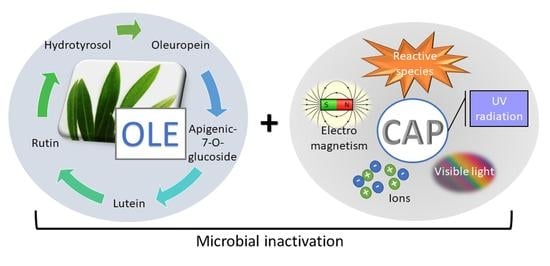
Combined Antimicrobial Effect of Bio-Waste Olive Leaf Extract and Remote Cold Atmospheric Plasma Effluent
Abstract
1. Introduction
2. Results
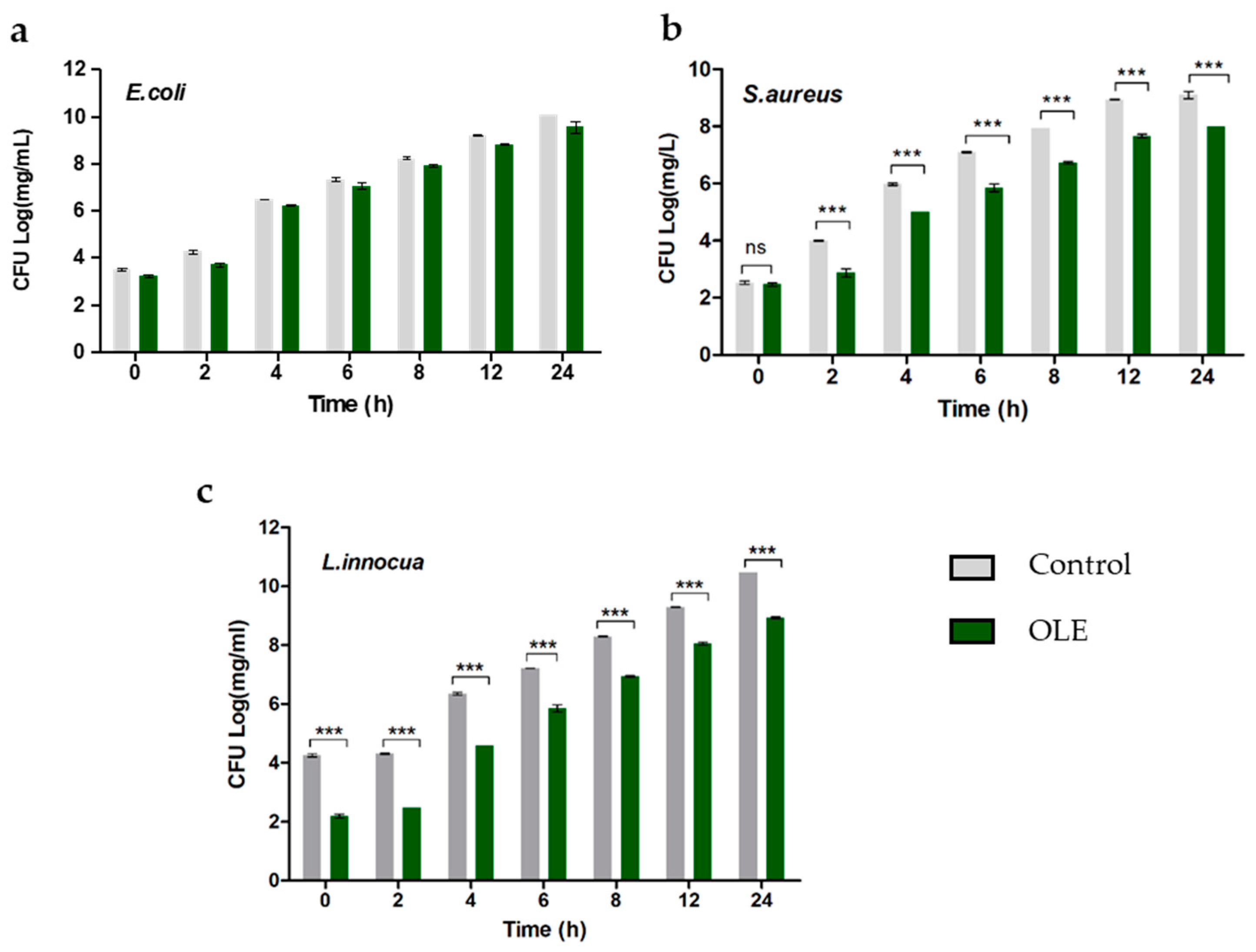
2.1. Effect of OLE on Bacterial Strains
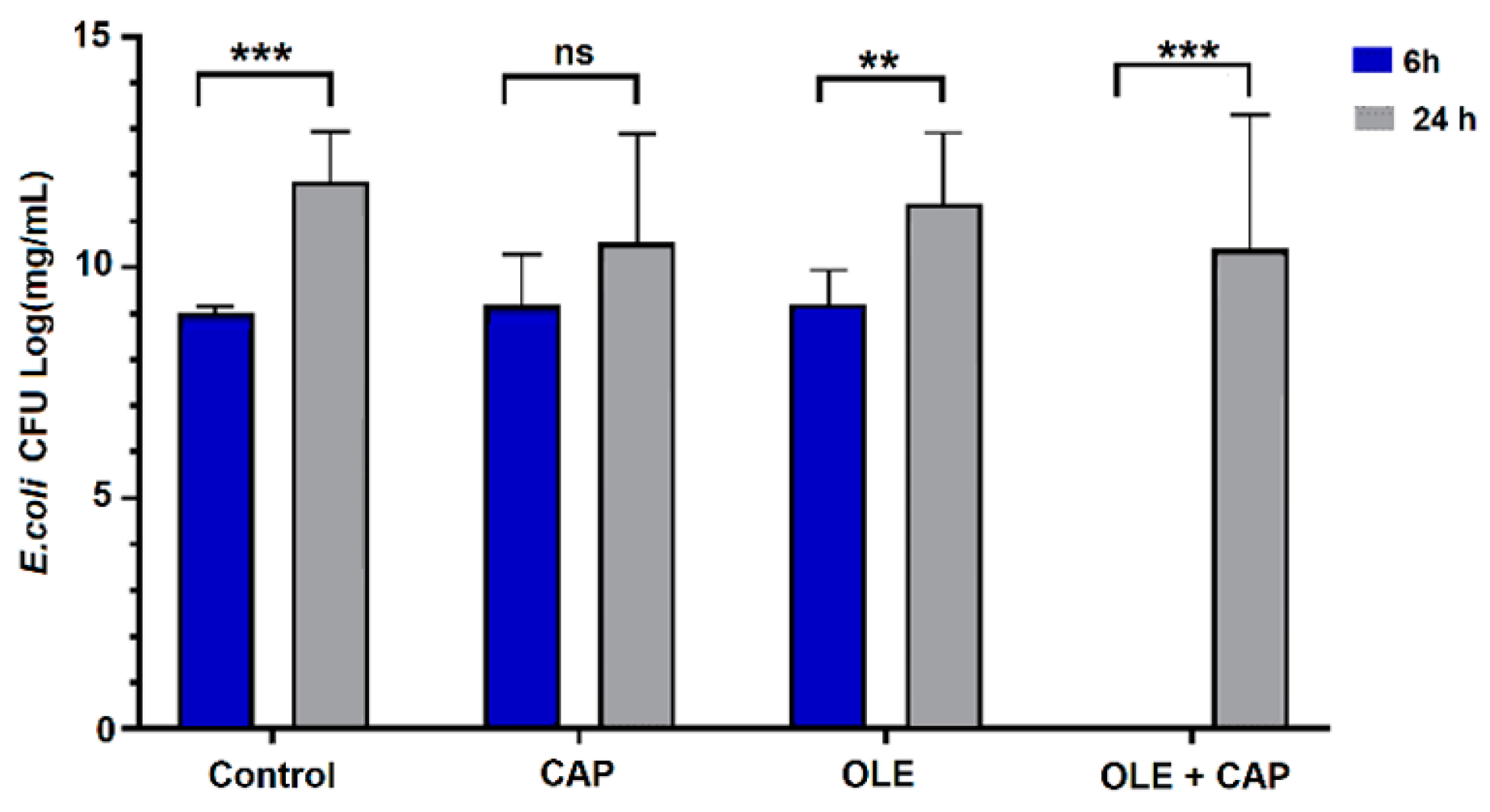
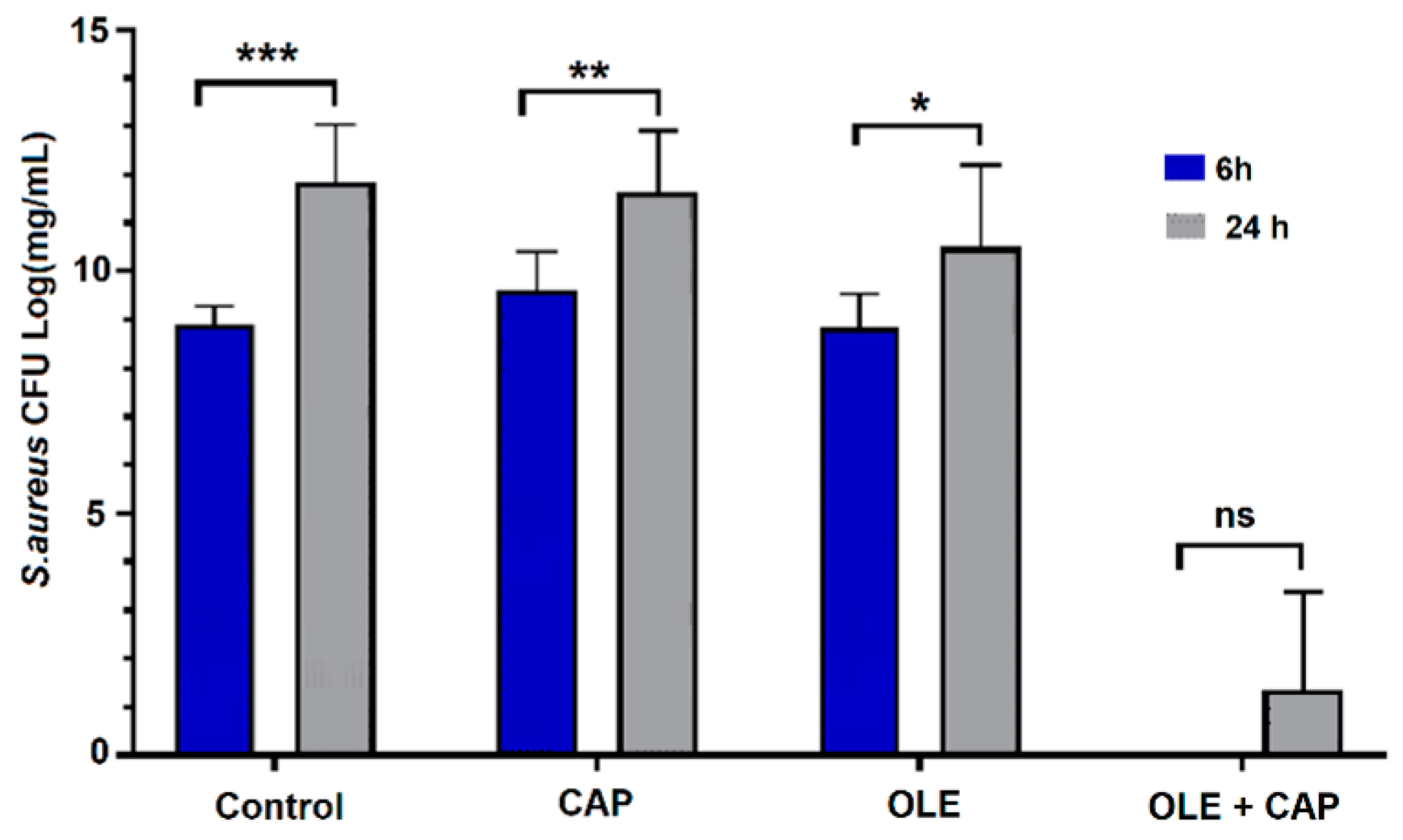
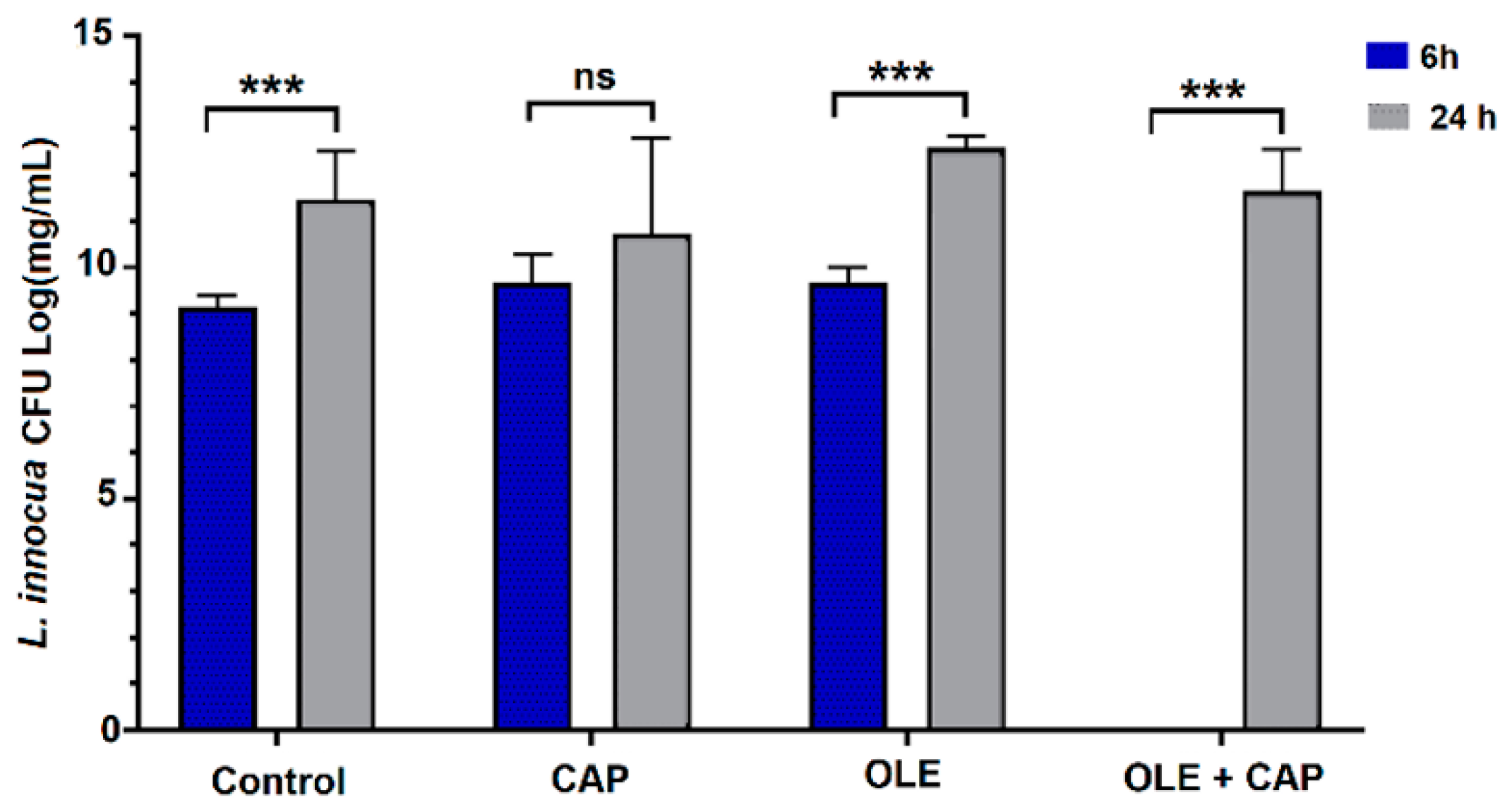
2.2. Effect of OLE + CAP on Bacterial Strains
3. Discussion
4. Materials and Methods
4.1. OLE Extraction and Characterization
4.2. CAP Technology
4.3. In Vitro Tests
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Guillard, V.; Gaucel, S.; Fornaciari, C.; Angellier-Coussy, H.; Buche, P.; Gontard, N. The next generation of sustainable food packaging to preserve our environment in a circular economy context. Front. Nutr. 2018, 5, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Isbary, G.; Shimizu, T.; Li, Y.; Stolz, W.; Tomas, H.M.; Morfill, G.; Zimmermmann, J. Cold atmospheric plasma devices for medical issues. Exp. Rev. Med. Dev. 2013, 10, 367–377. [Google Scholar] [CrossRef]
- Ben-Othman, S.; Jõudu, I.; Bhat, R. Bioactives from agri-food wastes: Present insights and future challenges. Molecules 2020, 25, 510. [Google Scholar] [CrossRef] [PubMed]
- Al-Hashimi, A.G. Antioxidant and antibacterial activities of Hibiscus sabdariffa L. extracts. Afr. J. Food Sci. 2012, 6, 506–511. [Google Scholar] [CrossRef]
- Amaral, G.P.; Mizdal, C.R.; Stefanello, S.T.; Mendez, A.S.L.; Puntel, R.L.; de Campos, M.M.A.; Soares, F.A.A.; Fachinetto, R. Antibacterial and antioxidant effects of Rosmarinus officinalis L. extract and its fractions. J. Tradit. Complement. Med. 2018, 9, 383–392. [Google Scholar] [CrossRef]
- Moemenbellah-Fard, M.D.; Abdollahi, A.; Ghanbariasad, A.; Osanloo, M. Antibacterial and leishmanicidal activities of Syzygium aromaticum essential oil versus its major ingredient, eugenol. Flavour Fragr. J. 2020, 35, 534–540. [Google Scholar] [CrossRef]
- Fani, M.; Kohanteb, J. In vitro antimicrobial activity of thymus vulgaris essential oil against major oral pathogens. J. Evidence-Based Complement. Altern. Med. 2017, 22, 660–666. [Google Scholar] [CrossRef]
- Ahmed, S.; Moni, B.M.; Ahmed, S.; Gomes, D.J.; Shohael, A.M. Comparative phytochemical, antioxidant, and antibacterial study of different parts of Doigota plants (Bixa orellana L.). Bull. Natl. Res. Cent. 2020, 44, 1–10. [Google Scholar] [CrossRef]
- Sharifi-Rad, M.; Nazaruk, J.; Polito, L.; Morais-Braga, M.F.B.; Rocha, J.E.; Coutinho, H.D.M.; Salehi, B.; Tabanelli, G.; Montanari, C.; del Mar Contreras, M.; et al. Matricaria genus as a source of antimicrobial agents: From farm to pharmacy and food applications. Microbiol. Res. 2018, 215, 76–88. [Google Scholar] [CrossRef] [PubMed]
- Heck, C.I.; De Mejia, E.G. Yerba mate tea (Ilex paraguariensis): A comprehensive review on chemistry, health implications, and technological considerations. J. Food Sci. 2007, 77, R138–R151. [Google Scholar] [CrossRef] [PubMed]
- Razavi, S.M.; Zarrini, G.; Molavi, G.; Ghasemi, G. Bioactivity of Malva sylvestris L., a medicinal plant from Iran. Iran. J. Basic Med. Sci. 2011, 14, 574–579. [Google Scholar] [PubMed]
- Fayera, S.; Babu, G.N.; Dekebo, A.; Bogale, Y. Phytochemical investigation and antimicrobial study of leaf extract of Plantago lanceolata. Nat. Prod. Chem. Res. 2018, 6, 1000311. [Google Scholar] [CrossRef]
- Kolodziejczyk-Czepas, J.; Liudvytska, O. Rheum rhaponticum and Rheum rhabarbarum: A review of phytochemistry, biological activities and therapeutic potential. Phytochem. Rev. 2020. [Google Scholar] [CrossRef]
- Fufa, B.K. Anti-bacterial and anti-fungal properties of garlic extract (Allium sativum): A Review. Microbiol. Res. J. Int. 2019, 28, 1–5. [Google Scholar] [CrossRef]
- Seow, Y.X.; Yeo, C.R.; Chung, H.L.; Yuk, H.G. Plant essential oils as active antimicrobial agents. Crit. Rev. Food Sci. Nutr. 2014, 54, 625–644. [Google Scholar] [CrossRef]
- Lee, O.-H.; Lee, B.-Y. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour. Technol. 2010, 101, 3751–3754. [Google Scholar] [CrossRef]
- Salomone, R.; Ioppolo, G. Environmental impacts of olive oil production: A Life Cycle Assessment case study in the province of Messina (Sicily). J. Clean. Prod. 2012, 28, 88–100. [Google Scholar] [CrossRef]
- Lafka, T.-I.; Lazou, A.; Sinanoglou, V.; Lazos, E. Phenolic extracts from wild olive leaves and their potential as edible oils antioxidants. Foods 2013, 2, 18–31. [Google Scholar] [CrossRef]
- Rombaut, N.; Tixier, A.-S.; Bily, A.; Chemat, F. Green extraction processes of natural products as tools for biorefinery. Biofuels Bioprod. Bioref. 2014, 8, 530–544. [Google Scholar] [CrossRef]
- Özcan, M.M.; Matthäus, B. A review: Benefit and bioactive properties of olive (Olea europaea L.) leaves. Eur. Food Res. Technol. 2017, 243, 89–99. [Google Scholar] [CrossRef]
- Nediani, C.; Ruzzolini, J.; Romani, A.; Calorini, L. Oleuropein, a bioactive compound from Olea europaea L., as a potential preventive and therapeutic agent in non-communicable diseases. Antioxidants 2019, 8, 578. [Google Scholar] [CrossRef]
- Hashmi, M.A.; Khan, A.; Hanif, M.; Farooq, U.; Perveen, S. Traditional uses, phytochemistry, and pharmacology of Olea europaea (Olive). Evidence-Based Complement. Altern. Med. 2015, 2015, 541591. [Google Scholar] [CrossRef] [PubMed]
- Şahin, S.; Bilgin, M. Olive tree (Olea europaea L.) leaf as a waste by-product of table olive and olive oil industry: A review. J. Sci. Food Agric. 2018, 98, 1271–1279. [Google Scholar] [CrossRef] [PubMed]
- Poudyal, H.; Campbell, F.; Brown, L. Olive leaf extract attenuates cardiac, hepatic, and metabolic changes in high carbohydrate-, high fat-fed rats. J. Nutr. 2010, 140, 946–953. [Google Scholar] [CrossRef] [PubMed]
- Sudjana, A.N.; D’Orazio, C.; Ryan, V.; Rasool, N.; Ng, J.; Islam, N.; Riley, T.V.; Hammer, K.A. Antimicrobial activity of commercial Olea europaea (olive) leaf extract. Int. J. Antimicrob. Agents 2009, 33, 461–463. [Google Scholar] [CrossRef] [PubMed]
- Djenane, D.; Gómez, D.; Yangüela, J.; Roncalés, P.; Ariño, A. Olive leaves extract from Algerian oleaster (Olea europaea var. sylvestris) on microbiological safety and shelf-life stability of raw Halal minced beef during display. Foods 2019, 8, 10. [Google Scholar] [CrossRef]
- Albertos, I.; Avena-Bustillos, R.J.; Martín-Diana, A.B.; Du, W.X.; Rico, D.; McHugh, T.H. Antimicrobial Olive Leaf Gelatin films for enhancing the quality of cold-smoked Salmon? Food Packag. Shelf Life 2017, 13, 49–55. [Google Scholar] [CrossRef]
- Pereira, A.P.; Ferreira, I.C.F.R.; Marcelino, F.; Valentão, P.; Andrade, P.B.; Eabra, R.; Estevinho, L.; Bento, A.; Pereira, J.A. Phenolic compounds and antimicrobial activity of olive (Olea europaea L. Cv. Cobrançosa) leaves. Molecules 2007, 12, 1153–1162. [Google Scholar] [CrossRef] [PubMed]
- Tranter, H.S.; Tassou, S.C.; Nychas, G.J. The effect of the olive phenolic compound, oleuropein, on growth and enterotoxin B production by Staphylococcus aureus. J. Appl. Bacteriol. 1993, 74, 253–259. [Google Scholar] [CrossRef]
- Ghomari, O.; Sounni, F.; Massaoudi, Y.; Ghanam, J.; Drissi Kaitouni, L.B.; Merzouki, M.; Benlemlih, M. Phenolic profile (HPLC-UV) of olive leaves according to extraction procedure and assessment of antibacterial activity. Biotechnol. Rep. 2019, 23, e00347. [Google Scholar] [CrossRef] [PubMed]
- Bourke, P.; Ziuzina, D.; Han, L.; Cullen, P.J.; Gilmore, B.F. Microbiological interactions with cold plasma. J. Appl. Microbiol. 2017, 123, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Cheng, J.-H.; Sun, D.-W. Cold plasma-mediated treatments for shelf life extension of fresh produce: A review of recent research developments. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1312–1326. [Google Scholar] [CrossRef] [PubMed]
- Graves, D. The emerging role of reactive oxygen and nitrogen species in redox biology and some implications for plasma applications to medicine and biology. J. Phys. D Appl. Phys. 2012, 45, 263001. [Google Scholar] [CrossRef]
- Djenane, D.; Aboudaou, M.; Djenane, F.; García-Gonzalo, D.; Pagán, R. Improvement of the shelf-life status of modified atmosphere packaged camel meat using nisin and Olea europaea subsp. laperrinei leaf extract. Foods 2020, 9, 1336. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Huang, K.; Wang, J. Bactericidal effect of various non-thermal plasma agents and the influence of experimental conditions in microbial inactivation: A review. Food Control 2015, 50, 482–490. [Google Scholar] [CrossRef]
- Cullen, P.J.; Lalor, J.; Scally, L.; Boehm, D.; Milosavljević, V.; Bourke, P.; Keener, K. Translation of plasma technology from the lab to the food industry. Plasma Process. Polym. 2018, 15, 1–11. [Google Scholar] [CrossRef]
- Chang, Y.; Tu, P.; Wu, M.; Hsueh, T.; Hsu, S. A study on chitosan modification of polyester fabrics by atmospheric pressure plasma and its antibacterial effects. Fibers Polym. 2008, 9, 307–311. [Google Scholar] [CrossRef]
- Nikiforov, A.; Deng, X.; Xiong, Q.; Cvelbar, U.; Degeyter, N.; Morent, R.; Leys, C. Non-thermal plasma technology for the development of antimicrobial surfaces: A review. J. Phys. D Appl. Phys. 2016, 49, 204002. [Google Scholar] [CrossRef]
- Karam, L.; Jama, C.; Mamede, A.-S.; Fahs, A.; Louarn, G.; Dhulster, P.; Chihib, N.-E. Study of nisin adsorption on plasma-treated polymer surfaces for setting up materials with antibacterial properties. Reactive Funct. Polym. 2013, 73, 1473–1479. [Google Scholar] [CrossRef]
- Duday, D.; Vreuls, C.; Moreno, M.; Frache, G.; Boscher, N.D.; Zocchi, G.; Archambeau, C.; Van De Weerdt, C.; Martial, J.; Choquet, P. Atmospheric pressure plasma modified surfaces for immobilization of antimicrobial nisin peptides. Surf. Coat. Technol. 2013, 218, 152–161. [Google Scholar] [CrossRef]
- Schmid, M.; Dallmann, K.; Bugnicourt, E.; Cordoni, D.; Wild, F.; Lazzeri, A.; Noller, K. Properties of whey-protein-coated films and laminates as novel recyclable food packaging materials with excellent barrier properties. Int. J. Polym. Sci. 2012, 2012, 5–7. [Google Scholar] [CrossRef]
- Tihminlioglu, F.; Atik, I.D.; Özen, B. Water vapor and oxygen-barrier performance of corn-zein coated polypropylene films. J. Food Eng. 2010, 96, 342–347. [Google Scholar] [CrossRef]
- Laroussi, M.; Leipold, F. Evaluation of the roles of reactive species, heat, and UV radiation in the inactivation of bacterial cells by air plasmas at atmospheric pressure. Int. J. Mass Spectrom. 2004, 233, 81–86. [Google Scholar] [CrossRef]
- McKeen, L. Introduction to food irradiation and medical sterilization. In Film Properties of Plastics and Elastomers, 3rd ed.; McKeen, L., Ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012; pp. 1–40. ISBN 978-1-4557-2551-9. [Google Scholar]
- De la Ossa, J.G.; Felice, F.; Azimi, B.; Salsano, J.E.; Digiacomo, M.; Macchia, M.; Danti, S.; Di Stefano, R. Waste autochthonous tuscan olive leaves (Olea europaea var. olivastra seggianese) as antioxidant source for biomedicine. Int. J. Mol. Sci. 2019, 20, 5918. [Google Scholar] [CrossRef]
- Liu, Y.; McKeever, L.C.; Malik, N.S.A. Assessment of the antimicrobial activity of olive leaf extract against foodborne bacterial pathogens. Front. Microbiol. 2017, 8, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Costello, K.M.; Gutierrez-Merino, J.; Bussemaker, M.; Ramaioli, M.; Baka, M.; Van Impe, J.F.; Velliou, E.G. Modelling the microbial dynamics and antimicrobial resistance development of Listeria in viscoelastic food model systems of various structural complexities. Int. J. Food Microbiol. 2018, 286, 15–30. [Google Scholar] [CrossRef]
- Costello, K.M.; Gutierrez-Merino, J.; Bussemaker, M.; Smet, C.; Van Impe, J.F.; Velliou, E.G. A multi-scale analysis of the effect of complex viscoelastic models on Listeria dynamics and adaptation in co-culture systems. AIChE J. 2020, 66, 1–15. [Google Scholar] [CrossRef]
- Zhou, J.; Velliou, E.; Hong, S.H. Investigating the effects of nisin and free fatty acid combined treatment on Listeria monocytogenes inactivation. LWT-Food Sci. Technol. 2020, 133, 110115. [Google Scholar] [CrossRef]
- Difonzo, G.; Russo, A.; Trani, A.; Paradiso, V.M.; Ranieri, M.; Pasqualone, A.; Summo, C.; Tamma, G.; Silletti, R.; Caponio, F. Green extracts from Coratina olive cultivar leaves: Antioxidant characterization and biological activity. J. Funct. Foods 2017, 31, 63–70. [Google Scholar] [CrossRef]
- Mai-Prochnow, A.; Clauson, M.; Hong, J.; Murphy, A.B. Gram positive and Gram negative bacteria differ in their sensitivity to cold plasma. Sci. Rep. 2016, 6, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Boehm, D.; Patil, S.; Cullen, P.J.; Bourke, P. Assessing stress responses to atmospheric cold plasma exposure using Escherichia coli knock-out mutants. J. Appl. Microbiol. 2016, 121, 352–363. [Google Scholar] [CrossRef]
- Dobrynin, D.; Fridman, G.; Friedman, G.; Fridman, A. Physical and biological mechanisms of direct plasma interaction with living tissue. New J. Phys. 2009, 11, 115020. [Google Scholar] [CrossRef]
- Surowsky, B.; Fröhling, A.; Gottschalk, N.; Schlüter, O.; Knorr, D. Impact of cold plasma on Citrobacter freundii in apple juice: Inactivation kinetics and mechanisms. Int. J. Food Microbiol. 2014, 174, 63–71. [Google Scholar] [CrossRef]
- Yang, D.C.; Blair, K.M.; Salama, N.R. Staying in shape: The impact of cell shape on bacterial survival in diverse environments. Microbiol. Mol. Biol. Rev. 2016, 80, 187–203. [Google Scholar] [CrossRef] [PubMed]
- von Woedtke, T.; Metelmann, H.-R.; Weltmann, K.-D. Clinical plasma medicine: State and perspectives of in vivo application of cold atmospheric plasma. Contrib. Plasma Phys. 2014, 54, 104–117. [Google Scholar] [CrossRef]
- Stoffels, E.; Kieft, I.E.; Sladek, R.E.J.; Van Den Bedem, L.J.M.; Van Der Laan, E.P.; Steinbuch, M. Plasma needle for in vivo medical treatment: Recent developments and perspectives. Plasma Sources Sci. Technol. 2006, 15, S169–S180. [Google Scholar] [CrossRef]
- Yusupov, M.; Bogaerts, A.; Huygh, S.; Snoeckx, R.; Van Duin, A.C.T.; Neyts, E.C. Plasma-induced destruction of bacterial cell wall components: A reactive molecular dynamics simulation. J. Phys. Chem. C 2013, 117, 5993–5998. [Google Scholar] [CrossRef]
- Costello, K.M.; Smet, C.; Gutierrez-Merino, J.; Bussemaker, M.; Van Impe, J.F.; Velliou, E.G. The impact of food model system structure on the inactivation of Listeria innocua by cold atmospheric plasma and nisin combined treatments. Int. J. Food Microbiol. 2021, 337, 108948. [Google Scholar] [CrossRef]
- Milazzo, M.; Gallone, G.; Marcello, E.; Mariniello, M.D.; Bruschini, L.; Roy, I.; Danti, S. Biodegradable polymeric micro/nano-structures with intrinsic antifouling/antimicrobial properties: Relevance in damaged skin and other biomedical applications. J. Funct. Biomater. 2020, 11, 60. [Google Scholar] [CrossRef]
- Danti, S.; Azimi, B.; Candito, M.; Fusco, A.; Sorayani Bafqi, M.S.; Ricci, C.; Milazzo, M.; Cristallini, C.; Latifi, M.; Donnarumma, G.; et al. Lithium niobate nanoparticles as biofunctional interface material for inner ear devices. Biointerphases 2020, 15, 31004. [Google Scholar] [CrossRef]
- Romano, L.; Portone, L.; Coltelli, M.B.; Patti, F.; Saija, R.; Iatì, M.A.; Gallone, G.; Lazzeri, A.; Danti, S.; Marago, O.; et al. Intelligent non-colorimetric indicators for the perishable supply chain by non-wovens with photo-programmed thermal response. Nat. Commun. 2020, 11, 5991. [Google Scholar] [CrossRef] [PubMed]
| Bacterial Species | Olive Variety and Origin | OLE Extraction Method | OLE Concentration | Reference |
|---|---|---|---|---|
| K. pneumoniae | Olea europaea (Turkey, west Anatolian) | Aqueous | 30 µL OLE; 15% (w/v) | [22,30] |
| S. aureus | Olea europaea (Portugal) | Aqueous | 5 mg/mL | [28,29] |
| B. cereus | Olea europaea (Portugal) | Aqueous | 5 mg/mL | [28] |
| B. subtilis | Olea europaea | Ethanol | 27.2 ± 0.99 mg/g | [28] |
| P. aeruginosa | Olea europaea (Portugal) | Aqueous | 5 mg/mL | [28] |
| C. jejuni | Olea europaea (Australia) | n.a. | n.a. | [27] |
| H. pylori | Olea europaea (Australia) | n.a. | n.a. | [27] |
| E. coli | Olea europaea (Several countries) | Water, ethanol. | Variable | [28,30,31] |
| S. enterica | Olea europaea subsp. europaea var. Sylvestris (Algeria) | Methanol/water | 198.7 ± 3.6 mg GAE/g | [27,28,30] |
| L. monocytogenes | Commercial extract (USA) | Water/ethanol | 62.5 mg/mL | [27,30] |
| OLE Composition | Concentration (mg/g OLE) |
|---|---|
| Oleuropein | 32.64 ± 3.06 |
| Luteolin-7-O-glucoside | 6.97 ± 0.24 |
| Rutin | 3.37 ± 0.33 |
| Apigenin-7-O-glucoside | 1.97 ± 0.17 |
| Hydroxy-tyrosol | 0.85 ± 0.08 |
| Caffeic acid | 0.18 ± 0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
De la Ossa, J.G.; El Kadri, H.; Gutierrez-Merino, J.; Wantock, T.; Harle, T.; Seggiani, M.; Danti, S.; Di Stefano, R.; Velliou, E. Combined Antimicrobial Effect of Bio-Waste Olive Leaf Extract and Remote Cold Atmospheric Plasma Effluent. Molecules 2021, 26, 1890. https://doi.org/10.3390/molecules26071890
De la Ossa JG, El Kadri H, Gutierrez-Merino J, Wantock T, Harle T, Seggiani M, Danti S, Di Stefano R, Velliou E. Combined Antimicrobial Effect of Bio-Waste Olive Leaf Extract and Remote Cold Atmospheric Plasma Effluent. Molecules. 2021; 26(7):1890. https://doi.org/10.3390/molecules26071890
Chicago/Turabian StyleDe la Ossa, Jose Gustavo, Hani El Kadri, Jorge Gutierrez-Merino, Thomas Wantock, Thomas Harle, Maurizia Seggiani, Serena Danti, Rossella Di Stefano, and Eirini Velliou. 2021. "Combined Antimicrobial Effect of Bio-Waste Olive Leaf Extract and Remote Cold Atmospheric Plasma Effluent" Molecules 26, no. 7: 1890. https://doi.org/10.3390/molecules26071890
APA StyleDe la Ossa, J. G., El Kadri, H., Gutierrez-Merino, J., Wantock, T., Harle, T., Seggiani, M., Danti, S., Di Stefano, R., & Velliou, E. (2021). Combined Antimicrobial Effect of Bio-Waste Olive Leaf Extract and Remote Cold Atmospheric Plasma Effluent. Molecules, 26(7), 1890. https://doi.org/10.3390/molecules26071890