In Vitro Evaluation of the Photoreactivity and Phototoxicity of Natural Polyphenol Antioxidants
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
3.1. Reagents
3.2. Spectral Absorption
3.3. Reactive Oxygen Species (ROS) Assay
3.4. Cell Culture
3.5. Phototoxicity Assay
3.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zillich, O.V.; Schweiggert-Weisz, U.; Eisner, P.; Kerscher, M. Polyphenols as active ingredients for cosmetic products. Int. J. Cosmet. Sci. 2015, 37, 455–464. [Google Scholar] [CrossRef]
- Jelena, C.H.; Giorgio, R.; Justyna, G.; Neda, M.-D.; Natasa, S.; Artur, B.; Giuseppe, G. Beneficial effects of polyphenols on chronic diseases and ageing. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Kidlington, UK, 2018; pp. 69–102. [Google Scholar]
- Cory, H.; Passarelli, S.; Szeto, J.; Tamez, M.; Mattei, J. The role of polyphenols in human health and food systems: A mini-review. Front. Nutr. 2018, 5, 87. [Google Scholar] [CrossRef] [Green Version]
- Fraga, C.G.; Croft, K.D.; Kennedy, D.O.; Tomás-Barberán, F.A. The effects of polyphenols and other bioactives on human health. Food Funct. 2019, 10, 514–528. [Google Scholar] [CrossRef] [Green Version]
- Bertelli, A.; Biagi, M.; Corsini, M.; Baini, G.; Cappellucci, G.; Miraldi, E. Polyphenols: From theory to practice. Foods 2021, 10, 2595. [Google Scholar] [CrossRef]
- Chen, K.; Lu, P.; Beeraka, N.M.; Sukocheva, O.A.; Madhunapantula, S.V.; Liu, J.; Sinelnikov, M.Y.; Nikolenko, V.N.; Bulygin, K.V.; Mikhaleva, L.M.; et al. Mitochondrial mutations and mitoepigenetics: Focus on regulation of oxidative stress-induced responses in breast cancers. Semin. Cancer Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Forman, H.J.; Zhang, H. Targeting oxidative stress in disease: Promise and limitations of antioxidant therapy. Nat. Rev. Drug Discov. 2021, 20, 689. [Google Scholar] [CrossRef] [PubMed]
- Boo, Y.C. Can plant phenolic compounds protect the skin from airborne particulate matter? Antioxidants 2019, 8, 379. [Google Scholar] [CrossRef] [Green Version]
- Jesumani, V.; Du, H.; Pei, P.; Aslam, M.; Huang, N. Comparative study on skin protection activity of polyphenol-rich extract and polysaccharide-rich extract from Sargassum vachellianum. PLoS ONE 2020, 15, e0227308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraf, S.; Kaur, C.D. Phytoconstituents as photoprotective novel cosmetic formulations. Pharmacogn. Rev. 2010, 4, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liu-Smith, F.; Jia, J.; Zheng, Y. UV-induced molecular signaling differences in melanoma and non-melanoma skin cancer. Adv. Exp. Med. Biol. 2017, 996, 27–40. [Google Scholar] [PubMed]
- Afaq, F.; Katiyar, S.K. Polyphenols: Skin photoprotection and inhibition of photocarcinogenesis. Mini Rev. Med. Chem. 2011, 11, 1200–1215. [Google Scholar]
- Rodrigues, F.; Cádiz-Gurrea, M.d.l.L.; Nunes, M.A.; Pinto, D.; Vinha, A.F.; Linares, I.B.; Oliveira, M.B.P.P.; Carretero, A.S. Cosmetics. In Polyphenols: Properties, Recovery, and Applications; Galanakis, C.M., Ed.; Woodhead Publishing: Kidlington, UK, 2018; pp. 393–427. [Google Scholar]
- Gonçalo, M. Phototoxic and Photoallergic Reactions. In Contact Dermatitis, 5th ed.; Johansen, J.D., Frosch, P.J., Lepoittevin, J.-P., Eds.; Springer: Berlin, Germany, 2011; pp. 361–376. [Google Scholar]
- International Conference on Harmonization of Technical Requirements for Registration of Pharmaceuticals for Human Use, 2014. Photosafety Evaluation of Pharmaceuticals S10, Step 4. Available online: https://database.ich.org/sites/default/files/S10_Guideline.pdf (accessed on 22 December 2021).
- U.S. Department of Health and Human Services. S10 Photosafety Evaluation of Pharmaceuticals; Guidance for Industry Food and Drug Administration; U.S. Department of Health and Human Services: Bethesda, MD, USA, 2015. Available online: https://www.fda.gov/media/85076/download (accessed on 22 December 2021).
- Committee for Human Medicinal Products. ICH Guidance S10 on Photosafety Evaluation of Pharmaceuticals, Step 5; European Medicines Agency: London, UK, 2015. Available online: https://www.ema.europa.eu/en/documents/regulatory-procedural-guideline/ich-guideline-s10-photosafety-evaluation-pharmaceuticals-step-5_en.pdf (accessed on 22 December 2021).
- Regulation (EC) No 1223/2009 of the European Parliament and of the Council on Cosmetic Products. Official Journal of the European Union L342. 22 December 2009, p. 59. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:32009R1223&from=EN (accessed on 22 December 2021).
- Onoue, S.; Hosoi, K.; Wakuri, S.; Iwase, Y.; Yamamoto, T.; Matsuoka, N.; Nakamura, K.; Toda, T.; Takagi, H.; Osaki, N.; et al. Establishment and intra-/inter-laboratory validation of a standard protocol of reactive oxygen species assay for chemical photosafety evaluation. J. Appl. Toxicol. 2013, 33, 1241–1250. [Google Scholar] [CrossRef]
- Onoue, S.; Hosoi, K.; Toda, T.; Takagi, H.; Osaki, N.; Matsumoto, Y.; Kawakami, S.; Wakuri, S.; Iwase, Y.; Yamamoto, T.; et al. Intra-/inter-laboratory validation study on reactive oxygen species assay for chemical photosafety evaluation using two different solar simulators. Toxicol. In Vitro 2014, 28, 515–523. [Google Scholar] [CrossRef]
- Lynch, A.M.; Wilcox, P. Review of the performance of the 3T3 NRU in vitro phototoxicity assay in the pharmaceutical industry. Exp. Toxicol. Pathol. 2011, 63, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Park, H.; Lim, K.-M. Phototoxicity: Its mechanism and animal alternative test methods. Toxicol. Res. 2015, 31, 97–104. [Google Scholar] [CrossRef] [Green Version]
- OECD. In Vitro 3T3 NRU Phototoxicity Test; OECD Guideline for Testing of Chemicals No. 432; OECD: Paris, France, 2018; Available online: https://www.oecd.org/env/ehs/testing/TG432-TC-4dec-clean.pdf (accessed on 22 December 2021).
- Maciel, B.; Moreira, P.; Carmo, H.; Goncalo, M.; Lobo, J.M.S.; Almeida, I.F. Implementation of an in vitro methodology for phototoxicity evaluation in a human keratinocyte cell line. Toxicol. In Vitro 2019, 61, 104618. [Google Scholar] [CrossRef]
- Magnani, C.; Isaac, V.L.B.; Correa, M.A.; Salgado, H.R.N. Caffeic acid: A review of its potential use in medications and cosmetics. Anal. Methods 2014, 6, 3203–3210. [Google Scholar] [CrossRef]
- Choi, S.J.; Lee, S.N.; Kim, K.; Joo da, H.; Shin, S.; Lee, J.; Lee, H.K.; Kim, J.; Kwon, S.B.; Kim, M.J.; et al. Biological effects of rutin on skin aging. Int. J. Mol. Med. 2016, 38, 357–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boo, Y.C. p-Coumaric Acid as An Active Ingredient in Cosmetics: A Review Focusing on its Antimelanogenic Effects. Antioxidants 2019, 8, 275. [Google Scholar] [CrossRef] [Green Version]
- Aleixandre-Tudo, J.L.; du Toit, W. The Role of UV-visible spectroscopy for phenolic compounds quantification in winemaking. In Frontiers and New Trends in the Science of Fermented Food and Beverages; Solís-Oviedo, R.L., de la Cruz Pech-Canul, A., Eds.; IntechOpen: London, UK, 2018; Available online: https://www.intechopen.com/chapters/62738 (accessed on 22 December 2021).
- OECD. Reactive Oxygen Species (ROS) Assay for Photoreactivity; OECD Guidelines for The testing of Chemicals No. 495; OECD: Paris, France, 2019; Available online: https://www.oecd-ilibrary.org/environment/tg-495-ros-reactive-oxygen-species-assay-for-photoreactivity_915e00ac-en (accessed on 22 December 2021).
- Aitken, G.R.; Henderson, J.R.; Chang, S.C.; McNeil, C.J.; Birch-Machin, M.A. Direct monitoring of UV-induced free radical generation in HaCaT keratinocytes. Clin. Exp. Dermatol. 2007, 32, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Saija, A.; Tomaino, A.; Cascio, R.L.; Trombetta, D.; Proteggente, A.; De Pasquale, A.; Uccella, N.; Bonina, F. Ferulic and caffeic acids as potential protective agents against photooxidative skin damage. J. Sci. Food Agric. 1999, 79, 476–480. [Google Scholar] [CrossRef]
- Saija, A. in vitro and in vivo evaluation of caffeic and ferulic acids as topical photoprotective agents. Int. J. Pharm. 2000, 199, 39–47. [Google Scholar] [CrossRef]
- An, S.M.; Koh, J.S.; Boo, Y.C. p-coumaric acid not only inhibits human tyrosinase activity in vitro but also melanogenesis in cells exposed to UVB. Phytother. Res. 2010, 24, 1175–1180. [Google Scholar] [CrossRef]
- Seok, J.K.; Boo, Y.C. p-Coumaric Acid Attenuates UVB-Induced Release of Stratifin from Keratinocytes and Indirectly Regulates Matrix Metalloproteinase 1 Release from Fibroblasts. Korean J. Physiol. Pharmacol. 2015, 19, 241–247. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, K.P.; Biel, S.; Siegers, C.P. Role of flavonoids in controlling the phototoxicity of Hypericum perforatum extracts. Phytomedicine 2001, 8, 306–309. [Google Scholar] [CrossRef]
- Hinneburg, I.; Kempe, S.; Rüttinger, H.H.; Neubert, R.H.H. A CE method for measuring phototoxicity in vitro. Chromatographia 2005, 62, 325–329. [Google Scholar] [CrossRef]
- OECD. UV-VIS Absorption Spectra; OECD Test Guideline for the Testing of Chemicals No. 101; OECD: Paris, France, 1981. [Google Scholar] [CrossRef]
- Tyagi, N.; Bhardwaj, A.; Srivastava, S.K.; Arora, S.; Marimuthu, S.; Deshmukh, S.K.; Singh, A.P.; Carter, J.E.; Singh, S. Development and characterization of a novel in vitro progression model for UVB-induced skin carcinogenesis. Sci. Rep. 2015, 5, 13894. [Google Scholar] [CrossRef] [Green Version]
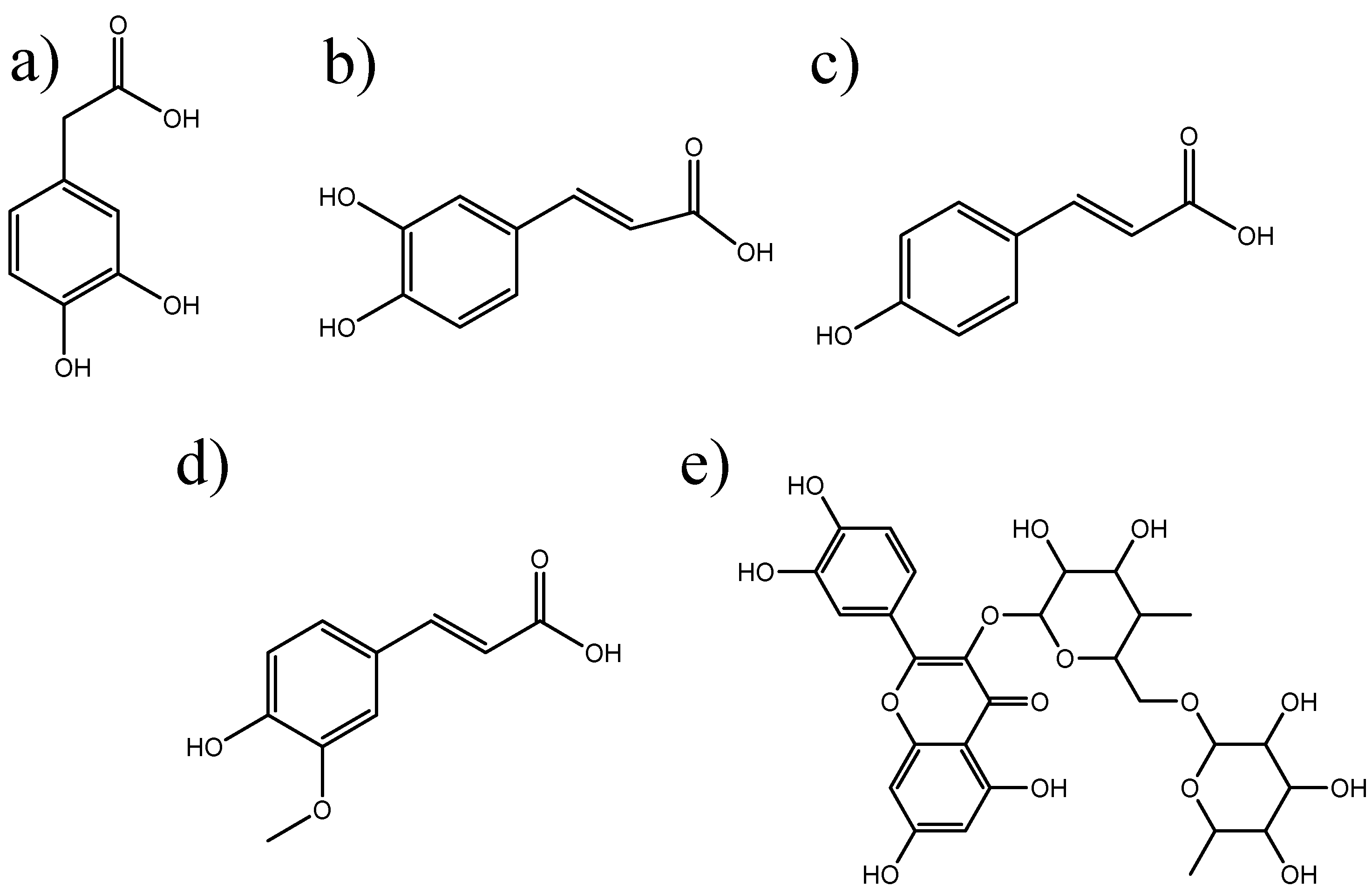


| Compounds | λmax (nm) | Absorbance | MEC (L mol−1 cm−1) |
|---|---|---|---|
| DOPAC | 286 | A286 = 0.246 | ℇ286 = 4129 |
| Caffeic acid | 331 | A331 = 1.112 | ℇ331 = 20,036 |
| p-Coumaric acid | 314 | A314 = 1.421 | ℇ314 = 23,335 |
| Rutin | 363 | A363 = 0.366 | ℇ363 = 22,354 |
| Ferulic acid | 327 | A327 = 1.011 | ℇ327 = 19,635 |
| Compounds | 1 | Photoreactivity | |
|---|---|---|---|
| Caffeic Acid | −2 | 4 | Non-photoreactive |
| Ferulic Acid | −9 | −1 | Non-photoreactive |
| p-Coumaric Acid | −3 | 2 | Non-photoreactive |
| DOPAC | 38 | 4 | Photoreactive |
| Rutin | 15 | 12 | Non-photoreactive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aguiar, B.; Carmo, H.; Garrido, J.; Sousa Lobo, J.M.; Almeida, I.F. In Vitro Evaluation of the Photoreactivity and Phototoxicity of Natural Polyphenol Antioxidants. Molecules 2022, 27, 189. https://doi.org/10.3390/molecules27010189
Aguiar B, Carmo H, Garrido J, Sousa Lobo JM, Almeida IF. In Vitro Evaluation of the Photoreactivity and Phototoxicity of Natural Polyphenol Antioxidants. Molecules. 2022; 27(1):189. https://doi.org/10.3390/molecules27010189
Chicago/Turabian StyleAguiar, Brandon, Helena Carmo, Jorge Garrido, José M. Sousa Lobo, and Isabel F. Almeida. 2022. "In Vitro Evaluation of the Photoreactivity and Phototoxicity of Natural Polyphenol Antioxidants" Molecules 27, no. 1: 189. https://doi.org/10.3390/molecules27010189
APA StyleAguiar, B., Carmo, H., Garrido, J., Sousa Lobo, J. M., & Almeida, I. F. (2022). In Vitro Evaluation of the Photoreactivity and Phototoxicity of Natural Polyphenol Antioxidants. Molecules, 27(1), 189. https://doi.org/10.3390/molecules27010189









