R. Green tea, black tea consumption and risk of lung cancer: A meta-analysis. Lung Cancer 2009, 65, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Haruma, K.; Kunihiro, M.; Nagata, S.; Kitadai, Y.; Manabe, N.; Sumii, M.; Yoshihara, M.; Kajiyama, G.; Chayama, K. Effects of aged garlic extract (AGE) on colorectal adenomas: A double-blinded study. Hiroshima J. Med. Sci. 2004, 53, 39–45. [Google Scholar] [PubMed]
- Miraghajani, M.; Rafie, N.; Hajianfar, H.; Larijani, B.; Azadbakht, L. Aged garlic and cancer: A systematic review. Int. J. Prev. Med. 2018, 9, 84. [Google Scholar]
- Tanaka, S.; Haruma, K.; Yoshihara, M.; Kajiyama, G.; Kira, K.; Amagase, H.; Chayama, K. Aged garlic extract has potential suppressive effect on colorectal adenomas in humans. J. Nutr. 2006, 136, 821S–826S. [Google Scholar] [CrossRef]
- Ishikawa, H.; Saeki, T.; Otani, T.; Suzuki, T.; Shimozuma, K.; Nishino, H.; Fukuda, S.; Morimoto, K. Aged garlic extract prevents a decline of NK cell number and activity in patients with advanced cancer. J. Nutr. 2006, 136, 816S–820S. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Li, H.-Q.; Wang, Y.; Xu, H.-X.; Fan, W.-T.; Wang, M.-L.; Sun, P.-H.; Xie, X.-Y. An intervention study to prevent gastric cancer by micro-selenium and large dose of allitridum. Chin. Med. J. 2004, 117, 1155–1160. [Google Scholar]
- Zhou, Y.; Zhuang, W.; Hu, W.; Liu, G.J.; Wu, T.X.; Wu, X.T. Consumption of large amounts of Allium vegetables reduces risk for gastric cancer in a meta-analysis. Gastroenterology 2011, 141, 80–89. [Google Scholar] [CrossRef]
- Nicastro, H.L.; Ross, S.A.; Milner, J.A. Garlic and Onions: Their Cancer Prevention PropertiesGarlic and Onions: Their Cancer Prevention Properties. Cancer Prev. Res. 2015, 8, 181–189. [Google Scholar] [CrossRef]
- Chen, Y.-K.; Lee, C.-H.; Wu, I.-C.; Liu, J.-S.; Wu, D.-C.; Lee, J.-M.; Goan, Y.-G.; Chou, S.-H.; Huang, C.-T.; Lee, C.-Y. Food intake and the occurrence of squamous cell carcinoma in different sections of the esophagus in Taiwanese men. Nutrition 2009, 25, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Hsing, A.W.; Chokkalingam, A.P.; Gao, Y.-T.; Madigan, M.P.; Deng, J.; Gridley, G.; Fraumeni Jr, J.F. Allium vegetables and risk of prostate cancer: A population-based study. J. Natl. Cancer Inst. 2002, 94, 1648–1651. [Google Scholar] [CrossRef]
- Chen, M.; Rao, Y.; Zheng, Y.; Wei, S.; Li, Y.; Guo, T.; Yin, P. Association between soy isoflavone intake and breast cancer risk for pre-and post-menopausal women: A meta-analysis of epidemiological studies. PLoS ONE 2014, 9, e89288. [Google Scholar]
- Trock, B.J.; Hilakivi-Clarke, L.; Clarke, R. Meta-analysis of soy intake and breast cancer risk. J. Natl. Cancer Inst. 2006, 98, 459–471. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Gong, P.; Madak-Erdogan, Z.; Martin, T.; Jeyakumar, M.; Carlson, K.; Khan, I.; Smillie, T.J.; Chittiboyina, A.G.; Rotte, S.C. Mechanisms enforcing the estrogen receptor β selectivity of botanical estrogens. FASEB J. 2013, 27, 4406–4418. [Google Scholar] [CrossRef] [PubMed]
- Lazarevic, B.; Boezelijn, G.; Diep, L.M.; Kvernrod, K.; Ogren, O.; Ramberg, H.; Moen, A.; Wessel, N.; Berg, R.E.; Egge-Jacobsen, W. Efficacy and safety of short-term genistein intervention in patients with localized prostate cancer prior to radical prostatectomy: A randomized, placebo-controlled, double-blind Phase 2 clinical trial. Nutr. Cancer 2011, 63, 889–898. [Google Scholar] [CrossRef]
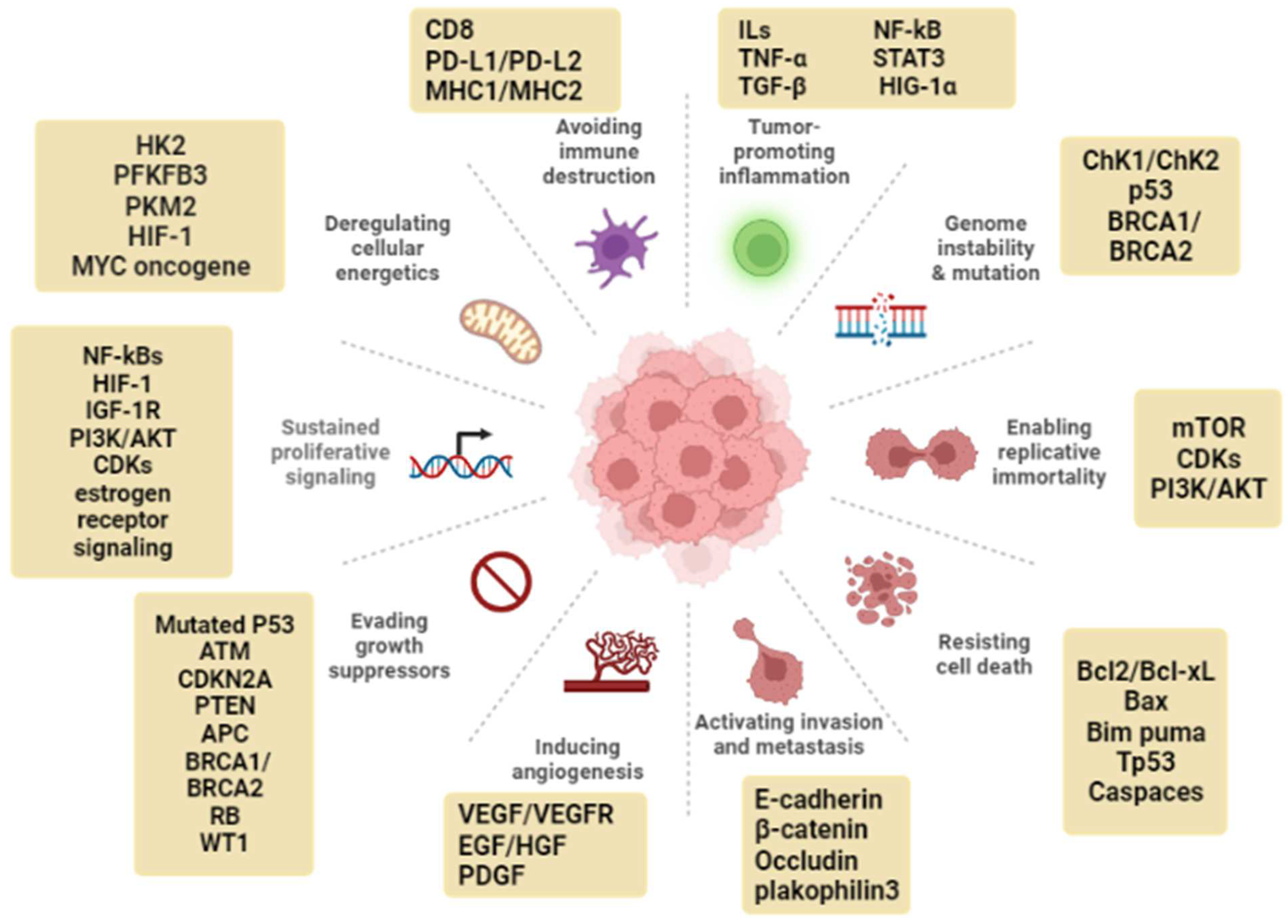
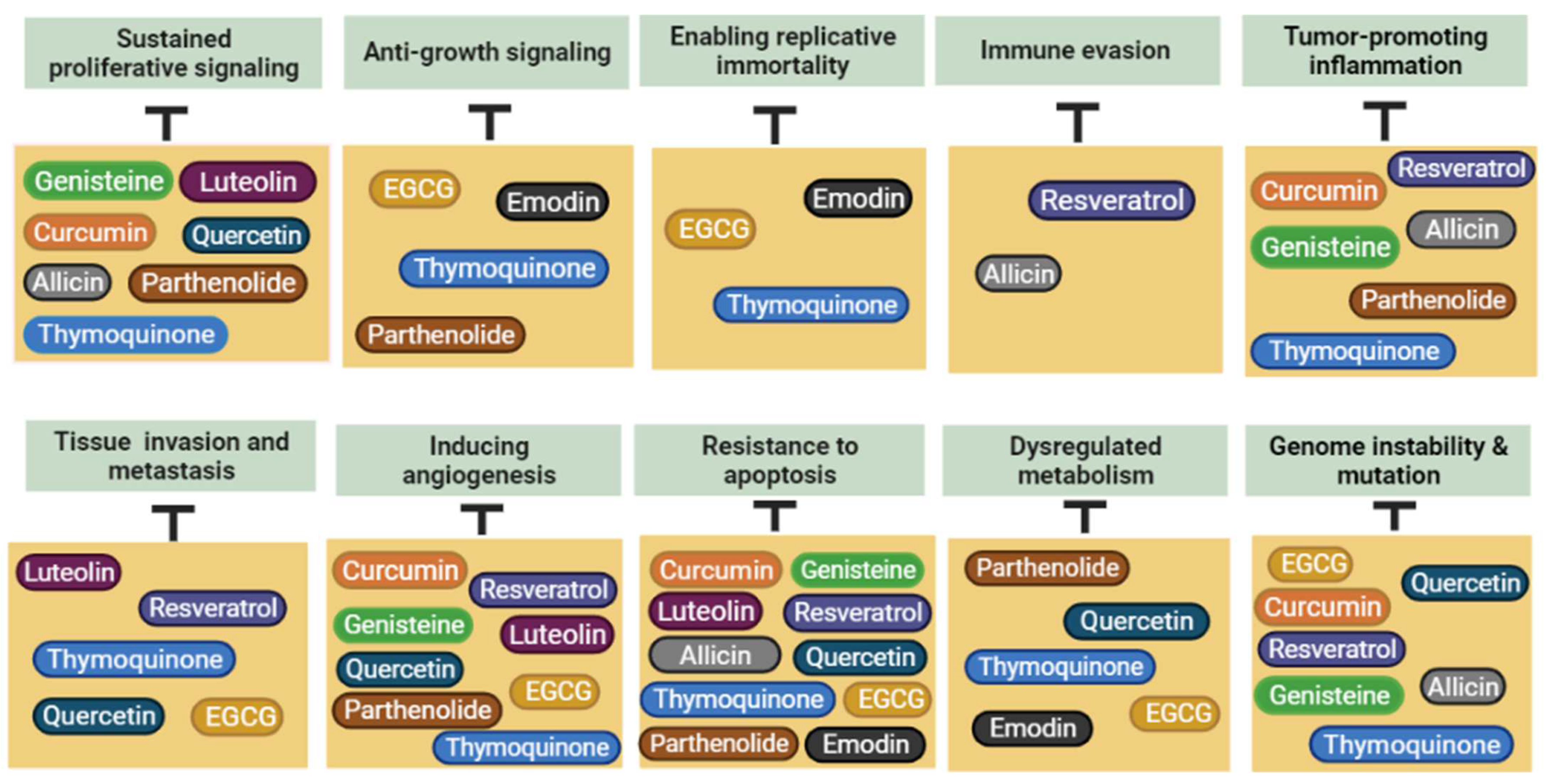
| Compounds | Natural Sources | Mechanisms of Anticancer Activity | References |
|---|---|---|---|
Curcumin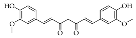 | Found in the rhizome of Curcuma longa and in others Curcuma multiple species | ↓ ROS ↑ Death receptor 5 ↑ caspase-3 and caspase-8 ↓ Bcl-2, NF-kB, EGFR ↑ JNK/ERK/AP1 pathway ↓ PKM2, Lactate production, glucose uptake ↓ miR-181b, miR-203, miR-9, miR-19, miR-21, miR203, miR-9, and miR-208 ↑ activity of chemotherapy (docetaxel, cisplatin, doxorubicin, vincristine) ↓ chemo-drug resistance | [87,88,89,90,92,93,97,102,103,104,105,107] |
Resveratrol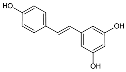 | Found in at least 72 plant species, such as mulberries, peanuts, cranberries, blueberries and grapes | ↑ SOD, CAT, GS-R-, GPx, GST ↑ chemo-sensitivity of temozolomide, cisplatin, doxorubicin, 5-fluorouracil, gemcitabine, docetaxel, and paclitaxel ↓ EFG, ERK, VEGF ↓ TNF-α, NF-kB, p65 ↑ caspase-3, caspase-9, Bcl-2 associated X protein, p53 ↓ AMPK/mTOR signaling pathway ↓ PI3K/Akt/NF-κB signaling pathway ↓ glioma-associated oncogene homolog 1 ↑ expression of activating receptors on natural killer (NK) cells | [118,120,122,125,126,127,128,130,131] |
Quercetin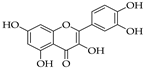 | Found in many foods such as capers, apples, berries, Brassica vegetables, grapes, pepper, asparagus, onions, broccoli, shallots, cherries, tea, and tomatoes | ↓ ROS ↑ caspase-3, caspase-9 ↓ PI3K/AKT/mTOR and STAT3 pathways ↓ c-FLIP, cyclin D1, and c-Myc ↓ P38MAPK, cyclin D 1, P21 ↓ VEGF, AKT, IGFIR, AR, PTHR1 ↑ c-Jun N-terminal kinase, ERK1/2, P38, and P90RSK ↓ S6, AKT, and P70S6K ↓ Hsp90 levels ↑ E-, N-cadherin, β-catenin, and snail ↓ MMPs | [145,146,147,148,149,151,153,154,156,157,158,159,160,161,164] |
EGCG | Found in cocoa-based products, nuts, some fruits, but green tea (Camellia sinensis Theaceae) is still considered as the main source of this product | ↑ caspase-3, caspase-9, PARP-1, Bax ↓ NF-κB, IL1β, IL-6, IL-8, TNF-α ↓ COX-2, iNOS, ↓ ABCG2, Bcl-2 ↓ ERK, EGFR, MAPK ↓ PI3K/Akt/mTOR pathway ↓ MMP-2, MMP-9 ↓ STAT3, AP-1 ↓ VEGF, HIF-1α ↑ P53, KDM2A | [174,175,176,177,178,179,180,181,182,183,184,187,189,190,192,194,195] |
Allicin | Extracted from garlic (Allium sativum L.) and other Allium species such as onion (Allium cepa L.) and shallot (Allium ascalonicum L.) | ↓ Akt/mTOR signaling pathway ↑ caspase-3, p53 ↓ VCAM-1 ↓ NRF2 ↑ Bcl-2/Bax mitochondrial pathway, MAPK/ERK signaling pathway ↓ PI3K/AKT signaling pathway ↓ chemo-drug resistance ↓ MDR1, MDR1, CD44, DKK1, WNT5A | [62,64,201,202,204,205,207,209,210] |
Thymoquinone | Obtained from the essential oil of black seed Nigella sativa L. | ↓ NF-κB, cell proliferation, hypoxia ↓ cyclin E, cyclin D ↑ p27 and p21, miR-125a-5p ↑ p21cip1/waf1 ↑ G1 phase cell cycle arrest ↓ TNF-α, IL-6, iNOS, COX-2 ↓ EGFR, JAK2, ↓ Jak2/STAT3 signaling pathway ↓ Bcl-2, Bcl-xL, survivin ↑ chemotherapy sensitivity ↓ STAT3 ↑ caspases 8, 9, 7, PPAR-γ ↓ CYP3A2 and CYP2C 11, CYP 3A4 enzymes ↑ PTEN mRNA, p53 ↓ androgen receptor expression and E2F-1 t ↓ miR-877-5p/PD-L1 ↓ Integrin-β1, VEGF, MMP-2 and MMP-9 | [217,220,221,222,223,224,225,228,229,230,231,232,233,234,235,236,238,239,240,244,245,246,248,249,250] |
Emodin | Isolated from the roots and rhizomes of several plants such as Rheum palmatum, Polygonum cuspidatum, Polygonum multiflorum, Aloe vera, and Cassia obtusifolia, as well as different fungal species, including Aspergillus ochraceus and Aspergillus wentii | ↓ HER2/neu, CKII kinase, CKII kinase ↓ ERK, VEGFA ↓ TGF-β ↓ Aurora kinase A ↓ survivin, β-catenin ↓ DNA repair ↓ casein kinase II ↓ GLUT1, PI3K/AKT signaling pathway ↑ HIF-1, intracellular SOD ↓ MDR-1, NF-κB, Bcl-2, XIAP ↑ Bax, cytochrome-C, caspase-9 and -3 ↓ MRP1 ↑ chemo-sensitivity | [256,260,261,262,263,264,265,266,269,178] |
Genistein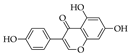 | Isolated from Genista tinctoria and the main secondary metabolite of the Trifolium species and in Glycine max (soybean) | ↓ histone deacetylase ↓ Hsp90 chaperones ↓ COX-2, NF-kB, AP-1 ↑ p21, caspase-3, p38MAPK ↓ cyclin D1 ↑ G2/M cell cycle arrest ↓ MMP-2, VEGF, Bcl-2, uPA, Bcl-xL ↓ AKT, IL-6/STAT3 pathway ↓ DNA-PKcs/Akt2/Rac1 pathway ↑ chemo-sensitivity | [272,282,290,291,296,297] |
Parthenolide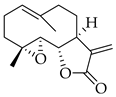 | Extracted from leaves of the medicinal plant feverfew (Tanacetum parthenium) | ↓ NF-Kb ↑ G0/G1 cell cycle arrest ↓ USP47 ↓ insulin-like growth factor 1 receptor, AKT, forkhead box O3α ↓ MAPK/Erk signaling pathway ↓ oncogenic characteristics ↓ PI3K/AKT/mTOR signal pathway ↓ FAK1-dependent signaling pathways ↑ E-cadherin protein ↓ TGFβ protein, TWIST1 gene ↓ STAT3 | [303,306,310,313,314,315,316,317,319,320,322] |
Luteolin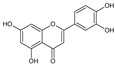 | Found in flowers (Reseda luteola and Chrysanthemums), herbs (parsley, peppermint, oregano, and thyme), vegetables (celery seeds, onion leaves, cabbages, sweet bell peppers, carrots, and broccoli), and spices (cardamom and anise) | ↓ DNA methyltransferases, some classical histone deacetylases, SIRT1 ↑ caspase-7, 8, 9 ↓ glutathione, IL-2, IFN-γ ↑ miR-124-3p, death receptor, MAPK ↑ caspase-3, Bax ↓ Bcl-2 ↑ chemo-sensitivity (5-FU) ↑ DNA double-strand breaks ↓ STAT3 ↓ TAM receptor tyrosine kinases ↑ Axl ↓ cyclin E, cyclin D ↓ Anoctamin 1 (ANO1) chloride channel activity ↓ Akt/mTOR/c-Myc signaling pathway | [331,336,340,341,343,346,347,348,349,350,352,353] |
| Natural Product | Experimental Design | Dosage | Comments | Reference |
|---|---|---|---|---|
| Curcumin | 160 patients with solid tumor were given Meriva as an adjuvant to chemotherapy. | 1500 mg/day of Meriva in three separate doses for six weeks. | Patients’ quality of life was improved, and systemic inflammation was significantly reduced. | [354] |
| Patients with colorectal cancer were orally provided curcuma extract. | Up to 2.2 g daily (equal to 180 mg of curcumin) for several months. | Curcumin was proven to accumulate at the colorectum and acquire the effective therapeutic concentration. | [356] | |
| 33 patients with benign prostatic hyperplasia were given Meriva. | 1000 mg/day in two divided doses) for 2 years. | Improvements in all categories of the International Prostate Symptom Score. | [357] | |
| Patients with familial adenomatous polyposis were given a combination of curcumin and quercetin. | 480 mg curcumin and 20 mg quercetin orally three times daily for six months. | The quantity and size of malignant polyps were dramatically reduced. | [359] | |
| 25 patients with advanced pancreatic cancer were given curcumin capsules. | 8 g of curcumin capsules daily, with restaging every eight weeks. | Oral curcumin had biological action and was safe in some pancreatic cancer patients. | [360] | |
| Resveratrol | 39 women with a high risk of breast cancer. | For 12 weeks, the participants were given two capsules per day containing either placebo, 5 mg of trans-resveratrol, or 50 mg of trans- resveratrol. | PGE2 levels were discovered to be reduced. | [365] |
| Patients with colon cancer were given low-dose resveratrol and resveratrol-containing freeze-dried grape powder. | Low-dose resveratrol (80 mg/d) and resveratrol-containing freeze-dried grape powder (80 g/day for 14 days of treatment). | There was an increase in the expression of Myc and cyclin D1 in colon cancer tissue. | [367] | |
| Micronized resveratrol (SRT501) was given to individuals with colorectal cancer and hepatic metastases. | Micronized resveratrol (SRT501) was given at a dose of 5 g/day for two weeks. | SRT501 was well tolerated and increased mean plasma resveratrol levels (3.6-fold) after a single dosage compared to non-micronized resveratrol. | [369] | |
| EGCG | 481,563 volunteers aged 51–71 years were given hot tea. | 1 cup/day or more of hot tea drinking for up to eight years of follow-up. | This study revealed a statistically significant inverse connection between hot tea drinking and risk of pharyngeal cancer. | [374] |
| 59 patients with oral mucosa leukoplakia were given green tea extract. | 3 g mixed tea oral administration and topical treatment for six months. | 37.9% of patients who received green tea treatment had smaller oral lesions. | [375] | |
| Assessment the relationship between green tea consumption and colorectal cancer risk on 69,710 Chinese women aged 40 to 70 years. | 2–3 cups/day of green tea for up to 3 years of follow-up. | The study indicated that drinking tea on a regular basis considerably lowered the incidence of colorectal cancer. | [377] | |
| 10 female patients (38–55 years old) with locally advanced noninflammatory breast cancer undergoing radiation were given EGCG capsules. | EGCG capsules (400 mg) were orally provided three times daily for two to eight weeks. | EGCG was discovered to increase the efficacy of radiotherapy in breast cancer patients, raising the possibility of EGCG being used as a therapeutic adjuvant in the treatment of metastatic breast cancer. | [378] | |
| 42 patients with androgen-independent prostate cancer were given green tea. | 6 g/day of green tea were provided orally in 6 divided doses for 2 months. | One of the patients demonstrated a 50% decrease in prostate-specific antigen (PSA) level. | [380] | |
| Patients with prostate cancer were prescribed green tea extract capsules. | Green tea extract capsules were given at a dose level of 250 mg twice daily for 2 months. | 40% of patients who finished the treatment showed delayed disease progression. | [381] | |
| 451 patients with pancreatic cancer were given green tea. | 200 g/month of green tea were provided for 3 years. | The study lowered the risk of pancreatic cancer. | [383] | |
| Allicin | 51 patients with colorectal adenomas were given aged garlic extract. | 2.4 mL/d of aged garlic extract for 12 months. | Aged garlic extract was related with a significantly lower risk of developing new colorectal adenomas. | [388] |
| 2526 persons with family history of stomach cancer were given allitridum and selenium. | 200 mg synthetic allitridum every day and 100 microg selenium every other day for 2 years. | High dosages of allitridum and microdoses of selenium have been found to prevent stomach cancer, particularly in men. | [390] | |
| 343 patients with esophageal squamous cell carcinoma and 755 cancer-free controls ingested raw garlic/onions. | Raw garlic/onions were given at least once per week for 10 years. | Raw onions/garlic were significantly protective against esophageal squamous cell carcinoma. | [393] | |
| Genistein | 9000 breast cancer patients were given soy food. | 19.1 mg/day of soy food was given for up to 10 years. | Study found that increasing the genistein dose reduced breast cancer risk. | [396] |
| 23 prostate cancer patients received genistein before radical prostatectomy. | 30 mg synthetic genistein was given daily for up to 6 weeks. | The level of the prostate cancer biomarker prostate-specific antigen in blood was reduced. | [398] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talib, W.H.; Daoud, S.; Mahmod, A.I.; Hamed, R.A.; Awajan, D.; Abuarab, S.F.; Odeh, L.H.; Khater, S.; Al Kury, L.T. Plants as a Source of Anticancer Agents: From Bench to Bedside. Molecules 2022, 27, 4818. https://doi.org/10.3390/molecules27154818
Talib WH, Daoud S, Mahmod AI, Hamed RA, Awajan D, Abuarab SF, Odeh LH, Khater S, Al Kury LT. Plants as a Source of Anticancer Agents: From Bench to Bedside. Molecules. 2022; 27(15):4818. https://doi.org/10.3390/molecules27154818
Chicago/Turabian StyleTalib, Wamidh H., Safa Daoud, Asma Ismail Mahmod, Reem Ali Hamed, Dima Awajan, Sara Feras Abuarab, Lena Hisham Odeh, Samar Khater, and Lina T. Al Kury. 2022. "Plants as a Source of Anticancer Agents: From Bench to Bedside" Molecules 27, no. 15: 4818. https://doi.org/10.3390/molecules27154818
APA StyleTalib, W. H., Daoud, S., Mahmod, A. I., Hamed, R. A., Awajan, D., Abuarab, S. F., Odeh, L. H., Khater, S., & Al Kury, L. T. (2022). Plants as a Source of Anticancer Agents: From Bench to Bedside. Molecules, 27(15), 4818. https://doi.org/10.3390/molecules27154818







