Chitosan Membranes Filled with Cyclosporine A as Possible Devices for Local Administration of Drugs in the Treatment of Breast Cancer
Abstract
1. Introduction
2. Results and Discussion
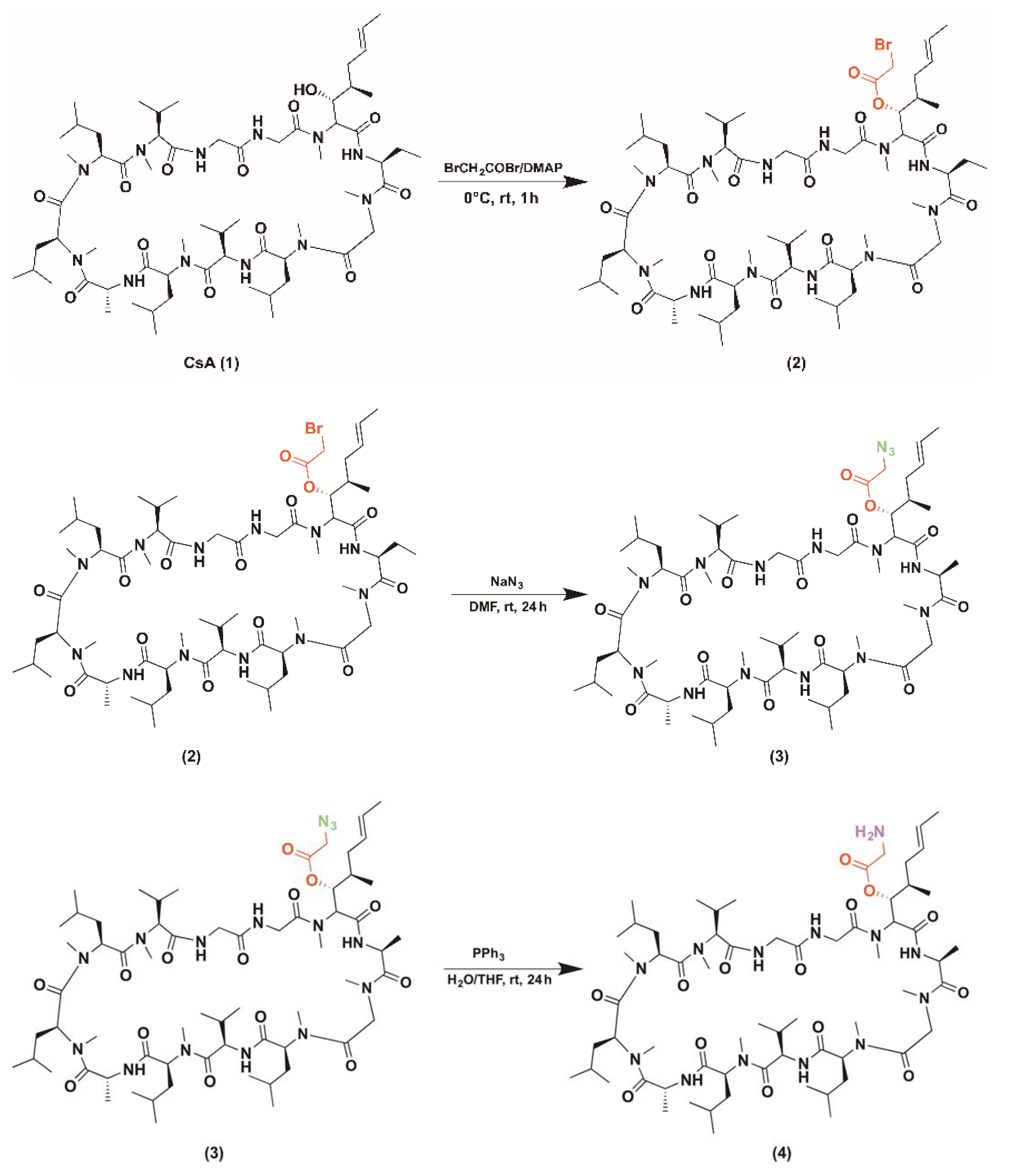
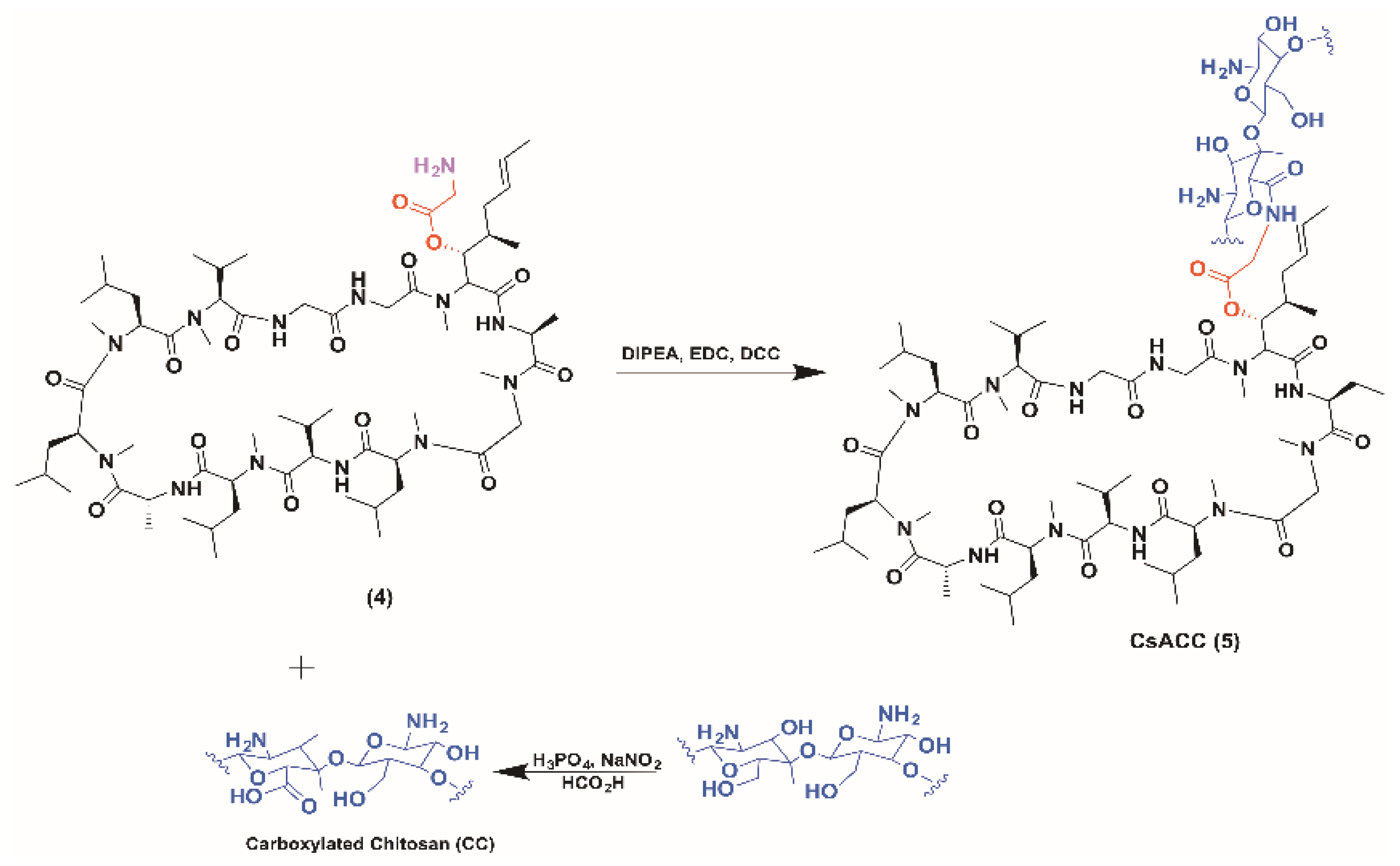
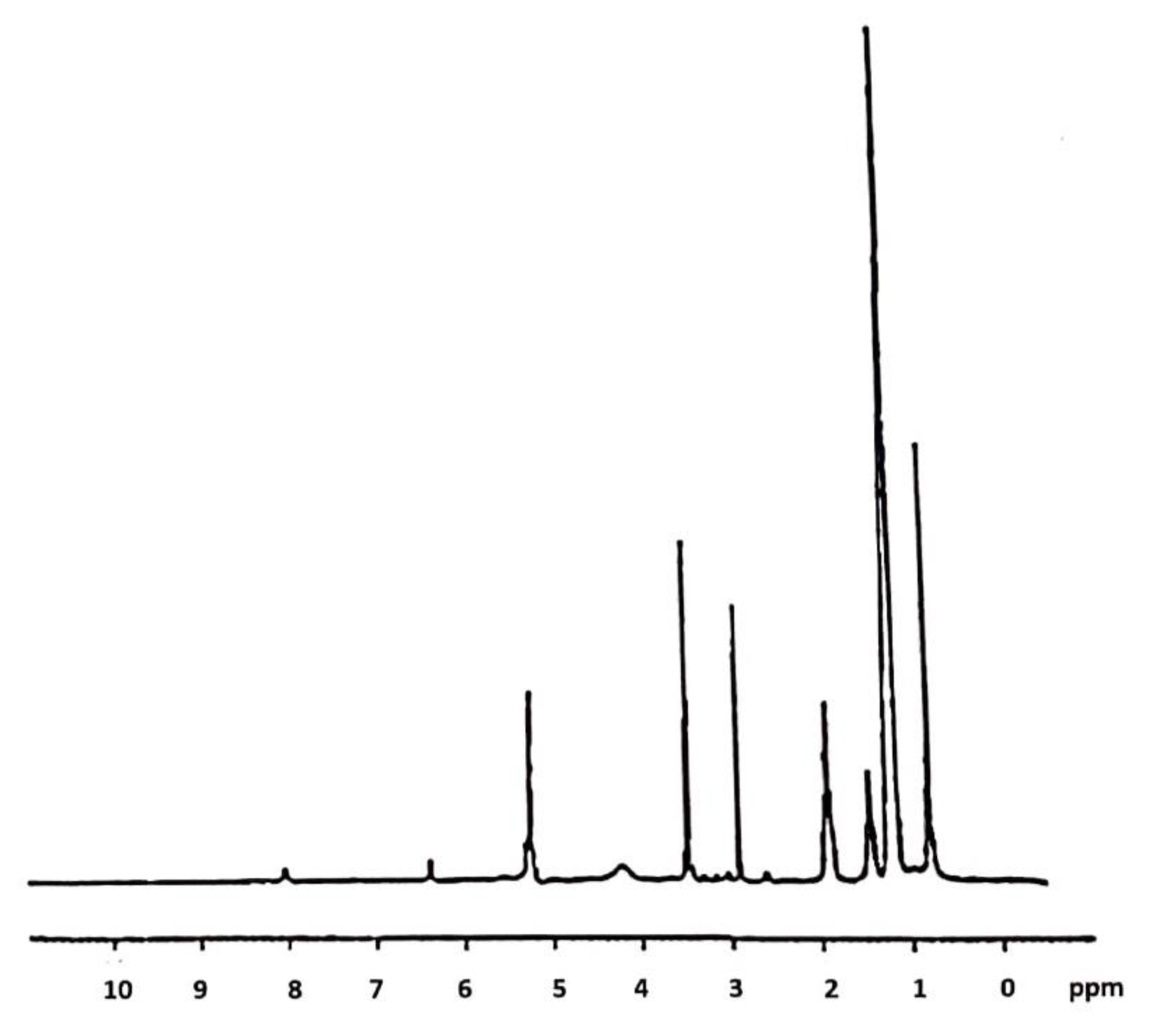
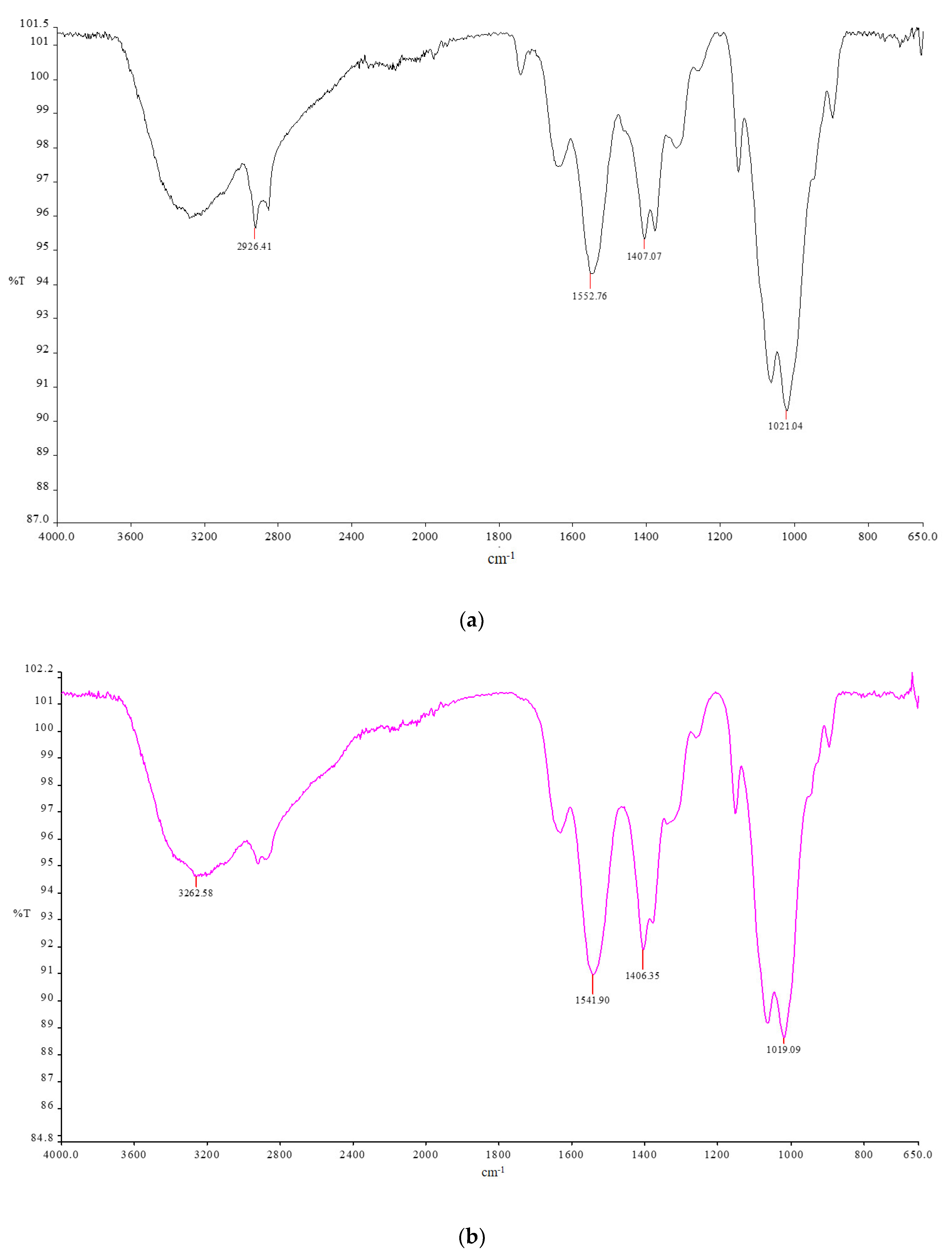
2.1. CsACC Synthesis and Characterization
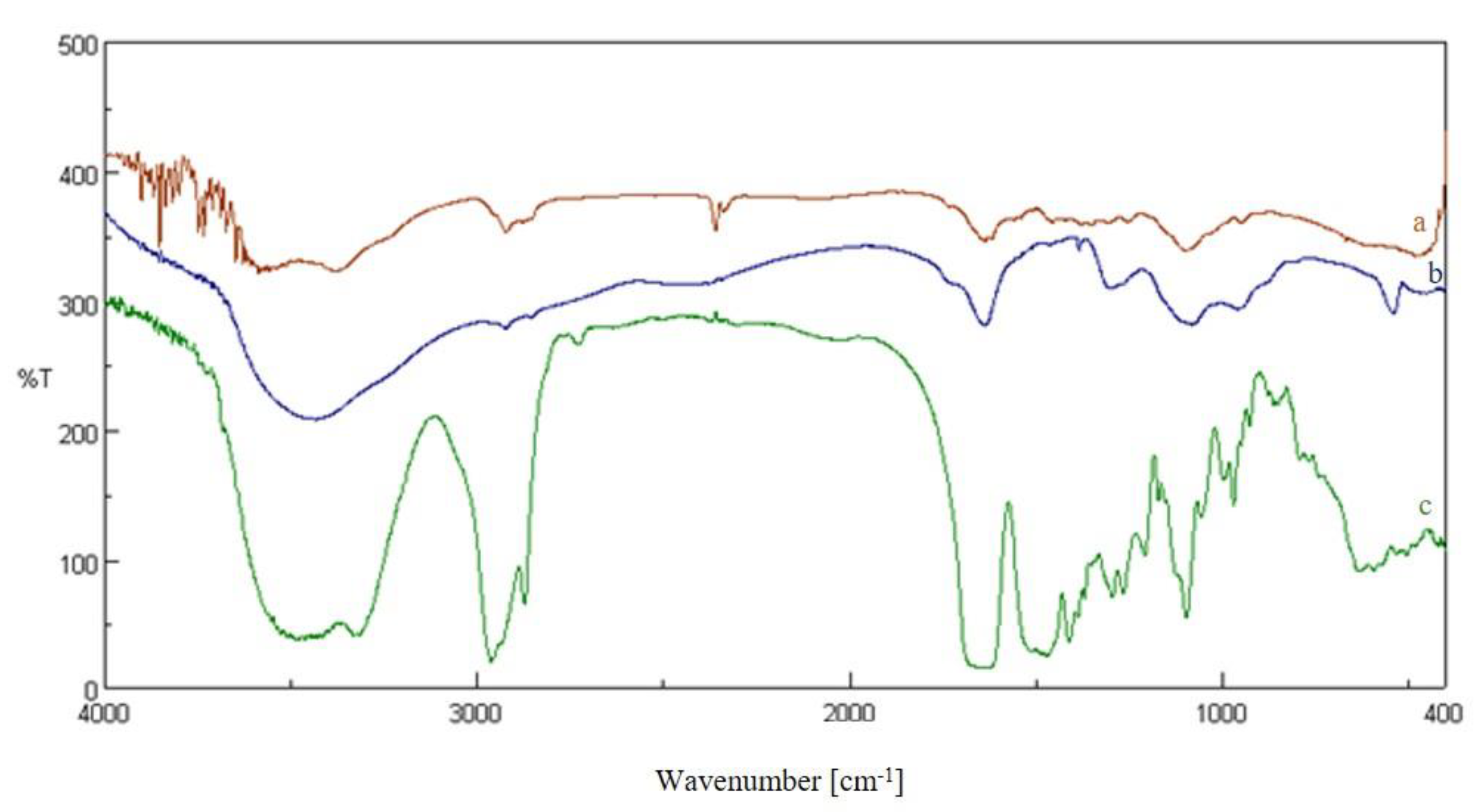
2.2. Characterization of Membranes
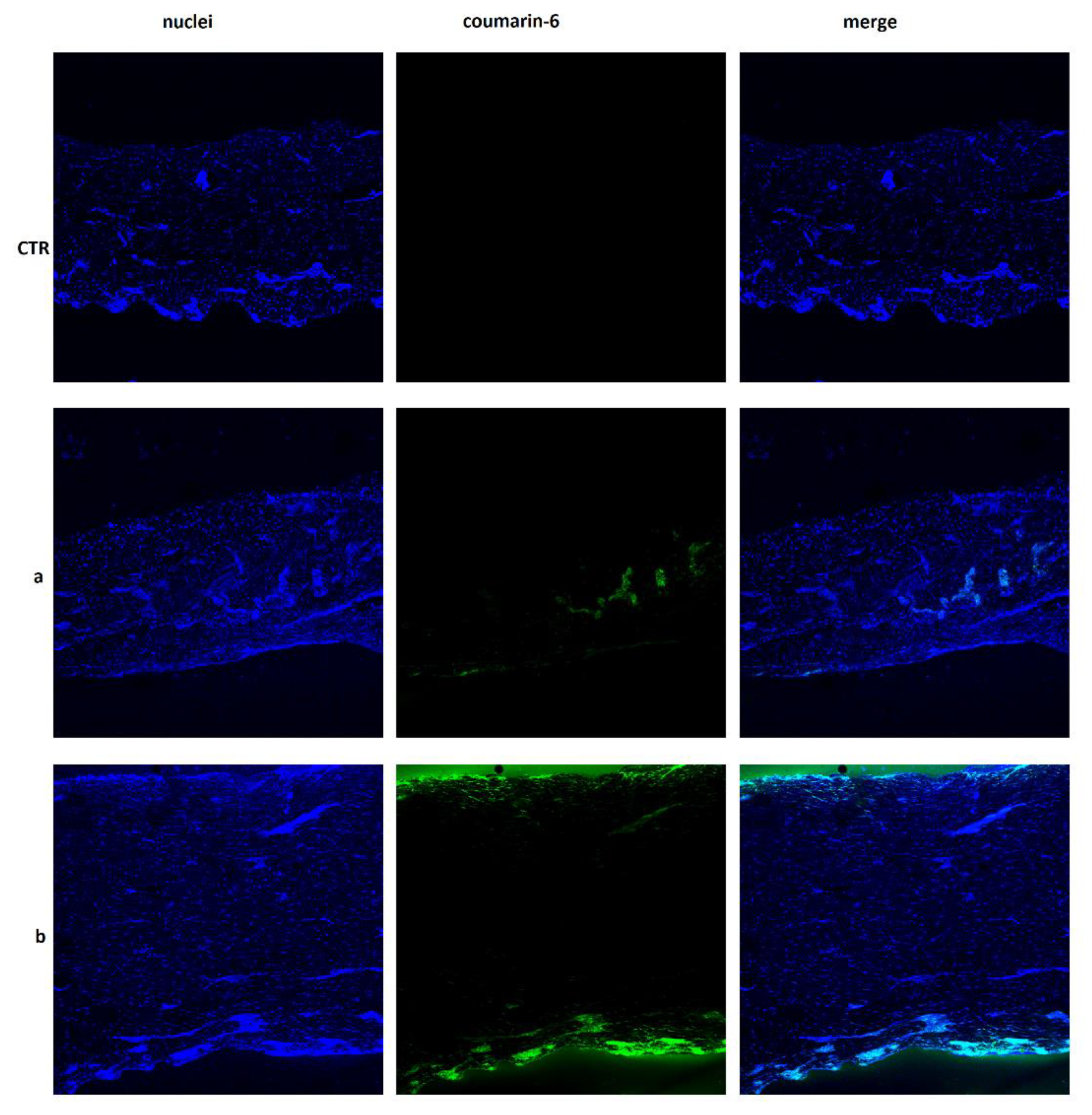
2.3. Skin Permeation Studies
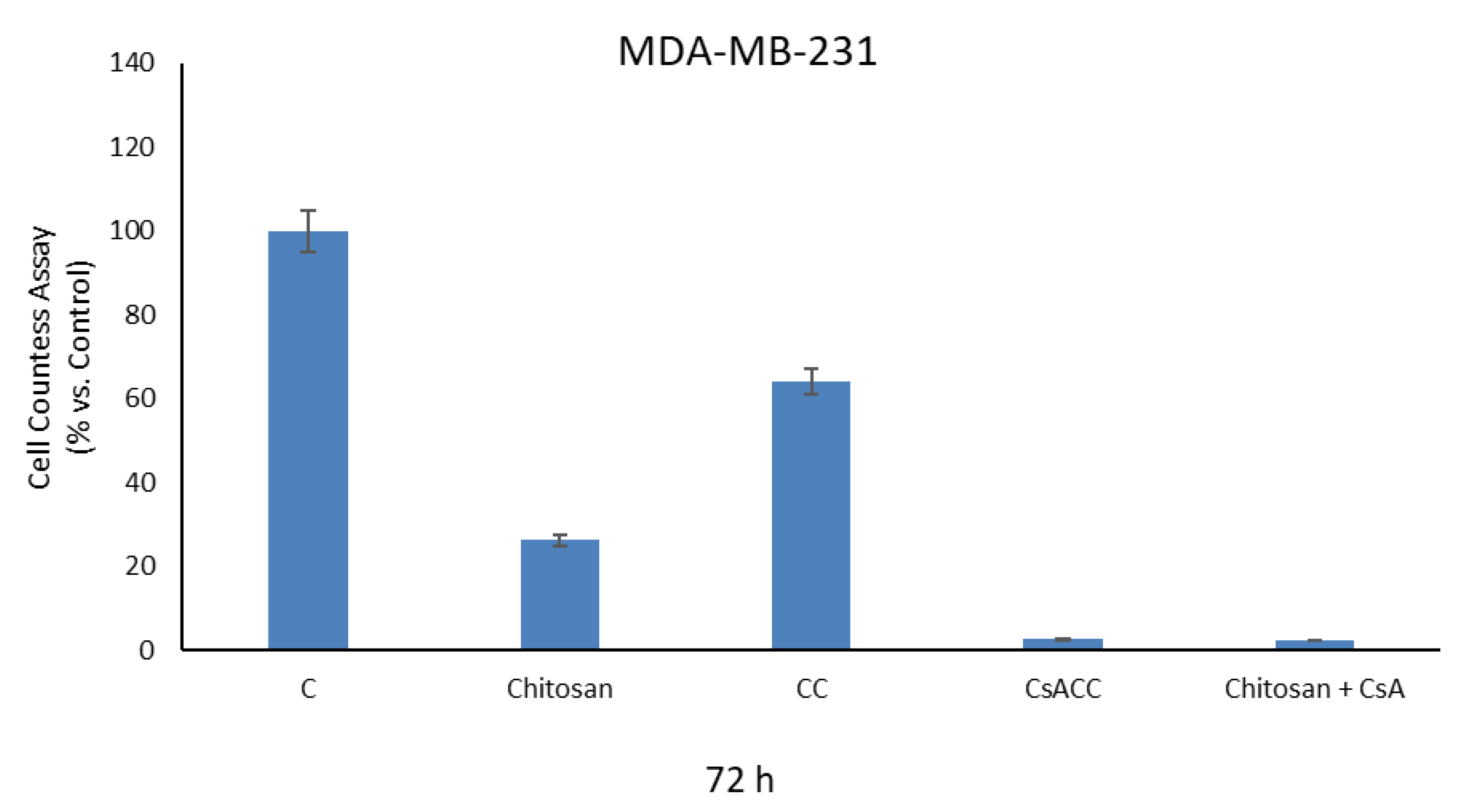
2.4. Cell Proliferation Assays
3. Materials and Methods
3.1. Materials
3.2. Cell Culture
3.3. Instruments
3.4. Synthesis of Carboxylated Chitosan (CC)
3.5. Determination of Carboxylic Groups Content
3.6. Synthesis of CSA-CC
3.7. Preparation of Chitosan (CM) and Membranes Chitosan (CMCsCC) Membranes
3.8. In Vitro Skin Permeation Studies
3.9. Localization of CsA in Skin (CLSM Study)
3.10. Cell Proliferation Assays
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- Budker, V.G.; Monahan, S.D.; Subbotin, V.M. Loco-regional cancer drug therapy: Present approaches and rapidly reversible hydrophobization (RRH) of therapeutic agents as the future direction. Drug Discov. Today 2014, 19, 1855–1870. [Google Scholar] [CrossRef] [PubMed]
- Chou, H.S.; Larsson, M.; Hsiao, M.H.; Chen, Y.C.; Röding, M.; Nydén, M.; Liu, D.M. Injectable insulin-lysozyme-loaded nanogels with enzymatically-controlled degradation and release for basal insulin treatment: In vitro characterization and in vivo observation. J. Control. Release 2016, 224, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, M.H.; Chiou, S.H.; Larsson, M.; Hung, K.H.; Wang, Y.L.; Liu, C.J.; Liu, D.M. A temperature-induced and shear-reversible assembly of latanoprost-loaded amphiphilic chitosan colloids: Characterization and in vivo glaucoma treatment. Acta Biomater. 2014, 10, 3188–3196. [Google Scholar] [CrossRef]
- Mathew, A.P.; Uthaman, S.; Cho, K.H.; Cho, C.S.; Park, I.K. Injectable hydrogels for delivering biotherapeutic molecules. Int. J. Biol. Macromol. 2018, 110, 17–29. [Google Scholar] [CrossRef]
- Rossi, S.M.; Murray, T.; McDonough, L.; Kelly, H. Loco-regional drug delivery in oncology: Current clinical applications and future translational opportunities. Expert. Opin. Drug Deliv. 2020. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Wang, P.; Zhang, J.; Tian, H.; Park, K.; Chen, X. Pulmonary codelivery of doxorubicin and siRNA by pH-sensitive nanoparticles for therapy of metastatic lung cancer. Small 2015, 11, 4321–4333. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Mehta, M.; Dhanjal, D.S.; Kaur, S.; Gupta, G.; Singh, H.; Thangavelu, L.; Rajeshkumar, S.; Tambuwala, M.; Bakshi, H.A.; et al. Emerging trends in the novel drug delivery approaches for the treatment of lung cancer. Chemico-Biol. Interact. 2019, 309, 108720. [Google Scholar] [CrossRef]
- Lee, H.-Y.; Mohammed, K.A.; Nasreen, N. Nanoparticle-based targeted gene therapy for lung cancer. Am. J. Cancer Res. 2016, 6, 1118–1134. [Google Scholar]
- Ruan, R.; Chen, M.; Sun, S.; Wei, P.; Zou, L.; Liu, J.; Gao, D.; Wen, L.; Ding, W. Topical and targeted delivery of siRNAs to melanoma cells using a fusion peptide carrier. Sci. Rep. 2016, 6, 29159. [Google Scholar] [CrossRef]
- Fahmy, H.M.; Saad, E.A.E.M.S.; Sabra, N.M.; Sabra, N.M.; El-Gohary, A.A.; Mohamed, F.F.; Gaber, M.H. Treatment merits of Latanoprost/Thymoquinone—Encapsulated liposome for glaucomatus rabbits. Int. J. Pharm. 2018, 548, 597–608. [Google Scholar] [CrossRef]
- Woodrow, K.A.; Cu, Y.; Booth, C.J.; Saucier-Sawyer, J.K.; Wood, M.J.; Saltzman, W.M. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat. Mater. 2009, 8, 526–533. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef]
- Tang, T.; Deng, Y.; Chen, J.; Zhao, Y.; Yue, R.; Choy, K.W.; Wang, C.C.; Du, Q.; Xu, Y.; Han, L.; et al. Local administration of siRNA through microneedle: Optimization, bio-distribution, tumor suppression and toxicity. Sci. Rep. 2016, 6, 30430. [Google Scholar] [CrossRef]
- Depieri, L.V.; Borgheti-Cardoso, L.N.; Campos, P.M.; Otaguiri, K.K.; de Carvalho Vicentini, F.T.; Lopes, L.B.; Fonseca, M.J.; Bentley, M.V. RNAi mediated IL-6 In vitro knockdown in psoriasis skin model with topical siRNA delivery system based on liquid crystalline phase. Eur. J. Pharm. Biopharm. 2016, 105, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Cullen, J.K.; Simmons, J.L.; Parsons, P.G.; Boyle, G.M. Topical treatments for skin cancer. Adv. Drug Deliv. Rev. 2020, 153, 54–64. [Google Scholar] [CrossRef] [PubMed]
- Ritprajak, P.; Hashiguchi, M.; Azuma, M. Topical application of cream-emulsified CD86 siRNA ameliorates allergic skin disease by targeting cutaneous dendritic cells. Mol. Ther. 2008, 16, 1323–1330. [Google Scholar] [CrossRef] [PubMed]
- Gulati, K.; Aw, M.S.; Losic, D. Nanoengineered drug-releasing Ti wires as an alternative for local delivery of chemotherapeutics in the brain. Int. J. Nanomed. 2012, 7, 2069–2076. [Google Scholar]
- Kaur, G.; Willsmore, T.; Gulati, K.; Zinonos, I.; Wang, Y.; Kurian, M.; Hay, S.; Losic, D.; Evdokiou, A. Titanium wire implants with nanotube arrays: A study model for localized cancer treatment. Biomaterials 2016, 101, 176–188. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Pillai, J. Implantable drug delivery systems: An overview. In Nanostructures for the Engineering of Cells, Tissues and Organs, 1st ed.; Grumezescu, A.M., Ed.; Elsevier: Amsterdam, The Netherlands, 2018; Chapter 18; pp. 473–511. ISBN 9780128136652. [Google Scholar]
- Benchetrit, S. Implantable Device for Injecting Medical Substances. U.S. Patent US 6,878,137, 12 April 2005. [Google Scholar]
- Arps, J. Implantable drug delivery devices. Pro. Med. Pharma. 2013, 1, 22. [Google Scholar]
- Park, H.; Park, K. Biocompatibility issues of implantable drug delivery systems. Pharm. Res. 1996, 13, 1770–1776. [Google Scholar] [CrossRef] [PubMed]
- Fournier, E.; Passirani, C.; Montero-Menei, C.N.; Benoi, J.P. Biocompatibility of implantable synthetic polymeric drug carriers: Focus on brain biocompatibility. Biomaterials 2003, 24, 3311–3331. [Google Scholar] [CrossRef]
- Bhatt, P.; Trehan, S.; Inamdar, N.; Mourya, V.K.; Misra, A. Polymers in Drug Delivery: An Update. In Applications of Polymers in Drug Delivery, 2nd ed.; Misra, A., Shahiwala, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; Chapter 2; pp. 1–42. ISBN 9780128196595. [Google Scholar]
- Caillol, S. Special Issue “Natural Polymers and Biopolymers II”. Molecules 2021, 26, 112. [Google Scholar] [CrossRef]
- Ottenbrite, R.M.; Kim, S.W. Polymeric Drugs, and Drug Delivery Systems, 1st ed.; CRC Press: Boca Raton, FL, USA, 2019. [Google Scholar]
- Amber, T.; Tabassum, S. Cyclosporin in dermatology: A practical compendium. Dermatol. Ther. 2020, 33, e13934. [Google Scholar] [CrossRef]
- Prodanovic, E.M.; Korman, N.J. Traditional systemic therapy: Metrotexate and cyclosporine. In Treatment of Psoriasis; Birkhäuser: Verlag, Switzerland, 2008; pp. 108–120. [Google Scholar]
- Dittmar, J.; Rothstein, R.J.; Reid, R.J.D.; Parsons, R.; Maurer, M.; Shaw, J.; Du, X. Methods to Treat Cancer Cyclosporine and Cyclosporine Derivates. U.S. Patent WO2012145427A1, 26 October 2012. [Google Scholar]
- Jiang, K.; He, B.; Lai, L.; Chen, Q.; Liu, Y.; Guo, Q.; Wang, Q. Cyclosporine a inhibits breast cancer cell growth by downregulating the expression of pyruvate kinase subtype M2. Int. J. Mol. Med. 2012, 30, 302–308. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Jiang, G.; Wang, Y.; Ding, F. Thermosensitive Chitosan Hydrogel for Implantable Drug Delivery: Blending PVA to Mitigate Body Response and Promote Bioavailability. J. Appl. Polym. Sci. 2012, 125, 2092–2101. [Google Scholar] [CrossRef]
- Islam, M.M.; Shahruzzaman, M.; Biswas, S.; Sakib, M.N.; Rashid, T.U. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact. Mater. 2020, 5, 164–183. [Google Scholar] [CrossRef]
- Peptu, C.; Humelnicu, A.C.; Rotaru, R.; Fortuna, M.E. Chitosan-based drug delivery systems. In Chitin and Chitosan: Properties and Applications, 1st ed.; van den Broek, L.A.M., Boeriu, C.G., Eds.; John Wiley & Sons Ltd.: New York, NY, USA, 2019. [Google Scholar]
- Denbanks, E.B.; Ottenbrite, R.M. Perspectives on: Chitosan Drug Delivery Systems Based on their Geometries. J. Bioact. Compat. Polym. 2006, 21, 351–368. [Google Scholar]
- Yee, G.C.; Gmur, D.J.; Kennedy, M.S. Liquid-chromatographic determination of cyclosporine in serum with use of a rapid extraction procedure. Clin. Chem. 1982, 28, 2269–2271. [Google Scholar] [CrossRef]
- Frei, R.W.; Zech, K. Selective Sample Handling and Detection in High-Performance Liquid Chromatography; Frel, R.W., Zech, K., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; p. 457. [Google Scholar]
- Moore, T.; Croy, S.; Mallapragada, S.; Pandit, N. Experimental investigation and mathematical modeling of Pluronic® F127 gel dissolution: Drug release in stirred systems. J. Control. Release 2000, 67, 191–202. [Google Scholar] [CrossRef]
- Pose-Vilarnovo, B.; Rodríguez-Tenreiro, C.; dos Santos, J.F.R.; Vázquez-Doval, J.; Concheiro, A.; Alvarez-Lorenzo, C.; Torres-Labandeira, J.J. Modulating drug release with cyclodextrins in hydroxypropyl methylcellulose gels and tablets. J. Control. Release 2004, 94, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Di Gioia, M.L.; Mellace, S.; Picci, N.; Trombino, S. Hemostatic gauzed based on chitosan and hydroquinone: Preparation, characterization and blood coagulation evaluation. J. Mater. Sci. Mater. Med. 2017, 28, 190. [Google Scholar] [CrossRef] [PubMed]
- Cassano, R.; Trombino, S.; Bloise, E.; Muzzalupo, R.; Iemma, F.; Chidichimo, G.; Picci, N. New broom fiber (Spartiumjunceum L.) derivatives: Preparation and characterization. J. Agric. Food Chem. 2007, 55, 9489–9495. [Google Scholar] [CrossRef] [PubMed]
- Hamel, A.R.; Hubler, F.; Carrupt, A.; Wenger, R.M.; Mutter, M. Cyclosporine A prodrug: Design, synthesis, and biophysical properties. J. Pept. Res. 2004, 63, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Foley, D.W.; Bermudez, I.; Baileys, P.D.; Meredith, D. A cyclosporine derivative is a substrate of the oligopeptide transporter PepT1. Med. Chem. Commun. 2016, 7, 999–1002. [Google Scholar] [CrossRef]
- Clasen, C.; Wilhelms, T.; Kulicke, W.M. Formation and characterization of chitosan membranes. Biomacromolecules 2006, 7, 3210–3222. [Google Scholar] [CrossRef]
- Cirillo, F.; Pellegrino, M.; Malivindi, R.; Rago, V.; Avino, S.; Muto, L.; Dolce, V.; Vivacqua, A.; Rigiracciolo, D.C.; De Marco, P.; et al. GPER is involved in the regulation of the estrogen-metabolizing CYP1B1 enzyme in breast cancer. Oncotarget 2017, 8, 106608–106624. [Google Scholar] [CrossRef]


| Membranes | Chitosan | Acetic Acid Solution (mL) | Csacc (g) |
|---|---|---|---|
| CM *1 | 0.15 g | 5 mL | - |
| CM *2 + Csacc | 0.15 g | 5 mL | 0.017 g |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Trombino, S.; Curcio, F.; Poerio, T.; Pellegrino, M.; Russo, R.; Cassano, R. Chitosan Membranes Filled with Cyclosporine A as Possible Devices for Local Administration of Drugs in the Treatment of Breast Cancer. Molecules 2021, 26, 1889. https://doi.org/10.3390/molecules26071889
Trombino S, Curcio F, Poerio T, Pellegrino M, Russo R, Cassano R. Chitosan Membranes Filled with Cyclosporine A as Possible Devices for Local Administration of Drugs in the Treatment of Breast Cancer. Molecules. 2021; 26(7):1889. https://doi.org/10.3390/molecules26071889
Chicago/Turabian StyleTrombino, Sonia, Federica Curcio, Teresa Poerio, Michele Pellegrino, Rossella Russo, and Roberta Cassano. 2021. "Chitosan Membranes Filled with Cyclosporine A as Possible Devices for Local Administration of Drugs in the Treatment of Breast Cancer" Molecules 26, no. 7: 1889. https://doi.org/10.3390/molecules26071889
APA StyleTrombino, S., Curcio, F., Poerio, T., Pellegrino, M., Russo, R., & Cassano, R. (2021). Chitosan Membranes Filled with Cyclosporine A as Possible Devices for Local Administration of Drugs in the Treatment of Breast Cancer. Molecules, 26(7), 1889. https://doi.org/10.3390/molecules26071889











