Characterization of Food Packaging Films with Blackcurrant Fruit Waste as a Source of Antioxidant and Color Sensing Intelligent Material
Abstract
1. Introduction
2. Results and Discussion
2.1. Physical, Chemical and Mechanical Characterization of Films
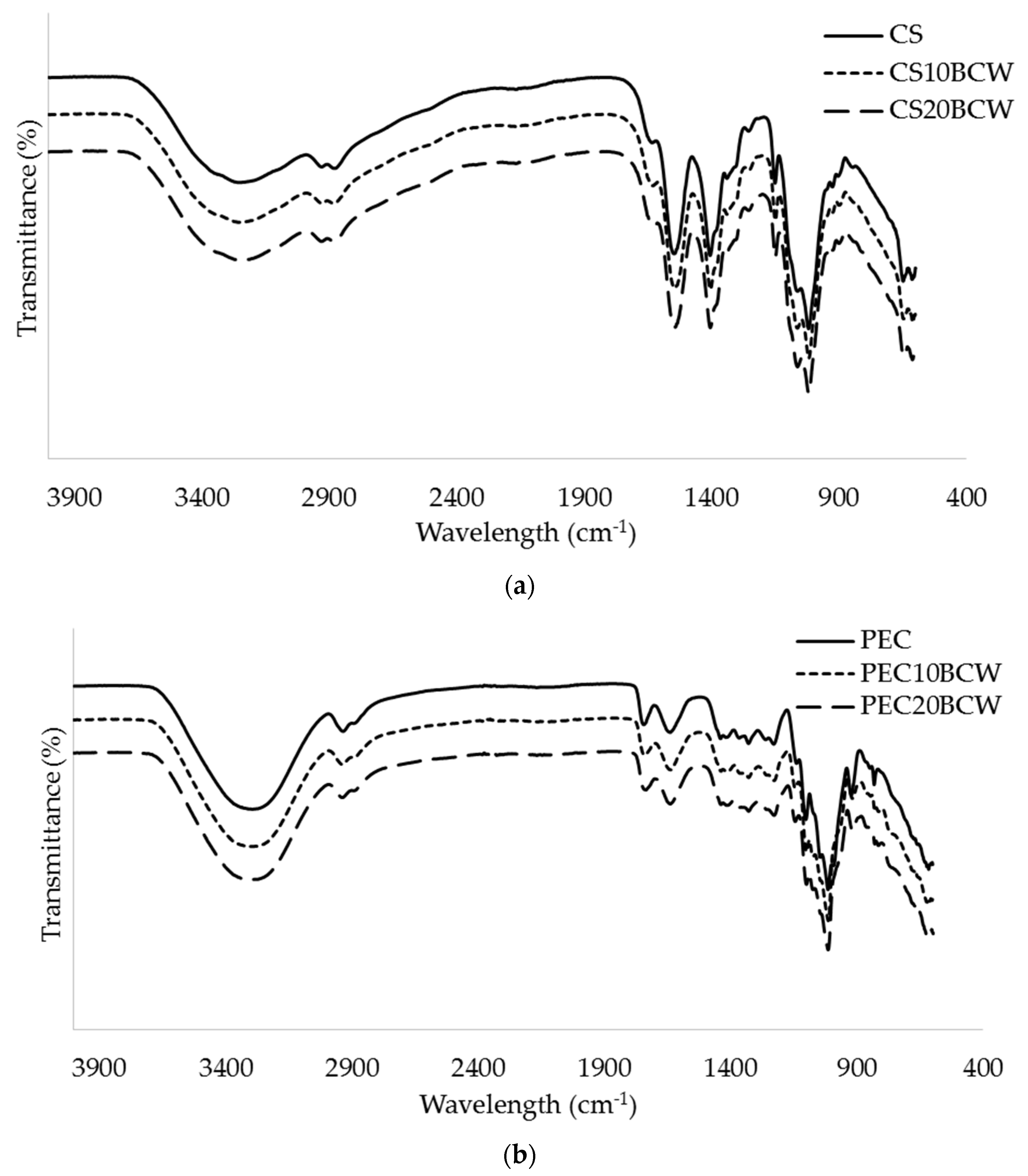
2.2. Molecular Interactions (FTIR-ATR)
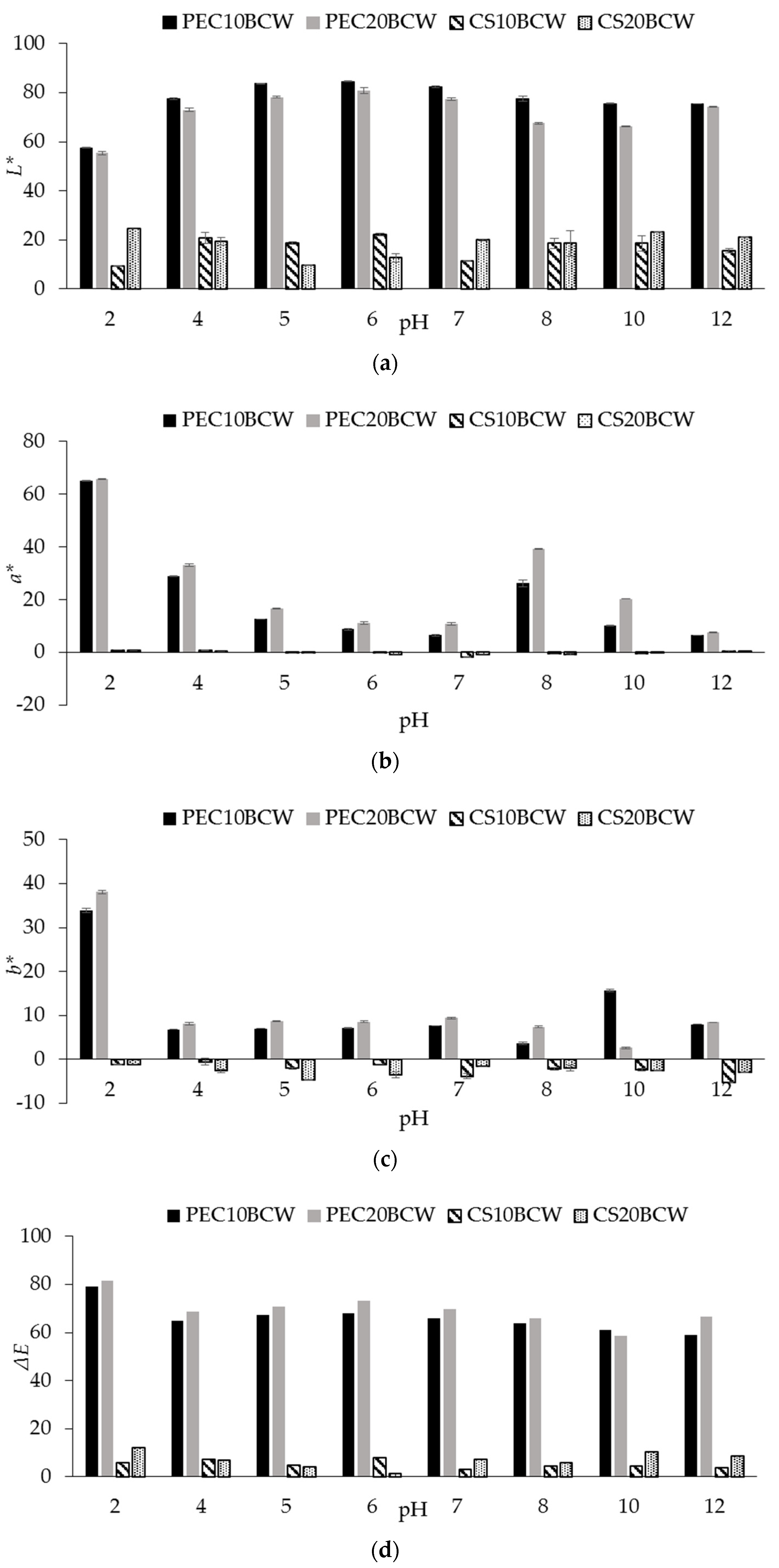
2.3. Appearance and Color Characterization
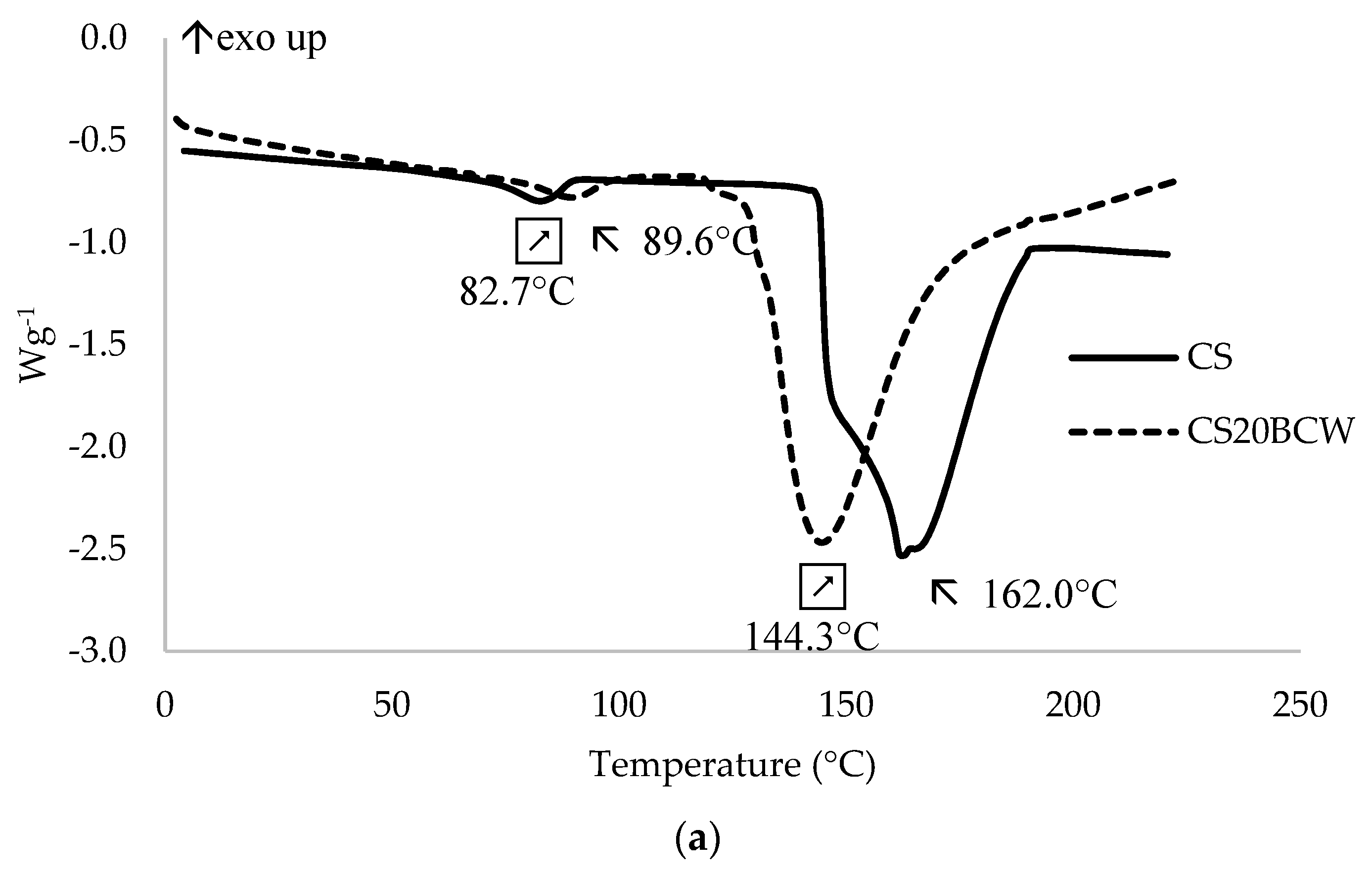
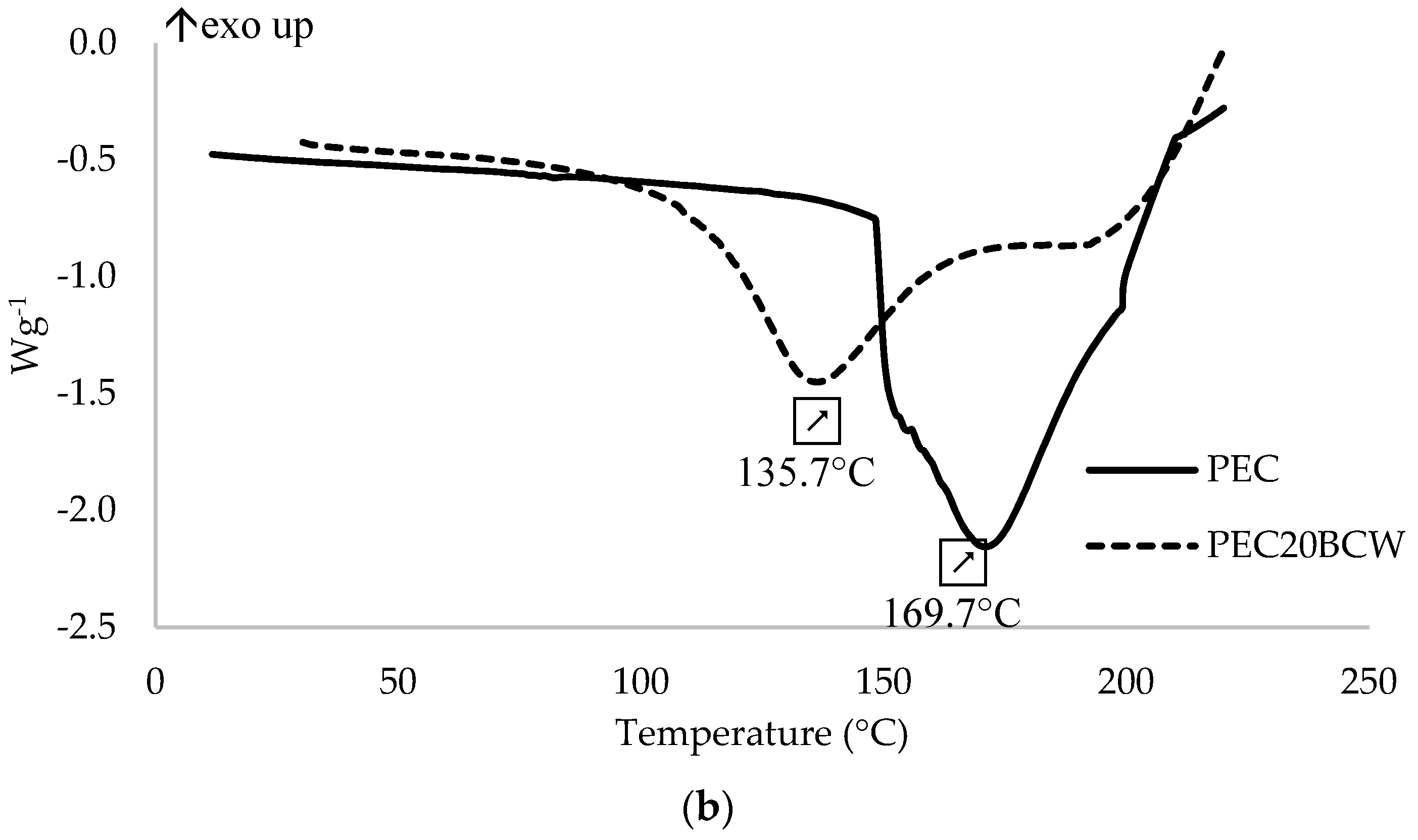
2.4. Thermal Properties
2.5. Antioxidant Film Properties
3. Materials and Methods
3.1. Materials and Reagents
3.2. Preparation of Blackcurrant Powder
3.3. Film Preparation
3.4. Physical and Chemical Characterization of Films
3.4.1. Film Thickness
3.4.2. Water Vapor Permeability (WVP) Measurements
3.4.3. Moisture Content and Water Solubility
3.4.4. Mechanical Properties
3.4.5. Film Color
3.4.6. FTIR Analysis
3.4.7. Differential Scanning Calorimetry (DSC) Analysis
3.5. Antioxidant Properties
3.5.1. Total Phenolic Content
3.5.2. Antioxidant Activity of the Films
3.6. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Heising, J.K.; van Boekel, M.A.J.S.; Dekker, M. Simulations on the prediction of cod (Gadus morhua) freshness from an intelligent packaging sensor concept. Food Packag. Shelf Life 2015, 3, 47–55. [Google Scholar] [CrossRef]
- Kuswandi, B.; Nurfawaidi, A. On-package dual sensors label based on pH indicators for real-time monitoring of beef freshness. Food Control 2017, 82, 91–100. [Google Scholar] [CrossRef]
- Kapasakalidis, P.G.; Rastall, R.A.; Gordon, M.H. Extraction of polyphenols from processed blackcurrant (Ribes nigrum L.) residues. J. Agric. Food Chem. 2006, 54, 4016–4021. [Google Scholar] [CrossRef]
- Schmidt, C.; Geweke, I.; Struck, S.; Zahn, S.; Rohm, H. Blackcurrant pomace from juice processing as partial flour substitute in savoury crackers: Dough characteristics and product properties. Int. J. Food Sci. Technol. 2018, 53, 237–245. [Google Scholar] [CrossRef]
- Alba, K.; Rizou, T.; Paraskevopoulou, A.; Campbell, G.M.; Kontogiorgos, V. Effects of blackcurrant fibre on dough physical properties and bread quality characteristics. Food Biophys. 2020, 15, 313–322. [Google Scholar] [CrossRef]
- Rohm, H.; Brennan, C.; Turner, C.; Günther, E.; Campbell, G.; Hernando, I.; Struck, S.; Kontogiorgos, V. Adding Value to fruit processing waste: Innovative ways to incorporate fibers from berry pomace in baked and extruded cereal-based foods—A SUSFOOD Project. Foods 2015, 4, 690–697. [Google Scholar] [CrossRef]
- Diez-Sánchez, E.; Quiles, A.; Llorca, E.; Reiβner, A.M.; Struck, S.; Rohm, H.; Hernando, I. Extruded flour as techno-functional ingredient in muffins with berry pomace. LWT 2019, 113. [Google Scholar] [CrossRef]
- Ju, J.; Xie, Y.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of edible coating with essential oil in food preservation. Crit. Rev. Food Sci. Nutr. 2019, 59, 2467–2480. [Google Scholar] [CrossRef] [PubMed]
- Shulga, O.; Prutulska, N.; Petrusha, O.; Smirnova, E. Edible coatings–environmental replacing of traditional candy paper wrapper of «Korivka» candies. J. Food Environ. Safety Suceava Univ. (Food Eng.) 2018, 27, 41–47. [Google Scholar]
- Pruksarojanakul, P.; Prakitchaiwattana, C.; Settachaimongkon, S.; Borompichaichartkul, C. Synbiotic edible film from konjac glucomannan composed of Lactobacillus casei-01® and Orafti®GR, and its application as coating on bread buns. J. Sci. Food Agric. 2020, 100, 2610–2617. [Google Scholar] [CrossRef] [PubMed]
- Lazaridou, A.; Biliaderis, C.G. Pectin: Technological and Physiological Properties; Kontogiorgos, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Kittur, F.S.; Kumar, K.R.; Tharanathan, R.N. Functional packaging properties of chitosan films. Eur. Food Res. Technol. 1998, 206, 44–47. [Google Scholar] [CrossRef]
- Nisar, T.; Wang, Z.C.; Yang, X.; Tian, Y.; Iqbal, M.; Guo, Y. Characterization of citrus pectin films integrated with clove bud essential oil: Physical, thermal, barrier, antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2018, 106, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Eça, K.S.; Sartori, T.; Menegalli, F.C. Films and edible coatings containing antioxidants-a review. Braz. J. Food Technol. 2014, 17, 98–112. [Google Scholar] [CrossRef]
- Kurek, M.; Garofulić, I.E.; Bakić, M.T.; Ščetar, M.; Uzelac, V.D.; Galić, K. Development and evaluation of a novel antioxidant and pH indicator film based on chitosan and food waste sources of antioxidants. Food Hydrocoll. 2018, 84, 238–246. [Google Scholar] [CrossRef]
- Shingel, K.I. Current knowledge on biosynthesis, biological activity, and chemical modification of the exopolysaccharide, pullulan. Carbohydr. Res. 2004, 339, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Bonilla, J.; Fortunati, E.; Atarés, L.; Chiralt, A.; Kenny, J.M. Physical, structural and antimicrobial properties of poly vinyl alcohol-chitosan biodegradable films. Food Hydrocoll. 2014, 35, 463–470. [Google Scholar] [CrossRef]
- Kurek, M.; Brachais, C.-H.; Nguimjeu, C.M.; Bonnotte, A.; Voilley, A.; Galić, K.; Couvercelle, J.-P.; Debeaufort, F. Structure and thermal properties of a chitosan coated polyethylene bilayer film. Polym. Degrad. Stab. 2012, 97, 1232–1240. [Google Scholar] [CrossRef]
- Queiroz, M.F.; Melo, K.R.T.; Sabry, D.A.; Sassaki, G.L.; Rocha, H.A.O. Does the use of chitosan contribute to oxalate kidney stone formation? Mar. Drugs 2015, 13, 141–158. [Google Scholar] [CrossRef] [PubMed]
- Sun-Waterhouse, D.; Nair, S.; Wibisono, R.; Wadhwa, S.S.; Massarotto, C.; Hedderley, D.I.; Zhou, J.; Jaeger, S.R.; Corrigan, V. Insights into smoothies with high levels of fibre & polyphenols: Factors influencing chemical, rheological & sensory properties. World Acad. Sci. Eng. Technol. 2010, 65, 276–285. [Google Scholar]
- Sivam, A.S.; Sun-Waterhouse, D.; Perera, C.O.; Waterhouse, G.I.N. Exploring the interactions between blackcurrant polyphenols, pectin and wheat biopolymers in model breads; a FTIR and HPLC investigation. Food Chem. 2012, 131, 802–810. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Kurek, M.; Bornaz, S.; Debeaufort, F. Barrier, structural and mechanical properties of bovine gelatin-chitosan blend films related to biopolymer interactions. J. Sci. Food Agric. 2014, 94, 2409–2419. [Google Scholar] [CrossRef]
- Srinivasa, P.C.; Ramesh, M.N.; Tharanathan, R.N. Effect of plasticizers and fatty acids on mechanical and permeability characteristics of chitosan films. Food Hydrocoll. 2007, 21, 1113–1122. [Google Scholar] [CrossRef]
- Rubilar, J.F.; Cruz, R.M.S.; Silva, H.D.; Vicente, A.A.; Khmelinskii, I.; Vieira, M.C. Physico-mechanical properties of chitosan films with carvacrol and grape seed extract. J. Food Eng. 2013. [Google Scholar] [CrossRef]
- Siripatrawan, U.; Harte, B.R. Physical properties and antioxidant activity of an active film from chitosan incorporated with green tea extract. Food Hydrocoll. 2010, 24, 770–775. [Google Scholar] [CrossRef]
- Khoo, H.E.; Azlan, A.; Tang, S.T.; Lim, S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017, 61, 1361779. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, X.; Lv, Y.; He, Q. Composition and color stability of anthocyanin-based extract from purple sweet potato. Food Sci. Technol. 2015, 35, 468–473. [Google Scholar] [CrossRef]
- Hui, A.H.; Sherkat, F. Handbook of Food Science, Technology, and Engineering; Hui, Y.H., Sherkat, F., Eds.; CRC Press: Boca Raton, FL, USA, 2005; Volume 4. [Google Scholar]
- Maciel, V.B.V.; Yoshida, C.M.P.; Franco, T.T. Chitosan/pectin polyelectrolyte complex as a pH indicator. Carbohydr. Polym. 2015, 132, 537–545. [Google Scholar] [CrossRef]
- Silva-Pereira, M.C.; Teixeira, J.A.; Pereira-Júnior, V.A.; Stefani, R. Chitosan/corn starch blend films with extract from Brassica oleraceae (red cabbage) as a visual indicator of fish deterioration. LWT Food Sci. Technol. 2015, 61, 258–262. [Google Scholar] [CrossRef]
- Yoshida, C.M.P.; Maciel, V.B.V.; Mendonça, M.E.D.; Franco, T.T. Chitosan biobased and intelligent films: Monitoring pH variations. LWT Food Sci. Technol. 2014, 55, 83–89. [Google Scholar] [CrossRef]
- Godeck, R.; Kunzek, H.; Kabbert, R. Thermal analysis of plant cell wall materials depending on the chemical structure and pretreatment prior drying. Eur. Food Res. Technol. 2001, 213, 395–404. [Google Scholar] [CrossRef]
- Iijima, M.; Nakamura, K.; Hatakeyama, T.; Hatakeyama, H. Phase transition of pectin with sorbed water. Carbohydr. Polym. 2000, 41. [Google Scholar] [CrossRef]
- Einhorn-Stoll, U.; Kunzek, H.; Dongowski, G. Thermal analysis of chemically and mechanically modified pectins. Food Hydrocoll. 2007, 21, 1101–1112. [Google Scholar] [CrossRef]
- Qin, Y.Y.; Zhang, Z.H.; Li, L.; Yuan, M.L.; Fan, J.; Zhao, T.R. Physio-mechanical properties of an active chitosan film incorporated with montmorillonite and natural antioxidants extracted from pomegranate rind. J. Food Sci. Technol. 2015, 52, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Viuda-Martos, M.; Ruiz-Navajas, Y.; Fernández-López, J.; Sendra, E.; Sayas-Barberá, E.; Pérez-Álvarez, J.A. Antioxidant properties of pomegranate (Punica granatum L.) bagasses obtained as co-product in the juice extraction. Food Res. Int. 2011, 44, 1217–1223. [Google Scholar] [CrossRef]
- Yen, M.T.; Yang, J.H.; Mau, J.L. Antioxidant properties of chitosan from crab shells. Carbohydr. Polym. 2008, 74, 840–844. [Google Scholar] [CrossRef]
- Tamer, T.M.; Valachová, K.; Mohyeldin, M.S.; Soltes, L. Free radical scavenger activity of chitosan and its aminated derivative. J. Appl. Pharm. Sci. 2016, 6, 195–201. [Google Scholar] [CrossRef]
- Pap, N.; Beszédes, S.; Pongrácz, E.; Myllykoski, L.; Gábor, M.; Gyimes, E.; Hodúr, C.; Keiski, R.L. Microwave-assisted extraction of anthocyanins from black currant marc. Food Bioprocess Technol. 2013, 6, 2666–2674. [Google Scholar] [CrossRef]
- Diaconeasa, Z.; Ştirbu, I.; Xiao, J.; Leopold, N.; Ayvaz, Z.; Danciu, C.; Ayvaz, H.; Stănilă, A.; Nistor, M.; Socaciu, C. Anthocyanins, vibrant color pigments, and their role in skin cancer prevention. Biomedicines 2020, 8, 336. [Google Scholar] [CrossRef] [PubMed]
- ASTM. ASTM E96-80 Standard Test Method for Water Vapor Transmission of Materials; ASTM B. Standard: West Conshohocken, PA, USA, 1980. [Google Scholar]
- Debeaufort, F.; Martin-Polo, M.; Voilley, A. Polarity homogeneity and structure affect water vapor permeability of model edible films. J. Food Sci. 1993, 58, 426–429. [Google Scholar] [CrossRef]
- Cuq, B.; Gontard, N.; Cuq, J.-L.; Guilbert, S. Functional properties of myofibrillar protein-based biopackaging as affected by film thickness. J. Food Sci. 1996, 61, 580–584. [Google Scholar] [CrossRef]
- ASTM. ASTM D882–97 ASTM. Standard Test Method for Tensile Properties of Thin Plastic Sheeting; ASTM B. Standard: West Conshohocken, PA, USA, 1997. [Google Scholar]
- Shortle, E.; O’Grady, M.N.; Gilroy, D.; Furey, A.; Quinn, N.; Kerry, J.P. Influence of extraction technique on the anti-oxidative potential of hawthorn (Crataegus monogyna) extracts in bovine muscle homogenates. Meat Sci. 2014, 98, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Fegredo, J.A.; Wong, M.C.Y.; Wiseman, H.; Preedy, V.R. Beer in Health and Disease Prevention; Preedy, V.R., Ed.; Academic Press: Cambridge, MA, USA, 2009; pp. 991–1002. [Google Scholar]
| Sample | Thickness (µm) | WVP (× 10−10 g m−1 s−1 Pa−1) | MC (%) | WS (%) |
|---|---|---|---|---|
| CS | 56.71 ± 1.93 c | 2.84 ± 0.13 c | 24.96 ± 0.04 a | 31.20 ± 1.20 b |
| CS10BCW | 47.83 ± 3.32 d | 2.43 ± 0.07 d | 22.13 ± 4.75 a | 35.68 ± 0.40 b |
| CS20BCW | 62.55 ± 3.79 c | 2.82 ± 0.05 c | 19.48 ± 0.62 a | 30.28 ± 1.25 b |
| PEC | 59.75 ± 2.29 c | 2.96 ± 0.08 c | 16.10 ± 0.15 b | 100.00 ± 0.00 a |
| PEC10BCW | 113.57 ± 3.20 b | 3.97 ± 0.04 a | 15.07 ± 1.19 b | 100.00 ± 0.00 a |
| PEC20BCW | 145.00 ± 4.99 a | 3.45 ± 0.09 b | 22.17 ± 1.03 a | 100.00 ± 0.00 a |
| Sample | E (%) | TS (MPa) | YM (MPa) |
|---|---|---|---|
| CS | 34.17 ± 0.01 a | 9.88 ± 0.02 b | 28.91 ± 0.05 c |
| CS10BCW | 16.61 ± 1.41 d | 13.32 ± 1.58 a | 79.62 ± 4.35 b |
| CS20BCW | 7.68 ± 0.69 e | 13.36 ± 1.31 a | 175.87 ± 15.41 a |
| PEC | 28.92 ± 0.45 b | 9.29 ± 0.53 b | 32.10 ± 1.57 c |
| PEC10BCW | 21.34 ± 1.34 c | 3.97 ± 0.04 c | 24.49 ± 4.42 c |
| PEC20BCW | 14.73 ± 1.45 d | 1.48 ± 0.14 d | 10.08 ± 0.77 d |
| Sample | L* | a* | b* | ΔE |
|---|---|---|---|---|
| CS | 16.77 ± 0.99 b,c | −1.23 ± 0.12 d | −3.05 ± 0.76 e | 0.00 ± 0.00 d |
| CS10BCW | 14.28 ± 2.34 b,c | −1.52 ± 0.20 d | −2.49 ± 0.34 d | 3.89 ± 1.15 c |
| CS20BCW | 12.77 ± 2.33 c | −1.29 ± 0.11 d | −2.57 ± 0.35 d | 4.30 ± 2.10 c |
| PEC | 96.66 ± 0.02 a | 0.07 ± 0.00 c | 4.98 ± 0.01 a | 0.00 ± 0.00 d |
| PEC10BCW | 16.93 ± 0.68 b | 6.61 ± 0.38 b | −0.38 ± 0.32 b,c | 80.18 ± 0.65 b |
| PEC20BCW | 8.15 ± 0.33 b–d | 11.61 ± 0.20 a | −0.09 ± 0.41 b,c | 89.40 ± 0.28 a |
| Sample | Tm (°C) | ΔHf (J g−1) |
|---|---|---|
| CS | 162.0 | 351.3 |
| CS20BCW | 144.3 | 291.6 |
| PEC | 169.7 | 246.5 |
| PEC20BCW | 135.7 | 146.4 |
| Sample | TPC (mg GAE g−1) | AOA (mg AEE g−1) |
|---|---|---|
| CS | 0.53 ± 0.53 d | 0.89 ± 0.01 f |
| CS10BCW | 2.58 ± 0.15 c | 2.51 ± 0.17 e |
| CS20BCW | 2.12 ± 0.64 c | 3.02 ± 0.46 d |
| PEC | 0.33 ± 0.06 d | 0.79 ± 0.01 f |
| PEC10BCW | 3.49 ± 0.23 b,c | 8.99 ± 0.28 c |
| PEC20BCW | 6.15 ± 0.59 b | 24.26 ± 0.55 b |
| BCW10% | 13.18 ± 0.71 a | 52.85 ± 3.25 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kurek, M.; Benbettaieb, N.; Ščetar, M.; Chaudy, E.; Repajić, M.; Klepac, D.; Valić, S.; Debeaufort, F.; Galić, K. Characterization of Food Packaging Films with Blackcurrant Fruit Waste as a Source of Antioxidant and Color Sensing Intelligent Material. Molecules 2021, 26, 2569. https://doi.org/10.3390/molecules26092569
Kurek M, Benbettaieb N, Ščetar M, Chaudy E, Repajić M, Klepac D, Valić S, Debeaufort F, Galić K. Characterization of Food Packaging Films with Blackcurrant Fruit Waste as a Source of Antioxidant and Color Sensing Intelligent Material. Molecules. 2021; 26(9):2569. https://doi.org/10.3390/molecules26092569
Chicago/Turabian StyleKurek, Mia, Nasreddine Benbettaieb, Mario Ščetar, Eliot Chaudy, Maja Repajić, Damir Klepac, Srećko Valić, Frédéric Debeaufort, and Kata Galić. 2021. "Characterization of Food Packaging Films with Blackcurrant Fruit Waste as a Source of Antioxidant and Color Sensing Intelligent Material" Molecules 26, no. 9: 2569. https://doi.org/10.3390/molecules26092569
APA StyleKurek, M., Benbettaieb, N., Ščetar, M., Chaudy, E., Repajić, M., Klepac, D., Valić, S., Debeaufort, F., & Galić, K. (2021). Characterization of Food Packaging Films with Blackcurrant Fruit Waste as a Source of Antioxidant and Color Sensing Intelligent Material. Molecules, 26(9), 2569. https://doi.org/10.3390/molecules26092569












