Model Systems for Evidencing the Mediator Role of Riboflavin in the UVA Cross-Linking Treatment of Keratoconus
Abstract
:1. Introduction
2. Results and Discussion
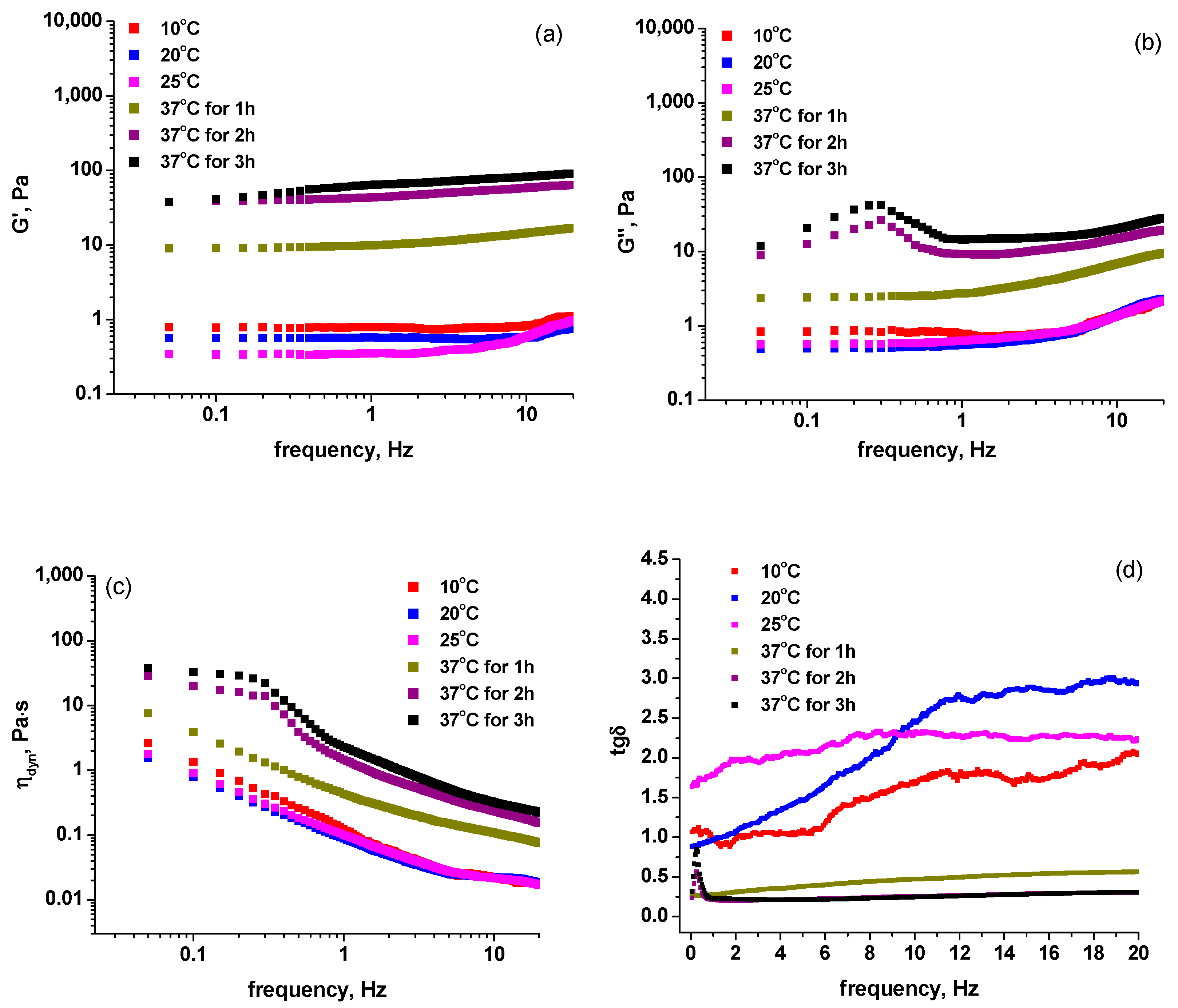
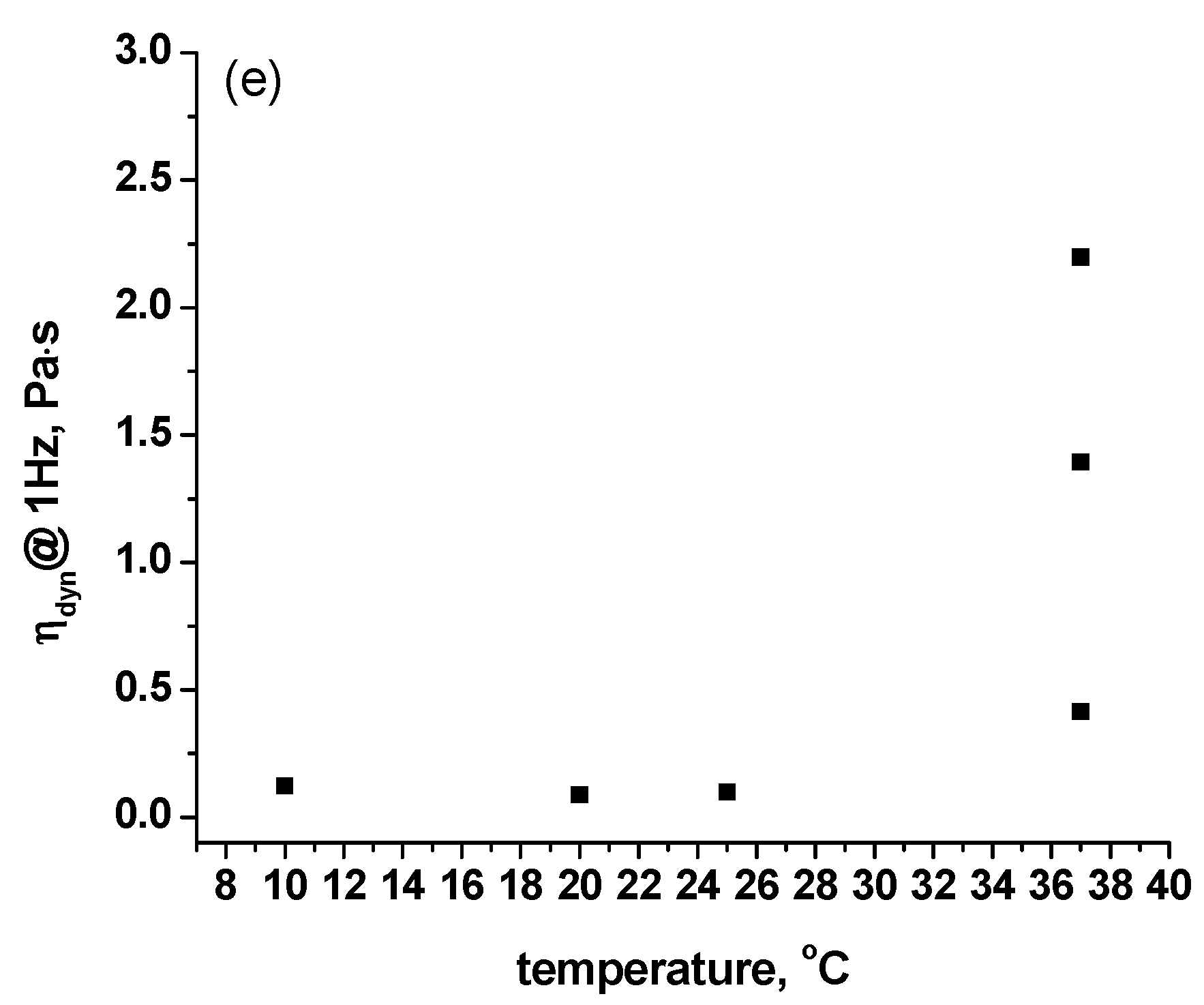
2.1. Rheological Measurements
2.2. STEM Images
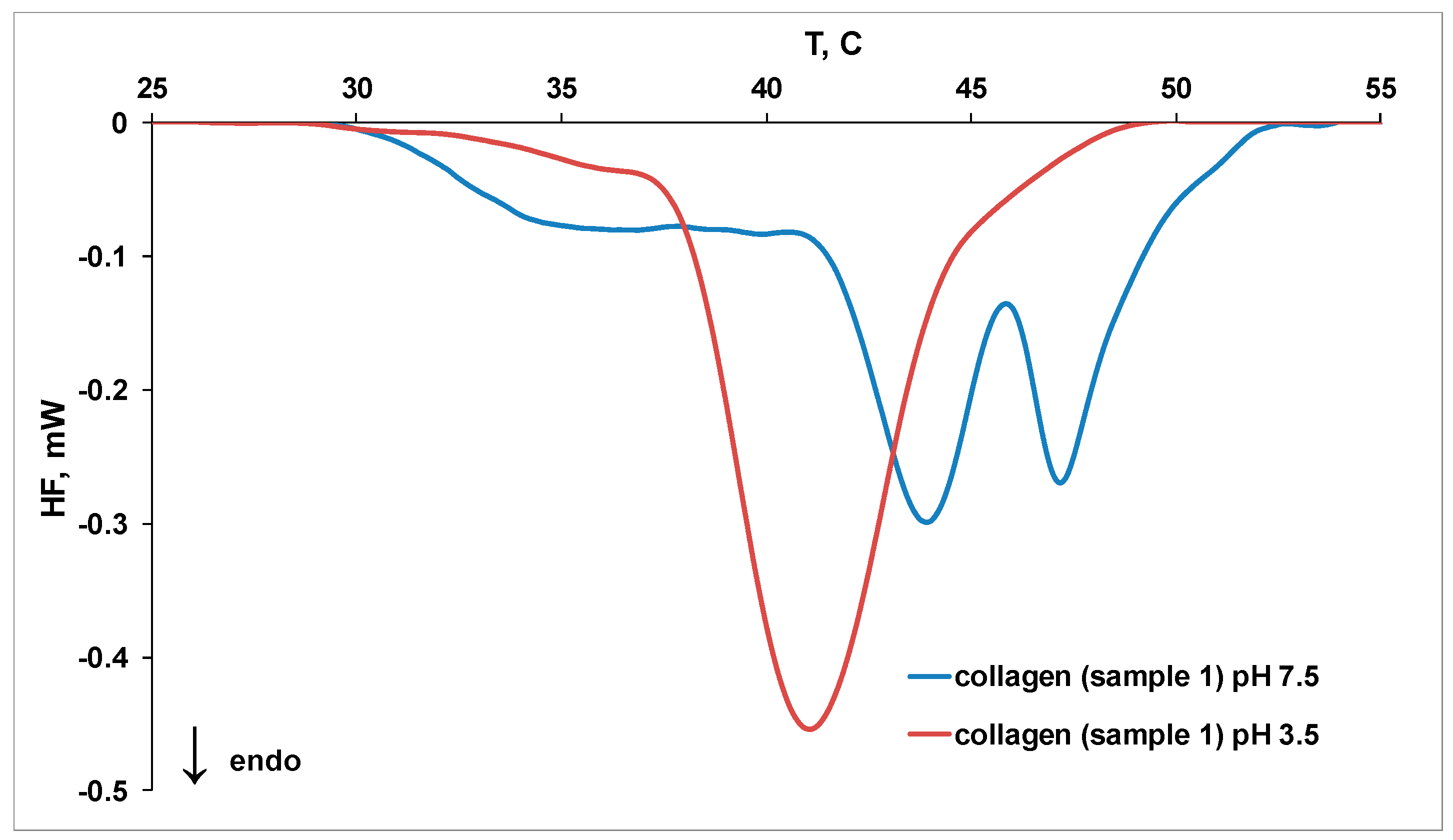
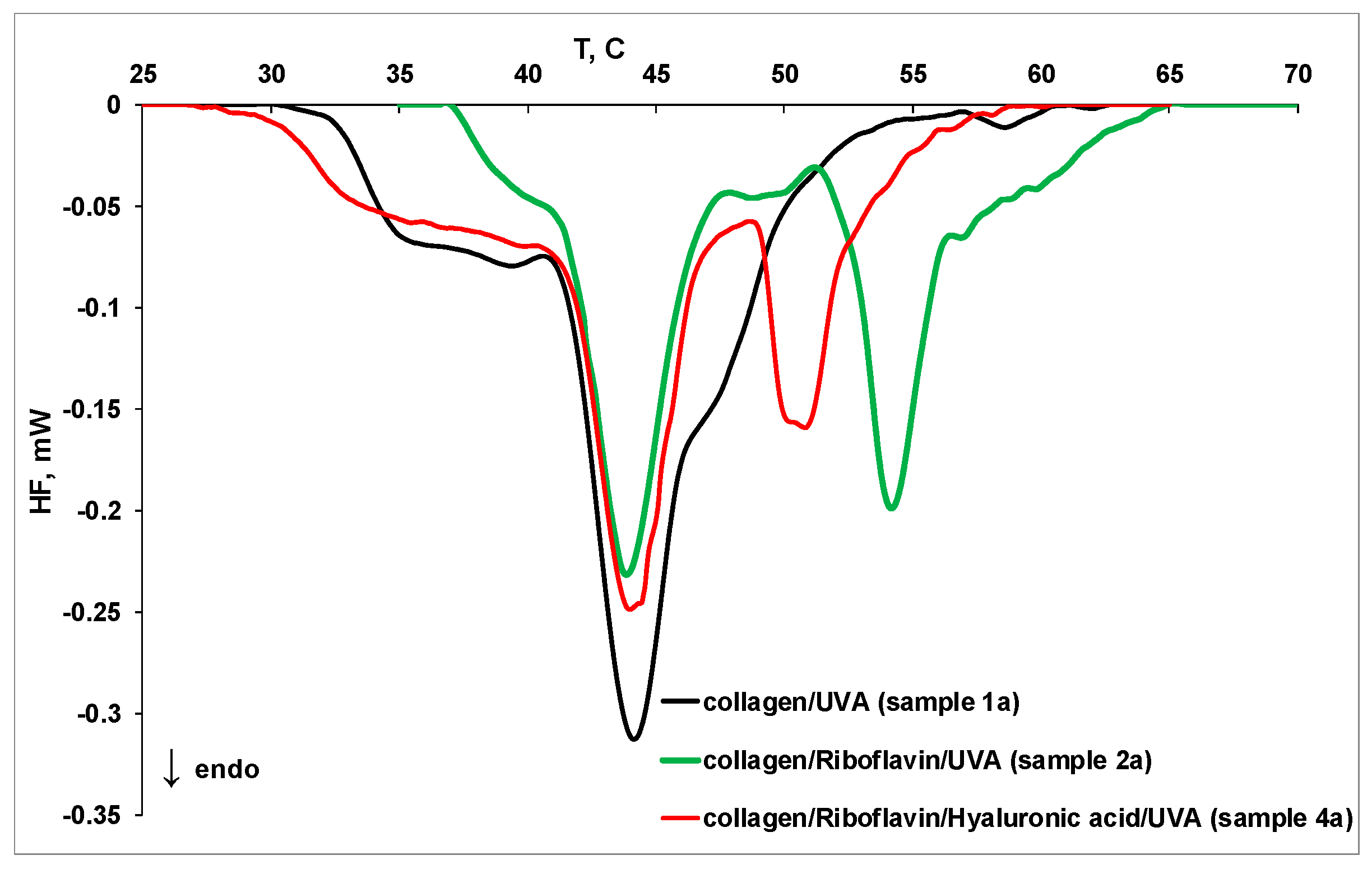
2.3. MicroDSC Measurements
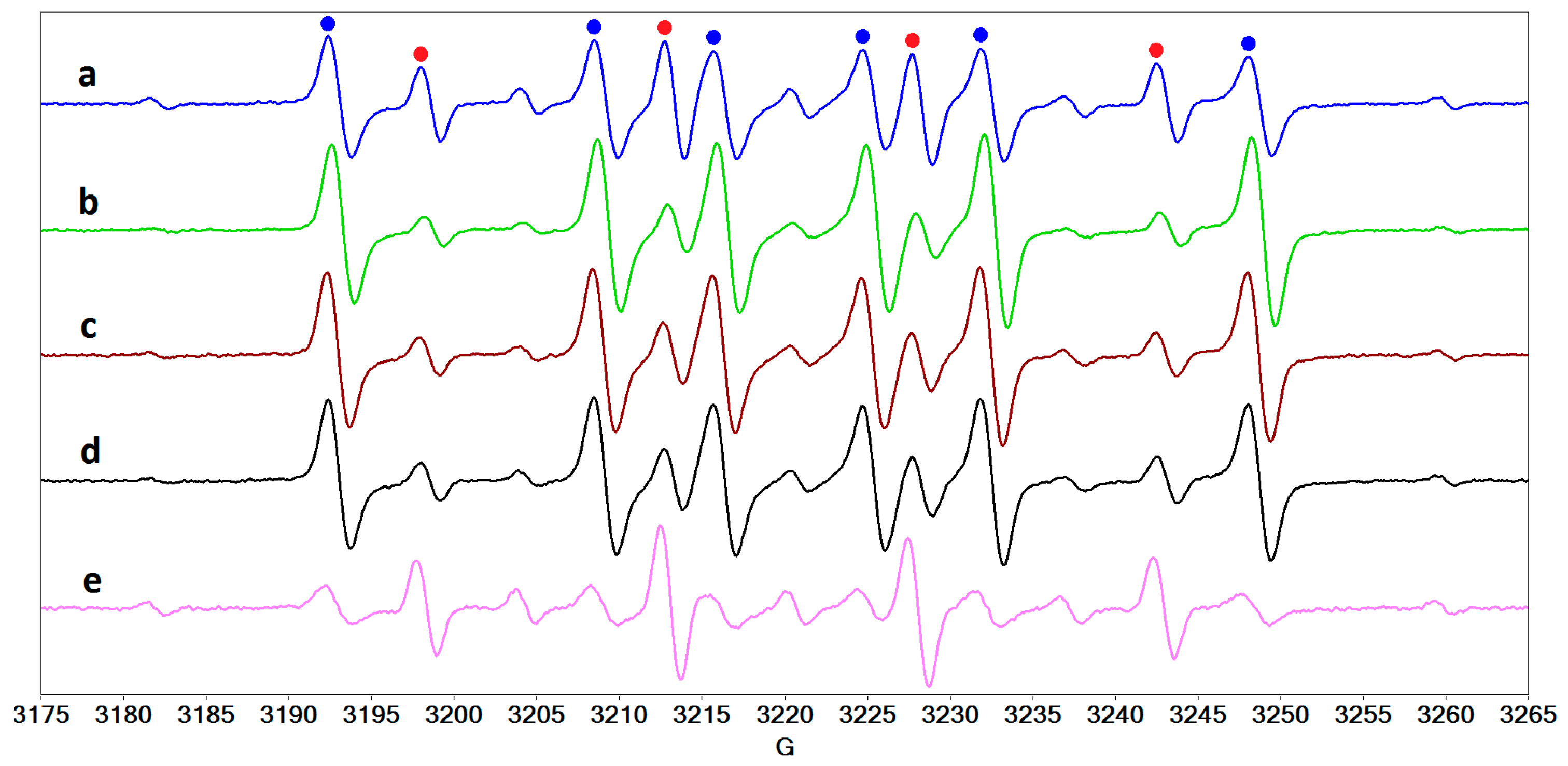
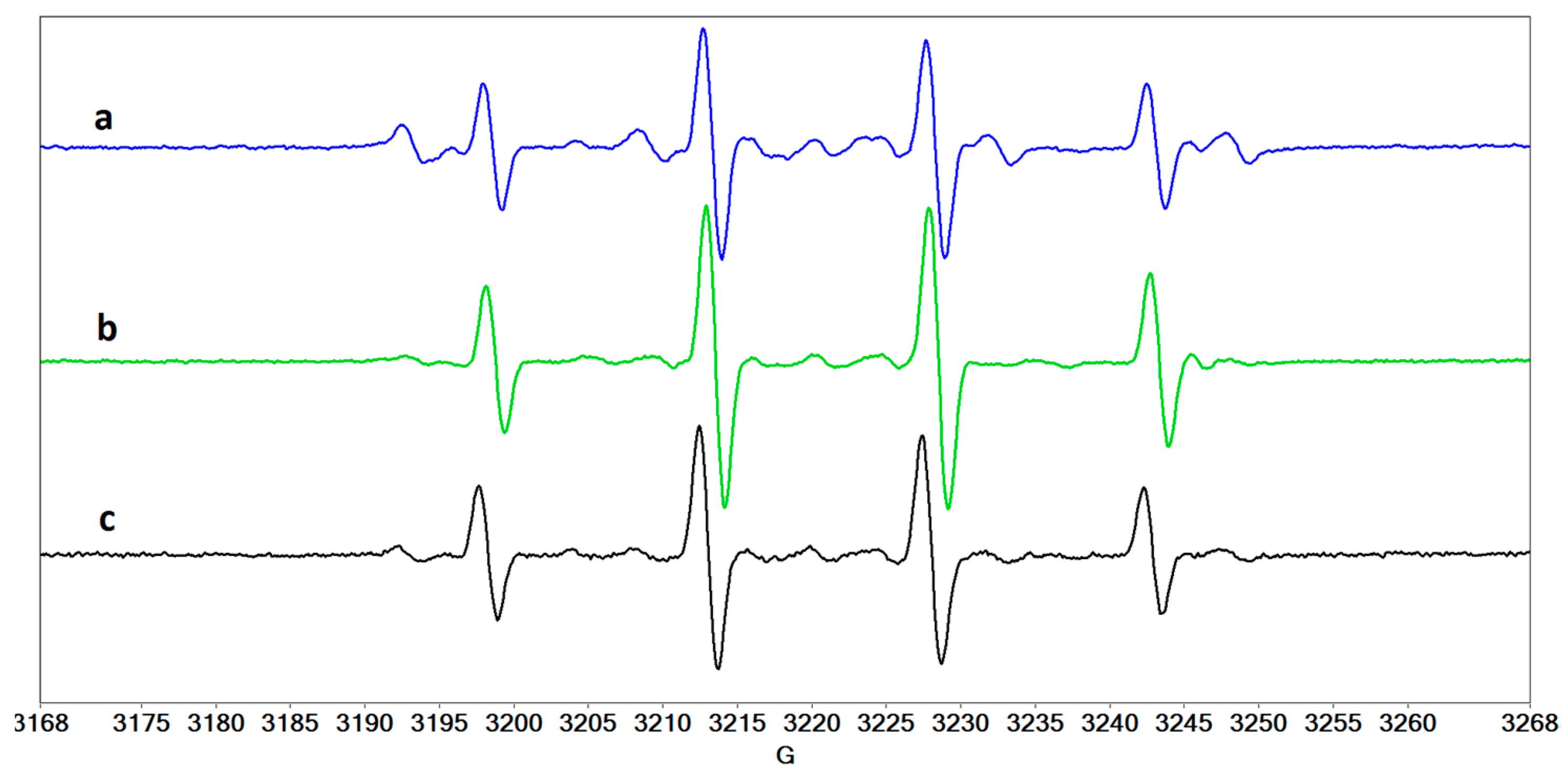
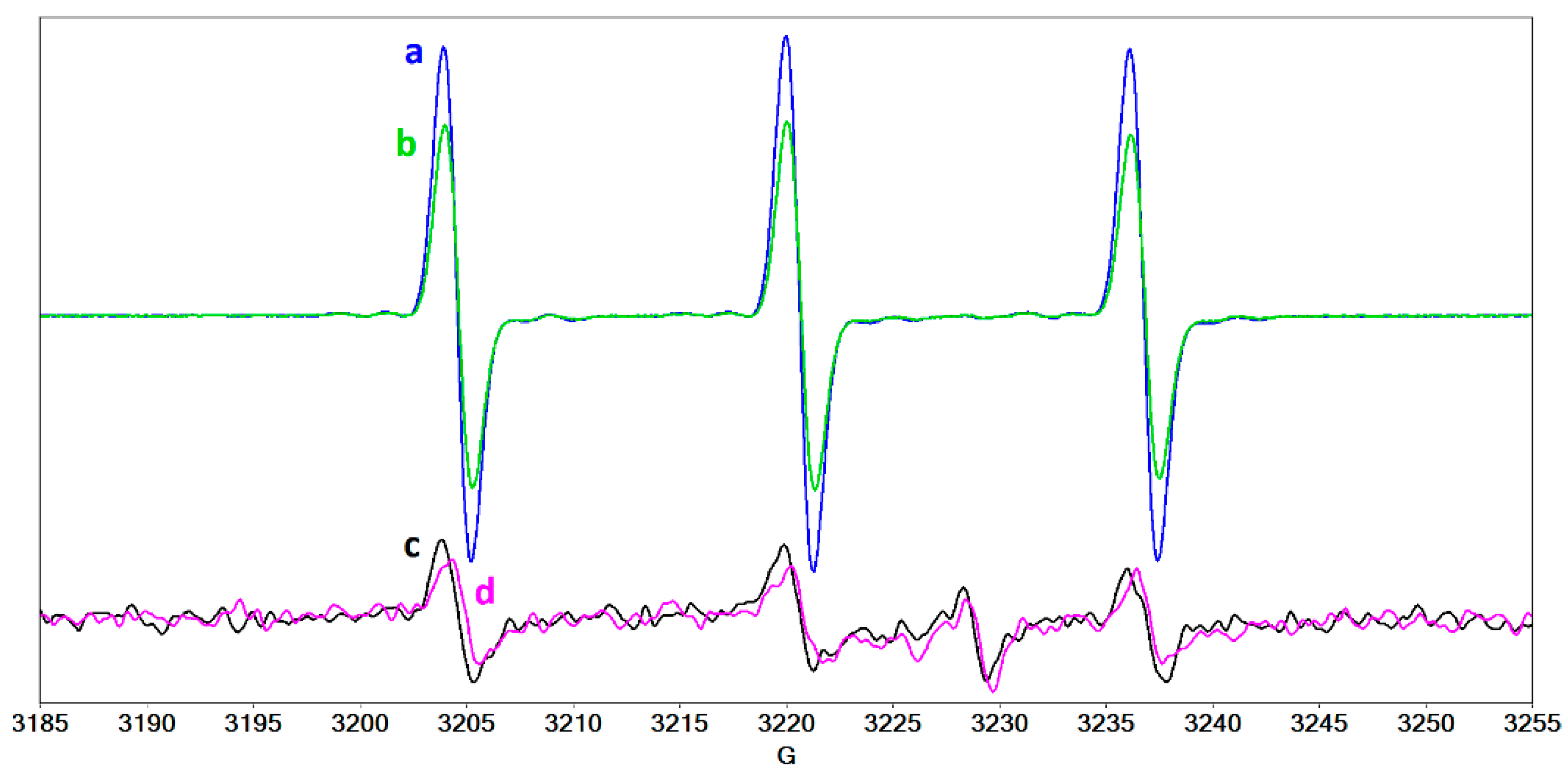
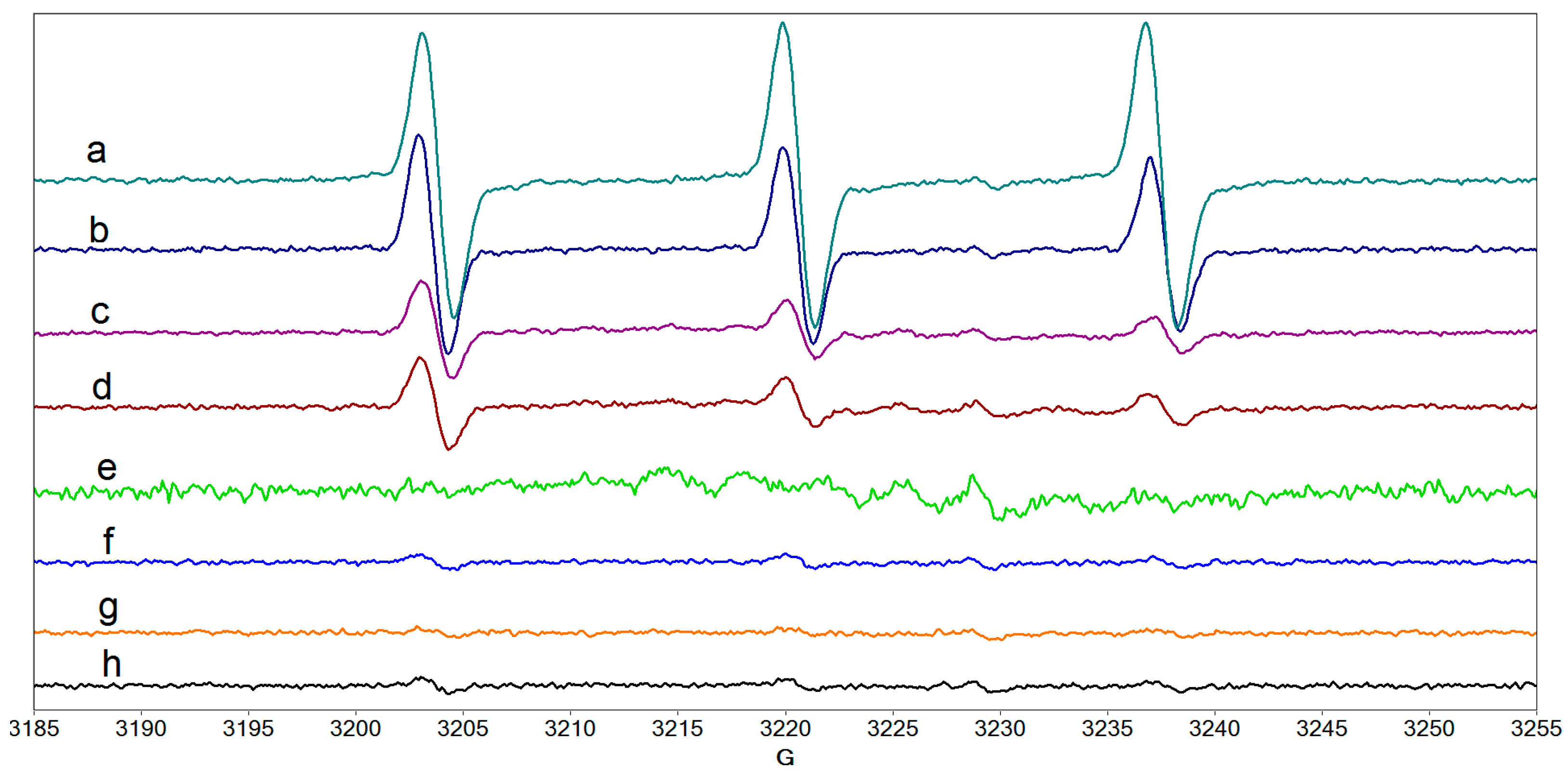
2.4. Radical Species Formed in Collagen/Riboflavin/UVA Systems
3. Materials and Methods
3.1. Collagen Extraction and Purification
3.2. Sample Preparation
3.3. Instruments
3.3.1. STEM
3.3.2. Rheometry
3.3.3. MicroDSC
3.3.4. EPR Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Kennedy, R.H.; Bourne, W.M.; Dyer, J.A. A 48-year clinical and epidemiologic study of keratoconus. Am. J. Ophthalmol. 1986, 101, 267–273. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Mohan, S.; Pye, D.C.; Willcox, M.D.P. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012, 90, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Carracedo, G.; Recchioni, A.; Alejandre-Alba, N.; Martin-Gil, A.; Crooke, A.; Morote, I.J.-A.; Pintor, J. Signs and symptoms of dry eye in keratoconus patients: A pilot study. Curr. Eye Res. 2015, 40, 1088–1094. [Google Scholar] [CrossRef] [PubMed]
- Wollensak, G.; Sperl, E.; Seiler, T. Riboflavin/ultraviolet-A–induced collagen crosslinking for the treatment of keratoconus. Am. J. Ophathalmol. 2003, 135, 620–627. [Google Scholar] [CrossRef]
- O’Brart, D.P.S. Corneal collagen cross-linking: A review. J. Optom. 2014, 7, 113–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frullini, A.; Manetti, L.; Di Cicco, E.; Fortuna, D. Photoinduced collagen cross-linking: A new approach to venous insufficiency. Dermatol. Surg. 2011, 37, 1113–1118. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Palamara, J.E.A.; Burrow, M.F. Effects of collagen crosslinkers on dentine: A literature review. Calcif. Tissue Int. 2018, 102, 265–279. [Google Scholar] [CrossRef]
- Spoerl, E.; Huhle, M.; Seiler, T. Induction of cross-links in corneal tissue. Exp. Eye Res. 1998, 66, 97–103. [Google Scholar] [CrossRef]
- Wollensak, G.; Iomdina, E.; Dittert, D.D.; Salamatina, O.; Stoltenburg, G. Cross-linking of scleral collagen in the rabbit using riboflavin and UVA. Acta Ophthalmol. Scand. 2005, 83, 477–482. [Google Scholar] [CrossRef]
- Spoerl, E.; Wollensak, G.; Seiler, T. Increased resistance of crosslinked cornea against enzymatic digestion. Curr. Eye Res. 2004, 29, 35–40. [Google Scholar] [CrossRef]
- Hsu, K.M.; Sugar, J. Keratoconus and other corneal diseases: Pharmacologic cross-linking and future therapy. Pharm. Log. Ther. Ocul. Dis. 2017, 242, 137–161. [Google Scholar] [CrossRef]
- Constantin, M.M.; Corbu, C.G.; Tanase, C.; Codrici, E.; Mihai, S.; Popescu, I.D.; Enciu, A.M.; Mocanu, S.; Matei, I.; Ionita, G. Spin probe method of electron paramagnetic resonance spectroscopy-a qualitative test for measuring the evolution of dry eye syndrome under treatment. Anal. Methods 2019, 11, 965–972. [Google Scholar] [CrossRef]
- Balasubramanian, S.A.; Pye, D.C.; Willcox, M.D.P. Levels of lactoferrin, secretory IgA and serum albumin in the tear film of people with keratoconus. Exp. Eye Res. 2012, 96, 132–137. [Google Scholar] [CrossRef]
- Glasgow, B.J.; Marshall, G.; Gasymov, O.K.; Abduragimov, A.R.; Yusifov, T.N.; Knobler, C.M. Tear lipocalins: Potential lipid scavengers for the corneal surface. Investig. Ophthalmol. Vis. Sci. 1999, 40, 3100–3107. [Google Scholar]
- Ohashi, Y.; Dogru, M.; Tsubota, K. Laboratory findings in tear fluid analysis. Clin. Chim. Acta 2006, 369, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Bron, A.J.; Mengher, L.S. The ocular surface in keratoconjunctivitis sicca. Eye 1989, 3, 428–437. [Google Scholar] [CrossRef]
- Bailey, A.J. Structure, function and ageing of the collagens of the eye. Eye 1987, 1, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Zheng, M.; Liu, X.; Yue, O.; Wang, X.; Jiang, H. Advanced collagen nanofibers-based functional bio-composites for high-value utilization of leather: A review. J. Sci. Adv. Mater. Devices 2021, 6, 153–166. [Google Scholar] [CrossRef]
- Williams, B.R.; Gelman, R.A.; Poppke, D.C.; Piez, K.A. Collagen fibril formation: Optimal in vitro conditions and preliminary kintic results. J. Biol. Chem. 1978, 253, 6578–6585. [Google Scholar] [CrossRef]
- Silver, F.H. Type I collagen fibrillogenesis in vitro: Additional evidence for the assembly mechanism. J. Biol. Chem. 1981, 256, 4973–4977. [Google Scholar] [CrossRef]
- Silver, F.H.; Birk, D.E. Kinetic analysis of collagen fibrillogenesis: I. Use of turbidity-time data. Collagen Rel. Res. 1983, 3, 393–405. [Google Scholar] [CrossRef]
- Birk, D.E.; Silver, F.H. Kinetic analysis of collagen fibrillogenesis: II. Corneal and scleral type I collagen. Collagen Rel. Res. 1984, 4, 265–277. [Google Scholar] [CrossRef]
- Hansen, U.; Bruckner, P. Macromolecular specificity of collagen fibrillogenesis: Fibrils of collagens I and XI contain a heteritypic alloyed core and a collagen I sheath. J. Biol. Chem. 2003, 278, 37352–37359. [Google Scholar] [CrossRef] [Green Version]
- Gobeaux, F.; Mosser, G.; Anglo, A.; Panine, P.; Davidson, P.; Giraud-Guille, M.M.; Belamie, E. Fibrillogenesis in dense collagen solutions: A physicochemical study. J. Biol. Chem. 2008, 376, 1509–1522. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; McBride, D.J.; Losert, W.; Leikin, S. Segregation of type I collagen homo- and heterotrimers in fibrils. J. Biol. Chem. 2008, 383, 122–132. [Google Scholar] [CrossRef] [Green Version]
- Sheraz, M.A.; Kazi, S.H.; Ahmed, S.; Anwar, Z.; Ahmad, I. Photo, thermal and chemical degradation of riboflavin. Beilstein J. Org. Chem. 2014, 10, 1999–2012. [Google Scholar] [CrossRef]
- Balazs, E.A. Interaction between cells, hyaluronic acid and collagen. Upsala J. Med. Sci. 1977, 82, 94. [Google Scholar] [CrossRef]
- Lapcik, L., Jr.; Omelka, L.; Kubena, K.; Galatik, A.; Kello, V. Photodegradation of hyaluronic acid and of the vitreous body. Gen. Physiol. Biophys. 1990, 9, 419–429. [Google Scholar] [PubMed]
- Marchini, M.; Morocutti, M.; Ruggeri, A.; Koch, M.H.J.; Bigi, A.; Roveri, N. Differences in the fibril structure of corneal and tendon collagen. An electron microscopy and X-ray diffraction investigation. Connect. Tissue Res. 1986, 15, 269–281. [Google Scholar] [CrossRef]
- Su, H.N.; Ran, L.Y.; Chen, Z.H.; Qin, Q.L.; Shi, M.; Song, X.Y.; Chen, X.L.; Zhang, Y.Z.; Xie, B.B. The ultrastructure of type I collagen at nanoscale: Large or small D-spacing distribution? Nanoscale 2014, 6, 8134–8139. [Google Scholar] [CrossRef]
- Komsa-Penkova, R.; Koynova, R.; Kostov, G.; Tenchov, B.G. Thermal stability of calf skin collagen type I in salt solutions. BBA Protein Struct. Mol. Enzymol. 1996, 1297, 171–181. [Google Scholar] [CrossRef]
- Mu, C.; Li, D.; Lin, W.; Ding, Y.; Zhang, G. Temperature induced denaturation of collagen in acidic solution. Biopolymers 2007, 86, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Flandin, F.; Buffevant, C.; Herbage, D. A differential scanning calorimetry analysis of the age-related changes in the thermal stability of rat skin collagen. Biochim. Biophys. Acta. 1984, 791, 205–211. [Google Scholar] [CrossRef]
- Wallace, D.G.; Condell, R.A.; Donovan, J.W.; Paivinen, A.; Rhee, W.M.; Wade, S.B. Multiple denaturational transitions in fibrillar collagen. Biopolymers 1986, 25, 1875–1895. [Google Scholar] [CrossRef]
- He, L.; Mu, C.; Li, D.; Lin, W. Revisit the pre-transition of type I collagen denaturation in dilute solution by ultrasensitive differential scanning calorimetry. Thermochim. Acta 2012, 548, 1–5. [Google Scholar] [CrossRef]
- Tiktopulo, E.I.; Kajava, A.V. Denaturation of type I collagen fibrils is an endothermic process accompanied by a noticeable change in the partial heat capacity. Biochemistry 1998, 37, 8147–8152. [Google Scholar] [CrossRef]
- Miles, C.A.; Avery, N.C.; Rodin, V.V.; Bailey, A.J. The increase in denaturation temperature following cross-linking of collagen is caused by dehydration of the fibres. J. Mol. Biol. 2005, 346, 551–556. [Google Scholar] [CrossRef]
- Choe, E.; Huang, R.; Min, D.B. Chemical reactions and stability of riboflavin in foods. J. Food Sci. 2005, 70, R28–R36. [Google Scholar] [CrossRef]
- Ionita, G.; Matei, I. Application of riboflavin photochemical properties in hydrogel synthesis. In Biophysical Chemistry—Advance Applications; Khalid, M.A.A., Ed.; IntechOpen: London, UK, 2019; pp. 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dikalov, S.; Grigorev, I.A.; Voinov, M.; Bassenge, E. Detection of superoxide radicals and peroxynitrite by 1-hydroxy-4-phosphonooxy-2,2,6,6-tetramethylpiperidine: Quantification of extracellular superoxide radicals formation. Biochem. Biophys. Res. Commun. 1998, 248, 211. [Google Scholar] [CrossRef]
- Bielski, B.H.J.; Cabelli, D.E.; Arudi, R.L.; Ross, A.B. Reactivity of HO2/O2 radicals in aqueous solution. J. Phys. Chem. Ref. Data 1985, 14, 1041–1100. [Google Scholar] [CrossRef]
- Collin, F. Chemical basis of reactive oxygen species reactivity and involvement in neurodegenerative diseases. Int. J. Mol. Sci. 2019, 20, 2407. [Google Scholar] [CrossRef] [Green Version]
- Fujii, T.; Kühn, K. Isolation and characterization of pepsin-treated type III collagen from calf skin. Hoppe-Seyler’s Z. Phys. Chem. 1975, 356, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Seyer, J.M.; Hutcheson, E.T.; Kang, A.H. Collagen polymorphism in normal and cirrhotic human liver. J. Clin. Investig. 1977, 59, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Seyer, J.M.; Hutcheson, E.T.; Kang, A.H. Collagen polymorphism in idiopathic chronic pulmonary fibrosis. J. Clin. Investig. 1976, 57, 1498–1507. [Google Scholar] [CrossRef] [Green Version]
- Siddiqui, Y.D.; Arief, E.M.; Yusoff, A.; Hamid, S.S.A.; Norani, T.Y.; Abdullah, M.Y.S. Extraction, purification and physical characterization of collagen from body wall of sea cucumber Bohadschia bivitatta. Health Environ. J. 2013, 4, 53–65. [Google Scholar]
- Gao, L.; Wang, Z.; Li, Z.; Zhang, C.; Zhang, D. The characterization of acid and pepsin soluble collagen from ovine bones (Ujumuqin sheep). J. Integr. Agric. 2018, 17, 704–711. [Google Scholar] [CrossRef] [Green Version]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Collagen extraction optimization from the skin of the small-spotted catshark (S. canicula) by response surface methodology. Mar. Drugs 2019, 17, 40. [Google Scholar] [CrossRef] [Green Version]
- Noorzai, S.; Verbeek, C.J.R.; Lay, M.C.; Swan, J. Collagen extraction from various waste bovine hide sources. Waste Biomass Valori. 2020, 11, 5687–5698. [Google Scholar] [CrossRef]
- Bachinger, H.P.; Mizuno, K.; Vranka, J.A.; Boudko, S.P. Collagen formation and structure. In Comprehensive Natural Products II. Chemistry and Biology, Aminoacids, Peptides and Proteins; Liu, H.-W., Mander, L., Eds.; Elsevier Ltd.: Amsterdam, The Netherlands, 2010; Volume 5, pp. 481–482. [Google Scholar]
- Chandrasekhar, S.; Laurie, G.W.; Cannon, F.B.; Martin, G.R.; Kleinman, H.K. In vitro regulation of cartilage matrix assembly by a Mr 54,000 collagen-binding proteins. Proc. Natl. Acad. Sci. USA 1986, 83, 5126–5130. [Google Scholar] [CrossRef] [Green Version]
- Birk, D.E.; Fitch, J.M.; Babiarz, J.P.; Doane, K.J.; Linsenmayer, T.F. Collagen fibrillogenesis in vitro: Interaction of types I and V collagen regulates fibril diameter. J. Cell Sci. 1990, 95, 649–657. [Google Scholar] [CrossRef]
- Li, Y.; Asadi, A.; Monroe, M.R.; Douglas, E.P. pH effects on collagen fibrillogenesis in vitro: Electrostatic interactions and phosphate binding. Mater. Sci. Eng. C 2009, 29, 1643–1649. [Google Scholar] [CrossRef]
- Micutz, M.; Matalon, E.; Staicu, T.; Angelescu, D.G.; Ariciu, A.M.; Rogozea, A.; Turcu, I.M.; Ionita, G. The influence of hydroxypropyl β-cyclodextrin on the micellar to gel transition in F127 solutions investigated at macro and nanoscale levels. New J. Chem. 2014, 38, 2801–2812. [Google Scholar] [CrossRef]
- Neacsu, M.V.; Matei, I.; Micutz, M.; Staicu, T.; Precupas, A.; Popa, V.T.; Salifoglou, A.; Ionita, G. Interaction between albumin and Pluronic F127 block copolymer by global and local physicochemical profiling. J. Phys. Chem. B 2016, 120, 4258–4267. [Google Scholar] [CrossRef] [PubMed]
- Staicu, T.; Ilis, M.; Circu, V.; Micutz, M. Influence of hydrocarbon moieties of partially fluorinated N-benzoyl thiourea compounds on their gelation properties. A detailed rheological study of complex viscoelastic behavior of decanol/N-benzoyl thiourea mixtures. J. Mol. Liq. 2018, 255, 297–312. [Google Scholar] [CrossRef]
- Staicu, T.; Circu, V.; Ionita, G.; Ghica, C.; Popa, V.T.; Micutz, M. Analysis of bimodal thermally-induced denaturation of type I collagen extracted from calfskin. RSC Adv. 2015, 5, 38391–38406. [Google Scholar] [CrossRef]
- Duling, D.R. Simulation of multiple isotropic spin-trap EPR spectra. J. Magn. Reson. B 1994, 104, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Duling, D.R. PEST Winsim; Version 0.96; National Institute of Environmental Health Sciences: Triangle Park, NC, USA, 1996. [Google Scholar]
- Kühn, K. The classical collagens: Types I, II, and III. In Structure and Function of Collagen Types; Mayne, R., Burgeson, R.E., Eds.; Academic Press: Orlando, Fl, USA, 1987; pp. 1–42. [Google Scholar]
- Ventre, M.; Padovani, M.; Covington, A.D.; Netti, P.A. Composition, structure and physical properties of foetal calf skin. In Proceedings of the II IULTCS Eurocongress: Innovation and New Technologies for the Future of Global Leather Industry, Istanbul, Turkey, 24–27 May 2006. [Google Scholar]




| Sample | Collagen 0.25% | Riboflavin 0.1% | Hyaluronic Acid 0.1% | UVA Exposure |
|---|---|---|---|---|
| 1 | x | - | - | - |
| 1a | x | - | - | x |
| 2 | x | x | - | - |
| 2a | x | x | - | x |
| 3 | x | - | x | - |
| 3a | x | - | x | x |
| 4 | x | x | x | - |
| 4a | x | x | x | x |
| Sample | 10 °C | 20 °C | 25 °C | 37 °C (1 h) | 37 °C (2 h) | 37 °C (3 h) |
|---|---|---|---|---|---|---|
| 1 | 0.79 | 0.57 | 0.36 | 9.90 | 43.43 | 63.88 |
| 1a | 0.66 | 0.66 | 0.53 | 14.16 | 77.04 | 149.10 |
| 2 | 0.77 | 0.49 | 0.34 | 40.08 | 72.16 | 113.26 |
| 2a | 0.60 | 0.39 | 0.47 | 1.38 | 19.36 | 45.00 |
| 3 | 0.67 | 0.66 | 0.42 | 62.39 | 104.70 | 137.52 |
| 3a | 0.51 | 0.51 | 0.43 | 41.11 | 75.05 | 94.90 |
| 4 | 0.59 | 0.49 | 0.24 | 49.88 | 50.11 | 52.88 |
| 4a | 0.61 | 0.52 | 0.43 | 8.28 | 46.73 | 71.83 |
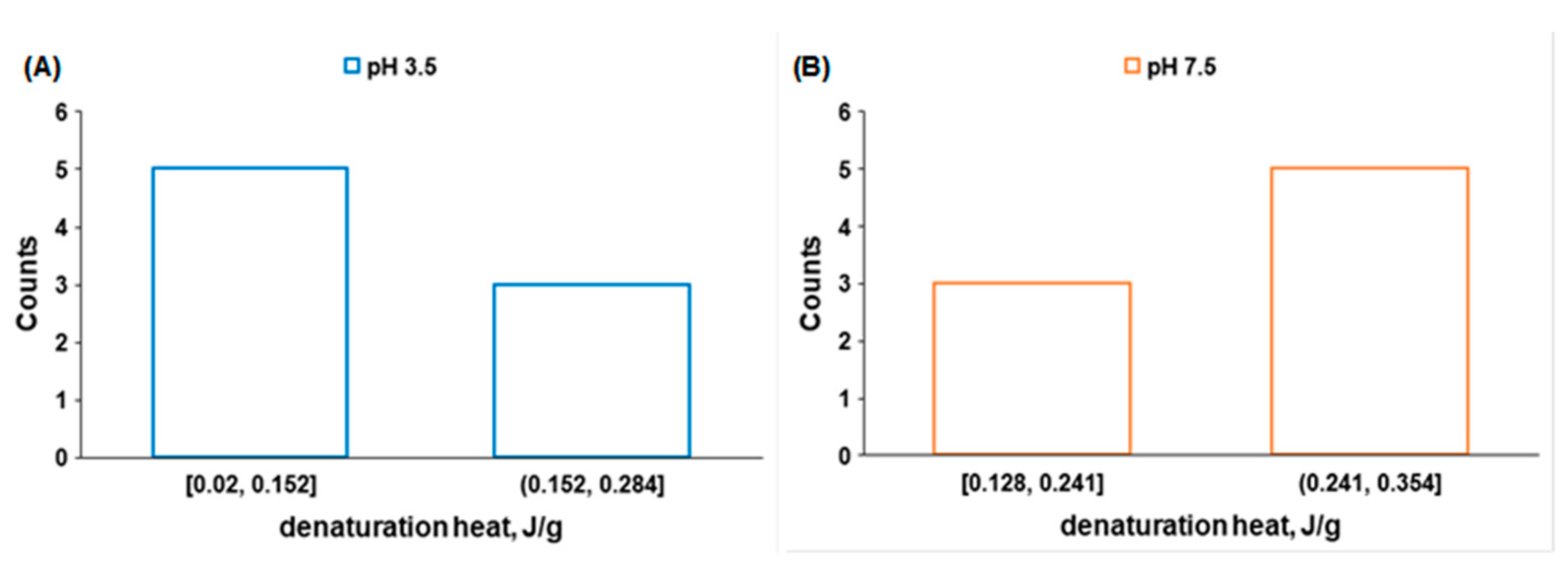
| Sample | Tpeak1, °C | Tpeak2, °C | Tpeak3, °C | Denaturation Heat, J/g |
|---|---|---|---|---|
| 1 (pH 3.5) | 36.10 | 41.06 | 45.82 | 0.27 |
| 1 (pH 7.5) | 36.74 | 43.93 | 47.15 | 0.29 |
| Sample | Tpeak1, °C | Tpeak2, °C | Tpeak3, °C | Tpeak4, °C | Denaturation Heat, J/g |
|---|---|---|---|---|---|
| 1 | 36.74 | 43.93 | 47.15 | - | 0.23 |
| 1a | 37.22 | 44.15 | 47.42 | - | 0.27 |
| 2 | 37.60 | 43.89 | 50.32 | - | 0.29 |
| 2a | 42.44 | 43.88 | 54.24 | 57.33 | 0.24 |
| 3 | 32.96 | 40.31 | 43.71 | 50.95 | 0.17 |
| 3a | 40.80 | 43.70 | 50.63 | 54.04 | 0.13 |
| 4 | 37.46 | 43.93 | 51.43 | 54.15 | 0.27 |
| 4a | 38.73 | 44.04 | 50.43 | - | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, M.M.; Corbu, C.G.; Mocanu, S.; Popescu, E.I.; Micutz, M.; Staicu, T.; Şomoghi, R.; Trică, B.; Popa, V.T.; Precupas, A.; et al. Model Systems for Evidencing the Mediator Role of Riboflavin in the UVA Cross-Linking Treatment of Keratoconus. Molecules 2022, 27, 190. https://doi.org/10.3390/molecules27010190
Constantin MM, Corbu CG, Mocanu S, Popescu EI, Micutz M, Staicu T, Şomoghi R, Trică B, Popa VT, Precupas A, et al. Model Systems for Evidencing the Mediator Role of Riboflavin in the UVA Cross-Linking Treatment of Keratoconus. Molecules. 2022; 27(1):190. https://doi.org/10.3390/molecules27010190
Chicago/Turabian StyleConstantin, Mihaela Monica, Cătălina Gabriela Corbu, Sorin Mocanu, Elena Irina Popescu, Marin Micutz, Teodora Staicu, Raluca Şomoghi, Bogdan Trică, Vlad Tudor Popa, Aurica Precupas, and et al. 2022. "Model Systems for Evidencing the Mediator Role of Riboflavin in the UVA Cross-Linking Treatment of Keratoconus" Molecules 27, no. 1: 190. https://doi.org/10.3390/molecules27010190
APA StyleConstantin, M. M., Corbu, C. G., Mocanu, S., Popescu, E. I., Micutz, M., Staicu, T., Şomoghi, R., Trică, B., Popa, V. T., Precupas, A., Matei, I., & Ionita, G. (2022). Model Systems for Evidencing the Mediator Role of Riboflavin in the UVA Cross-Linking Treatment of Keratoconus. Molecules, 27(1), 190. https://doi.org/10.3390/molecules27010190














