Changes in Triterpenes in Alismatis rhizoma after Processing Based on Targeted Metabolomics Using UHPLC-QTOF-MS/MS
Abstract
1. Introduction
2. Results
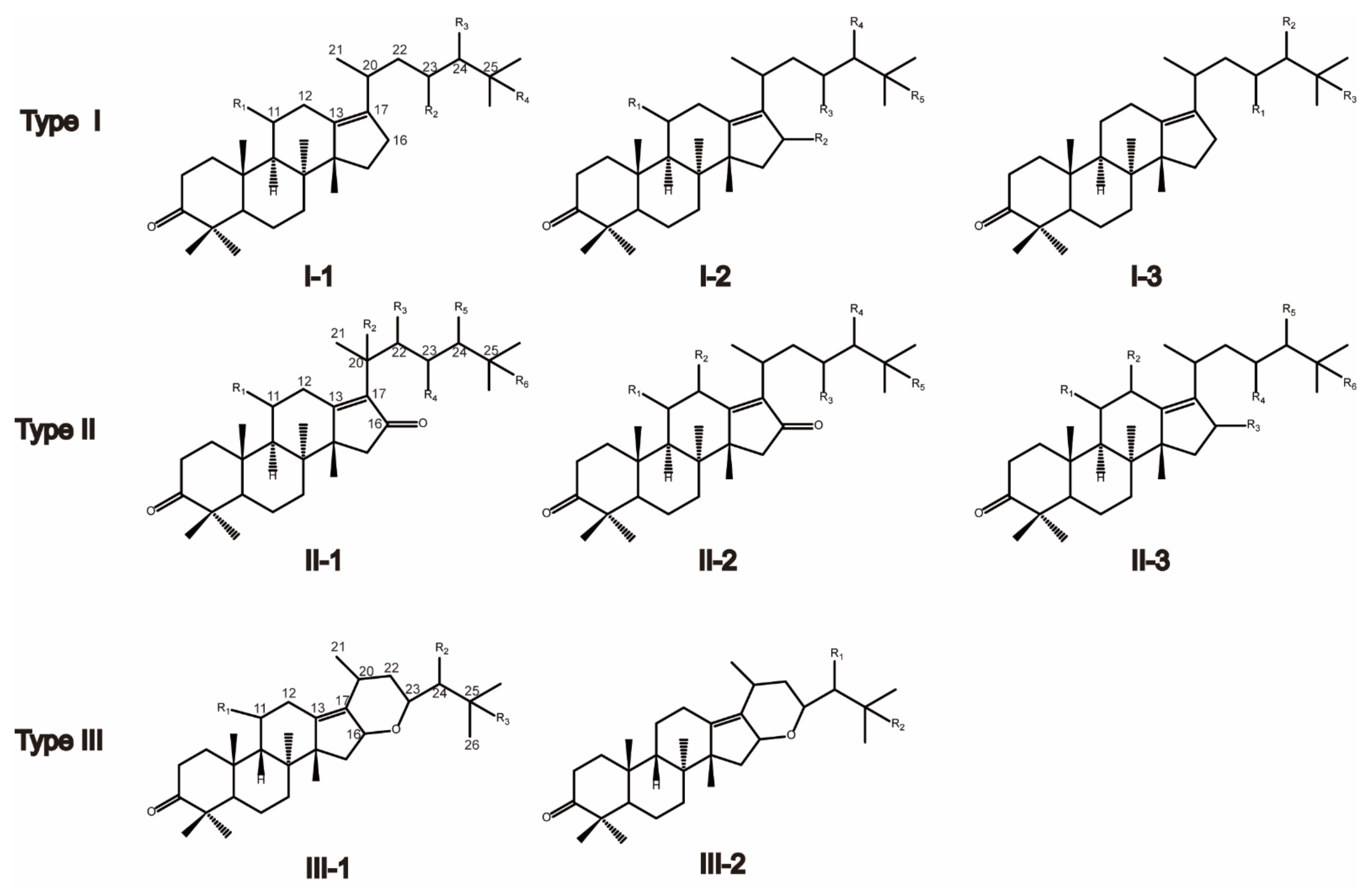
2.1. Qualitative Analysis of Triterpenes in AR and Processed AR
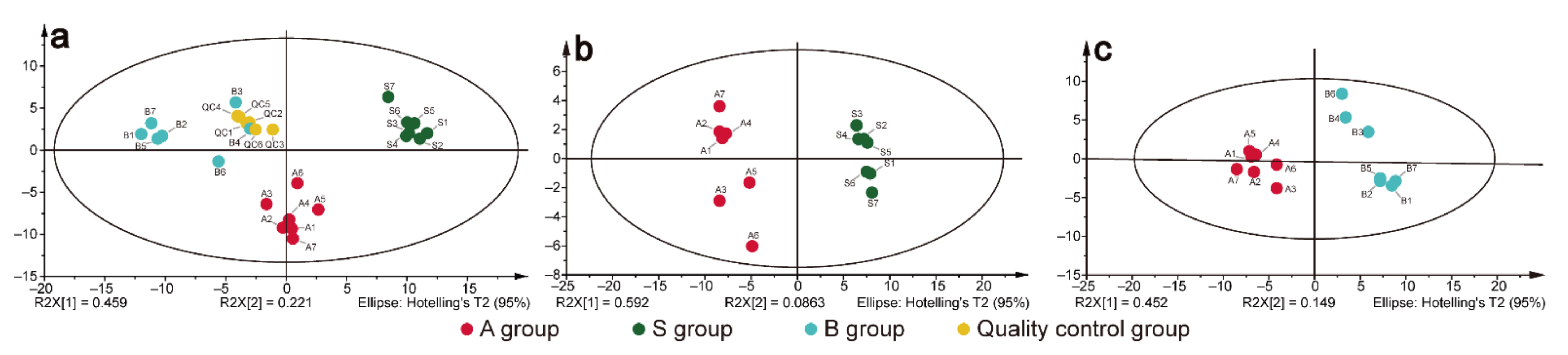
2.2. Targeted Metabolomic Analysis of Triterpenes
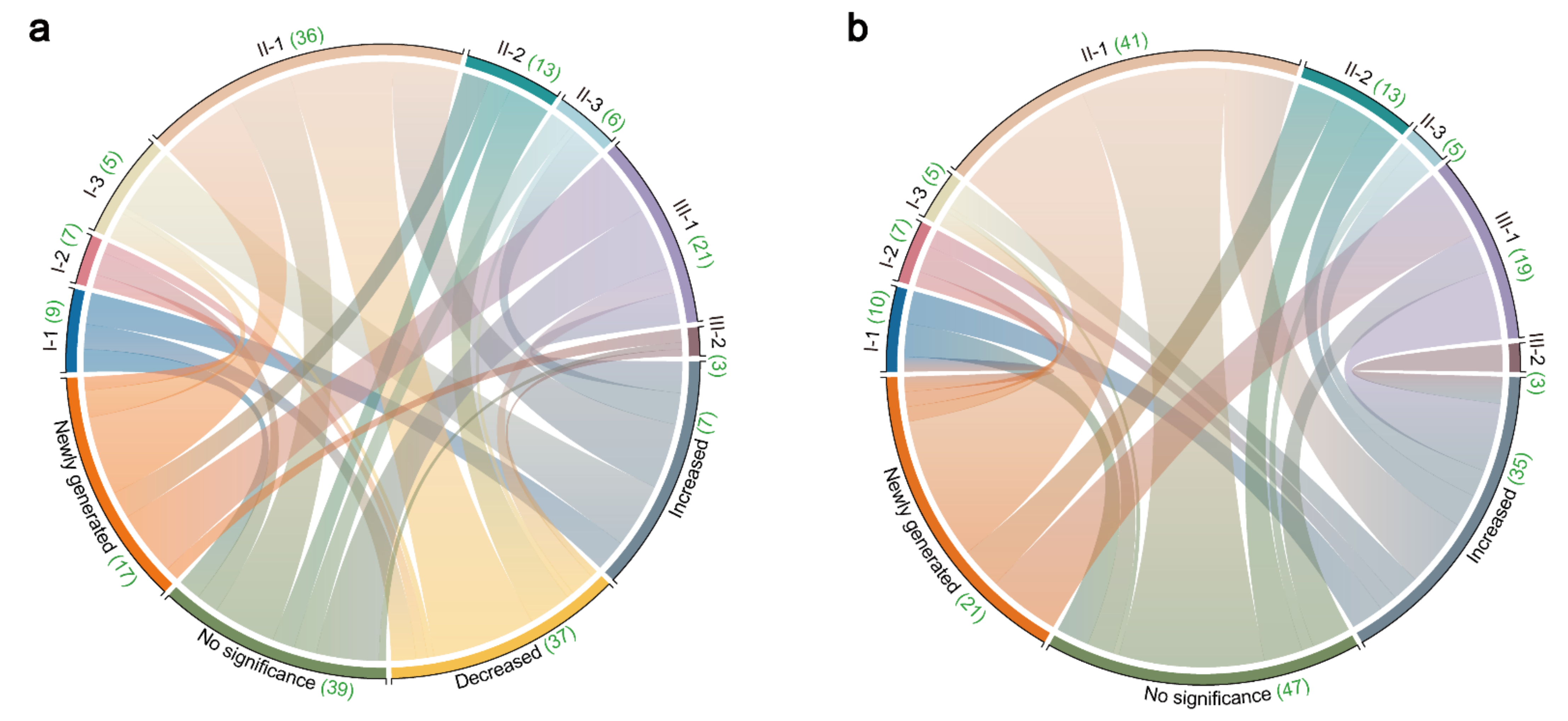
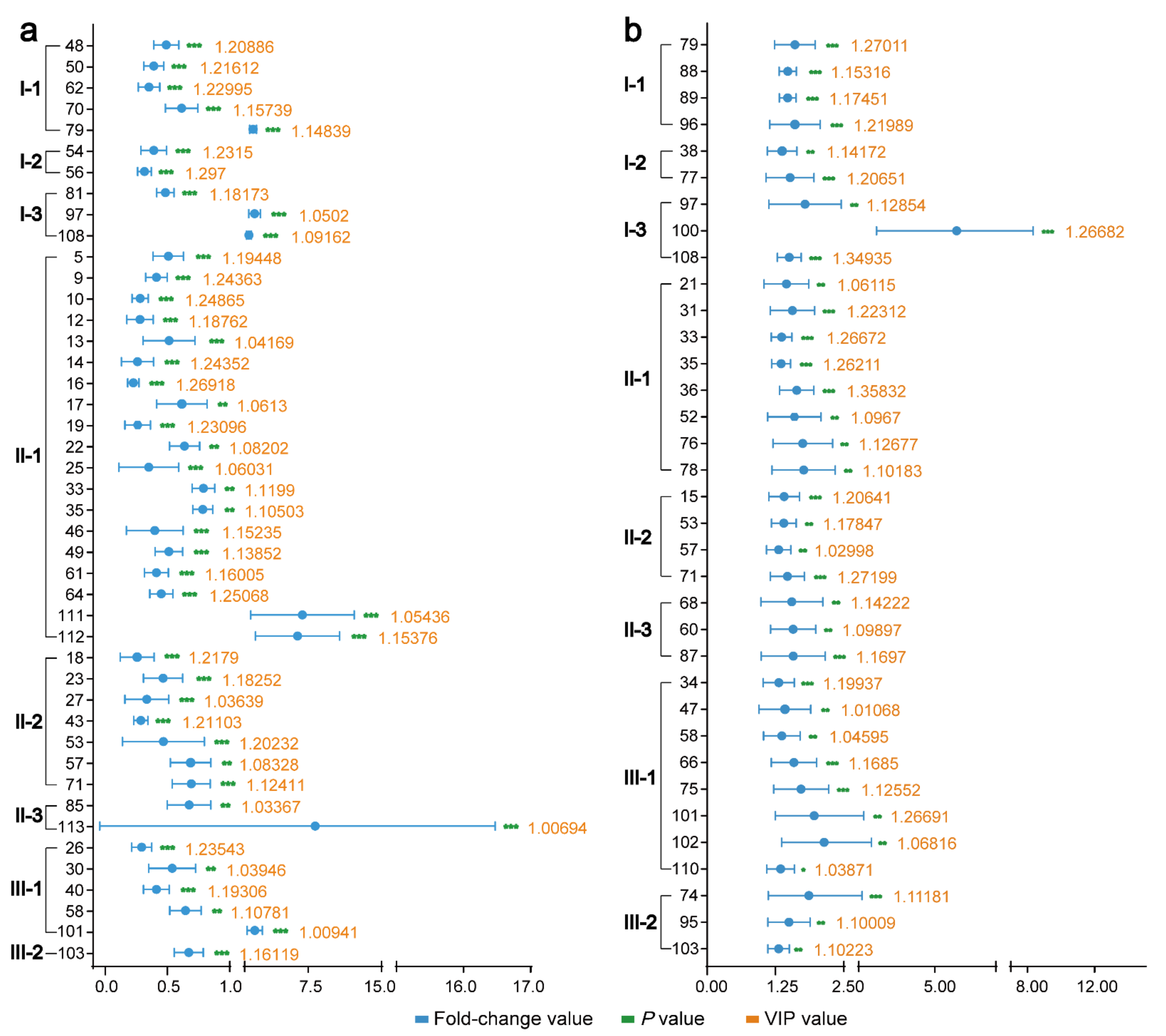
2.3. Discovery the Changes in Triterpenes in SAR and BAR
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Preparation of SAR and BAR
4.3. Preparation of Sample Solutions
4.4. Chromatography and Mass Spectrometric Analysis
4.5. Statistics Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
Abbreviations
| TCM | traditional Chinese Medicine; |
| AR | Alismatis rhizoma; |
| SAR | salt-processed AR; |
| BAR | bran-processed AR; |
| UHPLC-QTOF-MS/MS | ultra-high performance liquid chromatography coupled with quadrupole time-of-flight-mass spectrometer; |
| PCA | principal component analysis; |
| PLS-DA | partial least squares-discrimination analyses; |
| VIP | variable importance in the projection; |
| One-way ANOVA | one-way analysis of variance; |
| A group | raw AR group; |
| S group | salt-processed AR group; |
| B group | bran-processed AR group; |
| QC | quality control samples. |
References
- Wu, X.; Wang, S.; Lu, J.; Jing, Y.; Li, M.; Cao, J.; Bian, B.; Hu, C. Seeing the unseen of Chinese herbal medicine processing (Paozhi): Advances in new perspectives. Chin. Med. 2018, 13, 4. [Google Scholar] [CrossRef]
- Zhao, Z.; Liang, Z.; Chan, K.; Lu, G.; Lee, E.; Chen, H.; Li, L. A unique issue in the standardization of Chinese materia medica: Processing. Planta Med. 2010, 76, 1975–1986. [Google Scholar] [CrossRef]
- Jiang, H.; Yang, L.; Hou, A.; Zhang, J.; Wang, S.; Man, W.; Zheng, S.; Yu, H.; Wang, X.; Yang, B.; et al. Botany, traditional uses, phytochemistry, analytical methods, processing, pharmacology and pharmacokinetics of Bupleuri Radix: A systematic review. Biomed. Pharmacother. 2020, 131, 110679. [Google Scholar] [CrossRef]
- Shu, Z.; Pu, J.; Chen, L.; Zhang, Y.; Rahman, K.; Qin, L.; Zheng, C. Alisma orientale: Ethnopharmacology, Phytochemistry and Pharmacology of an Important Traditional Chinese Medicine. Am. J. Chin. Med. 2016, 44, 227–251. [Google Scholar] [CrossRef]
- Zhang, X.; Li, X.; Lin, N.; Zhao, W.; Huang, X.; Chen, Y.; Huang, M.; Xu, W.; Wu, S. Diuretic Activity of Compatible Triterpene Components of Alismatis rhizoma. Molecules 2017, 22, 1459. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Jiang, H.; Ju, J.; Li, Y.; Gong, L.; Wang, X.; Yang, W.; Deng, Y. A metabolomic study using HPLC-TOF/MS coupled with ingenuity pathway analysis: Intervention effects of Rhizoma Alismatis on spontaneous hypertensive rats. J. Pharm. Biomed. Anal. 2016, 117, 446–452. [Google Scholar] [CrossRef]
- Miao, H.; Zhang, L.; Chen, D.; Chen, H.; Zhao, Y.; Ma, S. Urinary biomarker and treatment mechanism of Rhizoma Alismatis on hyperlipidemia. Biomed. Chromatogr. 2017, 31, e3829. [Google Scholar] [CrossRef] [PubMed]
- The State Pharmacopoeia Commission of PRC. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2020; Volume 1, p. 239. [Google Scholar]
- Li, S.; Wang, L.; Du, Z.; Jin, S.; Song, C.; Jia, S.; Zhang, Y.; Jiang, H. Identification of the lipid-lowering component of triterpenes from Alismatis rhizoma based on the MRM-based characteristic chemical profiles and support vector machine model. Anal. Bioanal. Chem. 2019, 411, 3257–3268. [Google Scholar] [CrossRef]
- Liu, D.; Gong, Q.; Liu, Q.; Su, P.; Wang, J.; Zhong, L.; Zhang, J.; Hao, G.; Yu, H.; Wang, Y. Overview of Research on Harvest, Processing in Production Place, Processing and Quality Evaluation of Alismatis Rhizoma. Chin. J. Exp. Tradit. Med. Formulae 2018, 24, 203–211. [Google Scholar] [CrossRef]
- Liu, S.; Sheng, W.; Li, Y.; Zhang, S.; Zhu, J.; Gao, H.; Yan, L.; Wang, Z.; Gao, L.; Zhang, M. Chemical constituents from Alismatis Rhizoma and their anti-inflammatory activities in vitro and in vivo. Bioorg. Chem. 2019, 92, 103226. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Chen, H.; Tian, T.; Chen, D.; Zhao, Y.; Lin, R. Diuretic and anti-diuretic activities of the ethanol and aqueous extracts of Alismatis rhizoma. J. Ethnopharmacol. 2014, 154, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Jang, M.; Han, Y.; Nam, J.; Han, C.; Kim, B.; Jeong, H.; Ha, K.; Jung, M. Protective Effects of Alisma orientale Extract against Hepatic Steatosis via Inhibition of Endoplasmic Reticulum Stress. Int. J. Mol. Sci. 2015, 16, 26151–26165. [Google Scholar] [CrossRef]
- Lau, C.; Chan, C.; Chan, Y.; Lau, K.; Lau, T.; Lam, F.; Che, C.; Leung, P.; Fung, K.; Ho, Y.; et al. In vitro antidiabetic activities of five medicinal herbs used in Chinese medicinal formulae. Phytother. Res. 2008, 22, 1384–1388. [Google Scholar] [CrossRef]
- Tian, S.; Zhao, X.; Liu, S.; Feng, W.; Chen, L.; Yan, L.; Wang, Z. Regional differences analysis of Alismatis Rhizoma based on UPLC characteristic chromatogram and determination of eight terpenoids. Zhongguo Zhong Yao Za Zhi 2020, 45, 1545–1557. [Google Scholar] [CrossRef]
- Tao, Y.; Jiang, E.; Yan, J.; Cai, B. A biochemometrics strategy for tracing diuretic components of crude and processed Alisma orientale based on quantitative determination and pharmacological evaluation. Biomed. Chromatogr. 2020, 34, e4744. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, S.; Zhu, R. The extraordinary transformation of traditional Chinese medicine: Processing with liquid excipients. Pharm. Biol. 2020, 58, 561–573. [Google Scholar] [CrossRef]
- Yan, G.; Lan, M.; Qiu, J.; Li, X.; Xu, W.; Wu, S. Study on Changes of Chemical Composition and Diuretic Effect in Alismatis Rhizoma after Processing. Chin. J. Inf. TCM 2020, 27, 59–65. [Google Scholar] [CrossRef]
- Li, S.; Jin, S.; Song, C.; Jia, S.; Zhang, Y.; Feng, Y.; Du, Z.; Jiang, H. The strategy for establishment of the multiple reaction monitoring based characteristic chemical profile of triterpenes in Alismatis rhizoma using two combined tandem mass spectrometers. J. Chromatogr. A 2017, 1524, 121–134. [Google Scholar] [CrossRef]
- Tai, Y.; Zou, F.; Zhang, Q.; Wang, J.; Rao, R.; Xie, R.; Wu, S.; Chu, K.; Xu, W.; Li, X.; et al. Quantitative Analysis of Eight Triterpenoids and Two Sesquiterpenoids in Rhizoma Alismatis by Using UPLC-ESI/APCI-MS/MS and Its Application to Optimisation of Best Harvest Time and Crude Processing Temperature. J. Anal. Methods Chem. 2019, 2019, 8320171. [Google Scholar] [CrossRef] [PubMed]
- Dai, X. Research on the Processing Technology and Function of Alisma Orientale. Master’s Thesis, Liaoning University of Chinese Medicine, Shenyang, China, 2009. [Google Scholar]
- Liu, F.; Zhang, Z.; Lai, J.; Hu, B. Determination of four kinds of alkaloids from Rhizoma Coptis and processed Rhizoma Coptis by HPLC. Chin. Tradit. Patent Med. 2010, 32, 1925–1928. [Google Scholar]
- Zhan, J.; Zheng, K.; Zhu, K.; Bi, C.; Zhang, W.; Du, C.; Fu, Q.; Dong, T.; Choi, R.; Tsim, K.; et al. Chemical and biological assessment of Angelicae Sinensis Radix after processing with wine: An orthogonal array design to reveal the optimized conditions. J. Agric. Food Chem. 2011, 59, 6091–6098. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zhang, Z.; Li, S.; Ye, X.; Li, X.; He, K. Synergy effects of herb extracts: Pharmacokinetics and pharmacodynamic basis. Fitoterapia 2014, 92, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Caesar, L.; Cech, N. Synergy and antagonism in natural product extracts: When 1 + 1 does not equal 2. Nat. Prod. Rep. 2019, 36, 869–888. [Google Scholar] [CrossRef]
- Song, L.; Pan, R.; Tian, X.; He, M. Effect of salt-processing on anti fatty liver effect of Alismatis rhizoma. Lishizhen Med. Mater. Med. Res. 2018, 29, 92–94. [Google Scholar] [CrossRef]
- Lu, J.; Liu, L.; Zhu, X.; Wu, L.; Chen, Z.; Xu, Z.; Li, W. Evaluation of the Absorption Behavior of Main Component Compounds of Salt-Fried Herb Ingredients in Qing’e Pills by Using Caco-2 Cell Model. Molecules 2018, 23, 3321. [Google Scholar] [CrossRef]
- Wang, H.; Li, W.; Gao, Q.; Weng, Z.; Zhao, G.; Chen, Z.; Cai, B. Study on Contents of Twelve Indicative Components in Qing’e Pills Composed of Herbs Processed by Different Methods. Tradit. Chin. Drug Res. Clin. Pharmacol. 2016, 27, 684–688. [Google Scholar] [CrossRef]
- Imai, Y.; Matsumura, H.; Aramaki, Y. Hypocholesterolemic effect of alisol A-24-monoacetate and its related compounds in rats. Jpn. J. Pharmacol. 1970, 20, 222–228. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, F.; Yu, H.; Lu, C.; Chen, J.; Gu, W. The Cholesterol-Lowering Effect of Alisol Acetates Based on HMG-CoA Reductase and Its Molecular Mechanism. Evid. Based Complement. Alternat. Med. 2016, 2016, 4753852. [Google Scholar] [CrossRef]
- Ma, Q.; Han, L.; Bi, X.; Wang, X.; Mu, Y.; Guan, P.; Li, L.; Huang, X. Structures and biological activities of the triterpenoids and sesquiterpenoids from Alisma orientale. Phytochemistry 2016, 131, 150–157. [Google Scholar] [CrossRef]
- Zhang, Q.; Jiang, Z.; Luo, J.; Ma, Y.; Liu, J.; Guo, R.; Zhang, X.; Zhou, J.; Niu, W.; Du, F.; et al. Anti-HBV agents. Part 3: Preliminary structure-activity relationships of tetra-acylalisol A derivatives as potent hepatitis B virus inhibitors. Bioorg. Med. Chem. Lett. 2009, 19, 6659–6665. [Google Scholar] [CrossRef]
- Xu, F.; Chen, J.; Zhang, Y.; Wu, Q.; Shen, Y.; Gu, W.; Liu, S.; Lu, C.; Liao, H.; Bao, K. Molecular insight into the mechanism of lipid regulating effect of Alisma orientalis based on ACAT. Int. J. Biol. Macromol. 2020, 158, 1141–1162. [Google Scholar] [CrossRef]
- Xia, F.; Liu, C.; Wan, J. Characterization of the cold and hot natures of raw and processed Rehmanniae Radix by integrated metabolomics and network pharmacology. Phytomedicine 2020, 74, 153071. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Li, H. Hepatoprotective effect of Alismatis rhizoma and different processed products on acute liver injury in mice. J. Chin. Med. Mater. 2006, 29, 592–593. [Google Scholar] [CrossRef]
- The State Pharmacopoeia Commission of PRC. Pharmacopoeia of the People’s Republic of China; Chinese Medical Science and Technology Press: Beijing, China, 2020; Volume 4, pp. 31–32. [Google Scholar]
- Li, Y.; Jin, Y.; Yang, S.; Zhang, W.; Zhang, J.; Zhao, W.; Chen, L.; Wen, Y.; Zhang, Y.; Lu, K.; et al. Strategy for comparative untargeted metabolomics reveals honey markers of different floral and geographic origins using ultrahigh-performance liquid chromatography-hybrid quadrupole-orbitrap mass spectrometry. J. Chromatogr. A 2017, 1499, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Chagas-Paula, D.; Zhang, T.; Da Costa, F.; Edrada-Ebel, R. A Metabolomic Approach to Target Compounds from the Asteraceae Family for Dual COX and LOX Inhibition. Metabolites 2015, 5, 404–430. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dai, M.; Li, S.; Shi, Q.; Xiang, X.; Jin, Y.; Wei, S.; Zhang, L.; Yang, M.; Song, C.; Huang, R.; et al. Changes in Triterpenes in Alismatis rhizoma after Processing Based on Targeted Metabolomics Using UHPLC-QTOF-MS/MS. Molecules 2022, 27, 185. https://doi.org/10.3390/molecules27010185
Dai M, Li S, Shi Q, Xiang X, Jin Y, Wei S, Zhang L, Yang M, Song C, Huang R, et al. Changes in Triterpenes in Alismatis rhizoma after Processing Based on Targeted Metabolomics Using UHPLC-QTOF-MS/MS. Molecules. 2022; 27(1):185. https://doi.org/10.3390/molecules27010185
Chicago/Turabian StyleDai, Mengxiang, Sen Li, Qingxin Shi, Xingliang Xiang, Yuehui Jin, Sha Wei, Lijun Zhang, Min Yang, Chengwu Song, Rongzeng Huang, and et al. 2022. "Changes in Triterpenes in Alismatis rhizoma after Processing Based on Targeted Metabolomics Using UHPLC-QTOF-MS/MS" Molecules 27, no. 1: 185. https://doi.org/10.3390/molecules27010185
APA StyleDai, M., Li, S., Shi, Q., Xiang, X., Jin, Y., Wei, S., Zhang, L., Yang, M., Song, C., Huang, R., & Jin, S. (2022). Changes in Triterpenes in Alismatis rhizoma after Processing Based on Targeted Metabolomics Using UHPLC-QTOF-MS/MS. Molecules, 27(1), 185. https://doi.org/10.3390/molecules27010185






