Impaired Expression of Cytoplasmic Actins Leads to Chromosomal Instability of MDA-MB-231 Basal-Like Mammary Gland Cancer Cell Line
Abstract
1. Introduction
2. Results
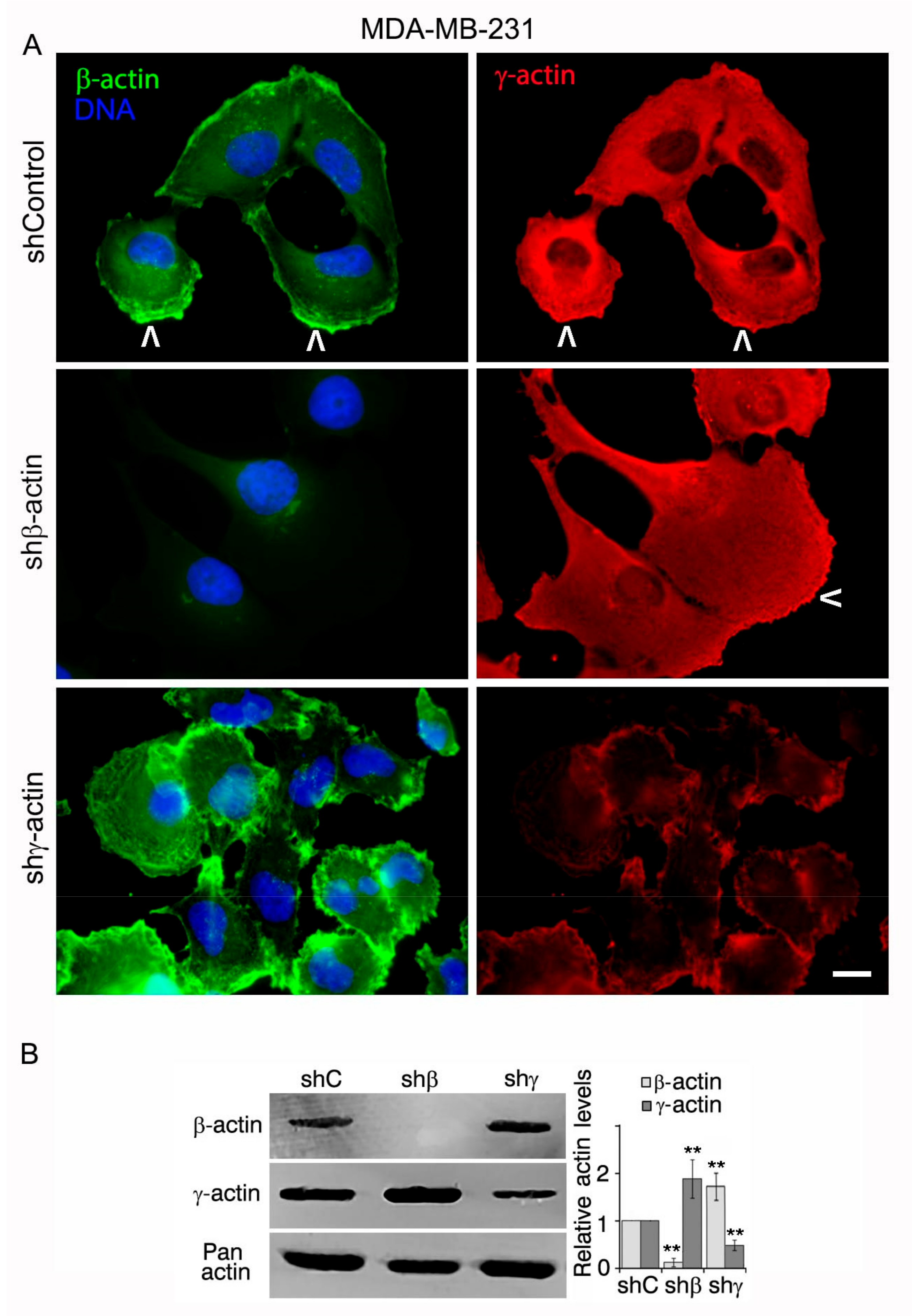
2.1. Downregulation of Cytoplasmic Actin Isoforms Alters the Phenotype of MDA-MB-231 Cell Line
2.2. Downregulation of Cytoplasmic Actin Isoforms Induces Cell Cycle Alterations in MDA-MB-231 Cells
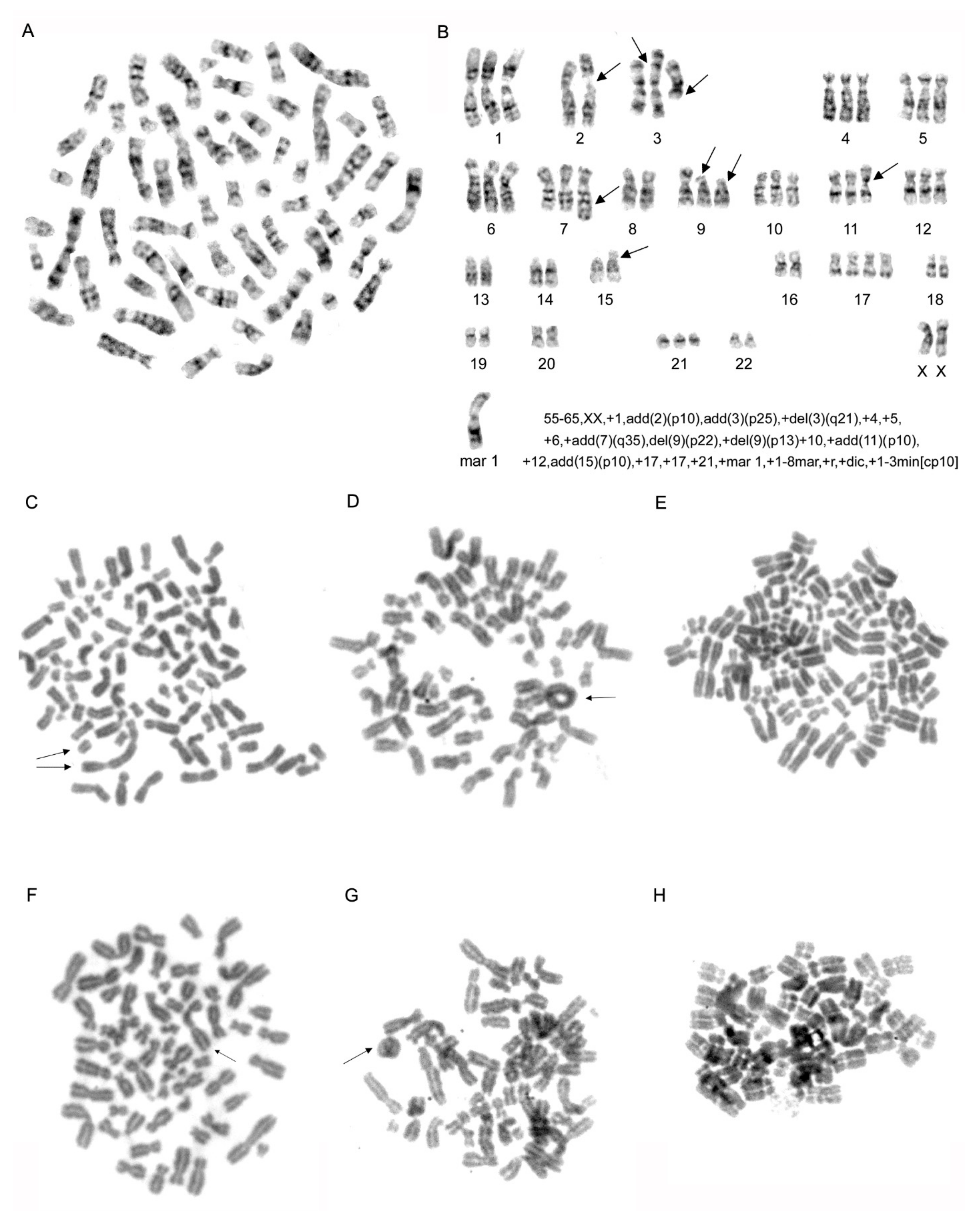
2.3. Karyotype Analysis of MDA-MB-231 Cell Line
2.4. Analysis of CENP-A, Histone H3, and Phospho-Histone H3 in MDA-MB-231 Cells after Downregulation of Cytoplasmic Actins
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. DNA Constructs
4.3. Viral Transfection and Cell Infection
4.4. Cell Cycle Analysis
4.5. Analysis of Chromosomal Aberrations
4.6. Western Blot Analysis
4.7. Antibodies
4.8. Immunofluorescence Microscopy
4.9. 3D-IF Microscopy (Wide-Field with Deconvolution)
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Percipalle, P.; Vartiainen, M. Cytoskeletal proteins in the cell nucleus: A special nuclear actin perspective. Mol. Biol. Cell 2019, 30, 1781–1785. [Google Scholar] [CrossRef]
- Lancaster, O.M.; Baum, B. Shaping up to divide: Coordinating actin and microtubule cytoskeletal remodelling during mitosis. Semin. Cell Dev. Biol. 2014, 34, 109–115. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.; Zwaenepoel, I.; Gabbiani, G.; Clement, S.; Chaponnier, C.; Clément, S.; Chaponnier, C. Beta and gamma-cytoplasmic actins display distinct distribution and functional diversity. J. Cell Sci. 2009, 122, 2980–2988. [Google Scholar] [CrossRef]
- Shagieva, G.S.; Alieva, I.B.; Chaponnier, C.; Dugina, V.B. Divergent impact of actin isoforms on division of epithelial cells. Biochem. 2020, 85, 1072–1081. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.; Khromova, N.; Rybko, V.; Blizniukov, O.; Shagieva, G.; Chaponnier, C.; Kopnin, B.; Kopnin, P. Tumor promotion by γ and suppression by β non-muscle actin isoforms. Oncotarget 2015, 6, 14556–14571. [Google Scholar] [CrossRef] [PubMed]
- Dugina, V.; Shagieva, G.; Khromova, N.; Kopnin, P. Divergent impact of actin isoforms on cell cycle regulation. Cell Cycle 2018, 17, 2610–2621. [Google Scholar] [CrossRef]
- Rajagopalan, H.; Lengauer, C. Aneuploidy and cancer. Nature 2004, 432, 338–341. [Google Scholar] [CrossRef] [PubMed]
- Nechemia-Arbely, Y.; Fachinetti, D.; Miga, K.H.; Sekulic, N.; Soni, G.V.; Kim, D.H.; Wong, A.K.; Lee, A.Y.; Nguyen, K.; Dekker, C.; et al. Human centromeric CENP-A chromatin is a homotypic, octameric nucleosome at all cell cycle points. J. Cell Biol. 2017, 216, 607–621. [Google Scholar] [CrossRef]
- Ohzeki, J.; Larionov, V.; Earnshaw, W.C.; Masumoto, H. De novo formation and epigenetic maintenance of centromere chromatin. Curr. Opin. Cell Biol. 2019, 58, 15–25. [Google Scholar] [CrossRef]
- Bunnell, T.M.; Ervasti, J.M. Delayed embryonic development and impaired cell growth and survival in Actg1 null mice. Cytoskeleton 2010, 67, 564–572. [Google Scholar] [CrossRef]
- Lyubimova, A.; Bershadsky, A.D.; Ben-Zeev, A. Autoregulation of actin synthesis requires the 3’-UTR of actin mRNA and protects cells from actin overproduction. J. Cell. Biochem. 1999, 76, 1–12. [Google Scholar] [CrossRef]
- Latham, S.L.; Ehmke, N.; Reinke, P.Y.A.; Taft, M.H.; Eicke, D.; Reindl, T.; Stenzel, W.; Lyons, M.J.; Friez, M.J.; Lee, J.A.; et al. Variants in exons 5 and 6 of ACTB cause syndromic thrombocytopenia. Nat. Commun. 2018, 9, 4250. [Google Scholar] [CrossRef] [PubMed]
- Malek, N.; Mrówczyńska, E.; Michrowska, A.; Mazurkiewicz, E.; Pavlyk, I.; Mazur, A.J. Knockout of ACTB and ACTG1 with CRISPR/Cas9(D10A) technique shows that non-muscle β and γ actin are not equal in relation to human melanoma cells’ motility and focal adhesion formation. Int. J. Mol. Sci. 2020, 21, 2746. [Google Scholar] [CrossRef] [PubMed]
- Siciliano, M.J.; Barker, P.E.; Cailleau, R. Mutually exclusive genetic signatures of human breast tumor cell lines with a common chromosomal marker. Cancer Res. 1979, 39, 919–922. [Google Scholar]
- Satya-Prakash, K.L.; Pathak, S.; Hsu, T.C.; Olivé, M.; Cailleau, R. Cytogenetic analysis on eight human breast tumor cell lines: High frequencies of 1q, 11q and HeLa-like marker chromosomes. Cancer Genet. Cytogenet. 1981, 3, 61–73. [Google Scholar] [CrossRef]
- Skaland, I.; Janssen, E.A.M.; Gudlaugsson, E.; Hui Ru Guo, L.; Baak, J.P.A. The prognostic value of the proliferation marker Phosphohistone H3 (PPH3) in luminal, basal-like and triple negative phenotype invasive lymph node-negative breast cancer. Cell. Oncol. 2009, 31, 261–271. [Google Scholar] [CrossRef]
- Dugina, V.B.; Chipysheva, T.A.; Ermilova, V.D.; Gabbiani, D.; Chaponnier, C.; Vasilev, I.M. Distribution of actin isoforms in normal, dysplastic, and tumorous human breast cells. Arkh. Patol. 2008, 70, 28–31. [Google Scholar]
- Dugina, V.B.; Ermilova, V.D.; Chemeris, G.I.; Chipysheva, T.A. Actins and keratins in the diagnosis of human basal-like breast cancer. Arkh. Patol. 2010, 72, 12–15. [Google Scholar] [PubMed]
- Shagieva, G.S.; Domnina, L.V.; Chipysheva, T.A.; Ermilova, V.D.; Chaponnier, C.; Dugina, V.B. Actin isoforms and reorganization of adhesion junctions in epithelial-to-mesenchymal transition of cervical carcinoma cells. Biochemistry 2012, 77, 1266–1276. [Google Scholar] [CrossRef]
- Sørlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef]
- Nielsen, T.O.; Hsu, F.D.; Jensen, K.; Cheang, M.; Karaca, G.; Hu, Z.; Hernandez-Boussard, T.; Livasy, C.; Cowan, D.; Dressler, L.; et al. Immunohistochemical and clinical characterization of the basal-like subtype of invasive breast carcinoma. Clin. Cancer Res. 2004, 10, 5367–5374. [Google Scholar] [CrossRef]
- Thompson, S.L.; Bakhoum, S.F.; Compton, D.A. Mechanisms of chromosomal instability. Curr. Biol. 2010, 20, R285–R295. [Google Scholar] [CrossRef]
- Duesberg, P.; Fabarius, A.; Hehlmann, R. Aneuploidy, the primary cause of the multilateral genomic instability of neoplastic and preneoplastic cells. IUBMB Life 2004, 56, 65–81. [Google Scholar] [CrossRef]
- Fox, D.T.; Duronio, R.J. Endoreplication and polyploidy: Insights into development and disease. Development 2013, 140, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Swanton, C.; Nicke, B.; Schuett, M.; Eklund, A.C.; Ng, C.; Li, Q.; Hardcastle, T.; Lee, A.; Roy, R.; East, P.; et al. Chromosomal instability determines taxane response. Proc. Natl. Acad. Sci. USA 2009, 106, 8671–8676. [Google Scholar] [CrossRef] [PubMed]
- Riffell, J.L.; Zimmerman, C.; Khong, A.; McHardy, L.M.; Roberge, M. Effects of chemical manipulation of mitotic arrest and slippage on cancer cell survival and proliferation. Cell Cycle 2009, 8, 3029–3042. [Google Scholar] [CrossRef]
- Nakayama, Y.; Inoue, T. Antiproliferative fate of the tetraploid formed after mitotic slippage and its promotion; a novel target for cancer therapy based on microtubule poisons. Molecules 2016, 21, 663. [Google Scholar] [CrossRef]
- Voutsadakis, I.A. Clinical implications of chromosomal instability (CIN) and kinetochore abnormalities in breast cancers. Mol. Diagnosis Ther. 2019, 23, 707–721. [Google Scholar] [CrossRef]
- Sun, X.; Clermont, P.L.; Jiao, W.; Helgason, C.D.; Gout, P.W.; Wang, Y.; Qu, S. Elevated expression of the centromere protein-A(CENP-A)-encoding gene as a prognostic and predictive biomarker in human cancers. Int. J. Cancer 2016, 139, 899–907. [Google Scholar] [CrossRef]
- McGovern, S.L.; Qi, Y.; Pusztai, L.; Symmans, W.F.; Buchholz, T.A. Centromere protein-A, an essential centromere protein, is a prognostic marker for relapse in estrogen receptor-positive breast cancer. Breast Cancer Res. 2012, 14, R72. [Google Scholar] [CrossRef]
- Yuen, K.W.Y.; Montpetit, B.; Hieter, P. The kinetochore and cancer: What’s the connection? Curr. Opin. Cell Biol. 2005, 17, 576–582. [Google Scholar] [CrossRef]
- Stangeland, B.; Mughal, A.A.; Grieg, Z.; Sandberg, C.J.; Joel, M.; Nygård, S.; Meling, T.; Murrell, W.; Vik Mo, E.O.; Langmoen, I.A. Combined expressional analysis, bioinformatics and targeted proteomics identify new potential therapeutic targets in glioblastoma stem cells. Oncotarget 2015, 6, 26192–26215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Mao, J.H.; Zhu, W.; Jain, A.K.; Liu, K.; Brown, J.B.; Karpen, G.H. Centromere and kinetochore gene misexpression predicts cancer patient survival and response to radiotherapy and chemotherapy. Nat. Commun. 2016, 7, 12619. [Google Scholar] [CrossRef]
- Shrestha, R.L.; Ahn, G.S.; Staples, M.I.; Sathyan, K.M.; Karpova, T.S.; Foltz, D.R.; Basrai, M.A. Mislocalization of centromeric histone H3 variant CENP-A contributes to chromosomal instability (CIN) in human cells. Oncotarget 2017, 8, 46781–46800. [Google Scholar] [CrossRef] [PubMed]
- Tomonaga, T.; Matsushita, K.; Yamaguchi, S.; Oohashi, T.; Shimada, H.; Ochiai, T.; Yoda, K.; Nomura, F. Overexpression and mistargeting of centromere protein-a in human primary colorectal cancer. Cancer Res. 2003, 63, 3511–3516. [Google Scholar] [PubMed]
- Vardabasso, C.; Hasson, D.; Ratnakumar, K.; Chung, C.Y.; Duarte, L.F.; Bernstein, E. Histone variants: Emerging players in cancer biology. Cell. Mol. Life Sci. 2014, 71, 379–404. [Google Scholar] [CrossRef]
- Fujiwara, T.; Bandi, M.; Nitta, M.; Ivanova, E.V.; Bronson, R.T.; Pellman, D. Cytokinesis failure generating tetraploids promotes tumorigenesis in p53-null cells. Nature 2005, 437, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Mogessie, B.; Schuh, M. Actin protects mammalian eggs against chromosome segregation errors. Science 2017, 357, eaal1647. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.; Arora, P.D.; McCulloch, C.A.; Wilde, A. Cytokinesis requires localized β-actin filament production by an actin isoform specific nucleator. Nat. Commun. 2017, 8, 1530. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dugina, V.; Shagieva, G.; Novikova, M.; Lavrushkina, S.; Sokova, O.; Kireev, I.; Kopnin, P. Impaired Expression of Cytoplasmic Actins Leads to Chromosomal Instability of MDA-MB-231 Basal-Like Mammary Gland Cancer Cell Line. Molecules 2021, 26, 2151. https://doi.org/10.3390/molecules26082151
Dugina V, Shagieva G, Novikova M, Lavrushkina S, Sokova O, Kireev I, Kopnin P. Impaired Expression of Cytoplasmic Actins Leads to Chromosomal Instability of MDA-MB-231 Basal-Like Mammary Gland Cancer Cell Line. Molecules. 2021; 26(8):2151. https://doi.org/10.3390/molecules26082151
Chicago/Turabian StyleDugina, Vera, Galina Shagieva, Mariya Novikova, Svetlana Lavrushkina, Olga Sokova, Igor Kireev, and Pavel Kopnin. 2021. "Impaired Expression of Cytoplasmic Actins Leads to Chromosomal Instability of MDA-MB-231 Basal-Like Mammary Gland Cancer Cell Line" Molecules 26, no. 8: 2151. https://doi.org/10.3390/molecules26082151
APA StyleDugina, V., Shagieva, G., Novikova, M., Lavrushkina, S., Sokova, O., Kireev, I., & Kopnin, P. (2021). Impaired Expression of Cytoplasmic Actins Leads to Chromosomal Instability of MDA-MB-231 Basal-Like Mammary Gland Cancer Cell Line. Molecules, 26(8), 2151. https://doi.org/10.3390/molecules26082151






