Polyphenols, L-Ascorbic Acid, and Antioxidant Activity in Wines from Rose Fruits (Rosa rugosa)
Abstract
1. Introduction
2. Results and Discussion
2.1. Physicochemical Characteristics of the Varieties of Rosa rugosa Wines Produced
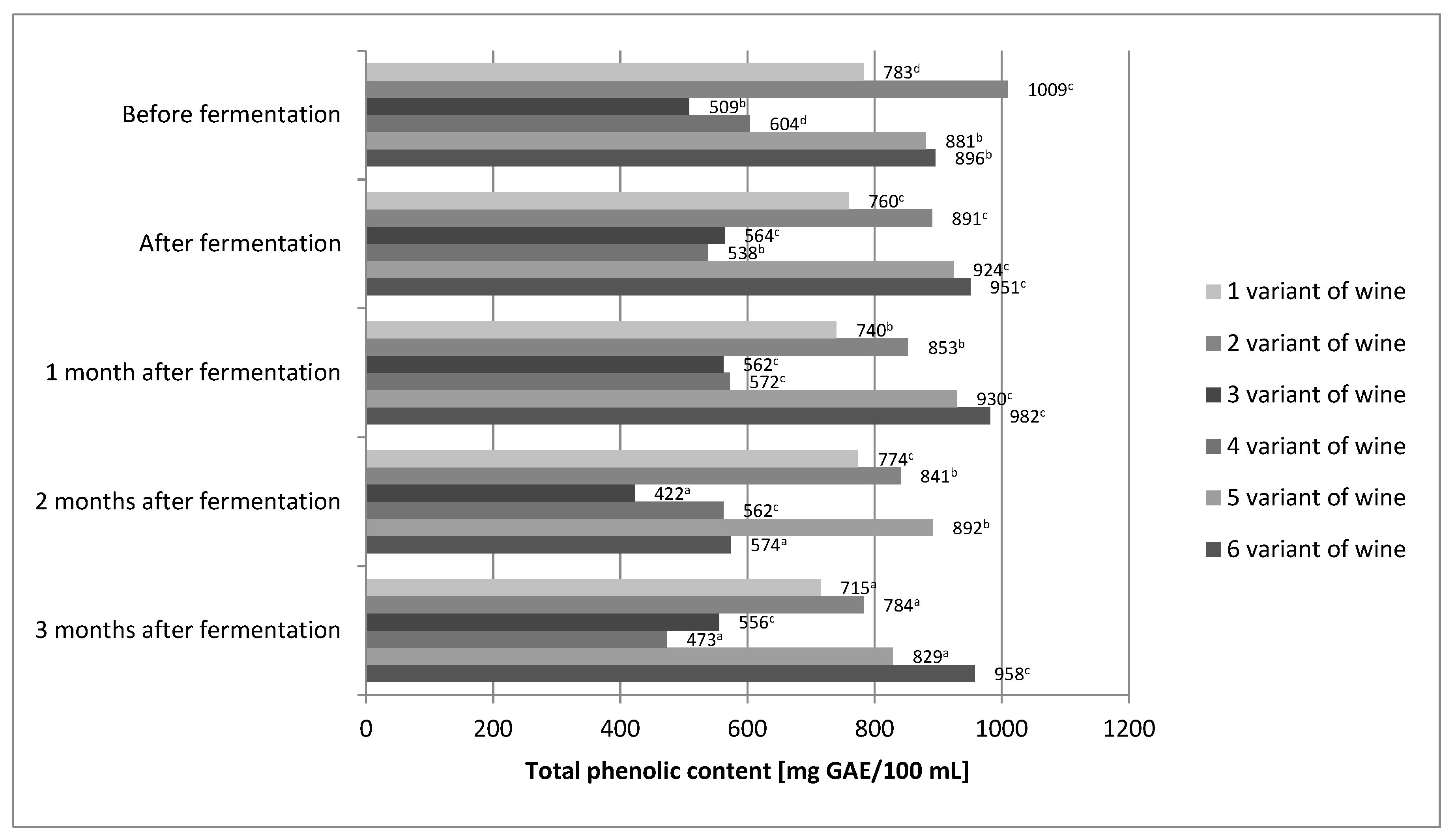
2.2. Total Phenolic Content
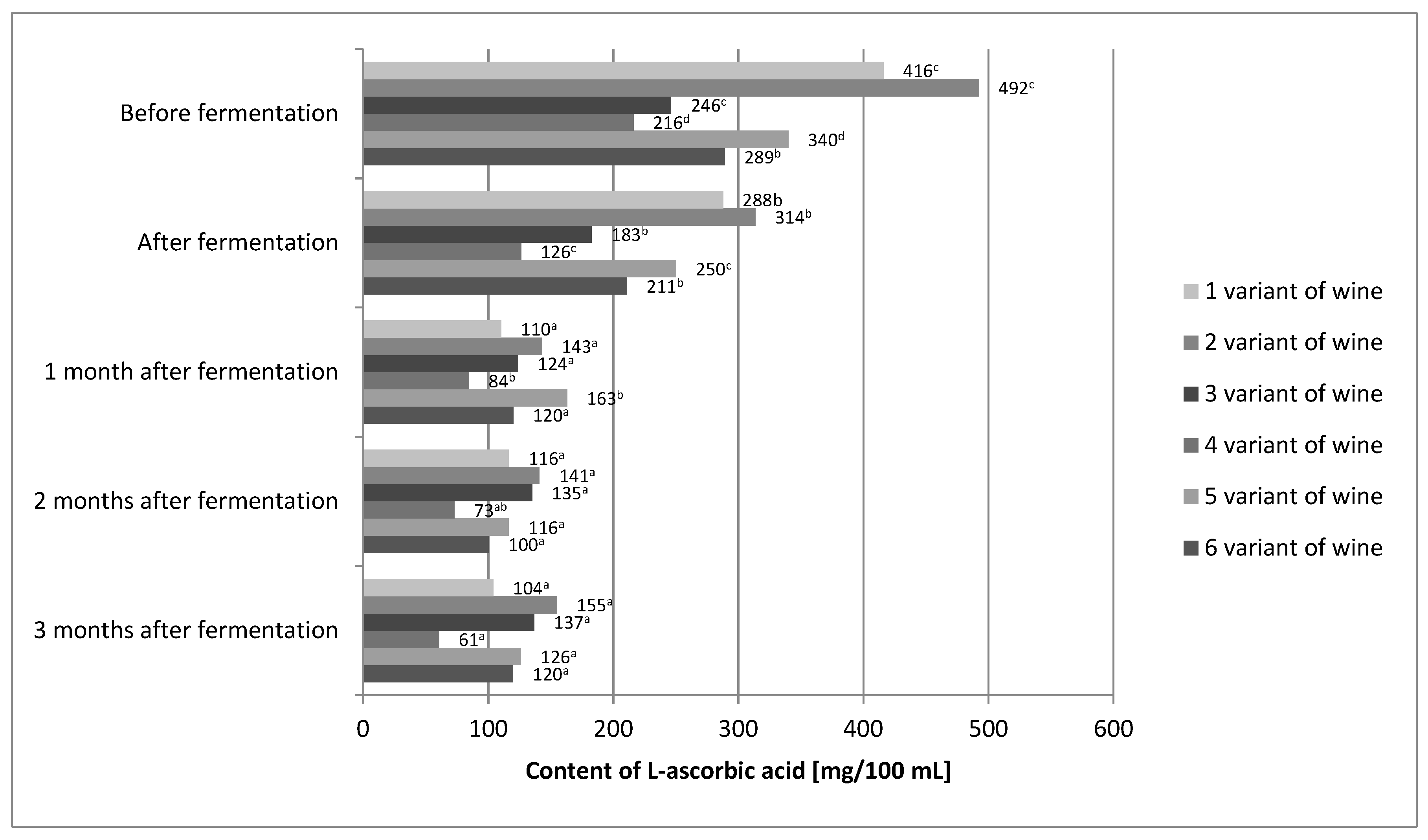
2.3. L-Ascorbic Acid Content
2.4. Antioxidant Capacity
3. Materials and Methods
3.1. Chemicals and Reagents
3.2. Plant Material
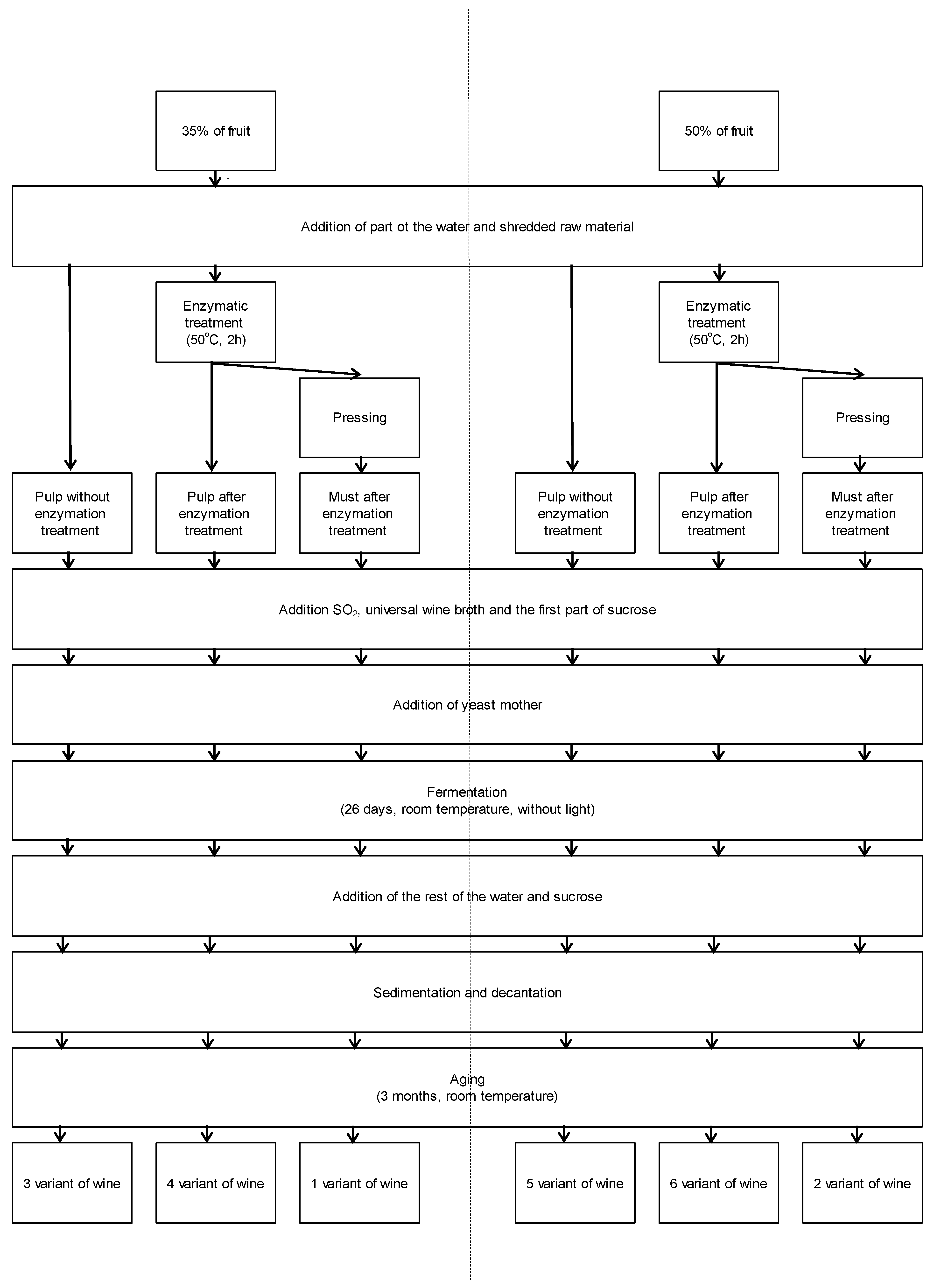
3.3. Winemaking Procedure
3.4. Physicochemical Analysis
3.5. Determination of Total Phenolic Content
3.6. Determinations of L-Ascorbic Acid
3.7. DPPH Assay
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Components | 1 Variant | 2 Variant | 3 Variant | 4 Variant | 5 Variant | 6 Variant |
|---|---|---|---|---|---|---|
| Starting mass for the prepared wine | 1800 | 1845 | 3000 | 3000 | 3000 | 3000 |
| Mass of must | 930 | 1230 | absence | absence | absence | absence |
| Sucrose | 363 | 340 | 605 | 605 | 553 | 553 |
| SO2 * | in must | in must | 6.0 | 6.0 | 6.0 | 6.0 |
| Universal wine broth * | 7.2 | 7.4 | 12.0 | 12.0 | 12.0 | 12.0 |
| Enzyme ** | in must | in must | absence | 2.1 | absence | 3.0 |
| Yeast mother | 90 | 92 | 150 | 150 | 150 | 150 |
| Water | 406 | 172 | 1177 | 1175 | 779 | 776 |
| Syrup *** | 679 | 420 | 1132 | 1130 | 682 | 679 |
| The second part of sucrose | 90 | 92 | 134 | 134 | 128 | 128 |
| Additional addition of sucrose | 83 | 57 | absence | absence | absence | absence |
| An additional addition of spirit | absence | absence | 31 | 31 | 31 | 31 |
| Concentrations (%) | |||||||
|---|---|---|---|---|---|---|---|
| Standard Substance | Trolox | 0.125 | 0.063 | 0.031 | 0.016 | 0.008 | 0.004 |
| Methanol extracts | 4 variants of pulp and 2 variants of musts and 1–4 variant of the finished wine | 20 | 10 | 5 | 2.5 | 1.25 | 0.625 |
| 5–6 variant of the finished wine | 10 | 5 | 2.5 | 1.25 | 0.625 | 0.313 | |
References
- Lindley, M.G. The impact of food processing on antioxidants in vegetable oils, fruits and vegetables. Trends Food Sci. Technol. 1998, 9, 336–340. [Google Scholar] [CrossRef]
- Helyes, L.; Lugasi, A. Formation of certain compounds having technological and nutritional importance in tomato fruits during maturation. Acta Aliment. 2006, 35, 183–193. [Google Scholar] [CrossRef]
- Howard, A.; Chopra, M.; Thurnham, D.I.; Strain, J.J.; Fuhrman, B.; Aviram, M. Red wine con-sumption and inhibition of LDL oxidation: What are the important components? Med. Hypoth. 2002, 59, 101–104. [Google Scholar] [CrossRef]
- Kumar, Y.S.; Prakasam, R.S. Optimization of fermentation conditions for mango (Mangifera indica L.) wine production by employing response surface methodology. Int. J. Food Sci. Technol. 2009, 44, 2320–2327. [Google Scholar] [CrossRef]
- Akubor, P.I.; Obio, S.O.; Nwadomere, K.A.; Obiomah, E. Production and quality evaluation of banana wine. Plant Foods Hum. Nutr. 2003, 58, 1–6. [Google Scholar]
- Sun, X.Y.; Li, L.; Ma, T.T.; Zhao, F.; Yu, D.; Huang, W.D.; Zhan, J.C. High hydrostatic pressure treatment: An artificial accelerating aging method which did not change the region and variety non-colored phenolic characteristic of red wine. Innov. Food Sci. Emerg. Technol. 2016, 33, 123–134. [Google Scholar] [CrossRef]
- Ma, T.T.; Sun, X.Y.; Gao, G.T.; Wang, X.Y.; Liu, X.Y.; Du, G.R.; Zhan, J.C. Phenolic characterisation and antioxidant capacity of young wines made from different grape varieties grown in Helanshan Donglu wine zone (China). S. Afr. J. Enol. Vitic. 2014, 35, 321–331. [Google Scholar] [CrossRef]
- Isabelle, M.; Lee, B.; Ong, C.; Liu, X.; Huang, D. Peroxyl radical scavenging capacity, polyphenolics, and lipophilic antioxidant profiles of mulberry fruits cultivated in southern China. J. Agric. Food Chem. 2009, 56, 9410–9416. [Google Scholar] [CrossRef]
- Sun, X.; Ma, T.; Han, L.; Huang, W.; Zhan, J. Effects of Copper Pollution on the Phenolic Compound Content, Color, and Antioxidant Activity of Wine. Molecules 2017, 22, 726. [Google Scholar]
- Gorinstein, S.; Goldblum, A.; Kitov, S.; Deutsch, J.; Loinger, C.; Cohen, S.; Tabakman, H.; Stiller, A.; Zykerman, A.; Loinger, C.; et al. The relationship between metals, polyphenols, nitogenous substances and treatment of red and white wines. Am. J. Enol. Vitic. 1984, 35, 9–15. [Google Scholar]
- Sun, X.Y.; Li, L.; Ma, T.T.; Liu, Y.Y.; Huang, W.D.; Zhan, J.C. Profiles of phenolic acids and flavan-3-ols for select Chinese red wines: A comparison and differentiation according to geographic origin and grape variety. J. Food Sci. 2015, 80, 2170–2179. [Google Scholar] [CrossRef]
- Robinson, N.; Winge, D. Copper metallochaperones. Annu. Rev. Biochem. 2010, 79, 537–562. [Google Scholar] [CrossRef]
- Clark, A.; Grant-Preece, P.; Cleghorn, N.; Scollary, G. Copper(II) addition to white wines containing hydrogen sulfide: Residual copper concentration and activity. Aust. J. Grape Wine Res. 2015, 21, 30–39. [Google Scholar] [CrossRef]
- Clark, A.; Wilkes, E.; Scollary, G. Chemistry of copper in white wine: A review. Aust. J. Grape Wine Res. 2015, 21, 339–350. [Google Scholar] [CrossRef]
- Stępniewska, A.; Czech, A.; Malik, A.; Chałabis-Mazurek, A.; Ognik, K. The influence of winemaking on the content of natural antioxidants and mineral elements in wines made from berry fruits. J. Elem. 2015, 21, 871–880. [Google Scholar] [CrossRef]
- Demir, F.; Ozcan, M. Chemical and technological properties of rose (Rosa canina L.) fruits grown wild in Turkey. J. Food Eng. 2001, 47, 333–336. [Google Scholar] [CrossRef]
- Altıner, D.; Kılıçgün, H. The antioxidant effect of Rosa rugosa. Drug Metabol Drug Interact. 2008, 23, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Ercisli, S. Chemical composition of fruits in some rose (Rosa spp.) species. Food Chem. 2007, 104, 1379–1384. [Google Scholar] [CrossRef]
- Fecka, I. Qualitative and quantitative determination of hydrolysable tannins and other polyphenols in herbal products from meadowsweet and dog rose. Phytochem. Anal. 2009, 20, 177–190. [Google Scholar] [CrossRef]
- Kazaz, S.; Baydar, H.; Erbas, S. Variations in chemical compositions of Rosa damascena Mill. and Rosa canina L. fruits. Czech J. Food Sci. 2009, 27, 178–184. [Google Scholar] [CrossRef]
- Türkben, C.; Uylaser, V.; Incedayi, B. Influence of traditional processing on some compounds of Rose Hip (Rosa canina L.) fruits collected from habitat in Bursa, Turkey. Asian J. Chem. 2010, 22, 2309–2318. [Google Scholar]
- Koczka, N.; Stefanovits-Bányai, É.; Ombódi, A. Total Polyphenol Content and Antioxidant Capacity of Rosehips of Some Rosa Species. Medicines 2018, 5, 84. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Fang, Y.; Gao, J.; Qiao, L.; Zhang, A.; Guo, Z.; Qin, M.; Huang, J.; Hu, Y.; Zhuang, X. Phenolics composition and antioxidant activity of wine produced from Spine Grape (Vitis davidii Foex) and Cherokee Rose (Rosa laevigata Michx.) fruits from South China. J. Food Sci. 2012, 71, C8–C14. [Google Scholar] [CrossRef] [PubMed]
- Czyżowska, A.; Klewicka, E.; Pogorzelski, E.; Nowak, A. Polyphenols, vitamin C and antioxidant activity in wines from Rosa canina L. and Rosa rugosa Thunb. J. Food Compos. Anal. 2015, 39, 62–68. [Google Scholar] [CrossRef]
- Villano, D.; Fernández-Pachón, M.S.; Troncoso, A.M.; García-Parrilla, M.C. Influence of enological practices on the antioxidant capacity and total polyphenols. Food Chem. 2006, 95, 394–404. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: Central role of iron and copper. Am. J. Enol. Viticult. 2003, 54, 73–85. [Google Scholar]
- Di Majo, D.; La Guardia, M.; Giammanco, S.; La Neve, L.; Giammanco, M. The antioxidant capacity of red wine in relationship with its polyphenolic constituents. Food Chem. 2008, 111, 45–49. [Google Scholar] [CrossRef]
- Wzorek, W.; Pogorzelski, E. Technology of Fruit and Grape Winemaking; Wydawnictwo Sigma-Not: Warsaw, Poland, 1998; pp. 1–288. [Google Scholar]
- Margalit, Y. Wine Production Technology; Powszechne Wydawnictwo Rolnicze i Leśne: Warsaw, Poland, 2014; pp. 41–70. [Google Scholar]
- Sacchi, K.L.; Bisson, L.F.; Adams, D.O. A review of the effect of winemaking techniques on phenolic extraction in red wines. Am. J. Enol. Vitic. 2005, 56, 3. [Google Scholar]
- Pretorius, I.S. Conducting wine symphonics with the aid of yeast genomics. Beverages 2016, 2, 36. [Google Scholar] [CrossRef]
- Grajek, W.; Szymanowska, D. The influence of environmental stresses on Saccharomyces cerevisiae yeast in ethanol fermentation. Biotechnlogia 2008, 3, 47–62. [Google Scholar]
- Szwedziak, K.; Polańczyk, E.; Dąbrowska-Molenda, M.; Wolak, J. The use of bioactive substances in the wine production technology. Postępy Tech. Przetwórstwa Spożywczego 2018, 2, 25–27. [Google Scholar]
- Banel, A.; Zygmunt, B. Volatile fatty acids in a landfill—Occurrence and determination. Ecol. Chem. Eng. S 2009, 16, 193–206. [Google Scholar]
- Gnilomedova, N.V.; Anikina, N.S.; Gerzhikova, V.G. Profile of sugars in a grape wine system as the identifying indicator of the authenticity of wine products. Foods Raw Mater. 2018, 6, 191–200. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef]
- Sokół-Łętowska, A.; Kucharska, A.Z.; Wińska, K.; Szumny, A.; Nawirska-Olszańska, A.; Mizgier, P.; Wyspiańska, D. Composition and antioxidant activity of red fruit liqueurs. Food Chem. 2014, 157, 533–539. [Google Scholar] [CrossRef]
- Roman, I.; Stănilă, A.; Stănilă, S. Bioactive compounds and antioxidant activity of Rosa canina L. biotypes from spontaneous flora of Transylvania. Chem. Cent. J. 2013, 7, 73. [Google Scholar] [CrossRef]
- Laskowska, J.; Czyżycki, A.; Włodarczyk, M. Changes in vitamin C content in the process of obtaining fruit wines from wild rose. Przemysł Ferment. Owocowo-Warzywny 2000, 44, 22–24. [Google Scholar]
- Gheribi, E. The importance of polyphenolic compounds from fruits and vegetables in the diet therapy of atherosclerosis. Med. Rodz. 2011, 4, 149–152. [Google Scholar]
- Zieliński, J. Evaluation of the Fruit Yield of the Ruffled Rose (Rosa Rugosa) Grown on The Waste Recultivated by Various Methods. Rocz. A. R. Pozn. 2007, CCCLXXXIII, 247–250. [Google Scholar]
- Janda, K.; Kasprzak, M.; Wolska, J. Vitamin C—structure, properties, functions and occurrence. Pomeranian J. Life Sci. 2015, 61, 419–425. [Google Scholar]
- Dłużewski, M. Food ingredients and quality. In Food Technology; Wydawnictwo Szkolne i Pedagogiczne: Warsaw, Poland, 2008; Volume 1, pp. 209–213. [Google Scholar]
- Publications Office of the EU. Commission Directive 2008/100/EC of 28 October 2008 Amending Council Directive 90/496/EEC on Nutrition Labeling for Foodstuffs as Regards Recommended Daily Allowance, Energy Conversion Factors and Definitions; Publications Office of the EU: Luxembourg, 2008. [Google Scholar]
- Katalinic, V.; Milos, M.; Jukic, M. Screening of 70 medicinal plant extracts for antioxidant capacity and total phenols. Food Chem. 2006, 94, 550–557. [Google Scholar] [CrossRef]
- Wenzig, E.M.; Widowitz, U.; Kunert, O.; Chrubasik, S.; Bucar, F.; Knauder, E.; Bauer, R. Phytochemical composition and in vitro pharmacological activity of two rose hip (Rosa canina L.) preparations. Phytomedicine 2008, 15, 826–835. [Google Scholar] [CrossRef]
- Ghazghazi, H.; Miguel, M.G.; Hasnaoui, B.; Sebei, H.; Ksontini, M.; Figueiredo, A.C.; Pedro, L.G.; Barroso, J.G. Phenols, essential oils and carotenoids of Rosa canina from Tunisia and their antioxidant activities. Afr. J. Biotechnol. 2010, 9, 2709–2716. [Google Scholar]
- Szajdek, A.; Borowska, J. Antioxidant properties of plant food. Food. Sci. Technol. Qual. 2004, 4, 5–23. [Google Scholar]
- Gumul, D.; Korus, J.; Achremowicz, B. Effect of processing processes on the antioxidant activity of raw materials of plant origin. Food. Sci. Technol. Qual. 2005, 4, 41–48. [Google Scholar]
- Muzolf-Panek, M. Antioxidant and Pro-Oxidant Activity of Catechins Found in Green Tea. Ph.D. Thesis, Poznań University of Economics and Business, Poznań, Poland, 2009; pp. 21–56. Available online: https://www.wbc.poznan.pl/Content/120631/PDF/Muzolf-Panek_Malgorzata-rozprawa_doktorska.pdf (accessed on 12 March 2021).
- Claus, H.; Mojsov, K. Enzymes for wine fermentation: Current and perspective applications. Fermentation 2018, 4, 52. [Google Scholar] [CrossRef]
- Shahidi, F.; Wanasundara, J.P.D. Phenolic antioxidant. Crit. Rev. Food Sci. Nutr. 1992, 32, 67–103. [Google Scholar] [CrossRef] [PubMed]
- Gramza, A.; Korczak, J.; Amarowicz, R. Tea polyphenols—their antioxidant properties and biological activity—A review. Pol. J. Food Nutr. Sci. 2005, 14, 219–235. [Google Scholar]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Polish Standard PN-EN-1132: 1999. Fruit and Vegetable Juices—Determination of pH; Polish Committee for Standardization: Warsaw, Poland, 1999. [Google Scholar]
- Polish Standard PN-EN 12147: 2000. Fruit and Vegetable Juices—Determination of Titratable Acidity; Polish Committee for Standardization: Warsaw, Poland, 2000. [Google Scholar]
- Dziennik Ustaw Rzeczypospolitej Polskiej. Journal of Laws of the Republic of Poland. Dz.U. of 2005, No. 88, Item 748. Available online: https://www.dziennikustaw.gov.pl/D2005088074801.pdf (accessed on 12 March 2021).
- Gao, X.; Ohlander, M.; Jeppson, N.; Bjork, L.; Trajkovski, V. Changes in antioxidant effect and their relationship to phytonutrients in fruit of sea buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef] [PubMed]
- Gökmen, V.; Acar, J. Determination of total vitamin C; as ascorbic acid in foods using HPLC. Eur. Food Chem. Viii 1995, 2, 345–348. [Google Scholar]
- Polish Standard PN-EN 14130:2004. Food Items—Determination of Vitamin C. by HPLC; Polish Committee for Standardization: Warsaw, Poland, 2004. [Google Scholar]
- Annegowda, H.V.; Anwar, L.N.; Mordi, M.N.; Ramanathan, S.; Mansor, S.M. Influence of sonication on the phenolic content and antioxidant activity of Terminalia catappa L. leaves. Pharmacognosy Res. 2010, 2, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2003, 26, 211–219. [Google Scholar]

| Variant of Wine | Alcohol Content, % | pH | Titratable Acidity, g/100 mL | Volatile Acidity, g/L | Sugars, g/L |
|---|---|---|---|---|---|
| 1 | 14.60 ± 0.07 | 3.65 ± 0.01 | 12.40 ± 0.02 | 1.278 ± 0.02 | 51.95 ± 1.23 |
| 2 | 13.76 ± 0.02 | 3.65 ± 0.01 | 14.16 ± 0.02 | 1.137 ± 0.01 | 51.87 ± 1.89 |
| 3 | 12.78 ± 0.06 | 3.63 ± 0.01 | 10.14 ± 0.01 | 1.167 ± 0.01 | 51.51 ± 1.56 |
| 4 | 12.26 ± 0.10 | 3.60 ± 0.01 | 10.50 ± 0.02 | 1.195 ± 0.01 | 51.08 ± 1.61 |
| 5 | 12.78 ± 0.07 | 3.68 ± 0.01 | 13.67 ± 0.01 | 1.609 ± 0.02 | 51.68 ± 1.42 |
| 6 | 12.27 ± 0.10 | 3.61 ± 0.01 | 14.34 ± 0.01 | 1.726 ± 0.01 | 51.32 ± 1.21 |
| % Share of Fruit | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group | |
|---|---|---|---|---|---|---|
| 35 | 635 | 15.5 | 602 | 669 | A | |
| 50 | 806 | 15.5 | 772 | 840 | B | |
| Fermentation Environment | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group | |
| in the pulp | 704 | 11 | 680 | 728 | A | |
| in the must | 738 | 19 | 696 | 779 | A | |
| The Enzyme Treatment Used | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group | |
| without | 709 | 19 | 668 | 751 | A | |
| with | 732 | 11 | 709 | 756 | A | |
| % Share of Fruit | Fermentation Environment | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group |
| 35 | in the pulp | 515 | 15.5 | 481 | 548 | A |
| 50 | in the must | 719 | 26.9 | 661 | 778 | B |
| 35 | in the must | 756 | 26.9 | 698 | 815 | B |
| 50 | in the pulp | 893 | 15.5 | 860 | 927 | C |
| % Share of Fruit | The Enzyme Treatment Used | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group |
| 35 | with | 594 | 15.5 | 560 | 628 | A |
| 35 | without | 677 | 26.9 | 618 | 735 | AB |
| 50 | without | 742 | 26.9 | 683 | 801 | B |
| 50 | with | 871 | 15.5 | 837 | 904 | C |
| % Share of Fruit | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group | |
|---|---|---|---|---|---|---|
| 35 | 109 | 2.25 | 105 | 114 | A | |
| 50 | 141 | 2.25 | 136 | 145 | B | |
| Fermentation Environment | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group | |
| in the pulp | 111 | 1.59 | 107 | 114 | A | |
| in the must | 139 | 2.75 | 133 | 145 | B | |
| The Enzyme Treatment Used | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group | |
| with | 104 | 1.59 | 101 | 108 | A | |
| without | 146 | 2.75 | 140 | 152 | B | |
| % Share of Fruit | Fermentation Environment | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group |
| 35 | in the pulp | 98.8 | 2.25 | 93.9 | 104 | A |
| 35 | in the must | 120.1 | 3.89 | 111.6 | 129 | B |
| 50 | in the pulp | 122.9 | 2.25 | 118 | 128 | B |
| 50 | in the must | 158.1 | 3.89 | 149.7 | 167 | C |
| % Share of Fruit | The Enzyme Treatment Used | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group |
| 35 | with | 71.4 | 2.25 | 66.5 | 76.3 | A |
| 50 | with | 137.4 | 2.25 | 132.5 | 142.3 | B |
| 50 | without | 143.7 | 3.89 | 135.2 | 152.1 | B |
| 35 | without | 147.5 | 3.89 | 139 | 156 | B |
| % Share of Fruit | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group | |
|---|---|---|---|---|---|---|
| 35 | 26.1 | 0.54 | 25.0 | 27.3 | A | |
| 50 | 61.1 | 0.54 | 59.9 | 62.3 | B | |
| Fermentation Environment | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group | |
| in the must | 39.1 | 0.66 | 37.6 | 40.5 | A | |
| in the pulp | 48.2 | 0.38 | 47.4 | 49.0 | B | |
| The Enzyme Treatment Used | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group | |
| with | 40.3 | 0.38 | 39.5 | 41.2 | A | |
| without | 46.9 | 0.66 | 45.5 | 48.4 | B | |
| % Share of Fruit | Fermentation Environment | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group |
| 35 | in the pulp | 23.9 | 0.54 | 22.7 | 25.0 | A |
| 35 | in the must | 28.4 | 0.94 | 26.4 | 30.4 | B |
| 50 | in the must | 49.7 | 0.94 | 47.7 | 51.7 | C |
| 50 | in the pulp | 72.5 | 0.54 | 71.3 | 73.7 | D |
| % Share of Fruit | The Enzyme Treatment Used | Average | Standard Error | Start of the Confidence Interval (95%) | End of Confidence Interval (95%) | Homogeneous Group |
| 35 | without | 23.6 | 0.94 | 21.6 | 25.7 | A |
| 35 | with | 28.6 | 0.54 | 27.5 | 29.8 | B |
| 50 | with | 52.0 | 0.54 | 50.8 | 53.2 | C |
| 50 | without | 70.2 | 0.94 | 68.2 | 72.2 | D |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cendrowski, A.; Królak, M.; Kalisz, S. Polyphenols, L-Ascorbic Acid, and Antioxidant Activity in Wines from Rose Fruits (Rosa rugosa). Molecules 2021, 26, 2561. https://doi.org/10.3390/molecules26092561
Cendrowski A, Królak M, Kalisz S. Polyphenols, L-Ascorbic Acid, and Antioxidant Activity in Wines from Rose Fruits (Rosa rugosa). Molecules. 2021; 26(9):2561. https://doi.org/10.3390/molecules26092561
Chicago/Turabian StyleCendrowski, Andrzej, Marcin Królak, and Stanisław Kalisz. 2021. "Polyphenols, L-Ascorbic Acid, and Antioxidant Activity in Wines from Rose Fruits (Rosa rugosa)" Molecules 26, no. 9: 2561. https://doi.org/10.3390/molecules26092561
APA StyleCendrowski, A., Królak, M., & Kalisz, S. (2021). Polyphenols, L-Ascorbic Acid, and Antioxidant Activity in Wines from Rose Fruits (Rosa rugosa). Molecules, 26(9), 2561. https://doi.org/10.3390/molecules26092561






