Valorization of Bio-Residues from the Processing of Main Portuguese Fruit Crops: From Discarded Waste to Health Promoting Compounds
Abstract
1. Introduction
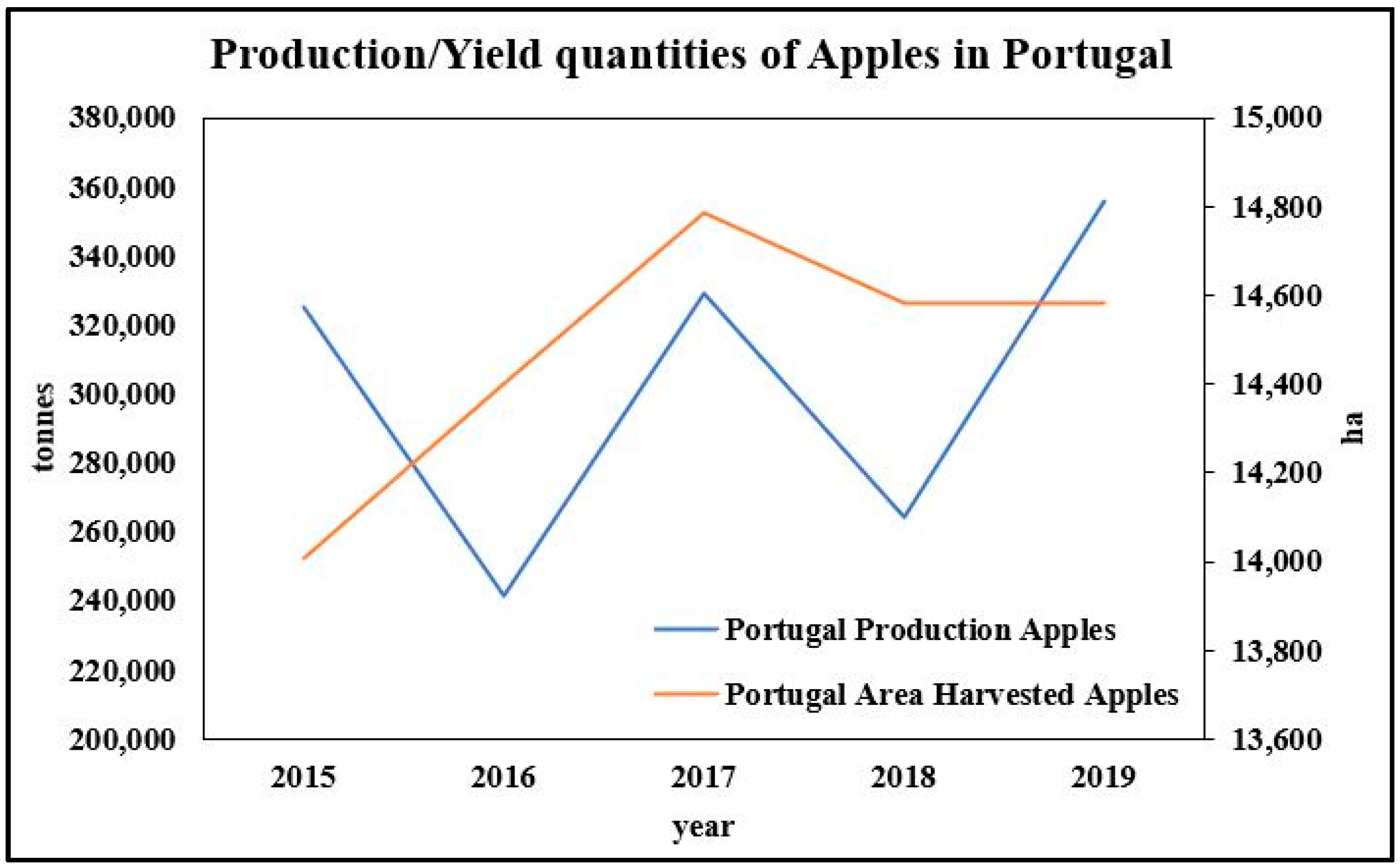
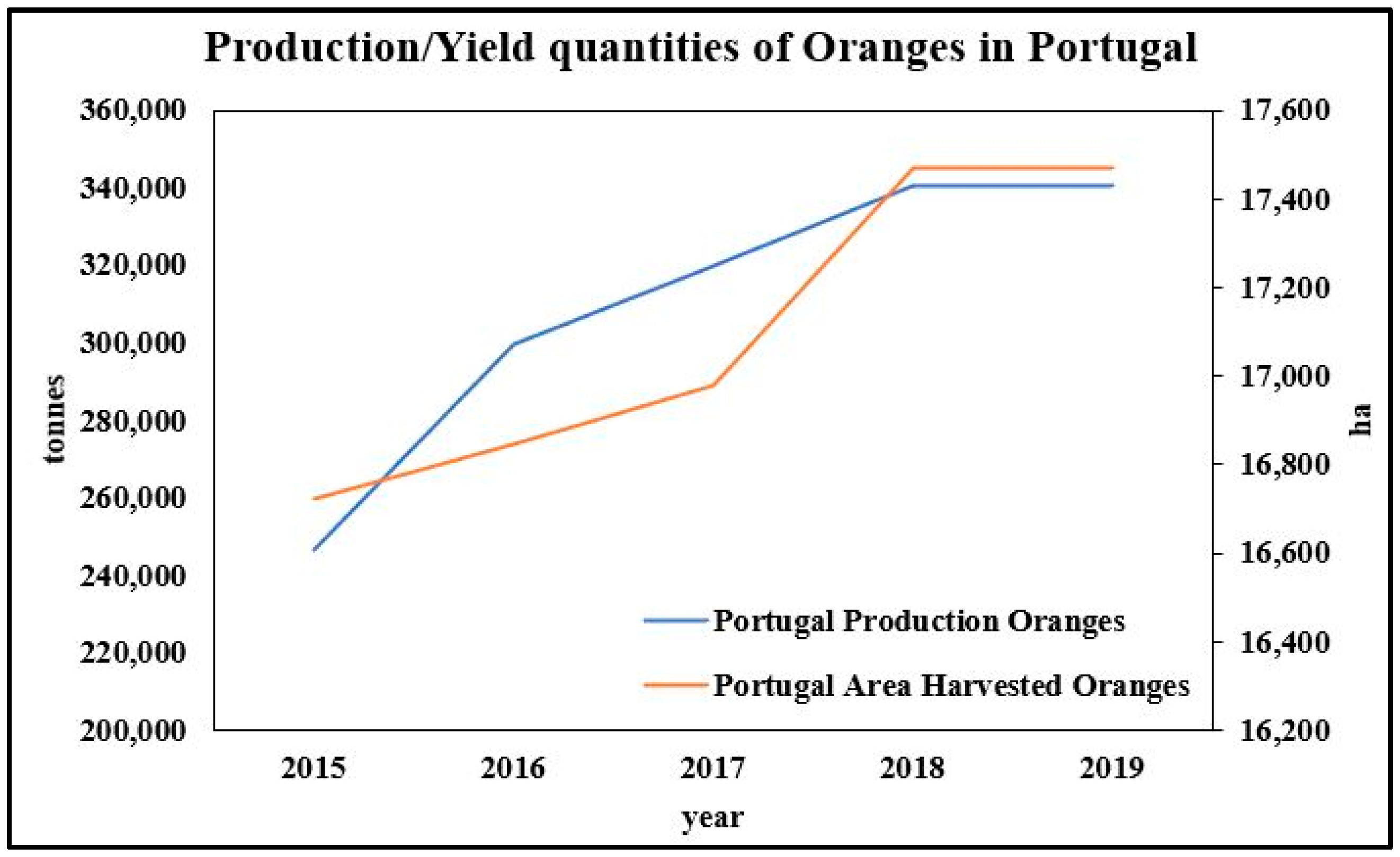
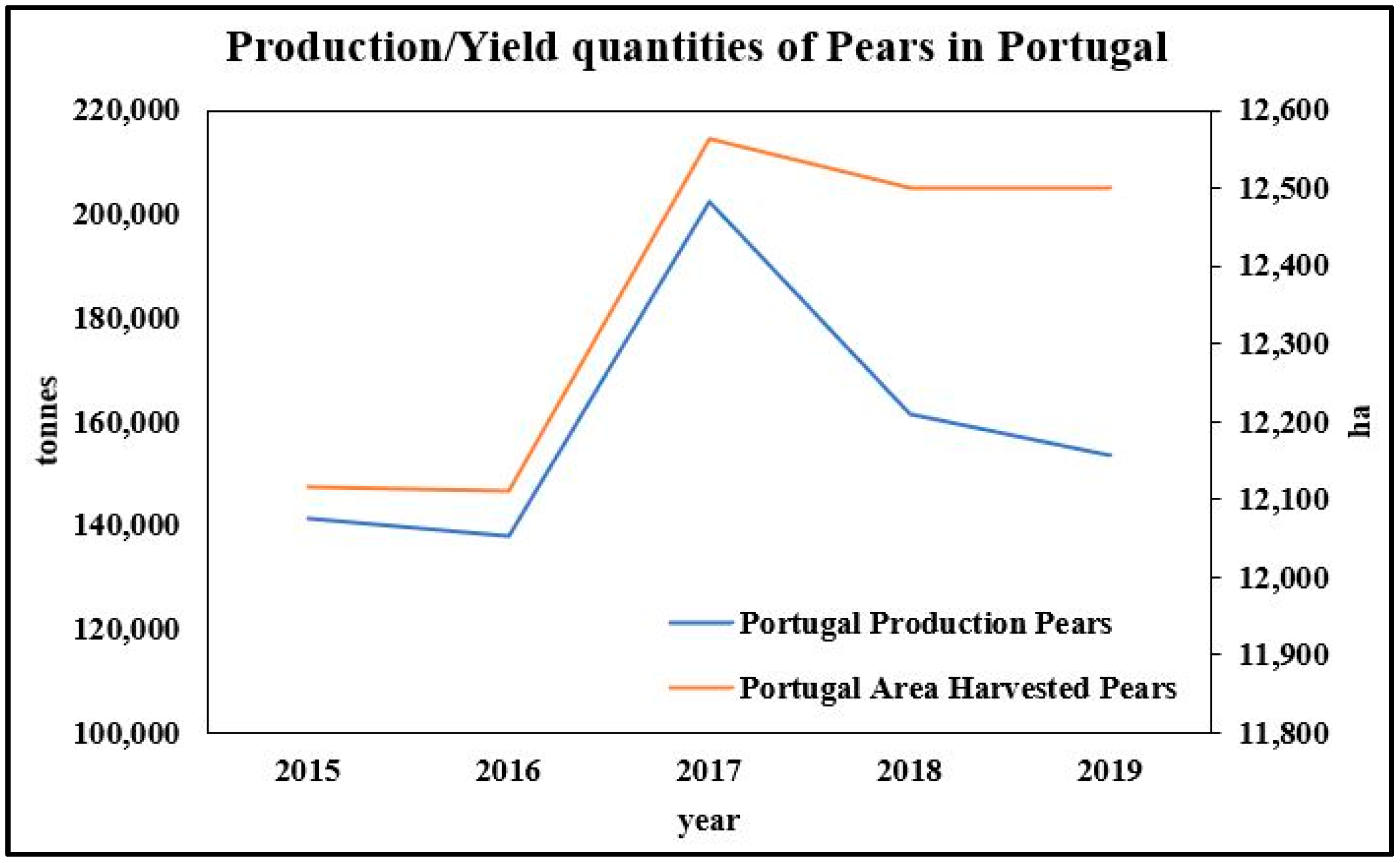
The Current Status of Fruit Production in Portugal
2. Bio-Residues from Main Portuguese Fruit Crops Processing as Sources of Bioactive/Functional Compounds
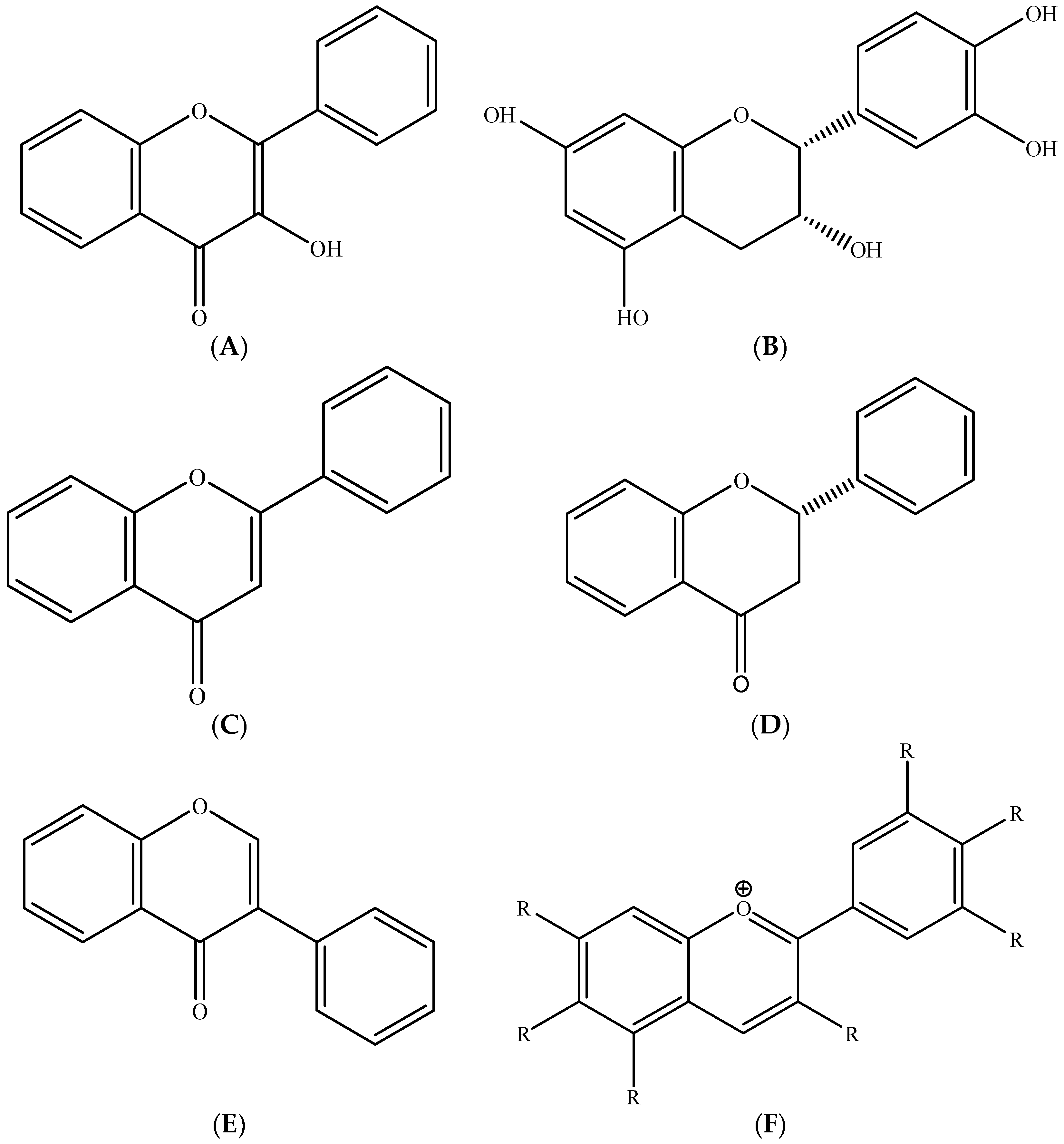
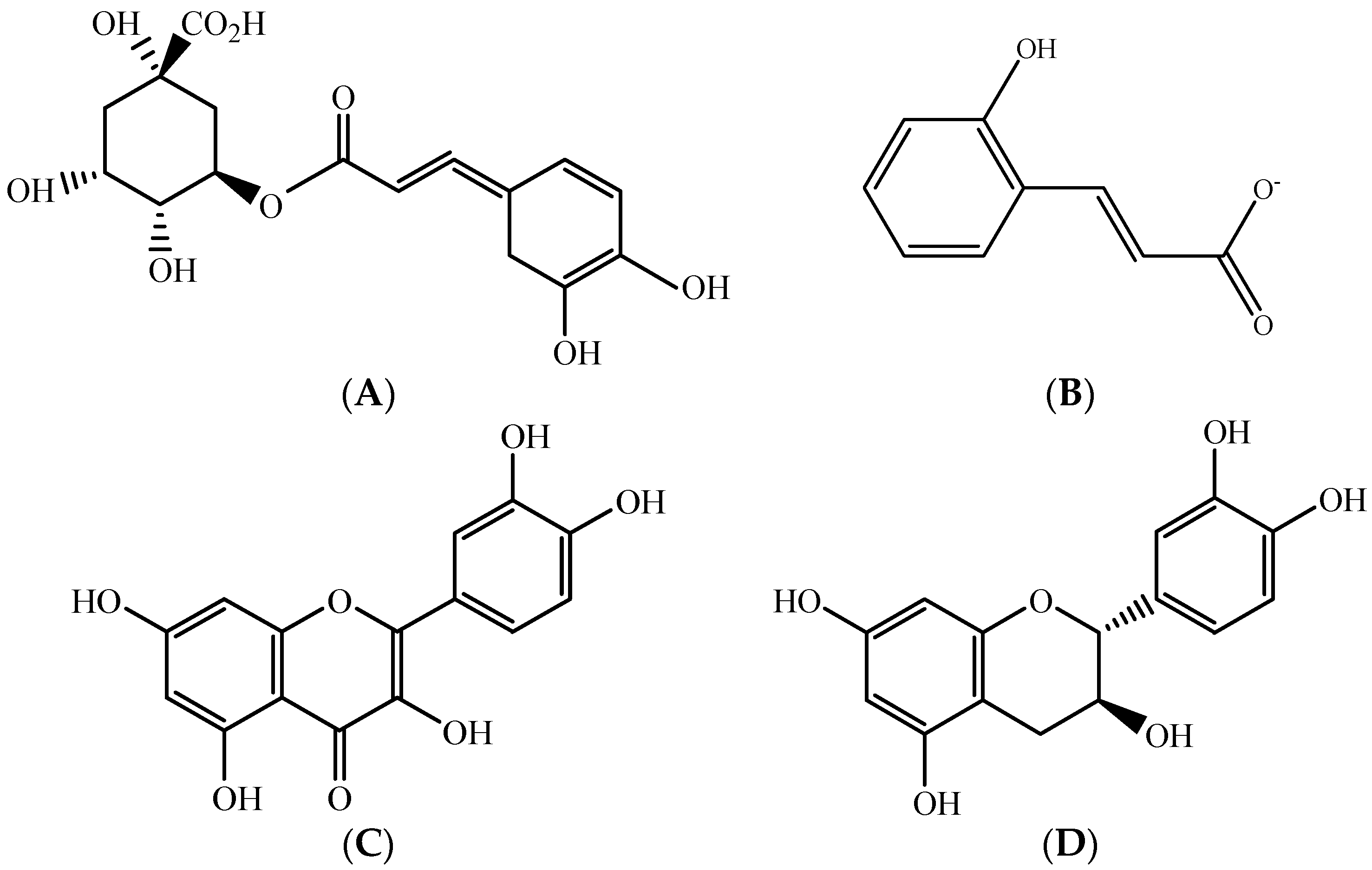
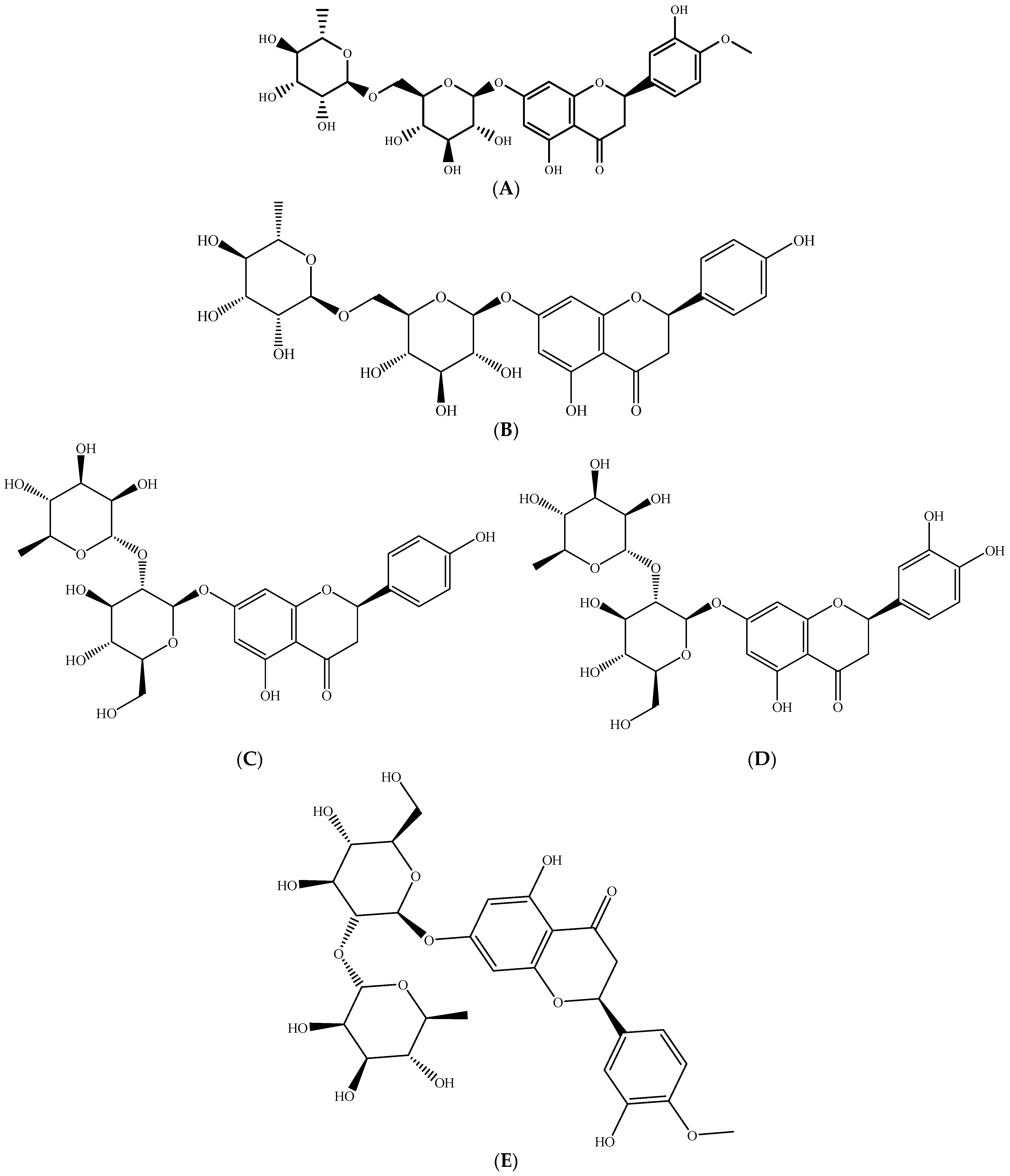
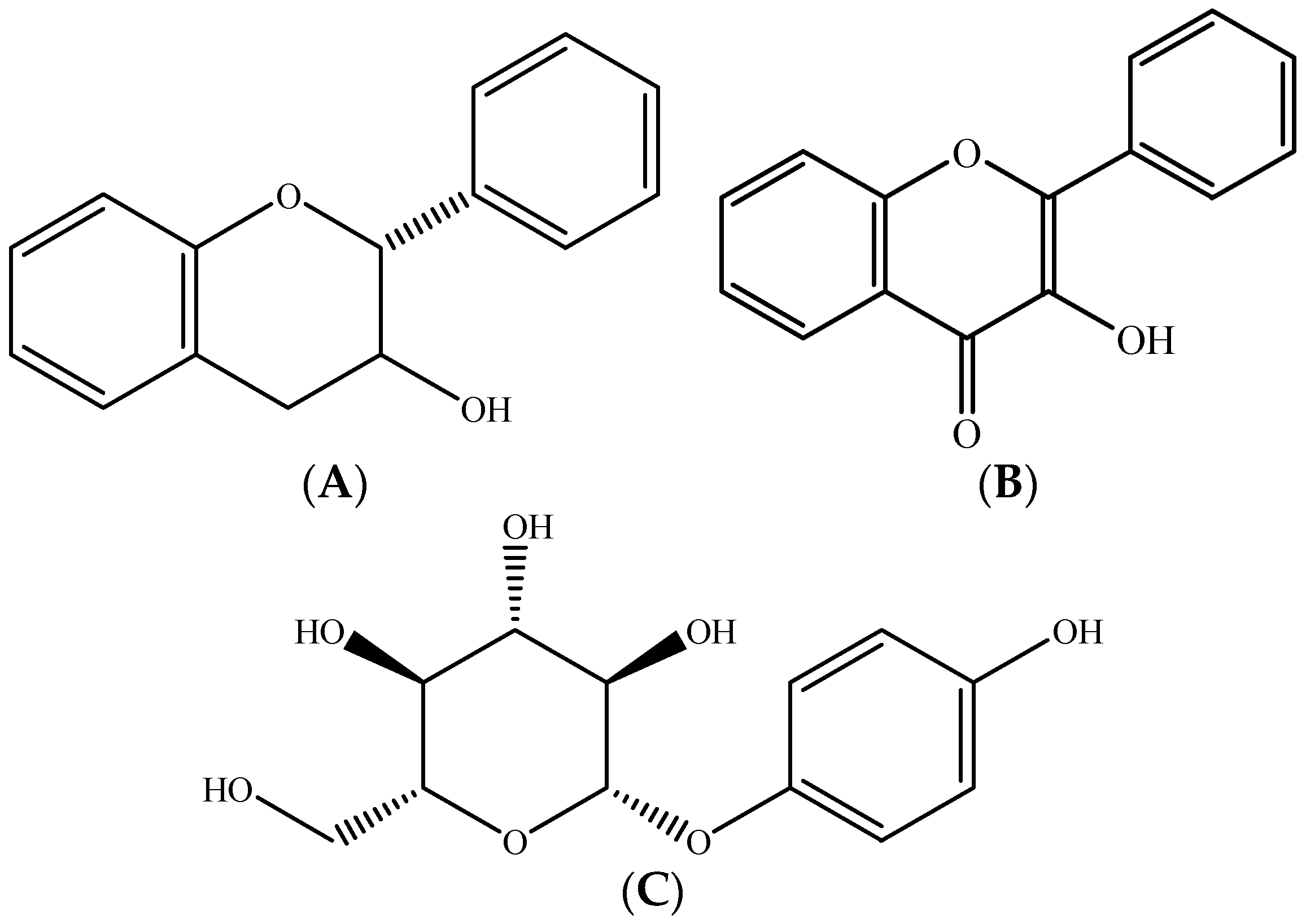
2.1. Polyphenols–Biomolecules Common to the Three Fruit Crops
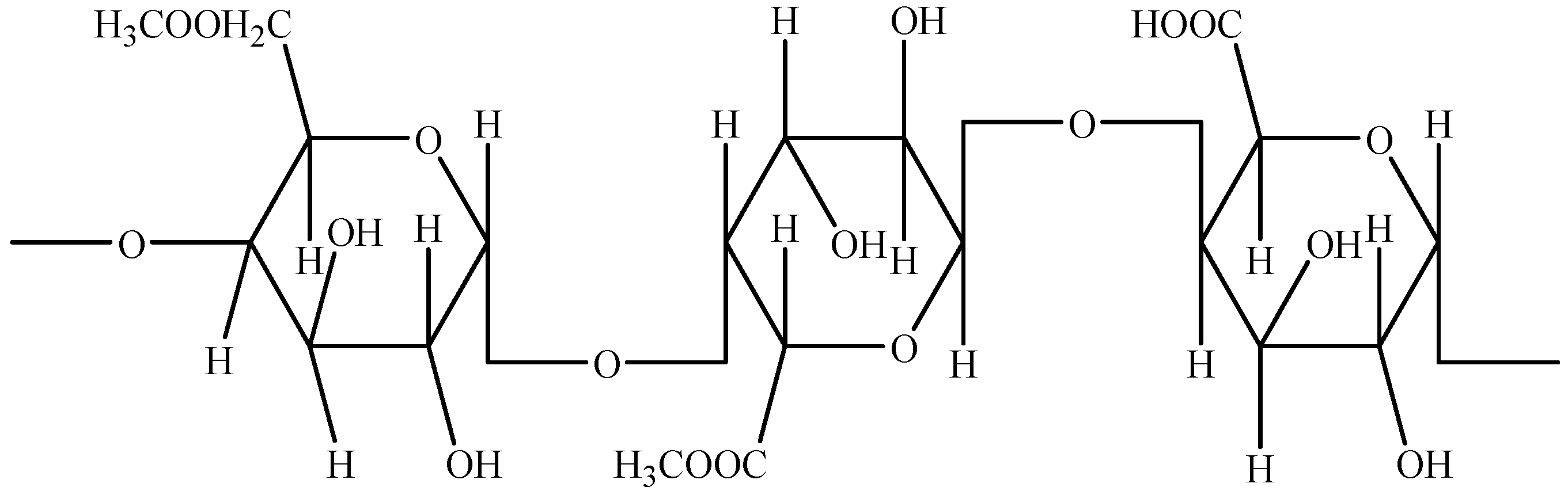
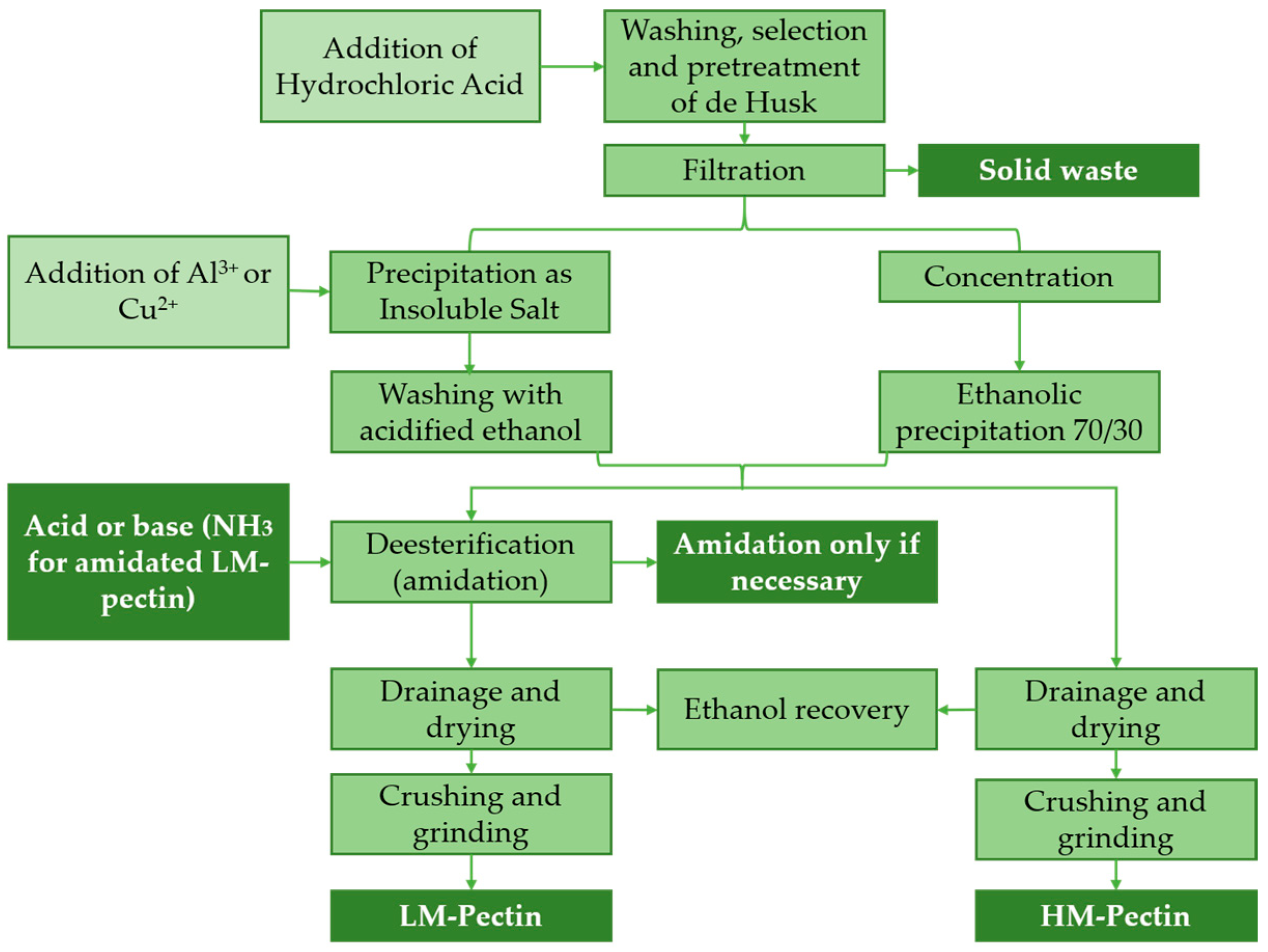
2.2. Pectins–Biomolecules Common for Apple and Orange Crops
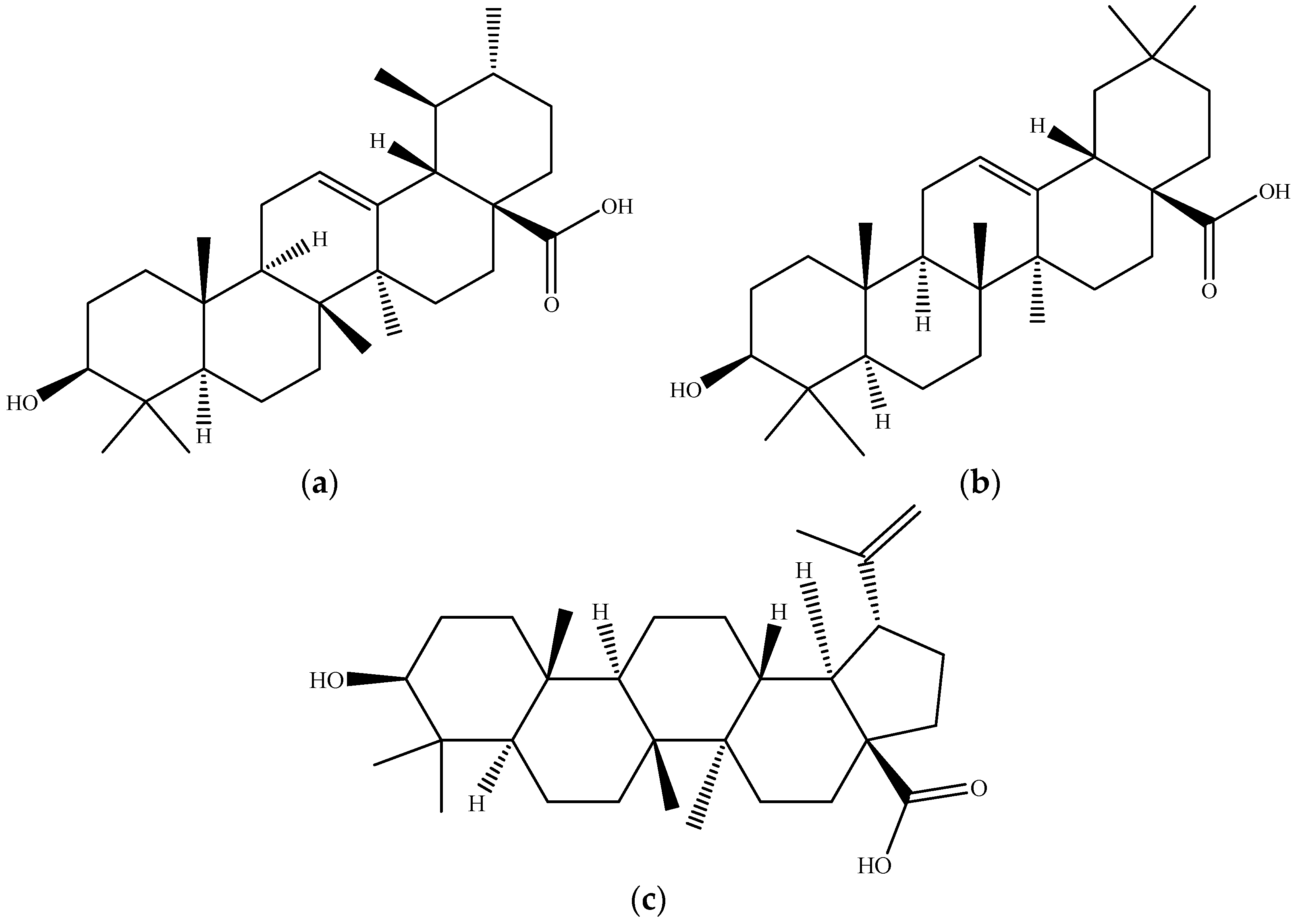
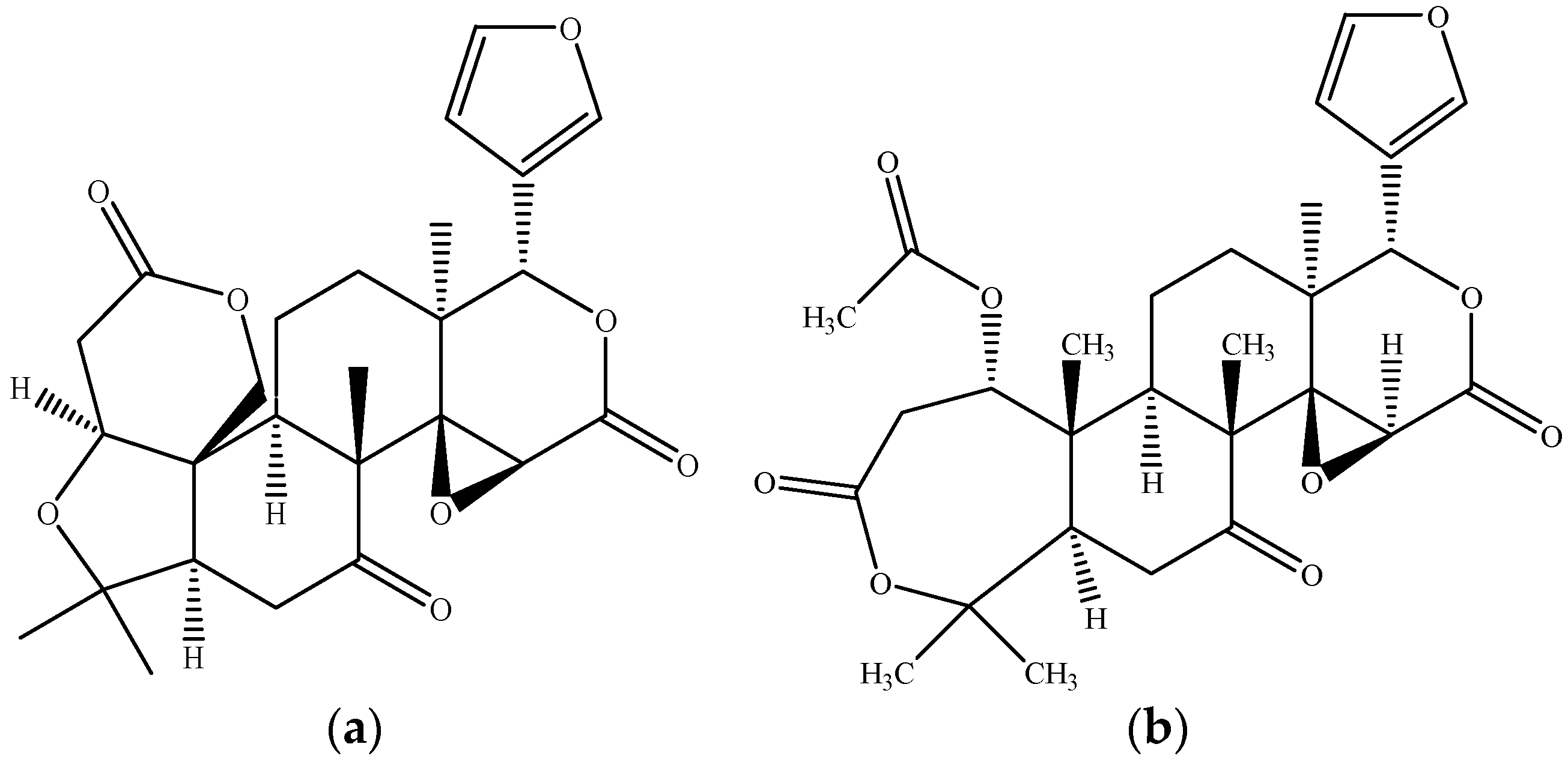
2.3. Triterpenoids–Biomolecules Common for Apple and Orange Crops
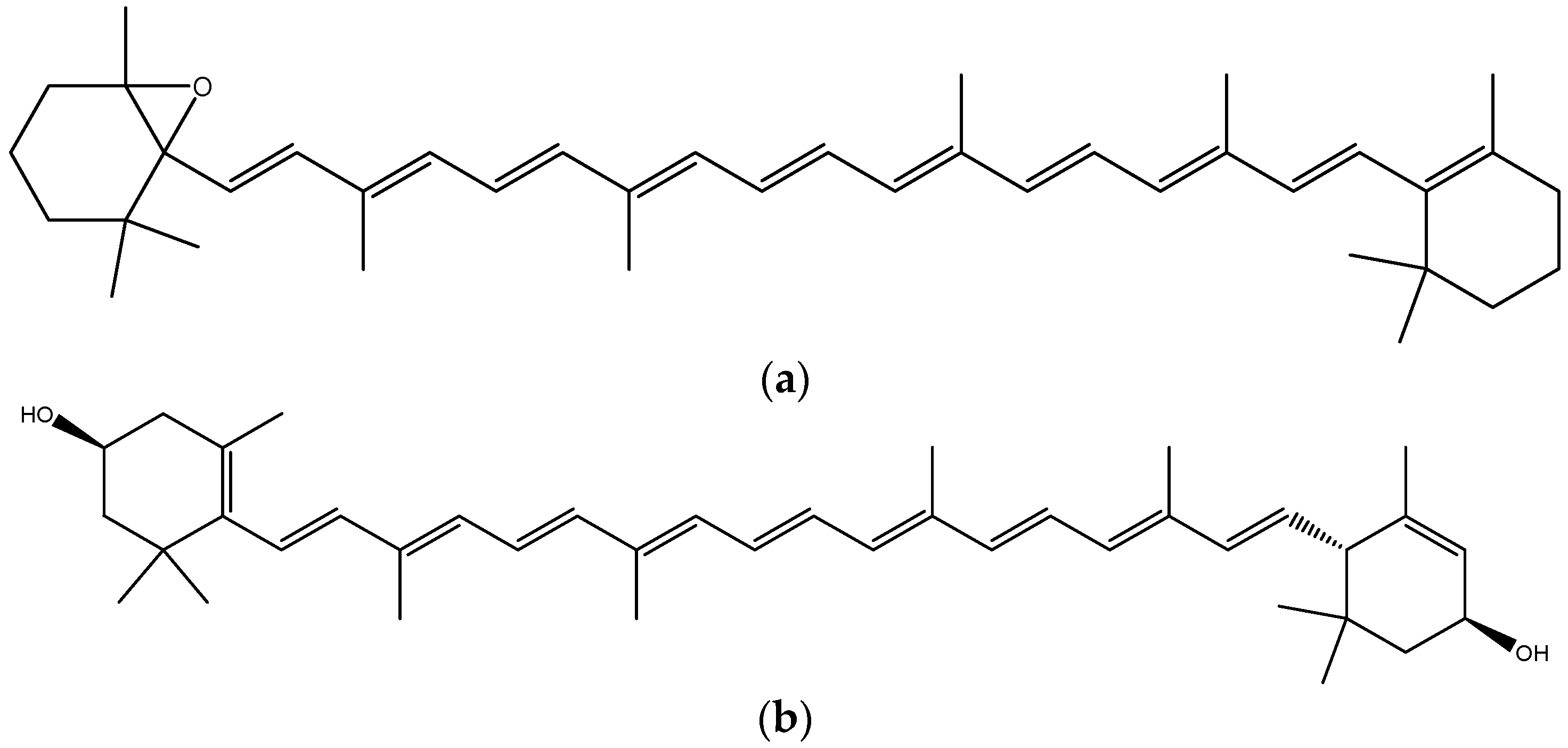
2.4. Carotenoids–Biomolecules Common for Apple and Orange Crops
2.5. Limonoids–Biomolecules Common for Orange Crops
3. Evidence-Based Bioactive and Therapeutic Potential of Bio-Residues Generated from Apple, Orange, and Pear Processing
3.1. Antioxidant Activity
3.2. Anti-Inflammatory Activity
3.3. Antimicrobial Activity
3.4. Other Bioactivities
4. Potential Applications in the Food and Nutraceutical Industry
5. Potential Applications in the Pharmaceutical and Cosmetic Industry
6. Concluding Remarks and Future Trends
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Marić, M.; Grassino, A.N.; Zhu, Z.; Barba, F.J.; Brnčić, M.; Rimac Brnčić, S. An Overview of the Traditional and Innovative Approaches for Pectin Extraction from Plant Food Wastes and By-Products: Ultrasound-, Microwaves-, and Enzyme-Assisted Extraction. Trends Food Sci. Technol. 2018, 76, 28–37. [Google Scholar] [CrossRef]
- Gustavsson, J.; Cederberg, C.; Sonesson, U. Global Food Losses and Food Waste; Food and Agriculture Organization of the United Nations: Düsseldorf, Germany, 2011. [Google Scholar]
- Andrade, M.A.; Lima, V.; Sanches Silva, A.; Vilarinho, F.; Castilho, M.C.; Khwaldia, K.; Ramos, F. Pomegranate and Grape By-Products and Their Active Compounds: Are They a Valuable Source for Food Applications? Trends Food Sci. Technol. 2019, 86, 68–84. [Google Scholar] [CrossRef]
- Ayala-Zavala, J.F.; Vega-Vega, V.; Rosas-Domínguez, C.; Palafox-Carlos, H.; Villa-Rodriguez, J.A.; Siddiqui, M.W.; Dávila-Aviña, J.E.; González-Aguilar, G.A. Agro-Industrial Potential of Exotic Fruit Byproducts as a Source of Food Additives. Food Res. Int. 2011, 44, 1866–1874. [Google Scholar] [CrossRef]
- Crawshaw, R. Food industry co-products as animal feeds. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Elsevier Inc.: Amsterdam, The Netherlands, 2009; Volume 2, pp. 391–411. ISBN 9781845697051. [Google Scholar]
- Martínez, R.; Torres, P.; Meneses, M.A.; Figueroa, J.G.; Pérez-Álvarez, J.A.; Viuda-Martos, M. Chemical, Technological and in Vitro Antioxidant Properties of Mango, Guava, Pineapple and Passion Fruit Dietary Fibre Concentrate. Food Chem. 2012, 135, 1520–1526. [Google Scholar] [CrossRef] [PubMed]
- Rezzadori, K.; Benedetti, S.; Amante, E.R. Proposals for the Residues Recovery: Orange Waste as Raw Material for New Products. Food Bioprod. Process. 2012, 90, 606–614. [Google Scholar] [CrossRef]
- Zuin, V.G.; Ramin, L.Z. Green and Sustainable Separation of Natural Products from Agro-Industrial Waste: Challenges, Potentialities, and Perspectives on Emerging Approaches. Top. Curr. Chem. 2018, 376, 3. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wang, M.; Chen, H.; Wang, Q.; Zhao, J.; Ren, X.; Li, D.; Awasthi, S.K.; Shen, F.; Li, R.; et al. Heterogeneity of Biochar Amendment to Improve the Carbon and Nitrogen Sequestration through Reduce the Greenhouse Gases Emissions during Sewage Sludge Composting. Bioresour. Technol. 2017, 224, 428–438. [Google Scholar] [CrossRef] [PubMed]
- Sivarathnakumar, S.; Jayamuthunagai, J.; Baskar, G.; Praveenkumar, R.; Selvakumari, I.A.E.; Bharathiraja, B. Bioethanol Production from Woody Stem Prosopis Juliflora Using Thermo Tolerant Yeast Kluyveromyces Marxianus and Its Kinetics Studies. Bioresour. Technol. 2019, 293, 122060. [Google Scholar] [CrossRef]
- Tian, Y.; Wang, F.; Djandja, J.O.; Zhang, S.L.; Xu, Y.P.; Duan, P.G. Hydrothermal Liquefaction of Crop Straws: Effect of Feedstock Composition. Fuel 2020, 265, 116946. [Google Scholar] [CrossRef]
- Barreira, J.C.M.; Arraibi, A.A.; Ferreira, I.C.F.R. Bioactive and Functional Compounds in Apple Pomace from Juice and Cider Manufacturing: Potential Use in Dermal Formulations. Trends Food Sci. Technol. 2019, 90, 76–87. [Google Scholar] [CrossRef]
- Lee, J.K.; Patel, S.K.S.; Sung, B.H.; Kalia, V.C. Biomolecules from Municipal and Food Industry Wastes: An Overview. Bioresour. Technol. 2020, 298, 122346. [Google Scholar] [CrossRef]
- Dahiya, S.; Kumar, A.N.; Shanthi Sravan, J.; Chatterjee, S.; Sarkar, O.; Mohan, S.V. Food Waste Biorefinery: Sustainable Strategy for Circular Bioeconomy. Bioresour. Technol. 2018, 248, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Qin, S.; Shekher Giri, B.; Kumar Patel, A.; Sar, T.; Liu, H.; Chen, H.; Juneja, A.; Kumar, D.; Zhang, Z.; Kumar Awasthi, M.; et al. Resource Recovery and Biorefinery Potential of Apple Orchard Waste in the Circular Bioeconomy. Bioresour. Technol. 2021, 321, 124496. [Google Scholar] [CrossRef] [PubMed]
- Agrotec. Maçã com Resíduos Zero Ao Nível de Aplicações Em Pós-Colheita—SafeApple|Agrotec.Pt. Available online: http://www.agrotec.pt/noticias/maca-com-residuos-zero-ao-nivel-de-aplicacoes-em-pos-colheita-safeapple/ (accessed on 8 January 2021).
- FAOSTAT. Available online: http://www.fao.org/faostat/es/#data/QC/ (accessed on 11 January 2021).
- Instituto Nacional de Estatística Boletim Mensal Da Agricultura e Pescas. Available online: https://www.ine.pt/xurl/pub/369849534 (accessed on 7 January 2021).
- Cargnin, S.T.; Gnoatto, S.B. Ursolic Acid from Apple Pomace and Traditional Plants: A Valuable Triterpenoid with Functional Properties. Food Chem. 2017, 220, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.H.; Chen, L.C.; Ho, Y.S. An Apple a Day to Prevent Cancer Formation: Reducing Cancer Risk with Flavonoids. J. Food Drug Anal. 2017, 25, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Vendruscolo, F.; Albuquerque, P.M.; Streit, F.; Esposito, E.; Ninow, J.L. Apple Pomace: A Versatile Substrate for Biotechnological Applications. Crit. Rev. Biotechnol. 2008, 28, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Bhushan, S.; Kalia, K.; Sharma, M.; Singh, B.; Ahuja, P.S. Processing of Apple Pomace for Bioactive Molecules. Crit. Rev. Biotechnol. 2008, 28, 285–296. [Google Scholar] [CrossRef]
- Cavaco, A.M.; Cruz, S.P.; Antunes, M.D.; Guerra, R.; Pires, R.; Afonso, A.M.; Brázio, A.; Silva, L.; Lucas, M.R.; Daniel, M.; et al. Spatiotemporal Modelling of the Quality and Ripening of Two Cultivars of “Algarve Citrus” Orchards at Different Edaphoclimatic Conditions. Postharvest Biol. Technol. 2021, 172, 111386. [Google Scholar] [CrossRef]
- Madeira, E.; Guerreiro, J.; Carvalho, A. Sapientia: Estratégias Técnicas e Institucionais Para o Desenvolvimento Da Citricultura Algarvia—O Caso Da IGP “Citrinos Do Algarve”. Spat. Organ. Dyn. 2010, 4, 57–71. [Google Scholar]
- Garcia-Castello, E.M.; Mayor, L.; Chorques, S.; Argüelles, A.; Vidal-Brotóns, D.; Gras, M.L. Reverse Osmosis Concentration of Press Liquid from Orange Juice Solid Wastes: Flux Decline Mechanisms. J. Food Eng. 2011, 106, 199–205. [Google Scholar] [CrossRef]
- Chen, X.M.; Tait, A.R.; Kitts, D.D. Flavonoid Composition of Orange Peel and Its Association with Antioxidant and Anti-Inflammatory Activities. Food Chem. 2017, 218, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Raffo, M.D.; Ponce, N.M.A.; Sozzi, G.O.; Vicente, A.R.; Stortz, C.A. Compositional Changes in Bartlett Pear (Pyrus communis L.) Cell Wall Polysaccharides as Affected by Sunlight Conditions. J. Agric. Food Chem. 2011, 59, 12155–12162. [Google Scholar] [CrossRef]
- Silva, G.J.; Souza, T.M.; Barbieri, R.L.; Costa de Oliveira, A. Origin, Domestication, and Dispersing of Pear (Pyrus Spp.). Adv. Agric. 2014, 2014, 541097. [Google Scholar] [CrossRef]
- Brahem, M.; Renard, C.M.G.C.; Eder, S.; Loonis, M.; Ouni, R.; Mars, M.; Le Bourvellec, C. Characterization and Quantification of Fruit Phenolic Compounds of European and Tunisian Pear Cultivars. Food Res. Int. 2017, 95, 125–133. [Google Scholar] [CrossRef]
- Sarkar, D.; Ankolekar, C.; Pinto, M.; Shetty, K. Dietary Functional Benefits of Bartlett and Starkrimson Pears for Potential Management of Hyperglycemia, Hypertension and Ulcer Bacteria Helicobacter Pylori While Supporting Beneficial Probiotic Bacterial Response. Food Res. Int. 2015, 69, 80–90. [Google Scholar] [CrossRef]
- Barbosa, A.C.L.; Sarkar, D.; Pinto, M.D.S.; Ankolekar, C.; Greene, D.; Shetty, K. Type 2 Diabetes Relevant Bioactive Potential of Freshly Harvested and Long-Term Stored Pears Using in Vitro Assay Models. J. Food Biochem. 2013, 37, 677–686. [Google Scholar] [CrossRef]
- Bhargava, N.; Sharanagat, V.S.; Mor, R.S.; Kumar, K. Active and Intelligent Biodegradable Packaging Films Using Food and Food Waste-Derived Bioactive Compounds: A Review. Trends Food Sci. Technol. 2020, 105, 385–401. [Google Scholar] [CrossRef]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory by-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar]
- Grigoras, C.G.; Destandau, E.; Fougère, L.; Elfakir, C. Evaluation of Apple Pomace Extracts as a Source of Bioactive Compounds. Ind. Crop. Prod. 2013, 49, 794–804. [Google Scholar] [CrossRef]
- Farneti, B.; Masuero, D.; Costa, F.; Magnago, P.; Malnoy, M.; Costa, G.; Vrhovsek, U.; Mattivi, F. Is There Room for Improving the Nutraceutical Composition of Apple? J. Agric. Food Chem. 2015, 63, 2750–2759. [Google Scholar] [CrossRef] [PubMed]
- Kalinowska, M.; Bielawska, A.; Lewandowska-Siwkiewicz, H.; Priebe, W.; Lewandowski, W. Apples: Content of Phenolic Compounds vs. Variety, Part of Apple and Cultivation Model, Extraction of Phenolic Compounds, Biological Properties. Plant Physiol. Biochem. 2014, 84, 169–188. [Google Scholar] [CrossRef]
- Marini, R. Fruit Color—Promoting Red Color Development in Apple. Available online: https://extension.psu.edu/fruit-color-promoting-red-color-development-in-apple (accessed on 24 February 2021).
- Cenário Hortifruti Maçã: Uma Das Frutas Mais Apreciadas Pela Humanidade. Available online: https://saberhortifruti.com.br/maca/ (accessed on 22 February 2021).
- Waldbauer, K.; McKinnon, R.; Kopp, B. Apple Pomace as Potential Source of Natural Active Compounds. Planta Med. 2017, 83, 994–1010. [Google Scholar] [CrossRef] [PubMed]
- Roussos, P.A. Phytochemicals and Antioxidant Capacity of Orange (Citrus Sinensis (L.) Osbeck Cv. Salustiana) Juice Produced under Organic and Integrated Farming System in Greece. Sci. Hortic. 2011, 129, 253–258. [Google Scholar] [CrossRef]
- Meléndez-Martínez, A.J.; Britton, G.; Vicario, I.M.; Heredia, F.J. The Complex Carotenoid Pattern of Orange Juices from Concentrate. Food Chem. 2008, 109, 546–553. [Google Scholar] [CrossRef]
- Widmer, W.; Zhou, W.; Grohmann, K. Pretreatment Effects on Orange Processing Waste for Making Ethanol by Simultaneous Saccharification and Fermentation. Bioresour. Technol. 2010, 101, 5242–5249. [Google Scholar] [CrossRef]
- Pak, T. The Orange Fruit and Its Products|Orange Book. Available online: https://orangebook.tetrapak.com/chapter/orange-fruit-and-its-products (accessed on 1 March 2021).
- Yim, S.H.; Nam, S.H. Physiochemical, Nutritional and Functional Characterization of 10 Different Pear Cultivars (Pyrus Spp.). J. Appl. Bot. Food Qual. 2016, 89, 73–81. [Google Scholar] [CrossRef]
- Tran, M.-L. Everything You Need to Know about Preparing and Storing in Season Oranges|Stories|Kitchen Stories. Available online: https://www.kitchenstories.com/en/stories/everything-you-need-to-know-about-preparing-and-storing-in-season-oranges (accessed on 1 March 2021).
- Schumann, S. Everything to Know about Cooking and Shopping for in Season Pears|Stories|Kitchen Stories. Available online: https://www.kitchenstories.com/en/stories/everything-you-need-to-know-about-cooking-and-shopping-for-in-season-pears (accessed on 1 March 2021).
- Waldron, K. Handbook of Waste Management and Co-Product Recovery in Food Processing. Available online: https://books.google.com/books?hl=pt-BR&lr=&id=ZQ6kAgAAQBAJ&oi=fnd&pg=PP1&ots=LVPCCj-mHZ&sig=A5dBLpMfj4GVuswbkGlc-IYL4es (accessed on 13 January 2021).
- Yang, Y.Y.; Ma, S.; Wang, X.X.; Zheng, X.L. Modification and Application of Dietary Fiber in Foods. J. Chem. 2017, 2017. [Google Scholar] [CrossRef]
- Farag, M.A.; Abib, B.; Ayad, L.; Khattab, A.R. Sweet and Bitter Oranges: An Updated Comparative Review of Their Bioactives, Nutrition, Food Quality, Therapeutic Merits and Biowaste Valorization Practices. Food Chem. 2020, 331, 127306. [Google Scholar] [CrossRef]
- Ghasemi, M.; Babaeian Jelodar, N.; Modarresi, M.; Bagheri, N.; Jamali, A. Increase of Chamazulene and α-Bisabolol Contents of the Essential Oil of German Chamomile (Matricaria Chamomilla L.) Using Salicylic Acid Treatments under Normal and Heat Stress Conditions. Foods 2016, 5, 56. [Google Scholar] [CrossRef]
- Kotnik, P.; Škerget, M.; Knez, Ž. Supercritical Fluid Extraction of Chamomile Flower Heads: Comparison with Conventional Extraction, Kinetics and Scale-Up. J. Supercrit. Fluids 2007, 43, 192–198. [Google Scholar] [CrossRef]
- Corrales, M.; Toepfl, S.; Butz, P.; Knorr, D.; Tauscher, B. Extraction of Anthocyanins from Grape By-Products Assisted by Ultrasonics, High Hydrostatic Pressure or Pulsed Electric Fields: A Comparison. Innov. Food Sci. Emerg. Technol. 2008, 9, 85–91. [Google Scholar] [CrossRef]
- Tan, Y.A.; Sambanthamurthi, R.; Sundram, K.; Wahid, M.B. Valorisation of Palm By-Products as Functional Components. Eur. J. Lipid Sci. Technol. 2007, 109, 380–393. [Google Scholar] [CrossRef]
- Tsouko, E.; Alexandri, M.; Fernandes, K.V.; Freire, D.M.G.; Mallouchos, A.; Koutinas, A.A. Extraction of Phenolic Compounds from Palm Oil Processing Residues and Their Application as Antioxidants. Food Technol. Biotechnol. 2019, 57, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Faggio, C.; Sureda, A.; Morabito, S.; Sanches-Silva, A.; Mocan, A.; Nabavi, S.F.; Nabavi, S.M. Flavonoids and Platelet Aggregation: A Brief Review. Eur. J. Pharmacol. 2017, 807, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Hollman, P.C.H.; Katan, M.B. Dietary Flavonoids: Intake, Health Effects and Bioavailability. Food Chem. Toxicol. 1999, 37, 937–942. [Google Scholar] [CrossRef]
- El Gharras, H. Polyphenols: Food Sources, Properties and Applications—A Review. Int. J. Food Sci. Technol. 2009, 44, 2512–2518. [Google Scholar] [CrossRef]
- Tsao, R. Chemistry and Biochemistry of Dietary Polyphenols. Nutrients 2010, 2, 1231–1246. [Google Scholar] [CrossRef]
- de Simón, B.F.; Pérez-Ilzarbe, J.; Hernández, T.; Gómez-Cordovés, C.; Estrella, I. Importance of Phenolic Compounds for the Characterization of Fruit Juices. J. Agric. Food Chem. 1992, 40, 1531–1535. [Google Scholar] [CrossRef]
- Andreotti, C.; Costa, G.; Treutter, D. Composition of Phenolic Compounds in Pear Leaves as Affected by Genetics, Ontogenesis and the Environment. Sci. Hortic. 2006, 109, 130–137. [Google Scholar] [CrossRef]
- Kolniak-Ostek, J. Identification and Quantification of Polyphenolic Compounds in Ten Pear Cultivars by UPLC-PDA-Q/TOF-MS. J. Food Compos. Anal. 2016, 49, 65–77. [Google Scholar] [CrossRef]
- Baiano, A.; Bevilacqua, L.; Terracone, C.; Contò, F.; Del Nobile, M.A. Single and Interactive Effects of Process Variables on Microwave-Assisted and Conventional Extractions of Antioxidants from Vegetable Solid Wastes. J. Food Eng. 2014, 120, 135–145. [Google Scholar] [CrossRef]
- Vrhovsek, U.; Rigo, A.; Tonon, D.; Mattivi, F. Quantitation of Polyphenols in Different Apple Varieties. J. Agric. Food Chem. 2004, 52, 6532–6538. [Google Scholar] [CrossRef]
- Guyot, S.; Marnet, N.; Sanoner, P.; Drilleau, J.F. Variability of the Polyphenolic Composition of Cider Apple (Malus domestica) Fruits and Juices. J. Agric. Food Chem. 2003, 51, 6240–6247. [Google Scholar] [CrossRef]
- Nogata, Y.; Sakamoto, K.; Shiratsuchi, H.; Ishii, T.; Yano, M.; Ohta, H. Flavonoid Composition of Fruit Tissues of Citrus Species. Biosci. Biotechnol. Biochem. 2006, 70, 178–192. [Google Scholar] [CrossRef]
- Manthey, J.; Guthrie, N.; Grohmann, K. Biological Properties of Citrus Flavonoids Pertaining to Cancer and Inflammation. Curr. Med. Chem. 2001, 8, 135–153. [Google Scholar] [CrossRef]
- Fayek, N.M.; Farag, M.A.; Abdel Monem, A.R.; Moussa, M.Y.; Abd-Elwahab, S.M.; El-Tanbouly, N.D. Comparative Metabolite Profiling of Four Citrus Peel Cultivars via Ultra-Performance Liquid Chromatography Coupled with Quadrupole-Time-of-Flight-Mass Spectrometry and Multivariate Data Analyses. J. Chromatogr. Sci. 2019, 57, 349–360. [Google Scholar] [CrossRef]
- Es-Safi, N.E.; Guyot, S.; Ducrot, P.H. NMR, ESI/MS, and MALDI-TOF/MS Analysis of Pear Juice Polymeric Proanthocyanidins with Potent Free Radical Scavenging Activity. J. Agric. Food Chem. 2006, 54, 6969–6977. [Google Scholar] [CrossRef] [PubMed]
- Öztürk, A.; Demirsoy, L.; Demirsoy, H.; Asan, A.; Gül, O. Phenolic Compounds and Chemical Characteristics of Pears (Pyrus Communis L.). Int. J. Food Prop. 2015, 18, 536–546. [Google Scholar] [CrossRef]
- Oleszek, W.; Amiot, M.J.; Aubert, S.Y. Identification of Some Phenolics in Pear Fruit. J. Agric. Food Chem. 1994, 42, 1261–1265. [Google Scholar] [CrossRef]
- Cui, T.; Nakamura, K.; Ma, L.; Li, J.Z.; Kayahara, H. Analyses of Arbutin and Chlorogenic Acid, the Major Phenolic Constituents in Oriental Pear. J. Agric. Food Chem. 2005, 53, 3882–3887. [Google Scholar] [CrossRef]
- Lin, L.Z.; Harnly, J.M. Phenolic Compounds and Chromatographic Profiles of Pear Skins (Pyrus Spp.). J. Agric. Food Chem. 2008, 56, 9094–9101. [Google Scholar] [CrossRef] [PubMed]
- Willats, W.G.T.; Knox, J.P.; Mikkelsen, J.D. Pectin: New Insights into an Old Polymer Are Starting to Gel. Trends Food Sci. Technol. 2006, 17, 97–104. [Google Scholar] [CrossRef]
- Kumar, A.; Chauhan, G.S. Extraction and Characterization of Pectin from Apple Pomace and Its Evaluation as Lipase (Steapsin) Inhibitor. Carbohydr. Polym. 2010, 82, 454–459. [Google Scholar] [CrossRef]
- Putnik, P.; Bursać Kovačević, D.; Režek Jambrak, A.; Barba, F.J.; Cravotto, G.; Binello, A.; Lorenzo, J.M.; Shpigelman, A. Innovative “Green” and Novel Strategies for the Extraction of Bioactive Added Value Compounds from Citruswastes—A Review. Molecules 2017, 22, 680. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.M.; Fu, X.; Luo, Z.G. Properties and Extraction of Pectin-Enriched Materials from Sugar Beet Pulp by Ultrasonic-Assisted Treatment Combined with Subcritical Water. Food Chem. 2015, 168, 302–310. [Google Scholar] [CrossRef]
- Ray, S. Development of a Process for the Extraction of Pectin from Citrus Fruit Wastes Viz. Lime Peel, Spent Guava Extract, Apple Pomace Etc. Int. J. Food Saf. 2011, 13, 391–397. [Google Scholar]
- Bondonno, N.P.; Bondonno, C.P.; Ward, N.C.; Hodgson, J.M.; Croft, K.D. The Cardiovascular Health Benefits of Apples: Whole Fruit vs. Isolated Compounds. Trends Food Sci. Technol. 2017, 69, 243–256. [Google Scholar] [CrossRef]
- Gulfi, M.; Arrigoni, E.; Amadò, R. The Chemical Characteristics of Apple Pectin Influence Its Fermentability In Vitro. LWT Food Sci. Technol. 2006, 39, 1001–1004. [Google Scholar] [CrossRef]
- Aprikian, O.; Duclos, V.; Guyot, S.; Besson, C.; Manach, C.; Bernalier, A.; Morand, C.; Rémésy, C.; Demigné, C. Apple Pectin and a Polyphenol-Rich Apple Concentrate Are More Effective Together than Separately on Cecal Fermentations and Plasma Lipids in Rats. J. Nutr. 2003, 133, 1860–1865. [Google Scholar] [CrossRef]
- Andoh, A.; Tsujikawa, T.; Fujiyama, Y. Role of Dietary Fiber and Short-Chain Fatty Acids in the Colon. Curr. Pharm. Des. 2005, 9, 347–358. [Google Scholar] [CrossRef]
- Yeoh, S.; Shi, J.; Langrish, T.A.G. Comparisons between Different Techniques for Water-Based Extraction of Pectin from Orange Peels. Desalination 2008, 218, 229–237. [Google Scholar] [CrossRef]
- Ruano, P.; Lazo Delgado, L.; Picco, S.; Villegas, L.; Tonelli, F.; Eduardo Aguilera Merlo, M.; Rigau, J.; Diaz, D.; Masuelli, M. Extraction and Characterization of Pectins From Peels of Criolla Oranges (Citrus sinensis): Experimental Reviews. In Pectins—Extraction, Purification, Characterization and Applications; IntechOpen: London, UK, 2020. [Google Scholar]
- Georgiev, Y.N.; Ognyanov, M.H.; Denev, P.N.; Kratchanova, M.G. Perspective Therapeutic Effects of Immunomodulating Acidic Herbal Heteropolysaccharides and Their Complexes in Functional and Dietary Nutrition. In Therapeutic Foods; Elsevier: Amsterdam, The Netherlands, 2018; pp. 285–327. [Google Scholar]
- Ferreira, S.S.; Passos, C.P.; Madureira, P.; Vilanova, M.; Coimbra, M.A. Structure-Function Relationships of Immunostimulatory Polysaccharides: A Review. Carbohydr. Polym. 2015, 132, 378–396. [Google Scholar] [CrossRef]
- Jung, C.; Hugot, J.P.; Barreau, F. Peyer’s Patches: The Immune Sensors of the Intestine. No Title. Int. J. Inflam. 2010. [Google Scholar] [CrossRef] [PubMed]
- Yamada, H.; Kiyohara, H. Immunomodulating Activity of Plant Polysaccharide Structures. Glycobiology 2007, 663–694. [Google Scholar] [CrossRef]
- Bishayee, A.; Ahmed, S.; Brankov, N.; Perloff, M. Triterpenoids as Potential Agents for the Chemoprevention and Therapy of Breast Cancer. Front. Biosci. 2011, 16, 980–996. [Google Scholar] [CrossRef] [PubMed]
- Domingues, R.; Guerra, A.; Duarte, M.; Freire, C.; Neto, C.; Silva, C.; Silvestre, A. Bioactive Triterpenic Acids: From Agroforestry Biomass Residues to Promising Therapeutic Tools. Mini-Rev. Org. Chem. 2014, 11, 382–399. [Google Scholar] [CrossRef]
- Mueller, D.; Triebel, S.; Rudakovski, O.; Richling, E. Influence of Triterpenoids Present in Apple Peel on Inflammatory Gene Expression Associated with Inflammatory Bowel Disease (IBD). Food Chem. 2013, 139, 339–346. [Google Scholar] [CrossRef]
- Pollastri, S.; Tsonev, T.; Loreto, F. Isoprene Improves Photochemical Efficiency and Enhances Heat Dissipation in Plants at Physiological Temperatures. J. Exp. Bot. 2014, 65, 1565–1570. [Google Scholar] [CrossRef]
- Babalola, I.T.; Shode, F.O. Ubiquitous Ursolic Acid: A Potential Pentacyclic Triterpene Natural Product. J. Pharmacogn. Phytochem. 2013, 2, 214–222. [Google Scholar]
- Álvarez, R.; Meléndez-Martínez, A.J.; Vicario, I.M.; Alcalde, M.J. Carotenoid and Vitamin A Contents in Biological Fluids and Tissues of Animals as an Effect of the Diet: A Review. Food Rev. Int. 2015, 31, 319–340. [Google Scholar] [CrossRef]
- Tang, G.; Russell, R.M. Carotenoids as Provitamin A. In Carotenoids; Birkhäuser: Basel, Switzerland, 2009; pp. 149–172. [Google Scholar]
- Johnson, E.J.; Krinsky, N.I. Carotenoids and Coronary Heart Disease. In Carotenoids; Birkhäuser: Basel, Switzerland, 2009; pp. 287–300. [Google Scholar]
- Schalch, W.; Landrum, J.T.; Bone, R.A. The Eye. In Carotenoids; Birkhäuser: Basel, Switzerland, 2009; pp. 301–334. [Google Scholar]
- Krinsky, N.I.; Johnson, E.J. Carotenoid Actions and Their Relation to Health and Disease. Mol. Asp. Med. 2005, 26, 459–516. [Google Scholar] [CrossRef]
- Stinco, C.M.; Escudero-Gilete, M.L.; Heredia, F.J.; Vicario, I.M.; Meléndez-Martínez, A.J. Multivariate Analyses of a Wide Selection of Orange Varieties Based on Carotenoid Contents, Color and In Vitro Antioxidant Capacity. Food Res. Int. 2016, 90, 194–204. [Google Scholar] [CrossRef]
- Madrera, R.R.; Bedriñana, R.P.; Valles, B.S. Production and Characterization of Aroma Compounds from Apple Pomace by Solid-State Fermentation with Selected Yeasts. LWT Food Sci. Technol. 2015, 64, 1342–1353. [Google Scholar] [CrossRef]
- Gualdani, R.; Cavalluzzi, M.; Lentini, G.; Habtemariam, S. The Chemistry and Pharmacology of Citrus Limonoids. Molecules 2016, 21, 1530. [Google Scholar] [CrossRef]
- Official Journal of the European Community European Commission, Regulation (EC) No. 1333/2008 of the European Parliament v.354. Available online: https://scholar.google.com/scholar_lookup?title=Regulation (EC) No 1333%2F2008&author=European Parliament and the Council of the European Union&publication_year=2008&pages=16-33 (accessed on 22 January 2021).
- Institute of Medicine (US) Panel on Dietary Antioxidants and Related Compounds. Dietary Reference Intakes for Vitamin C, Vitamin E, Selenium, and Carotenoids; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Branen, A.L.; Davidson, P.M.; Salminen, S.; Thorngate, J.H. Food Additives, Second Edition Revised and Expanded. In Food Additives; Marcel Dekker, Inc.: Basel, Switzerland, 2001; ISBN 0824793439. [Google Scholar]
- Petersen, M.; Simmonds, M.S.J. Rosmarinic Acid. Phytochemistry 2003, 62, 121–125. [Google Scholar] [CrossRef]
- Asghari, B.; Mafakheri, S.; Zarrabi, M.M.; Erdem, S.A.; Orhan, I.E.; Bahadori, M.B. Therapeutic Target Enzymes Inhibitory Potential, Antioxidant Activity, and Rosmarinic Acid Content of Echium Amoenum. S. Afr. J. Bot. 2019, 120, 191–197. [Google Scholar] [CrossRef]
- Modjinou, T.; Versace, D.L.; Abbad-Andallousi, S.; Bousserrhine, N.; Babinot, J.; Langlois, V.; Renard, E. Antibacterial Networks Based on Isosorbide and Linalool by Photoinitiated Process. ACS Sustain. Chem. Eng. 2015, 3, 1094–1100. [Google Scholar] [CrossRef]
- Ge, L.; Zhu, M.; Xu, Y.; Li, X.; Li, D.; Mu, C. Development of Antimicrobial and Controlled Biodegradable Gelatin-Based Edible Films Containing Nisin and Amino-Functionalized Montmorillonite. Food Bioprocess Technol. 2017, 10, 1727–1736. [Google Scholar] [CrossRef]
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; de Soares, N.F.F.; Mattoso, L.H.C. Antimicrobial and Physical-Mechanical Properties of Pectin/Papaya Puree/Cinnamaldehyde Nanoemulsion Edible Composite Films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Gómez-Estaca, J.; Balaguer, M.P.; López-Carballo, G.; Gavara, R.; Hernández-Muñoz, P. Improving Antioxidant and Antimicrobial Properties of Curcumin by Means of Encapsulation in Gelatin through Electrohydrodynamic Atomization. Food Hydrocoll. 2017, 70, 313–320. [Google Scholar] [CrossRef]
- Tran, T.N.; Athanassiou, A.; Basit, A.; Bayer, I.S. Starch-Based Bio-Elastomers Functionalized with Red Beetroot Natural Antioxidant. Food Chem. 2017, 216, 324–333. [Google Scholar] [CrossRef]
- Sartori, T.; Menegalli, F.C. Development and Characterization of Unripe Banana Starch Films Incorporated with Solid Lipid Microparticles Containing Ascorbic Acid. Food Hydrocoll. 2016, 55, 210–219. [Google Scholar] [CrossRef]
- Wang, L.; Dong, Y.; Men, H.; Tong, J.; Zhou, J. Preparation and Characterization of Active Films Based on Chitosan Incorporated Tea Polyphenols. Food Hydrocoll. 2013, 32, 35–41. [Google Scholar] [CrossRef]
- López-De-Dicastillo, C.; Gómez-Estaca, J.; Catalá, R.; Gavara, R.; Hernández-Muñoz, P. Active Antioxidant Packaging Films: Development and Effect on Lipid Stability of Brined Sardines. Food Chem. 2012, 131, 1376–1384. [Google Scholar] [CrossRef]
- Anal, A.K.; Anal, A.K. Bionanotechnology and Cellular Biomaterials. In Bionanotechnology; CRC Press: Boca Raton, FL, USA, 2018; pp. 1–10. [Google Scholar]
- Kusumawati, I.; Indrayanto, G. Natural antioxidants in cosmetics. In Studies in Natural Products Chemistry; Elsevier B.V.: Amsterdam, The Netherlands, 2013; Volume 40, pp. 485–505. ISBN 9780444596031. [Google Scholar]
- Lu, Y.; Yeap Foo, L. Antioxidant and Radical Scavenging Activities of Polyphenols from Apple Pomace. Food Chem. 2000, 68, 81–85. [Google Scholar] [CrossRef]
- Schieber, A.; Hilt, P.; Endreß, H.U.; Rentschler, C.; Carle, R. A New Process for the Combined Recovery of Pectin and Phenolic Compounds from Apple Pomace. Innov. Food Sci. Emerg. Technol. 2003, 4, 99–107. [Google Scholar] [CrossRef]
- Guyot, S.; Serrand, S.; Le Quéré, J.M.; Sanoner, P.; Renard, C.M.G.C. Enzymatic Synthesis and Physicochemical Characterisation of Phloridzin Oxidation Products (POP), a New Water-Soluble Yellow Dye Deriving from Apple. Innov. Food Sci. Emerg. Technol. 2007, 8, 443–450. [Google Scholar] [CrossRef]
- Ćetković, G.; Čanadanović-Brunet, J.; Djilas, S.; Savatović, S.; Mandić, A.; Tumbas, V. Assessment of Polyphenolic Content and in Vitro Antiradical Characteristics of Apple Pomace. Food Chem. 2008, 109, 340–347. [Google Scholar] [CrossRef]
- Dhillon, G.S.; Kaur, S.; Brar, S.K. Perspective of Apple Processing Wastes as Low-Cost Substrates for Bioproduction of High Value Products: A Review. Renew. Sustain. Energy Rev. 2013, 27, 789–805. [Google Scholar] [CrossRef]
- Escarpa, A.; González, M.C. High-Performance Liquid Chromatography with Diode-Array Detection for the Determination of Phenolic Compounds in Peel and Pulp from Different Apple Varieties. J. Chromatogr. A 1998, 823, 331–337. [Google Scholar] [CrossRef]
- Wijngaard, H.; Brunton, N. The Optimization of Extraction of Antioxidants from Apple Pomace by Pressurized Liquids. J. Agric. Food Chem. 2009, 57, 10625–10631. [Google Scholar] [CrossRef]
- Bellocco, E.; Barreca, D.; Laganà, G.; Calderaro, A.; El Lekhlifi, Z.; Chebaibi, S.; Smeriglio, A.; Trombetta, D. Cyanidin-3-O-Galactoside in Ripe Pistachio (Pistachia vera L. Variety Bronte) Hulls: Identification and Evaluation of Its Antioxidant and Cytoprotective Activities. J. Funct. Foods 2016, 27, 376–385. [Google Scholar] [CrossRef]
- Belwal, T.; Huang, H.; Li, L.; Duan, Z.; Zhang, X.; Aalim, H.; Luo, Z. Optimization Model for Ultrasonic-Assisted and Scale-up Extraction of Anthocyanins from Pyrus communis ‘Starkrimson’ Fruit Peel. Food Chem. 2019, 297, 124993. [Google Scholar] [CrossRef]
- Gautam, R.; Jachak, S.M. Recent Developments in Anti-Inflammatory Natural Products. Med. Res. Rev. 2009, 29, 767–820. [Google Scholar]
- Ikeda, Y.; Murakami, A.; Ohigashi, H. Ursolic Acid: An Anti- And pro-Inflammatory Triterpenoid. Mol. Nutr. Food Res. 2008, 52, 26–42. [Google Scholar] [PubMed]
- González, R.; Ballester, I.; López-Posadas, R.; Suárez, M.D.; Zarzuelo, A.; Martínez-Augustin, O.; Sánchez de Medina, F. Effects of Flavonoids and Other Polyphenols on Inflammation. Crit. Rev. Food Sci. Nutr. 2011, 51, 331–362. [Google Scholar] [PubMed]
- Ock, K.C.; Chung, S.J.; Claycombe, K.J.; Song, W.O. Serum C-Reactive Protein Concentrations Are Inversely Associated with Dietary Flavonoid Intake in U.S. Adults. J. Nutr. 2008, 138, 753–760. [Google Scholar] [CrossRef]
- Jung, M.; Triebel, S.; Anke, T.; Richling, E.; Erkel, G. Influence of Apple Polyphenols on Inflammatory Gene Expression. Mol. Nutr. Food Res. 2009, 53, 1263–1280. [Google Scholar] [CrossRef]
- Ravn-Haren, G.; Dragsted, L.O.; Buch-Andersen, T.; Jensen, E.N.; Jensen, R.I.; Németh-Balogh, M.; Paulovicsová, B.; Bergström, A.; Wilcks, A.; Licht, T.R.; et al. Intake of Whole Apples or Clear Apple Juice Has Contrasting Effects on Plasma Lipids in Healthy Volunteers. Eur. J. Nutr. 2013, 52, 1875–1889. [Google Scholar] [CrossRef]
- Barth, S.W.; Koch, T.C.L.; Watzl, B.; Dietrich, H.; Will, F.; Bub, A. Moderate Effects of Apple Juice Consumption on Obesity-Related Markers in Obese Men: Impact of Diet-Gene Interaction on Body Fat Content. Eur. J. Nutr. 2012, 51, 841–850. [Google Scholar] [CrossRef]
- North, C.J.; Venter, C.S.; Jerling, J.C. The Effects of Dietary Fibre on C-Reactive Protein, an Inflammation Marker Predicting Cardiovascular Disease. Eur. J. Clin. Nutr. 2009, 63, 921–933. [Google Scholar] [CrossRef]
- DuBois, A.B.; Brody, A.W.; Lewis, D.H.; Burgess, B.F. Oscillation Mechanics of Lungs and Chest in Man. J. Appl. Physiol. 1956, 8, 587–594. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wang, T.; Zhou, B.; Gao, W.; Cao, J.; Huang, L. Chemical Composition and Antioxidant and Anti-Inflammatory Potential of Peels and Flesh from 10 Different Pear Varieties (Pyrus Spp.). Food Chem. 2014, 152, 531–538. [Google Scholar] [CrossRef] [PubMed]
- Arslan, R.; Bektas, N.; Ozturk, Y. Antinociceptive Activity of Methanol Extract of Fruits of Capparis Ovata in Mice. J. Ethnopharmacol. 2010, 131, 28–32. [Google Scholar] [CrossRef]
- Wang, T.; Li, X.; Zhou, B.; Li, H.; Zeng, J.; Gao, W. Anti-Diabetic Activity in Type 2 Diabetic Mice and α-Glucosidase Inhibitory, Antioxidant and Anti-Inflammatory Potential of Chemically Profiled Pear Peel and Pulp Extracts (Pyrus Spp.). J. Funct. Foods 2015, 13, 276–288. [Google Scholar] [CrossRef]
- Martelli, G.; Giacomini, D. Antibacterial and Antioxidant Activities for Natural and Synthetic Dual-Active Compounds. Eur. J. Med. Chem. 2018, 158, 91–105. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing New Antimicrobial Therapies: Are Synergistic Combinations of Plant Extracts/Compounds with Conventional Antibiotics the Solution? Pharmacogn. Rev. 2017, 11, 57–72. [Google Scholar]
- Zang, L.L.; Wu, B.N.; Lin, Y.; Wang, J.; Fu, L.; Tang, Z.Y. Research Progress of Ursolic Acid’s Anti-Tumor Actions. Chin. J. Integr. Med. 2014, 20, 72–79. [Google Scholar] [CrossRef]
- Miklasínska-Majdanik, M.; Kępa, M.; Wojtyczka, R.D.; Idzik, D.; Wąsik, T.J. Phenolic Compounds Diminish Antibiotic Resistance of Staphylococcus aureus Clinical Strains. Int. J. Environ. Res. Public Health 2018, 15, 2321. [Google Scholar] [CrossRef]
- Fialova, S.; Rendekova, K.; Mucaji, P.; Slobodnikova, L. Plant Natural Agents: Polyphenols, Alkaloids and Essential Oils as Perspective Solution of Microbial Resistance. Curr. Org. Chem. 2017, 21. [Google Scholar] [CrossRef]
- Anani, K.; Adjrah, Y.; Ameyapoh, Y.; Karou, S.D.; Agbonon, A.; De Souza, C.; Gbeassor, M. Effects of Hydroethanolic Extracts of Balanites aegyptiaca (L.) Delile (Balanitaceae) on Some Resistant Pathogens Bacteria Isolated from Wounds. J. Ethnopharmacol. 2015, 164, 16–21. [Google Scholar] [CrossRef]
- Pinho, E.; Ferreira, I.C.F.R.; Barros, L.; Carvalho, A.M.; Soares, G.; Henriques, M. Antibacterial Potential of Northeastern Portugal Wild Plant Extracts and Respective Phenolic Compounds. BioMed Res. Int. 2014, 2014, 814590. [Google Scholar] [CrossRef]
- Zuk, M.; Dorotkiewicz-Jach, A.; Drulis-Kawa, Z.; Arendt, M.; Kulma, A.; Szopa, J. Bactericidal Activities of GM Flax Seedcake Extract on Pathogenic Bacteria Clinical Strains. BMC Biotechnol. 2014, 14. [Google Scholar] [CrossRef]
- Aziz, N.H.; Farag, S.E.; Mousa, L.A.A.; Abo-Zaid, M.A. Comparative Antibacterial and Antifungal Effects of Some Phenolic Compounds. Microbios 1998, 93, 43–54. [Google Scholar]
- Li, J.; Huang, S.Y.; Deng, Q.; Li, G.; Su, G.; Liu, J.; David Wang, H.M. Extraction and Characterization of Phenolic Compounds with Antioxidant and Antimicrobial Activities from Pickled Radish. Food Chem. Toxicol. 2020, 136, 111050. [Google Scholar] [CrossRef]
- Vaquero, M.J.R.; Alberto, M.R.; de Nadra, M.C.M. Antibacterial Effect of Phenolic Compounds from Different Wines. Food Control 2007, 18, 93–101. [Google Scholar] [CrossRef]
- Caponio, F.; Gomes, T.; Pasqualone, A. Phenolic Compounds in Virgin Olive Oils: Influence of the Degree of Olive Ripeness on Organoleptic Characteristics and Shelf-Life. Eur. Food Res. Technol. 2001, 212, 329–333. [Google Scholar] [CrossRef]
- Pereira, J.A.; Oliveira, I.; Sousa, A.; Valentão, P.; Andrade, P.B.; Ferreira, I.C.F.R.; Ferreres, F.; Bento, A.; Seabra, R.M.; Estevinho, L.; et al. Phenolic Profile in the Quality Control of Walnut (Juglans regia L.) Leaves. Food Chem. Toxicol. 2008, 46, 783–789. [Google Scholar] [CrossRef]
- Llorent-Martínez, E.J.; Ortega-Barrales, P.; Zengin, G.; Mocan, A.; Simirgiotis, M.J.; Ceylan, R.; Uysal, S.; Aktumsek, A. Evaluation of Antioxidant Potential, Enzyme Inhibition Activity and Phenolic Profile of Lathyrus Cicera and Lathyrus Digitatus: Potential Sources of Bioactive Compounds for the Food Industry. Food Chem. Toxicol. 2017, 107, 609–619. [Google Scholar] [CrossRef]
- Ahn, J.; Grün, I.U.; Fernando, L.N. Antioxidant Properties of Natural Plant Extracts Containing Polyphenolic Compounds in Cooked Ground Beef. J. Food Sci. 2002, 67, 1364–1369. [Google Scholar] [CrossRef]
- Ibrahium, M.; El-Ghany, M.E.A.; Ammar, M.S. Effect of Clove Essential Oil as Antioxidant and Antimicrobial Agent on Cake Shelf Life. World J. Dairy Food Sci. 2013, 8, 140–146. [Google Scholar]
- Caleja, C.; Barros, L.; Barreira, J.C.M.; Ciric, A.; Sokovic, M.; Calhelha, R.C.; Beatriz, M.; Oliveira, P.P.; Ferreira, I.C.F.R. Suitability of Lemon Balm (Melissa officinalis L.) Extract Rich in Rosmarinic Acid as a Potential Enhancer of Functional Properties in Cupcakes. Food Chem. 2018, 250, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Wolska, K.I.; Grudniak, A.M.; Fiecek, B.; Kraczkiewicz-Dowjat, A.; Kurek, A. Antibacterial Activity of Oleanolic and Ursolic Acids and Their Derivatives. Cent. Eur. J. Biol. 2010, 5, 543–553. [Google Scholar] [CrossRef]
- Zhou, L.; Ding, Y.; Chen, W.; Zhang, P.; Chen, Y.; Lv, X. The in Vitro Study of Ursolic Acid and Oleanolic Acid Inhibiting Cariogenic Microorganisms as Well as Biofilm. Oral Dis. 2013, 19, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Arellanes, A.; Luna-Herrera, J.; Cornejo-Garrido, J.; López-García, S.; Castro-Mussot, M.E.; Meckes-Fischer, M.; Mata-Espinosa, D.; Marquina, B.; Torres, J.; Hernández-Pando, R. Ursolic and Oleanolic Acids as Antimicrobial and Immunomodulatory Compounds for Tuberculosis Treatment. BMC Complement. Altern. Med. 2013, 13. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D. Chemical Profile, Antibacterial and Antioxidant Activity of Algerian Citrus Essential Oils and Their Application in Sardina pilchardus. Foods 2015, 4, 208–228. [Google Scholar] [CrossRef]
- Jin, S.; Sato, N. Benzoquinone, the Substance Essential for Antibacterial Activity in Aqueous Extracts from Succulent Young Shoots of the Pear Pyrus Spp. Phytochemistry 2003, 62, 101–107. [Google Scholar] [CrossRef]
- Fotschki, B.; Jurgoński, A.; Juśkiewicz, J.; Kołodziejczyk, K.; Sójka, M. Effects of Dietary Addition of a Low-Pectin Apple Fibre Preparation on Rats. Polish J. Food Nutr. Sci. 2014, 64, 193–199. [Google Scholar] [CrossRef]
- Leontowicz, M.; Gorinstein, S.; Bartnikowska, E.; Leontowicz, H.; Kulasek, G.; Trakhtenberg, S. Sugar Beet Pulp and Apple Pomace Dietary Fibers Improve Lipid Metabolism in Rats Fed Cholesterol. Food Chem. 2001, 72, 73–78. [Google Scholar] [CrossRef]
- Nishina, P.M.; Freedland, R.A. The Effects of Dietary Fiber Feeding on Cholesterol Metabolism in Rats. J. Nutr. 1990, 120, 800–805. [Google Scholar] [CrossRef]
- Rago, D.; Gürdeniz, G.; Ravn-Haren, G.; Dragsted, L.O. An Explorative Study of the Effect of Apple and Apple Products on the Human Plasma Metabolome Investigated by LC–MS Profiling. Metabolomics 2014, 11, 27–39. [Google Scholar] [CrossRef]
- Garcia-Diez, F.; Garcia-Mediavilla, V.; Bayon, J.E.; Gonzalez-Gallego, J. Pectin Feeding Influences Fecal Bile Acid Excretion, Hepatic Bile Acid and Cholesterol Synthesis and Serum Cholesterol in Rats. J. Nutr. 1996, 126, 1766–1771. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Ksouri, R.; Hamdi, S. Nanoemulsions as potential delivery systems for bioactive compounds in food systems: Preparation, characterization, and applications in food industry. In Emulsions; Elsevier: Amsterdam, The Netherlands, 2016; pp. 365–403. [Google Scholar]
- Liu, B.; Xu, H.; Zhao, H.; Liu, W.; Zhao, L.; Li, Y. Preparation and Characterization of Intelligent Starch/PVA Films for Simultaneous Colorimetric Indication and Antimicrobial Activity for Food Packaging Applications. Carbohydr. Polym. 2017, 157, 842–849. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural Antioxidants: Sources, Compounds, Mechanisms of Action, and Potential Applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Donsì, F.; Annunziata, M.; Sessa, M.; Ferrari, G. Nanoencapsulation of Essential Oils to Enhance Their Antimicrobial Activity in Foods. LWT Food Sci. Technol. 2011, 44, 1908–1914. [Google Scholar] [CrossRef]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Pérez-Álvarez, J. Antifungal Activity of Lemon (Citrus Lemon L.), Mandarin (Citrus Reticulata L.), Grapefruit (Citrus Paradisi L.) and Orange (Citrus Sinensis L.) Essential Oils. Food Control 2008, 19, 1130–1138. [Google Scholar] [CrossRef]
- Reda, S.Y.; Leal, E.S.; Batista, E.A.C.; Barana, A.C.; Schnitzel, E.; Carneiro, P.I.B. Caracterização Dos Óleos Das Sementes de Limão Rosa (Citrus Limonia Osbeck) e Limão Siciliano (Citrus Limon), Um Resíduo Agroindustrial. Ciência e Tecnol. Aliment. 2005, 25, 672–676. [Google Scholar] [CrossRef][Green Version]
- Yaşar, F.; Toǧrul, H.; Arslan, N. Flow Properties of Cellulose and Carboxymethyl Cellulose from Orange Peel. J. Food Eng. 2007, 81, 187–199. [Google Scholar] [CrossRef]
- Rivas, B.; Torrado, A.; Torre, P.; Converti, A.; Domínguez, J.M. Submerged Citric Acid Fermentation on Orange Peel Autohydrolysate. J. Agric. Food Chem. 2008, 56, 2380–2387. [Google Scholar] [CrossRef]
- Bortoluzzi, R.C.; Marangoni, C. Caracterização Da Fibra Dietética Obtida Da Extração Do Suco de Laranja. Rev. Bras. Prod. Agroind. 2006, 8, 61–66. [Google Scholar] [CrossRef]
- Bicu, I.; Mustata, F. Cellulose Extraction from Orange Peel Using Sulfite Digestion Reagents. Bioresour. Technol. 2011, 102, 10013–10019. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.A.; Kefalas, P.; Papageorgiou, V.P.; Assimopoulou, A.N.; Boskou, D. Radical Scavenging Activity of Various Extracts and Fractions of Sweet Orange Peel (Citrus sinensis). Food Chem. 2006, 94, 19–25. [Google Scholar] [CrossRef]
- Raeissi, S.; Diaz, S.; Espinosa, S.; Peters, C.J.; Brignole, E.A. Ethane as an Alternative Solvent for Supercritical Extraction of Orange Peel Oils. J. Supercrit. Fluids 2008, 45, 306–313. [Google Scholar] [CrossRef]
- de Moraes Crizel, T.; Jablonski, A.; de Oliveira Rios, A.; Rech, R.; Flôres, S.H. Dietary Fiber from Orange Byproducts as a Potential Fat Replacer. LWT Food Sci. Technol. 2013, 53, 9–14. [Google Scholar] [CrossRef]
- Sudha, M.L.; Baskaran, V.; Leelavathi, K. Apple Pomace as a Source of Dietary Fiber and Polyphenols and Its Effect on the Rheological Characteristics and Cake Making. Food Chem. 2007, 104, 686–692. [Google Scholar] [CrossRef]
- Rupasinghe, H.P.V.; Wang, L.; Huber, G.M.; Pitts, N.L. Effect of Baking on Dietary Fibre and Phenolics of Muffins Incorporated with Apple Skin Powder. Food Chem. 2008, 107, 1217–1224. [Google Scholar] [CrossRef]
- Mir, S.A.; Bosco, S.J.D.; Shah, M.A.; Santhalakshmy, S.; Mir, M.M. Effect of Apple Pomace on Quality Characteristics of Brown Rice Based Cracker. J. Saudi Soc. Agric. Sci. 2017, 16, 25–32. [Google Scholar] [CrossRef]
- Dryden, M.S. Skin and Soft Tissue Infection: Microbiology and Epidemiology. Int. J. Antimicrob. Agents 2009, 34. [Google Scholar] [CrossRef]
- Shoaib Khan, H.M.; Rasool, N.A.F.; Madni, A.; Saeed, T.; Khan, B.A.; Mahmood, T. Investigation of a New Sebum Control Cream Containing Apple Juice Extract. Asian J. Chem. 2011, 23, 810–812. [Google Scholar]
- Lee, K.E.; Youm, J.K.; Lee, W.J.; Kang, S.; Kim, Y.J. Polyphenol-Rich Apple Extract Inhibits Dexamethasone-Induced Sebaceous Lipids Production by Regulating SREBP1 Expression. Exp. Dermatol. 2017, 26, 958–960. [Google Scholar] [CrossRef]
- Nasri, H.; Bahmani, M.; Shahinfard, N.; Nafchi, A.M.; Saberianpour, S.; Kopaei, M.R. Medicinal Plants for the Treatment of Acne Vulgaris: A Review of Recent Evidences. Jundishapur J. Microbiol. 2015, 8, e25580. [Google Scholar] [CrossRef]
- Działo, M.; Mierziak, J.; Korzun, U.; Preisner, M.; Szopa, J.; Kulma, A. The Potential of Plant Phenolics in Prevention and Therapy of Skin Disorders. Int. J. Mol. Sci. 2016, 17, 160. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, M.; Shinoda, I.; Fukuwatari, Y.; Hayasawa, H. Arbutin Increases the Pigmentation of Cultured Human Melanocytes Through Mechanisms Other Than the Induction of Tyrosinase Activity. Pigment Cell Res. 1998, 11, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lademann, J.; Meinke, M.C.; Sterry, W.; Darvin, M.E. Carotenoids in Human Skin. Exp. Dermatol. 2011, 20, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Anunciato, T.P.; da Rocha Filho, P.A. Carotenoids and Polyphenols in Nutricosmetics, Nutraceuticals, and Cosmeceuticals. J. Cosmet. Dermatol. 2012, 11, 51–54. [Google Scholar] [CrossRef] [PubMed]
- Fiedor, J.; Burda, K. Potential Role of Carotenoids as Antioxidants in Human Health and Disease. Nutrients 2014, 6, 466–488. [Google Scholar] [CrossRef]
- Evans, J.A.; Johnson, E.J. The Role of Phytonutrients in Skin Health. Nutrients 2010, 2, 903–928. [Google Scholar] [CrossRef]
- Faria-Silva, C.; Ascenso, A.; Costa, A.M.; Marto, J.; Carvalheiro, M.; Ribeiro, H.M.; Simões, S. Feeding the Skin: A New Trend in Food and Cosmetics Convergence. Trends Food Sci. Technol. 2020, 95, 21–32. [Google Scholar] [CrossRef]

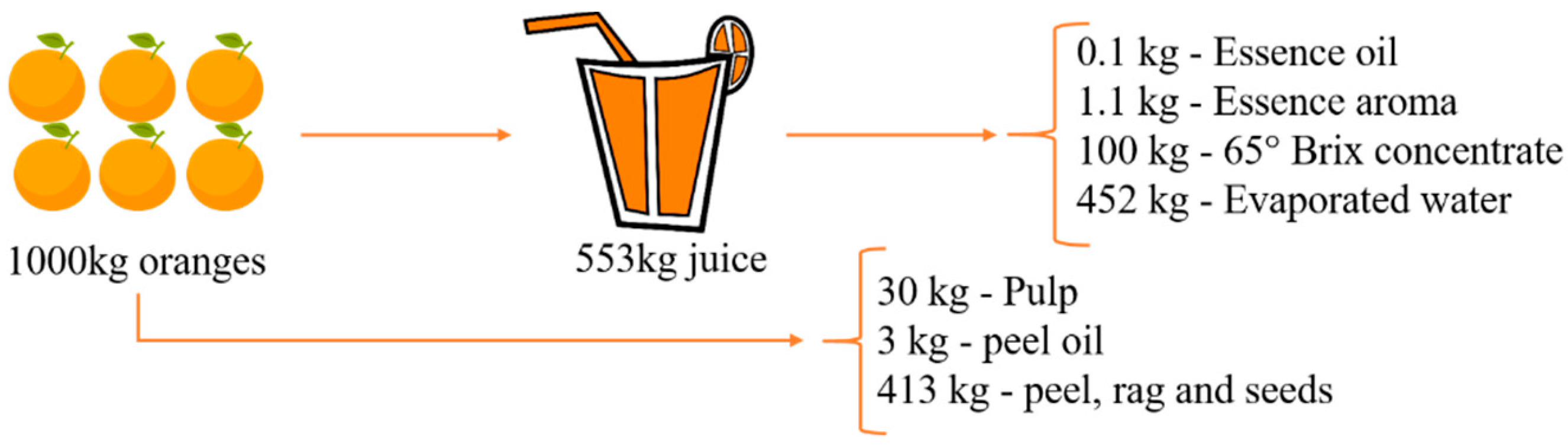

| Extractable Biomolecules | Substrate | Applications | Reference |
|---|---|---|---|
| Pectin | Citrus fruit peel, apple pomace, sunflower heads, sugar beet, and wastes from tropical fruits | Natural polymer for drug delivery, gel formation, water binder, mucoadhesive polymer, and gelling agents to improve food texture | [47] |
| Dietary fibres | Apple pomace, pear pomace, and orange pomace | Production of β-glucosidase from orange pomace, a key enzyme that can prevent the discoloration of fruit juices, and treatment of intestinal dysbiosis | [48,49] |
| Flavanones | Citrus fruit peels and residues after pressing segments and seeds | Phytomedicines, nutraceuticals, anticancer, and antimalarial | [29,47] |
| Essential oils (matricine, chamazulene, α-bisabolol) | Oranges and chamomile | Food, cosmetics, and pharmaceutical industries, treatment of aliments, including digestion, sleep disorders, wound healing, and skin infections | [50,51] |
| Anthocyanins | Grape skins like red apples | Cosmetic manufacturing, food processing, pharmaceutical industry, and solar cell development | [52] |
| Phenolic antioxidants | Apple, pear and oranges | Anti-radical, anti-aging, anti-cariogenic, hypocholesterolic agent, glycemia regulator, cosmetics (hair and skin care, anti-decay, anti-cellulite), and slimming products | [29,53,54] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pascoalino, L.A.; Reis, F.S.; Prieto, M.A.; Barreira, J.C.M.; Ferreira, I.C.F.R.; Barros, L. Valorization of Bio-Residues from the Processing of Main Portuguese Fruit Crops: From Discarded Waste to Health Promoting Compounds. Molecules 2021, 26, 2624. https://doi.org/10.3390/molecules26092624
Pascoalino LA, Reis FS, Prieto MA, Barreira JCM, Ferreira ICFR, Barros L. Valorization of Bio-Residues from the Processing of Main Portuguese Fruit Crops: From Discarded Waste to Health Promoting Compounds. Molecules. 2021; 26(9):2624. https://doi.org/10.3390/molecules26092624
Chicago/Turabian StylePascoalino, Liege A., Filipa S. Reis, Miguel A. Prieto, João C. M. Barreira, Isabel C. F. R. Ferreira, and Lillian Barros. 2021. "Valorization of Bio-Residues from the Processing of Main Portuguese Fruit Crops: From Discarded Waste to Health Promoting Compounds" Molecules 26, no. 9: 2624. https://doi.org/10.3390/molecules26092624
APA StylePascoalino, L. A., Reis, F. S., Prieto, M. A., Barreira, J. C. M., Ferreira, I. C. F. R., & Barros, L. (2021). Valorization of Bio-Residues from the Processing of Main Portuguese Fruit Crops: From Discarded Waste to Health Promoting Compounds. Molecules, 26(9), 2624. https://doi.org/10.3390/molecules26092624











