Comparative Study of The Yield and Physicochemical Properties of Collagen from Sea Cucumber (Holothuria scabra), Obtained through Dialysis and the Ultrafiltration Membrane
Abstract
1. Introduction
2. Results and Discussion
2.1. Yield, pH and Color of Collagen
2.2. Amino Acid Composition of Collagen
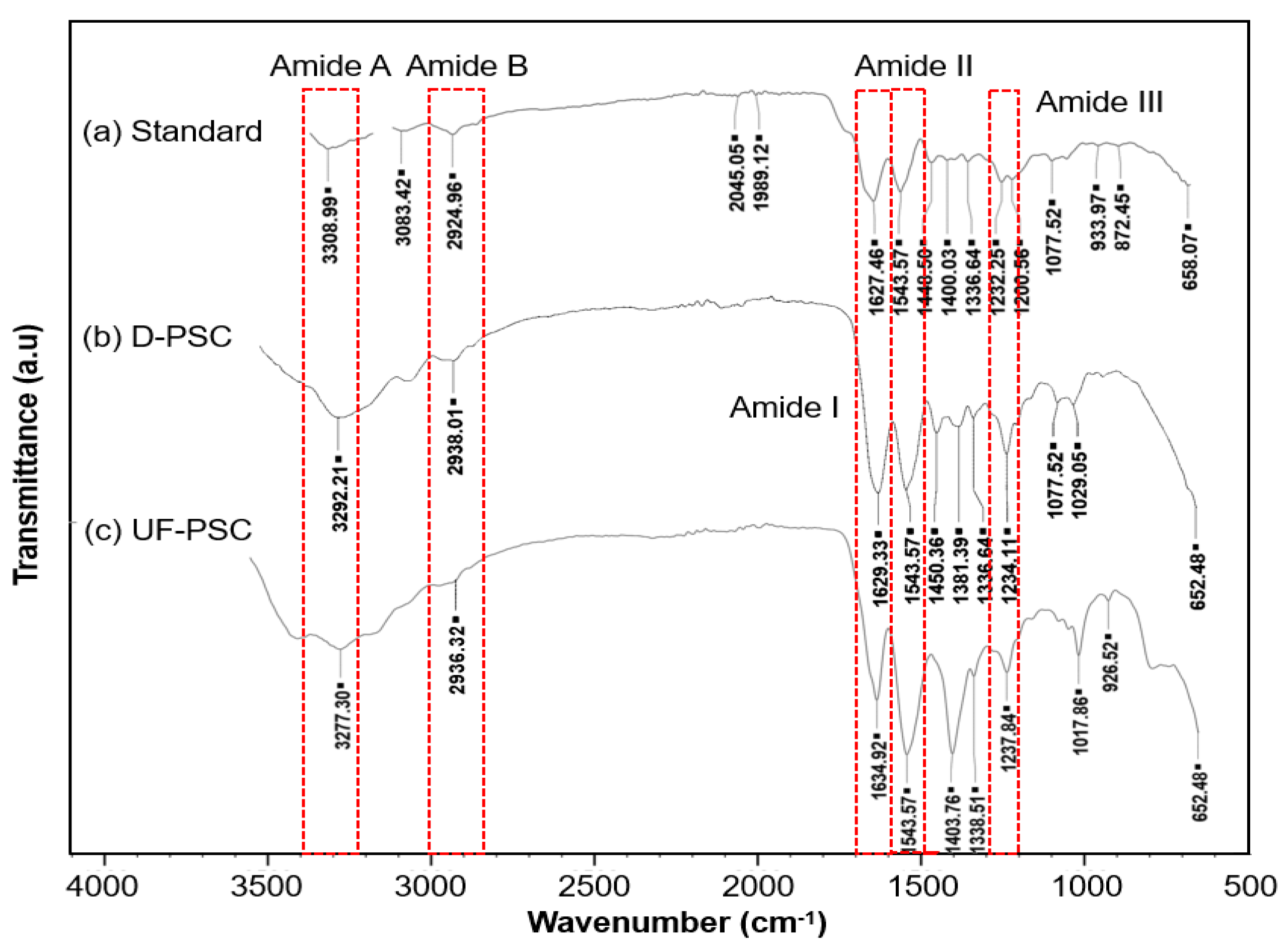
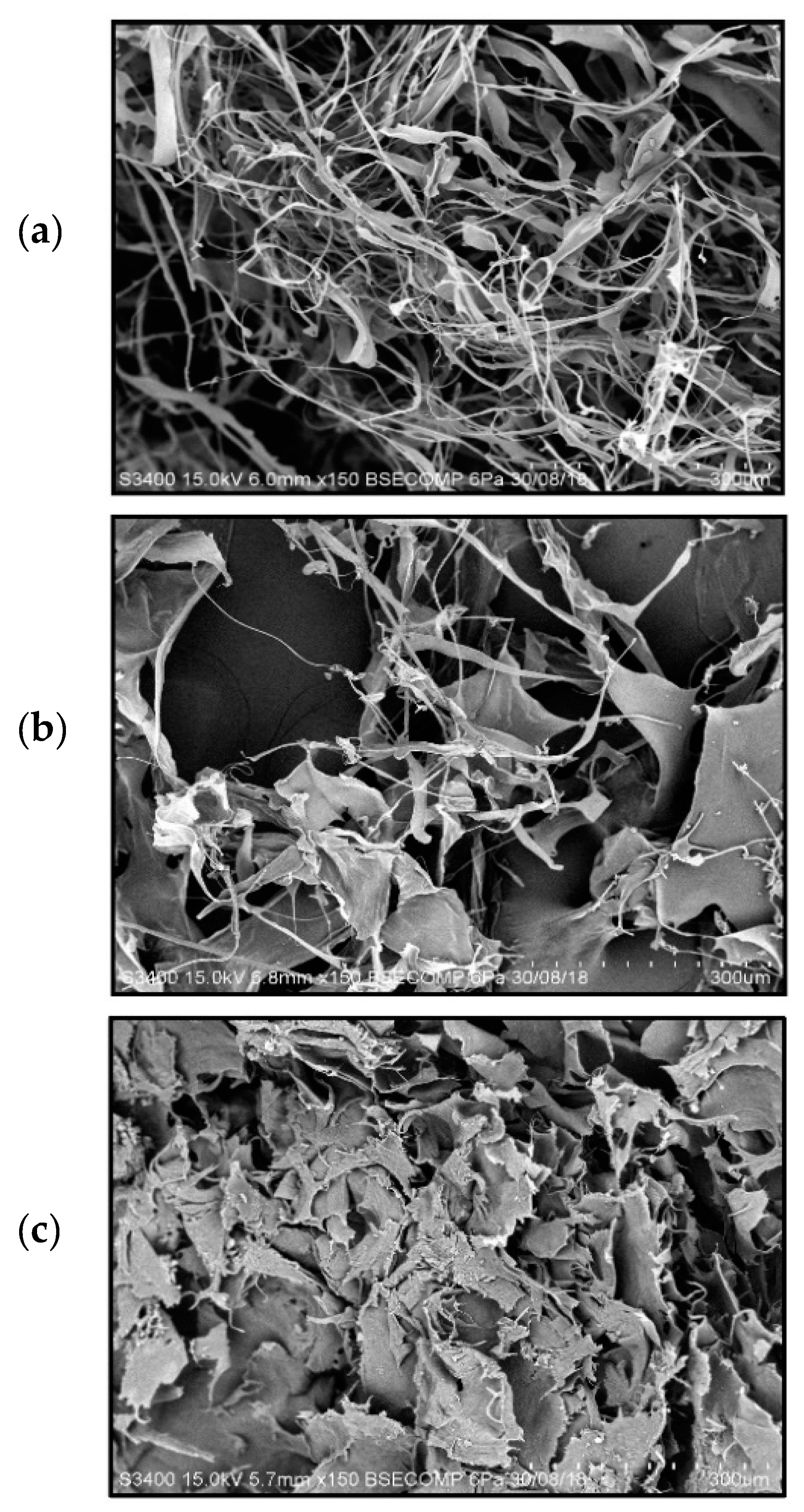
2.3. Characterization of Collagen
3. Materials and Methods
3.1. Sample Preparation
3.2. Extraction of Collagen
3.3. Isolation of Collagen Using Conventional Method
3.4. Isolation of Collagen Using the Ultrafiltration Membrane
3.5. Yield of Collagen
3.6. pH Determination
3.7. Color Determination
3.8. UV-Visible Measurement
3.9. Fourier Transform Infra-Red (FTIR) Spectroscopy
3.10. Determination of Amino Acid Compositions
3.11. Scanning Electron Microscopy (SEM)
3.12. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bordbar, S.; Anwar, F.; Saari, N. High-Value Components and Bioactives from Sea Cucumbers for Functional Foods. Rev. Mar. Drugs. 2011, 9, 1761–1805. [Google Scholar] [CrossRef]
- Choo, P.S. Population status, fisheries and trade of sea cucumbers in Asia. In Sea Cucumbers. A Global Review of Fisheries and Trade; Toral-Granda, V., Lovatelli, A., Vasconcellos, M., Eds.; FAO Fisheries and Aquaculture Technical Paper No. 516; FAO: Rome, Italy, 2008; pp. 81–118. [Google Scholar]
- Duan, X.; Zhang, M.; Mujumdar, A.S.; Huang, L.L.; Wang, H. A novel dielectric drying method of sea cucumber. Int. J. Food Sci. Technol. 2010, 45, 2538–2545. [Google Scholar] [CrossRef]
- Zhu, B.W.; Dong, X.P.; Zhou, D.Y.; Gao, Y.; Yang, J.F.; Li, D.M.; Zhou, X.K.; Ren, T.T.; Ye, W.X.; Tan, H.; et al. Physicochemical Properties and Radical Scavenging Capacities of Pepsin-Solubilized Collagen from Sea Cucumber Stichopus japonicas. Food Hydrocoll. 2012, 28, 182–188. [Google Scholar] [CrossRef]
- Kittiphattanabawon, P.; Benjakul, S.; Visessanguan, W.; Nagai, T.; Tanaka, M. Characterisation of acid-soluble collagen from skin and bone of bigeye snapper (Priacanthus tayenus). Food Chem. 2005, 89, 363–372. [Google Scholar] [CrossRef]
- Cui, F.X.; Xue, C.H.; Li, Z.J.; Zhang, Y.Q.; Don, G.P.; Fu, Z.Y.; Gao, X. Characterization and Subunit Composition of Collagen from the Body Wall of Sea Cucumber (Stichopus japonicas). Food Chem. 2007, 100, 1120–1125. [Google Scholar] [CrossRef]
- Saito, M.; Kunisaki, N.; Urano, N.; Kimura, S. Collagen as the Major Edible Component of Sea Cucumber (Stichopus japonicus). J. Food Sci. 2002, 67, 1319–1322. [Google Scholar] [CrossRef]
- Adibzadeh, N.; Aminzadeh, S.; Jamili, S.; Karkhane, A.A.; Farrokhi, N. Purification and Characterization of Pepsin-Solubilized Collagen from Skin of Sea Cucumber Holothuria parva. Appl. Biochem. Biotechnol. 2014, 173, 143–154. [Google Scholar] [CrossRef]
- Abedin, M.Z.; Karim, A.A.; Ahmed, F.; Latiff, A.A.; Gan, C.Y.; Ghazali, F.C.; Sarker, M.Z.I. Isolation and Characterization of Pepsin Solubilized Collagen from The Integument of Sea cucumber (Stichopus vastus). J. Sci. Food Agric. 2013, 93, 1083–1088. [Google Scholar] [CrossRef]
- Siddiqui, Y.D.; Arief, E.M.; Yusoff, A.; Hamid, S.S.A.; Norani, T.Y.; Abdullah, M.Y.S. Extraction, Purification and Physical Characterization of Collagen from Body wall of Sea cucumber Bohadschia bivitatta. Health Environ. J. 2013, 4, 53–65. [Google Scholar]
- Liu, Z.; Oliveira, A.C.M.; Su, Y.C. Purification and Characterization of Pepsin-Solubilized Collagen from Skin and Connective Tissue of Giant Red Sea Cucumber (Parastichopus californicus). J. Agric. Food Chem. 2010, 58, 1270–1274. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.N.; Li, D.D.; Jiang, F.Y.; Qiu, J.H.; Gao, C.J. Purification and concentration of collagen by charged ultrafiltration membrane of hydrophilic polyacrylonitrile blend. Sep. Purif. Technol. 2009, 66, 257–262. [Google Scholar] [CrossRef]
- Zhou, H.; Zhou, J.; Fan, H.J.; Chen, Y.; Yang, F.F.; Yuan, J.X.; Liu, R.W. Separation of NaCl, glycin from collagen solution by the thermal sensitive polyurethane membrane. Desalination 2009, 249, 843–849. [Google Scholar] [CrossRef]
- Ahsani, M.; Yegani, R. Study on the fouling behavior of silica nanocomposite modified polypropylene membrane in purification of collagen protein. Chem. Eng. Res. Des. 2015, 102, 261–273. [Google Scholar] [CrossRef]
- Jafarzadeh, Y.; Yegani, R. Analysis of fouling mechanism in TiO2 embedded high density polyethylene membranes for collagen. Chem. Eng. Res. Des. 2015, 93, 684–695. [Google Scholar] [CrossRef]
- Chen, S.; Chen, H.; Xie, Q.; Hong, B.; Chen, J.; Hua, F.; Bai, K.; He, J.; Yi, R.; Wu, H. Rapid isolation of high purity pepsin-soluble type I collagen from scales of red drum fish (Sciaenops ocellatus). Food Hydrocoll. 2016, 52, 468–477. [Google Scholar] [CrossRef]
- Park, S.Y.; Lim, H.K.; Lee, S.; Hwang, H.C.; Cho, S.K.; Cho, M. Pepsin-solubilized- collagen (PSC) from Red Sea cucumber (Stichopus japonicus) regulates cell cycle and the fibronectin synthesis in HaCat cell migration. Food Chem. 2011, 132, 487–492. [Google Scholar] [CrossRef]
- Peng, Y.; Glattauer, V.; Werkmeister, J.A.; Ramshaw, J.A.M. Evaluation for collagen products for cosmetic application. J. Cosmet. Sci. 2004, 55, 327–341. [Google Scholar] [CrossRef]
- Alhana; Suptijah, P.; Tarman, K. Ekstraksi dan karakteristik kolagen dari daging teripang gamma. J. Pengolah. Hasil Perikan. Indones. 2015, 18, 150–161. [Google Scholar] [CrossRef]
- Jamilah, B.; Umi Hartina, M.R.; Mat Hashim, D.; Sazili, A.Q. Properties of collagen from barramundi (Lates calcarifer) skin. Int. Food Res. J. 2013, 20, 835–842. [Google Scholar]
- Zhang, J.; Duan, R.; Tian, Y.; Konno, K. Characterisation of acid-soluble collagen from skin of silver carp (Hypophthalmichthys molitrix). Food Chem. 2009, 116, 318–322. [Google Scholar] [CrossRef]
- Safandowska, M.; Pietrucha, K. Effect of fish collagen modification on its thermal and rheological properties. Int. J. Biol. Macromol. 2013, 53, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, W.; Li, G.; Shi, B.; Miao, Y.; Wu, X. Isolation and partial characterization of pepsin-soluble collagen from the skin of grass carp (Ctenopharyngodon idella). Food Chem. 2007, 103, 906–912. [Google Scholar] [CrossRef]
- Sun, L.; Hou, H.; Li, B.; Zhang, Y. Characterization of acid-and pepsin-soluble collagen extracted from the skin of Nile tilapia (Oreochromis niloticus). Int. J. Biol Macromol. 2017, 99, 8–14. [Google Scholar] [CrossRef]
- Chuaychan, S.; Benjakul, S.; Kishimura, H. Characteristics of acid- and pepsin soluble collagens from scale of seabass (Lates calcarifer). LWT-Food Sci. Technol. 2015, 63, 71–76. [Google Scholar] [CrossRef]

| Properties | D-PSC | UF-PSC |
|---|---|---|
| Collagen Yield (%) | 5.15 ± 0.30 b | 11.39 ± 2.86 a |
| pH | 3.65 ± 0.42 b | 6.23 ± 0.49 a |
| Color | ||
| L* (lightness) | 80.90 ± 1.94 a | 77.44 ± 1.67 b |
| a* (green to red) | 0.37 ± 0.23 a | 0.48 ± 0.45 a |
| b* (blue to yellow) | 3.81 ± 1.50 b | 4.24 ± 1.58 b |
| Amino Acid Composition | D-PSC | UF-PSC |
|---|---|---|
| Glycine | 17.86 ± 0.06 | 18.39 ± 0.10 |
| Glutamic acid | 14.67 ± 0.10 | 15.77 ± 0.04 |
| Alanine | 10.15 ± 0.05 | 10.85 ± 0.08 |
| Proline | 10.42 ± 0.12 | 10.68 ± 0.03 |
| Arginine | 9.79 ± 0.02 | 9.05 ± 0.01 |
| Aspartic acid | 8.43 ± 0.10 | 9.04 ± 0.07 |
| Hydroxyproline | 8.05 ± 0.06 | 6.73 ± 0.10 |
| Threonine | 4.37 ± 0.03 | 4.19 ± 0.01 |
| Serine | 3.73 ± 0.05 | 3.52 ± 0.08 |
| Leucine | 3.07 ± 0.03 | 2.98 ± 0.01 |
| Valine | 2.86 ± 0.10 | 2.80 ± 0.02 |
| Isoleucine | 1.59 ± 0.03 | 1.53 ± 0.09 |
| Phenylalanine | 1.13 ± 0.04 | 0.99 ± 0.03 |
| Tyrosine | 0.96 ± 0.08 | 0.70 ± 0.02 |
| Histidine | 0.93 ± 0.01 | 0.82 ± 0.02 |
| Lysine | 0.90 ± 0.01 | 1.19 ± 0.02 |
| Methionine | 0.90 ± 0.01 | 0.73 ± 0.03 |
| Cysteine | 0.18 ± 0.01 | 0.13 ± 0.01 |
| Imino acids (Hyp + Pro) | 18.47 ± 0.19 a | 17.41 ± 0.13 b |
| Total Amino Acids | 100 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saallah, S.; Roslan, J.; Julius, F.S.; Saallah, S.; Mohamad Razali, U.H.; Pindi, W.; Sulaiman, M.R.; Pa’ee, K.F.; Mustapa Kamal, S.M. Comparative Study of The Yield and Physicochemical Properties of Collagen from Sea Cucumber (Holothuria scabra), Obtained through Dialysis and the Ultrafiltration Membrane. Molecules 2021, 26, 2564. https://doi.org/10.3390/molecules26092564
Saallah S, Roslan J, Julius FS, Saallah S, Mohamad Razali UH, Pindi W, Sulaiman MR, Pa’ee KF, Mustapa Kamal SM. Comparative Study of The Yield and Physicochemical Properties of Collagen from Sea Cucumber (Holothuria scabra), Obtained through Dialysis and the Ultrafiltration Membrane. Molecules. 2021; 26(9):2564. https://doi.org/10.3390/molecules26092564
Chicago/Turabian StyleSaallah, Suryani, Jumardi Roslan, Flavian Sheryl Julius, Sharinee Saallah, Umi Hartina Mohamad Razali, Wolyna Pindi, Mohd Rosni Sulaiman, Khairul Faizal Pa’ee, and Siti Mazlina Mustapa Kamal. 2021. "Comparative Study of The Yield and Physicochemical Properties of Collagen from Sea Cucumber (Holothuria scabra), Obtained through Dialysis and the Ultrafiltration Membrane" Molecules 26, no. 9: 2564. https://doi.org/10.3390/molecules26092564
APA StyleSaallah, S., Roslan, J., Julius, F. S., Saallah, S., Mohamad Razali, U. H., Pindi, W., Sulaiman, M. R., Pa’ee, K. F., & Mustapa Kamal, S. M. (2021). Comparative Study of The Yield and Physicochemical Properties of Collagen from Sea Cucumber (Holothuria scabra), Obtained through Dialysis and the Ultrafiltration Membrane. Molecules, 26(9), 2564. https://doi.org/10.3390/molecules26092564






