Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look
Abstract
1. Introduction
2. Results and Discussion
2.1. GBIF Data to Assess Species That Contain Alkaloid Abundances around the Globe
2.2. Marketed Alkaloids and Source Plants—An Update Based on GBIF2020
2.3. Nonmedicinal Alkaloids and the Exploration of Future Medicinal Potential of Alkaloids
2.4. Shifting Interests in Drug Discovery and Supplement Development
3. Conclusions
4. Materials/Methods
4.1. GBIF Analysis
4.2. Search Strategies for Used as Supplements/Botanicals
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Doak, B.C.; Over, B.; Giordanetto, F.; Kihlberg, J. Oral druggable space beyond the rule of 5: Insights from drugs and clinical candidates. Chem. Biol. 2014, 21, 1115–1142. [Google Scholar] [CrossRef] [PubMed]
- Lipinski, C.A. Drug-like properties and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249. [Google Scholar] [CrossRef]
- Hopkins, A.L.; Groom, C.R.; Alex, A. Ligand efficiency: A useful metric for lead selection. Drug Discov. Today 2004, 9. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 45, 2615–2623. [Google Scholar] [CrossRef] [PubMed]
- Hajar, R. History of Medicine Timeline. Heart Views 2015, 16, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M. Ethnopharmacology and Drug Discovery. In Comprehensive Natural Products II; Elsevier: Amsterdam, The Netherlands, 2010; pp. 351–381. [Google Scholar]
- Verpoorte, R. ALKALOIDS. In Encyclopedia of Analytical Science; Elsevier: Amsterdam, The Netherlands, 2005; pp. 56–61. [Google Scholar]
- Srivastava, S.; Srivastava, A.K. Biotechnology and Genetic Engineering for Alkaloid Production. In Natural Products: Phytochemistry, Botany and Metabolism of Alkaloids, Phenolics and Terpenes; Ramawat, K.G., Mérillon, J.-M., Eds.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 213–250. [Google Scholar]
- Yu, B.-W.; Chen, J.-Y.; Wang, Y.-P.; Cheng, K.-F.; Li, X.-Y.; Qin, G.-W. Alkaloids from Menispermum dauricum. Phytochemistry 2002, 61, 439–442. [Google Scholar] [CrossRef]
- Jilani, A.; Legseir, B.; Soulimani, R.; Dicko, A.; Younos, C. New extraction technique for alkaloids. J. Braz. Chem. Soc. 2006, 17. [Google Scholar] [CrossRef]
- Van der Heijden, R.; Jacobs, D.; Snoeijer, W.; Hallard, D.; Verpoorte, R. The Catharanthus alkaloids: Pharmacognosy and biotechnology. Curr. Med. Chem. 2004, 11, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Ganesan, A. The impact of natural products upon modern drug discovery. Curr. Opin. Chem. Biol. 2008, 12, 306–317. [Google Scholar] [CrossRef]
- Kaur, R.; Arora, S. Alkaloids—Important Therapeutic Secondary Metabolites of Plant Origin. J. Crit. Rev. 2015, 2, 1–8. [Google Scholar]
- Chik, S.C.C.; Or, T.C.T.; Luo, D.; Yang, C.L.H.; Lau, A.S.Y. Pharmacological Effects of Active Compounds on Neurodegenerative Disease with Gastrodia and Uncaria Decoction, a Commonly Used Poststroke Decoction. Sci. World J. 2013, 2013, 1–22. [Google Scholar] [CrossRef]
- Kurek, J. Introductory Chapter: Alkaloids—Their Importance in Nature and for Human Life. In Alkaloids—Their Importance in Nature and Human Life; IntechOpen: London, UK, 2019. [Google Scholar]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 28, 1–17. [Google Scholar]
- Global Biodiversity Information Facility. Global Biodiversity Information Facility Strategic Plan 2017–2021; GBIF: Copenhagen, Denmark, 2017. [Google Scholar]
- Amirkia, V.; Heinrich, M. Alkaloids as drug leads—A predictive structural and biodiversity-based analysis. Phytochem. Lett. 2014, 10. [Google Scholar] [CrossRef]
- Amirkia, V.; Heinrich, M. Natural products and drug discovery: A survey of stakeholders in industry and academia. Front. Pharmacol. 2015, 6, 237. [Google Scholar] [CrossRef] [PubMed]
- Chapman, A.D. Uses of Primary Species-Occurrence Data; Version 1.0; GBIF: Copenhagen, Denmark, 2005. [Google Scholar]
- Zizka, A.; Carvalho, F.A.; Calvente, A.; Baez-Lizarazo, M.R.; Cabral, A.; Coelho, J.F.R.; Colli-Silva, M.; Fantinati, M.R.; Fernandes, M.F.; Ferreira-Araújo, T.; et al. No one-size-fits-all solution to clean GBIF. PeerJ 2020, 8, e9916. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, V.; Plunkett, W.; Cortes, J.E. Omacetaxine: A Protein Translation Inhibitor for Treatment of Chronic Myelogenous Leukemia. Clin. Cancer Res. 2014, 20, 1735–1740. [Google Scholar] [CrossRef] [PubMed]
- Gambling, D.R.; Bender, M.; Faron, S.; Glaser, D.; Farrell, T.R. Prophylactic Intravenous Ephedrine to Minimize Fetal Bradycardia After Combined Spinal-epidural Labour Analgesia: A Randomized Controlled Study. Obstet. Anesth. Dig. 2016, 36, 144. [Google Scholar] [CrossRef]
- Aliane, J.; Dualé, C.; Guesmi, N.; Baud, C.; Rosset, E.; Pereira, B.; Bouvier, D.; Schoeffler, P. Compared effects on cerebral oxygenation of ephedrine vs phenylephrine to treat hypotension during carotid endarterectomy. Clin. Exp. Pharmacol. Physiol. 2017, 44, 739–748. [Google Scholar] [CrossRef]
- Higgins, N.; Fitzgerald, P.C.; van Dyk, D.; Dyer, R.A.; Rodriguez, N.; McCarthy, R.J.; Wong, C.A. The Effect of Prophylactic Phenylephrine and Ephedrine Infusions on Umbilical Artery Blood pH in Women with Preeclampsia Undergoing Cesarean Delivery with Spinal Anesthesia. Anesthesia Analg. 2018, 126, 1999–2006. [Google Scholar] [CrossRef]
- Tan, D.; Tay, S.A.; Loh, K.-L.; Chia, A. Topical Atropine in the Control of Myopia. Asia Pac. J. Ophthalmol. 2016, 5, 424–428. [Google Scholar] [CrossRef]
- Szántay, C.; Szabó, L.; Kalaus, G. Synthesis of vinca alkaloids and related compounds—II11Ref. 3 is considered as the first communication of the series: Stereoselective and enantioselective synthesis of (+)-vincamine. Tetrahedron 1977, 33, 1803–1808. [Google Scholar] [CrossRef]
- Trojánek, J.; Koblicová, Z.; Bláha, K. On alkaloids. XIX. Absolute configuration of vincamine and some related alkaloids; Optical rotatory dispersion. Collect. Czechoslov. Chem. Commun. 1968, 33, 2950–2961. [Google Scholar] [CrossRef]
- Stepp, J.R.; Moerman, D.E. The importance of weeds in ethnopharmacology. J. Ethnopharmacol. 2001, 75, 19–23. [Google Scholar] [CrossRef]
- Yeung, A.W.K.; Heinrich, M.; Atanasov, A.G. Ethnopharmacology—A Bibliometric Analysis of a Field of Research Meandering Between Medicine and Food Science? Front. Pharmacol. 2018, 9, 215. [Google Scholar] [CrossRef]
- Arnaud, E.; Castañeda-Álvarez, N.P.; Cossi, J.G.; Endresen, D.; Jahanshiri, E.; Vigouroux, Y. Final Report of the Task Group on GBIF Data Fitness for Use in Agrobiodiversity; GBIF: Copenhagen, Denmark, 2016; p. 112. [Google Scholar]
- National Center for Biotechnology Information. PubChem Compound Summary for CID 124434. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Calystegine-B2 (accessed on 30 December 2020).
- National Center for Biotechnology Information. PubChem Bioassay Record for Bioactivity AID 1125775-SID 103599768, Bioactivity for AID 1125775-SID 103599768. Available online: https://pubchem.ncbi.nlm.nih.gov/bioassay/1125775#sid=103599768 (accessed on 30 December 2020).
- Trapero, A.; Llebaria, A. Glucocerebrosidase inhibitors for the treatment of Gaucher disease. Futur. Med. Chem. 2013, 5. [Google Scholar] [CrossRef]
- Szymański, M.; Witkowska-Banaszczak, E.; Klak, N.; Marciniak, K.; Wołowiec, T.; Szymański, A. Effects of trace elements on polyphenolic compounds in Millefolii herba. Pol. J. Environ. Stud. 2014, 23, 459–466. [Google Scholar]
- Marzouk, A.M.; Deans, S.G.; Nash, R.J.; Gray, A.I. Transformed Root Cultures of Solanum dulcamara L.: A Model for Studying Production of Secondary Metabolites. In Genetic Transformation; IntechOpen: London, UK, 2011. [Google Scholar]
- Liu, L.; Yu, H.; Wu, H.; Yang, X.; Pan, Y.; Chen, Y.; Wang, K.; Wang, W.; Zhang, W.; Jin, Y.; et al. Toxic proteins from Croton tiglium L. exert a proinflammatory effect by inducing release of proinflammatory cytokines and activating the p38-MAPK signaling pathway. Mol. Med. Rep. 2017, 16, 631–638. [Google Scholar] [CrossRef]
- Hecker, E. Cocarcinogenic principles from the seed oil of Croton tiglium and from other Euphorbiaceae. Cancer Res. 1968, 28, 2338–2349. [Google Scholar]
- Qiu, H. Flora Reipublicae Popularis Sinicae; Editorial Committee of FRPS, Ed.; Science Press: Beijing, China, 1996; p. 130. [Google Scholar]
- Zhong Hua Ben Cao; Shanghai Ke Xue Ji Zhu Chu Ban She: Shanghai, China, 1999.
- Lee, H.; Jung, K.-H.; Park, S.; Kil, Y.-S.; Chung, E.Y.; Jang, Y.P.; Seo, E.-K.; Bae, H. Inhibitory effects of Stemona tuberosa on lung inflammation in a subacute cigarette smoke-induced mouse model. BMC Complement Altern. Med. 2014, 20, 513. [Google Scholar] [CrossRef]
- Liang, Y.; Wu, J.-L.; Li, X.; Guo, M.-Q.; Leung, E.L.-H.; Zhou, H.; Liu, L.; Li, N. Anti-cancer and anti-inflammatory new vakognavine-type alkaloid from the roots of Aconitum carmichaelii. Tetrahedron Lett. 2016, 57, 5881–5884. [Google Scholar] [CrossRef]
- Patnala, S.; Kanfer, I. Medicinal use of Sceletium: Characterization of Phytochemical Components of Sceletium Plant Species using HPLC with UV and Electrospray Ionization—Tandem Mass Spectroscopy. J. Pharm. Pharm. Sci. 2015, 18, 414. [Google Scholar] [CrossRef][Green Version]
- Smith, C. The effects of Sceletium tortuosum in an in vivo model of psychological stress. J. Ethnopharmacol. 2011, 133, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Harvey, A.L.; Young, L.C.; Viljoen, A.M.; Gericke, N.P. Pharmacological actions of the South African medicinal and functional food plant Sceletium tortuosum and its principal alkaloids. J. Ethnopharmacol. 2011, 137, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Murbach, T.S.; Hirka, G.; Szakonyiné, I.P.; Gericke, N.; Endres, J.R. A toxicological safety assessment of a standardized extract of Sceletium tortuosum (Zembrin®) in rats. Food Chem. Toxicol. 2014, 74, 190–199. [Google Scholar] [CrossRef]
- Plants of the World Online, Facilitated by the Royal Botanic Gardens, Kew. Available online: http://www.plantsoftheworldonline.org/ (accessed on 26 December 2020).
- Somchit, M.N. Zerumbone isolated from Zingiber zerumbet inhibits inflammation and pain in rats. J. Med. Plants Res. 2012, 16, 6. [Google Scholar] [CrossRef]
- Kasolo, J.; Bimenya, G.S.; Ojok, L.; Ochieng, J.; Ogwal-Okeng, J. Phytochemicals and uses of Moringa oleifera leaves in Ugandan rural communities. J. Med. Plants Res. 2010, 16, 753–757. [Google Scholar]
- Faizi, S.; Siddiqui, B.S.; Saleem, R.; Siddiqui, S.; Aftab, K.; Gilani, A.-U.-H. Fully acetylated carbamate and hypotensive thiocarbamate glycosides from Moringa oleifera. Phytochemistry 1995, 38, 957–963. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A.; Sharma, P.; Sharma, A. Cardioprotective potential of N,α-l-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorganic Med. Chem. Lett. 2013, 23, 959–962. [Google Scholar] [CrossRef]
- Ahmad, A.; Husain, A.; Mujeeb, M.; Khan, S.A.; Najmi, A.K.; Siddique, N.A.; Damanhouri, Z.A.; Anwar, F. A review on therapeutic potential of Nigella sativa: A miracle herb. Asian Pac. J. Trop. Biomed. 2013, 3, 337–352. [Google Scholar] [CrossRef]
- Koshak, A.; Koshak, E.; Heinrich, M. Medicinal benefits of Nigella sativa in bronchial asthma: A literature review. Saudi Pharm. J. 2017, 25, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Morikawa, T.; Xu, F.; Ninomiya, K.; Matsuda, H.; Yoshikawa, M. Nigellamines A3, A4, A5, and C, New Dolabellane-Type Diterpene Alkaloids, with Lipid Metabolism-Promoting Activities from the Egyptian Medicinal Food Black Cumin. Chem. Pharm. Bull. 2004, 52, 494–497. [Google Scholar] [CrossRef]
- Koshak, A.E.; Koshak, E.A.; Mobeireek, A.F.; Badawi, M.A.; Wali, S.O.; Malibary, H.M.; Atwah, A.F.; Alhamdan, M.M.; Almalki, R.A.; Madani, T.A. Nigella sativa supplementation to treat symptomatic mild COVID-19, A structured summary of a protocol for a randomised, controlled, clinical trial. Trials 2020, 21, 703. [Google Scholar] [CrossRef]
- Hassan, G.; Ghafoor, S.; Chaudhry, S.; Khan, Z.A. Salivary Interleukin-1 Levels in Chronic Periodontitis Patients after use of Nigella Sativa (Kalonji) Oil. J. Pak. Dent. Assoc. 2020, 29, 205–210. [Google Scholar] [CrossRef]
- Alobaidi, A. Effect of Nigella sativa and Allium sativum Coadminstered with Simvastatin in Dyslipidemia Patients: A Prospective, Randomized, Double-Blind Trial. Antiinflamm. Antiallergy Agents Med. Chem. 2014, 13, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Koshak, A.; Wei, L.; Koshak, E.; Wali, S.; Alamoudi, O.; Demerdash, A.; Qutub, M.; Pushparaj, P.N.; Heinrich, M. Nigella sativa Supplementation Improves Asthma Control and Biomarkers: A Randomized, Double-Blind, Placebo-Controlled Trial. Phytotherapy Res. 2017, 31, 403–409. [Google Scholar] [CrossRef] [PubMed]
- El-Shanshory, M.; Hablas, N.M.; Aboonq, M.S.; Fakhreldin, A.R.; Attia, M.; Arafa, W.; Mariah, R.A.; Baghdadi, H.; Ayat, M.; Zolaly, M.; et al. Nigella sativa improves anemia, enhances immunity and relieves iron overload-induced oxidative stress as a novel promising treatment in children having beta-thalassemia major. J. Herb. Med. 2019, 16, 100245. [Google Scholar] [CrossRef]
- Brinker, A.M.; Ma, J.; Lipsky, P.E.; Raskin, I. Medicinal chemistry and pharmacology of genus Tripterygium (Celastraceae). Phytochemistry 2007, 68, 732–766. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Y.; Wu, L.; Zhang, X. Isolation and insecticidal activity of sesquiterpenes alkaloids from Tripterygium wilfordii Hook f. Ind. Crop. Prod. 2014, 52, 642–648. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Tian, K.; Tang, Z.-H.; Chen, X.-J.; Bian, Z.-X.; Wang, Y.-T.; Lu, J.-J. Phytochemistry and Pharmacology of Carthamus tinctorius L. Am. J. Chin. Med. 2016, 44, 197–226. [Google Scholar] [CrossRef]
- Yu, M.; Yang, Y.-X.; Shu, X.-Y.; Huang, J.; Hou, D.-B. Aconitum carmichaelii Debeaux, cultivated as a medicinal plant in western China. Genet. Resour. Crop. Evol. 2016, 63, 919–924. [Google Scholar] [CrossRef]
- Chen, R.C.; Sun, G.B.; Zhang, Q.; Ye, Z.G.; Sun, X.B. Advances in studies on toxicity of aconite. China J. Chin. Mater. Med. 2013, 38, 1126–1129. [Google Scholar]
- Li, X.-J.; Jiang, Z.-Z.; Zhang, L. Triptolide: Progress on research in pharmacodynamics and toxicology. J. Ethnopharmacol. 2014, 155, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Wang, J.; Cui, Y.; Wu, X. Pharmacological effects of Chinese herb aconite (Fuzi) on cardiovascular system. J. Tradit. Chin. Med. 2012, 32, 308–313. [Google Scholar] [CrossRef]
- Hazawa, M.; Wada, K.; Takahashi, K.; Mori, T.; Kawahara, N.; Kashiwakura, I. Suppressive effects of novel derivatives prepared from Aconitum alkaloids on tumor growth. Investig. New Drugs 2009, 27, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Michl, J.; Kite, G.C.; Wanke, S.; Zierau, O.; Vollmer, G.; Neinhuis, C.; Simmonds, M.S.J.; Heinrich, M. LC-MS- and 1 H NMR-Based Metabolomic Analysis and in Vitro Toxicological Assessment of 43 Aristolochia Species. J. Nat. Prod. 2016, 79, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Hassan, Z.; Muzaimi, M.; Navaratnam, V.; Yusoff, N.H.M.; Suhaimi, F.W.; Vadivelu, R.; Vicknasingam, B.K.; Amato, D.; von Hörsten, S.; Ismail, N.I.; et al. From Kratom to mitragynine and its derivatives: Physiological and behavioural effects related to use, abuse, and addiction. Neurosci. Biobehav. Rev. 2013, 37, 138–151. [Google Scholar] [CrossRef]
- Beaumont, R.E.; Cordery, P.; James, L.J.; Watson, P. Supplementation with a low-dose of octopamine does not influence endurance cycling performance in recreationally active men. J. Sci. Med. Sport 2017, 20, 952–956. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Xing, Y.; Chen, J.; Zhang, D.; Guo, S.; Wang, C. Antimicrobial activities of endophytic fungi isolated from Ophiopogon japonicus (Liliaceae). BMC Complement Altern. Med. 2012, 12, 1198. [Google Scholar] [CrossRef]
- Applequist, W.L.; Brinckmann, J.A.; Cunningham, A.B.; Hart, R.E.; Heinrich, M.; Katerere, D.R.; van Andel, T. Scientists warning on climate change and medicinal plants. Planta Med. 2020, 86. [Google Scholar] [CrossRef]
| Data 2014 | Data 2020 | Folds-Increase | |
|---|---|---|---|
| Overall alkaloid: 24,325 | |||
| Average occurrences of a species per alkaloid compound | 1295 | 11,210 | 8.66 |
| Standard Deviation of occurrences for a species per alkaloid | 8703 | 49,503 | 8.33 |
| Medicinal alkaloids only data: 52 | |||
| Average occurrences of a species per alkaloid | 17,952 | 60,991 | 3.39 |
| Standard Deviation of occurrences for a species per alkaloid compound | 35,595 | 125,243 | 3.55 |
| Non-Medicine alkaloids only data: 24,273 | |||
| Average occurrences of a species per alkaloid | 1257 | 11,099 | 8.83 |
| Standard Deviation of occurrences of a species per alkaloid | 8509 | 72,261 | 8.5 |
| Alkaloid | Therapeutic Indications | Source; Other Uses of the Source |
|---|---|---|
| Aconitine | Rheumatism, neuralgia, sciatica | Aconitum napellus L. and others; reduce fever, pneumonia, laryngitis |
| Antiviral agent, pharmaceutical aid used to extend shelf-life of whole blood | Widespread throughout animal and plant tissues, many uses, especially of its derivatives as antiviral agents | |
| Ajmaline | Antiarrhythmic agent | Rauvolfia serpentina (L.) Benth. ex Kurz and others; hypertension, ‘insanity’, increase uterine contraction |
| Atropine | Antispasmodic, anti-parkinson, cycloplegic drug | Atropa bella-donna L. and others; to treat peptic ulcers, relives intestinal colic, pupil dilation agents |
| Berberine | Eye irritations, AIDS, hepatitis | Berberis vulgaris L. and others; anti-cancer, anti-inflammatory, antioxidant |
| Boldine | Cholelithiasis, vomiting, constipation | Lindera aggregata (Sims) Kosterm., Peumus boldus Molina and others; aromatic, decongestant, diuretic |
| Caffeine | Neonatal apnea, atopic dermatitis | Theobroma cacao L. and others; food additive, emollient, angina treatment, hypertension treatment |
| Canescine | Antihypertensive agent | R. serpentina and others; anti-inflammatory agent, uterine contractions agents |
| Cathine | Anorectic drug | Catha edulis (Vahl) Endl. and others; wakefulness, psychostimulatory effects |
| Cinchonidine | Increases reflexes, epileptiform convulsions | Cinchona tucujensis H.Karst. and others; fever treatment, malaria treatment |
| Cocaine | Local anaesthetic | Erythroxylum coca Lam. and others; GI symptoms treatment, altitude sickness treatment |
| Codeine | Antitussive, analgesic | Papaver somniferum L. and others; antioxidant, antimutagenic, and anticarcinogenic effects |
| Colchicine | Amyloidosis treatment, acute gout | Colchicum autumnale L. and others; analgesic, antirheumatic, cathartic and emetic |
| Diethanolamine | Base used in pharmaceuticals, etc. | For example, in Schinopsis balansae Eng.; used in dermatological products, as a surfactant, not an active pharmaceutical ingredient |
| Emetine | Intestinal amoebiasis, expectorant drug | Alangium lamarckii Thwaites and others; emetic, anthelmintic, purgative |
| Ephedrine | Nasal decongestant, bronchodilator | Ephedra distachya L. and others; fruit additive, antiviral agent, allergy treatment |
| Ergometrine | Postpartum/postabortal hemorrhage | Claviceps urpurea var. purpurea (Fr.) Tul. and others; migraine treatment, Parkinson’s disease treatment, antitumor agent |
| Ergotamine | Migraine treatment | Claviceps purpurea var. purpurea (Fr.) Tul. and others; migraine, Parkinson’s diseases. Antitumor |
| Eserine | Ophthalmology, antidote/poisoning | Physostigma venenosum Balf. and others; glaucoma, myasthenia gravis treatment |
| Galanthamine | Muscle relaxant, Alzheimer’s | Galanthus woronowii Losinsk. and many other species of the Amarylidaceae; emmenagogue, treatment of traumatic injuries to nervous system |
| Hydrastine | Gastrointestinal disorders | Corydalis fimbrillifera Korsh. and others; treatment for depression, hypertension, intestinal spasms |
| Hyoscine | Motion sickness | Datura stramonium L. and others; analgesic, anthelmintic and anti-inflammatory |
| Hyoscyamine | Antispasmodic, antiparkinson, cycloplegic drug | Hyoscyamus niger L. and others; pain killer, sedation, diuretic |
| Lobeline | Anti-smoking, asthma, cough | Lobelia inflata L. and others; antispasmodic, respiratory stimulant |
| Morphine | Pain relief, diarrhoea | Papaver somniferum L. and others; antispasmodic, expectorant, antitussive |
| Narceine | Cough suppressant | Papaver somniferum L. and others; antispasmodic, expectorant, antitussive |
| Nicotine | Anti-smoking | Nicotiana tabacum L. and others; relaxant, antispasmodic, discutient, diuretic |
| Noscapine | Cough suppressant | P. somniferum and others; antispasmodic, expectorant, antituissive |
| Omacetaxine mepesuccinate | chronic myeloid leukaemia (CML) | Cephalotaxus fortune and others; anti-cancer activities |
| Papaverine | Vasodilator, gastrointestinal disorders | P. somniferum. and others; antispasmodic, expectorant, antitussive |
| Pelletierine | Tenia infestations | Punica granatum L. and others; astringent, anti-bacterial, antiviral |
| Pilocarpine | Miotic in treatment of glaucoma, leprosy | Pilocarpus microphyllus Stapf ex Wardlew. and others; hair tonic, epilepsy, GI disorders treatment |
| Quinidine | Ventricular and supraventricular arrhythmias, malaria, cramping | Cinchona officinalis L. and others; fever, spasm relax, neuralgia |
| Quinine | Malaria, babesiosis, myotonic disorders | Cinchona officinalis L. and others; fever, spasm relax, neuralgia |
| Raubasine | Vascular disorders | Catharanthus roseus (L.) G.Don and others; anticancer, hypoglycaemic agent, emetic |
| Rescinnamine | Hypertension | R. serpentina and others; hypnotic, increase urine contractions, treat wounds and itches |
| Reserpine | Hypertension, psychoses | R. serpentina and others; hypnotic, increase urine contractions, treat wounds and itches |
| Rotundine | Analgesic, sedative, hypnotic agent | Stephania epigaea H.S.Lo and others; reduce fever |
| Sanguinarine | Antiplaque agent | Sanguinaria canadensis L. and others; fever, rheumatism, expectorant |
| Sparteine | Uterine contractions, cardiac arrhythmias | Lupinus pusillus Pursh var. pusillus and others; treatment for haemostatic, ears and eyes disorders |
| Strychnine | Eye disorders | Strychnos wallichiana Steud. ex A.DC. and others; leprosy, antidote for rabies, ulcers treatment, rheumatism treatment |
| Synephrine | Vasoconstrictor, conjunctival decongestant, weight loss | Citrus x aurantium L. and others; food, stimulant, appetite suppressant, trat. nausea |
| Taxol | Mamma and ovary carcinoma | Taxus brevifolia Nutt. and others; treatment for diabetes, cancer treatment |
| Theobromine | Asthma, diuretic agent | Theobroma cacao L. and others; food, emollient, angina treatment, hypertension treatment |
| Theophylline | Asthma, bronchospasms | Camellia sinensis (L.) Kuntze and others; cancer prevention, lower cholesterol, anti-parkinsons |
| Turbocuranine | Muscle relaxant | Chondrodendron tomentosum Ruiz and Pav.; oedema, kidney stones, persistent urinary tract infections |
| Vinblastine | Hodgkin’s disease, testicular cancer, blood disorders | Catharanthus roseus (L.) G.Don and others; anticancer, hypoglycaemic agent, emetic |
| Vincamine | Vasodilator | Vinca minor—L. and others.; arteriosclerosis, dementia, cerebral stimulant |
| Vincristine | Burkitt’s lymphoma | Catharanthus roseus (L.) G.Don and others; anticancer, hypoglycaemic agent, emetic |
| Vindesine | Chemotherapy | C. roseus and others; anticancer, hypoglycaemic agent, emetic |
| Yohimbine | Aphrodisiac, urinary incontinence | Corynanthe johimbe K. Schum. and others; cereberal stimulant, anti-diuretic, local anaesthetic |
| Alkaloid | Species | Pharmacological Studies Using the Alkaloid | Occurrences of the Species | Total Occurrences (Species and Genus Level) |
|---|---|---|---|---|
| 4-Hydroxy-1,1-dimethylpyrrolidinium-2-carboxylate/achillein | Achillea millefolium L., Betonica officinalis L., Marrubium vulgare L., etc. | 72 | 1,292,871 | 2,278,697 |
| 2,6-Benzoxazolediol; 6-Me-ether | Coix lacryma L., Triticum aestivum L., Zea mays L., Secale cereale L., etc. | 12 | 1,292,114 | 1,263,403 |
| 2,4-Undecadiene-8,10-diynoic acid; (2E,4E)-form, 2-Methylpropylamide | Chrysanthemum frutescens L., Achillea macrophylla L., Achillea ptarmica L., etc. | 2 | 1,032,568 | 1,360,771 |
| 8-Azabicyclo[3.2.1]octane-1,2,3,4-tetrol/calystegine | Atropa belladonna L., Solanum tuberosum L., Solanum dulcamara L., etc. | 160 | 1,018,893 | 2,129,193 |
| 2,4-Undecadiene-8,10-diynoic acid; (2E,4E)-form, 2,3-Didehydropiperidide | Otanthus maritimus (L.) Hoffmanns. and Link, Achillea millefolium L. and Achillea ptarmica L. | 8 | 1,016,004 | 1,310,187 |
| Homostachydrine; (S)-form | Medicago sativa L. and Achillea millefolium L. | 54 | 1,003,339 | 2,303,110 |
| Species | Alkaloid | Occurrences | Species Distribution | Reported Articles on the Species in WoS (2014–2020) | Search for Compounds |
|---|---|---|---|---|---|
| Bellendena montana R.Br. | Bellendine | 1141 | New Zealand | 1 | 4 |
| Bruguiera sexangula (Lour.) Poir. 2 | Tropine 1,2-dithiolane-3-carboxylate | 832 | Southeast Asia | 37 | 2 |
| Croton tiglium L. (syn.: Phragmanthera capitata (Spreng.) Balle) 2 | Crotonine | 1002 | Southeast Asia | 50 (11 under syn.) | 1 |
| Stemona tuberosa Lour.2 | Croomine | 614 | Southeast Asia | 60 | 40 1 |
| Mesembryanthemum tortuosum L. (syn.: Sceletium tortuosum (L.) N.E.Br.) 2 | Mesembrine | 288 | South Africa | 71 (69 under syn.) | 163 1 |
| Schizanthus pinnatus Ruiz and Pav. | Schizanthine A | 690 | South America | 1 | 4 |
| Stapelia hirsuta L. | N-Acetylhordenine | 212 | South Africa | 2 | 0 |
| Ulex jussiaei Webb | Jussiaeiine A | 331 | Portugal | 3 | 2 |
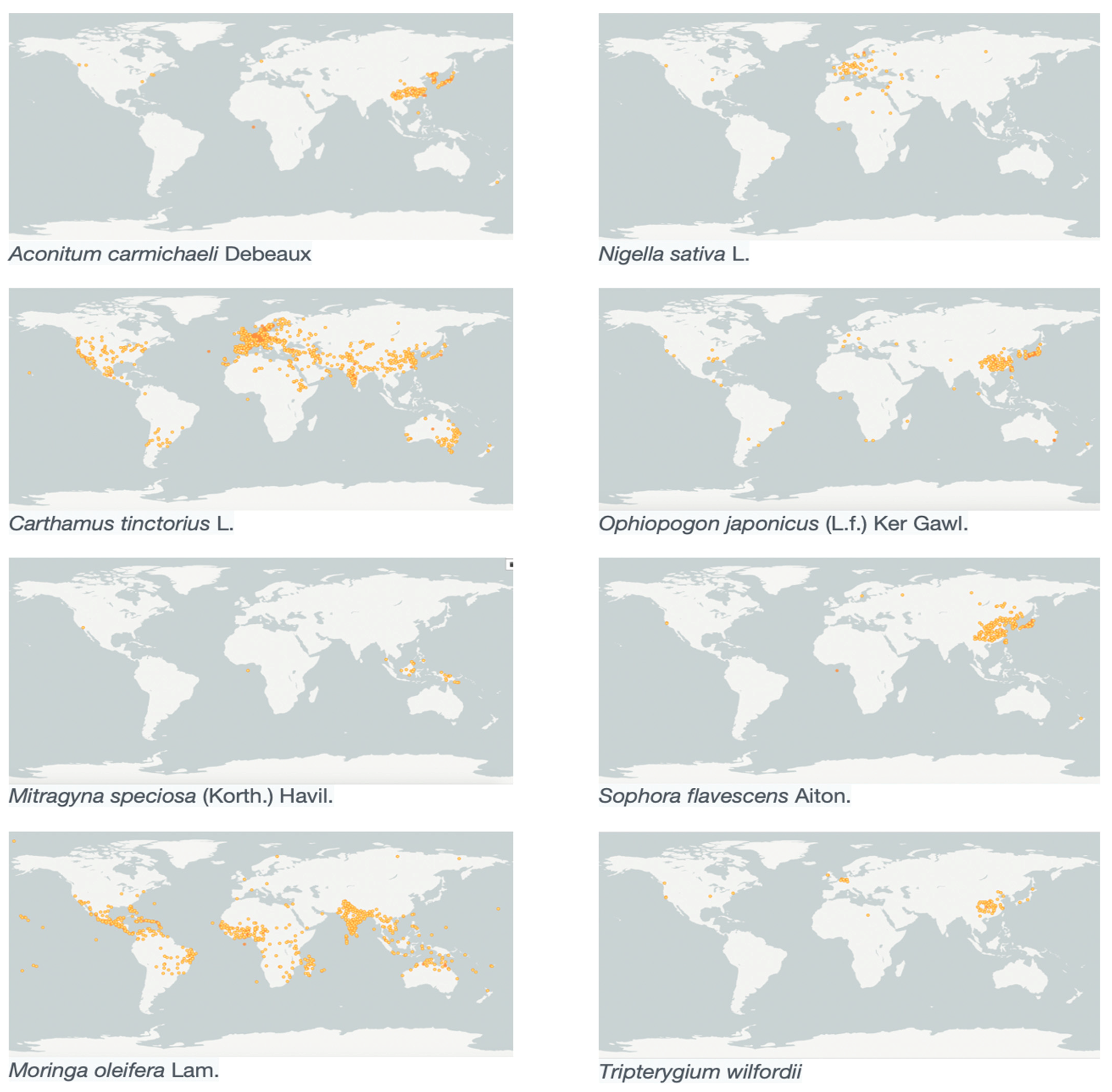
| Source Species | GBIF 2014 | GBIF 2020 | GBIF Increases | Published Ethnopharma-Cological Papers/Total Papers in WoS Core Collection (2014–2020) | Examples of Alkaloids in the Species | Clinical Trials (n) |
|---|---|---|---|---|---|---|
| Aconitum carmichaelii Debeaux * | 266 | 336 | 70 | 99/114 | C19- and C20-diterpenoid alkaloids | 1 |
| Carthamus tinctorius L. * | 3225 | 10967 | 7742 | 409/749 | Tetrahydro-β-carboline | 35 |
| Mitragyna speciosa Korth. ✞ | 85 | 147 | 62 | 204/230 | Mitragynine | 2 |
| Moringa oleifera Lam. * | 1720 | 2349 | 629 | 2544/2931 | Marumoside A and marumoside B | 12 |
| Nigella sativa L. * | 191 | 580 | 389 | 2206/3073 | Nigeglanine | 16 |
| Ophiopogon japonicus (Thunb.) Ker Gawl. * | 3270 | 4270 | 1000 | 149/192 | Octopamine and Ancistrobrevine B | 1 |
| Sophora flavescens Aiton * | 2601 | 2622 | 21 | 339/422 | Matrine-type, cytisine-type, anagyrine-type, and lupinine-type alkaloids; Sophora flavescent alkaloids (SFAs) | 1 |
| Tripterygium wilfordii Hook. f. ✞ | 2359 | 2713 | 354 | 641/694 | Wilfordine, wilforine, wilfornine, wilfortrine, ebenifoline E-II and cangorinine E-1 | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Heinrich, M.; Mah, J.; Amirkia, V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules 2021, 26, 1836. https://doi.org/10.3390/molecules26071836
Heinrich M, Mah J, Amirkia V. Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules. 2021; 26(7):1836. https://doi.org/10.3390/molecules26071836
Chicago/Turabian StyleHeinrich, Michael, Jeffrey Mah, and Vafa Amirkia. 2021. "Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look" Molecules 26, no. 7: 1836. https://doi.org/10.3390/molecules26071836
APA StyleHeinrich, M., Mah, J., & Amirkia, V. (2021). Alkaloids Used as Medicines: Structural Phytochemistry Meets Biodiversity—An Update and Forward Look. Molecules, 26(7), 1836. https://doi.org/10.3390/molecules26071836