New Antiproliferative Triflavanone from Thymelaea hirsuta—Isolation, Structure Elucidation and Molecular Docking Studies
Abstract
1. Introduction
2. Results and Discussion
2.1. Isolation of Compounds 1–10
2.2. Identification of Compounds 1–10
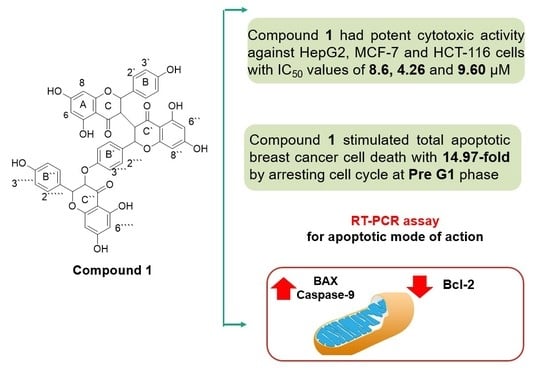
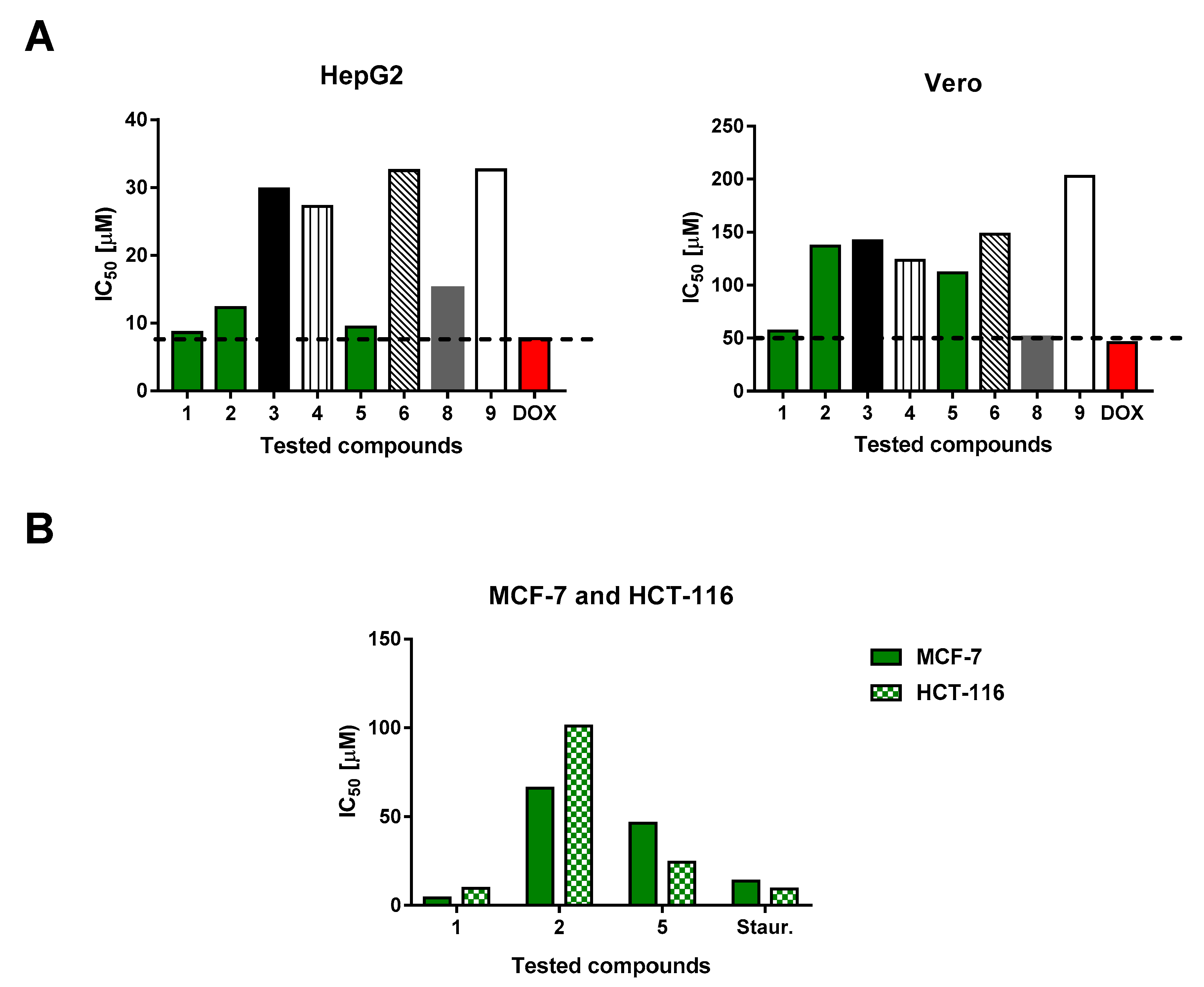
2.3. Cytotoxic Activities
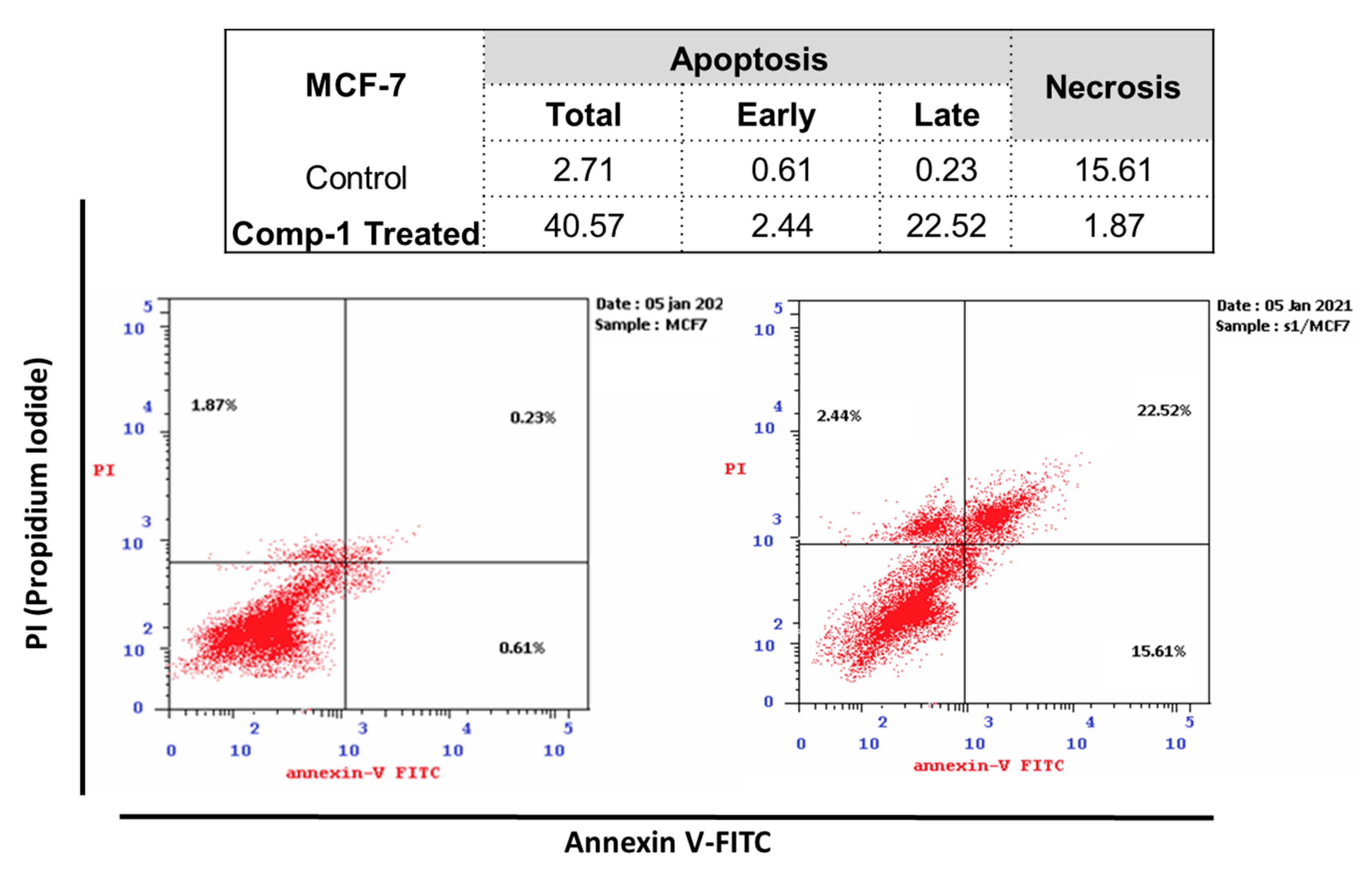
2.4. Cell Cycle Analysis
2.4.1. Annexin V/PI Staining
2.4.2. Cell Cycle Analysis
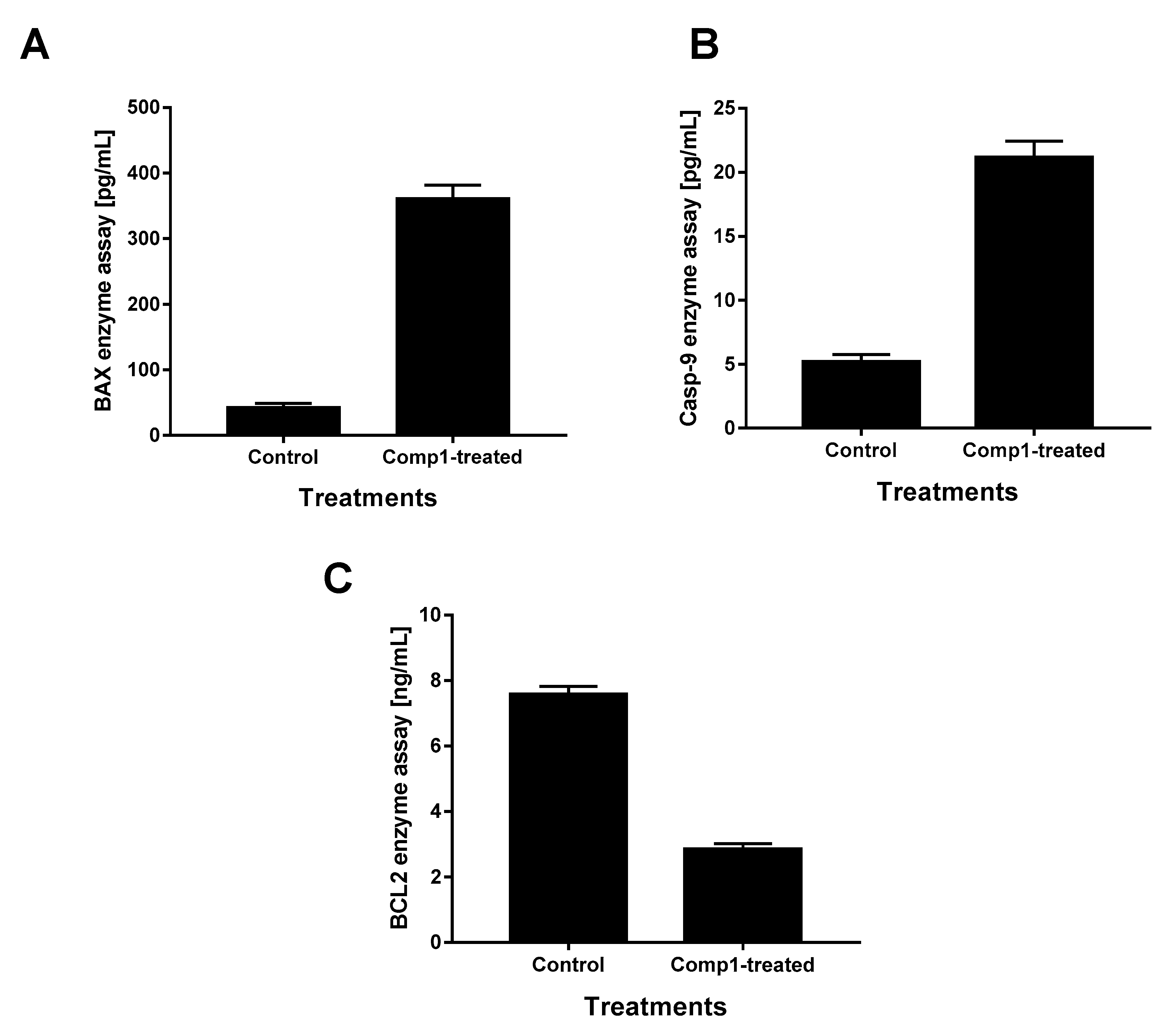
2.5. Enzymatic Assays for Apoptotic Markers
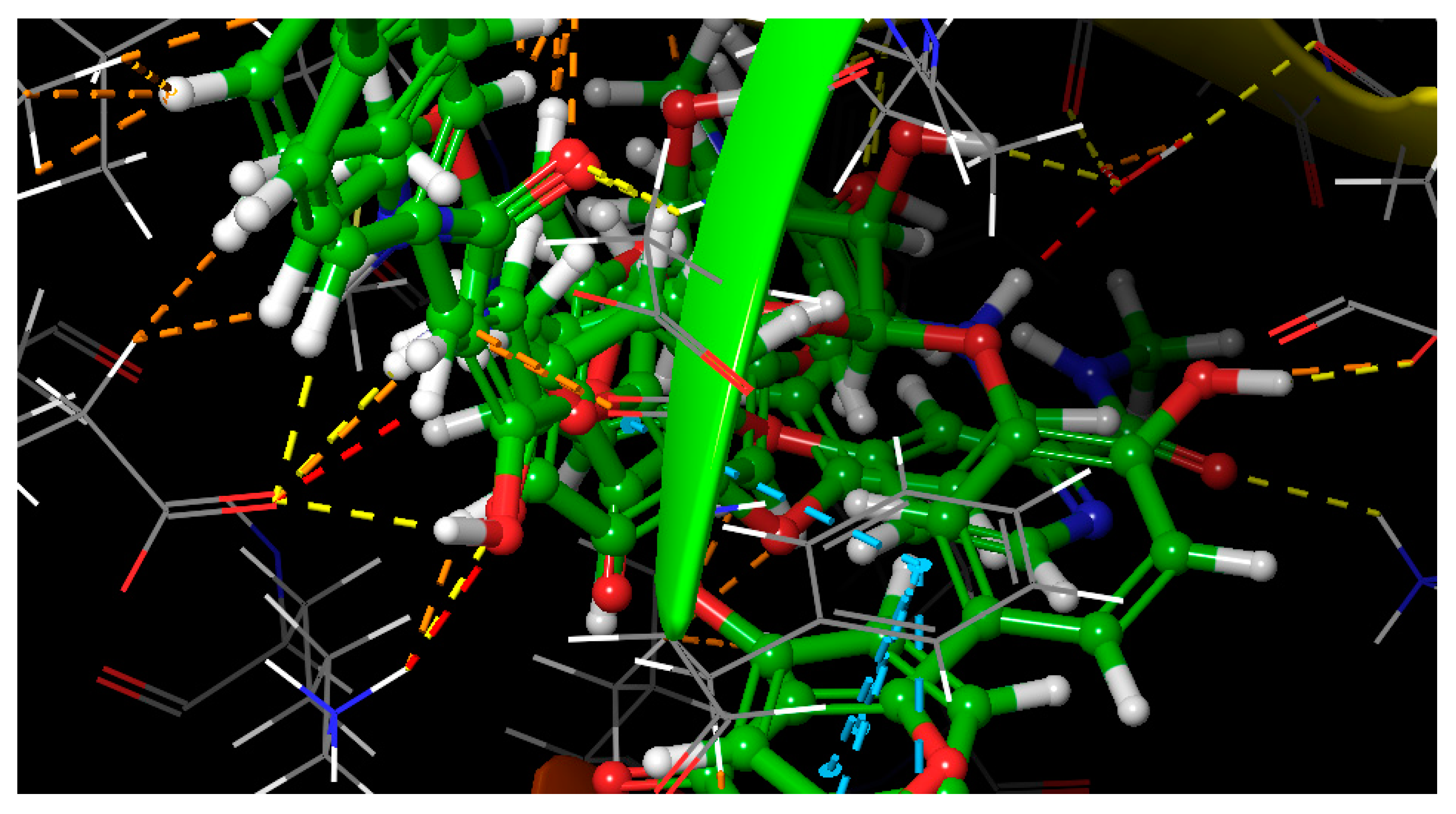
2.6. Molecular Docking
3. Materials and Methods
3.1. Plant Material
3.2. Extraction and Isolation
3.3. Cytotoxic Activity
3.3.1. Cell Lines
3.3.2. Procedure
3.3.3. Investigation of Apoptosis
Annexin V/PI Staining and Cell Cycle Analysis
Enzymatic Assays for Apoptotic Markers
3.4. Molecular Modeling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Markham, M.J.; Wachter, K.; Agarwal, N.; Bertagnolli, M.M.; Chang, S.M.; Dale, W.; Diefenbach, C.S.M.; Rodriguez-Galindo, C.; George, D.J.; Gilligan, T.D.; et al. Clinical Cancer Advances 2020: Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J. Clin. Oncol. 2020, 38, 1081. [Google Scholar] [CrossRef]
- Cragg, G.M.; Newman, D.J. Natural products: A continuing source of novel drug leads. Biochim. Biophys. Acta 2013, 1830, 3670–3695. [Google Scholar] [CrossRef]
- Cragg, G.M.; Pezzuto, J.M. Natural Products as a Vital Source for the Discovery of Cancer Chemotherapeutic and Chemopreventive Agents. Med. Princ. Pract. 2016, 25, 41–59. [Google Scholar] [CrossRef]
- Han, S.; Li, L.-Z.; Song, S.-J. Daphne giraldii Nitsche (Thymelaeaceae): Phytochemistry, pharmacology and medicinal uses. Phytochemistry 2020, 171, 112231. [Google Scholar] [CrossRef]
- Adam, A.; Lee, S.Y.; Mohamed, R. Pharmacological properties of agarwood tea derived from Aquilaria (Thymelaeaceae) leaves: An emerging contemporary herbal drink. J. Herb. Med. 2017, 10, 37–40. [Google Scholar] [CrossRef]
- Yan, Z.; Guo, H.; Yang, J.; Liu, Q.; Jin, H.; Xu, R.; Cui, H.; Qin, B. Phytotoxic flavonoids from roots of Stellera chamaejasme L. (Thymelaeaceae). Phytochemistry 2014, 106, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.J.; Santana, L.; Uriarte, E.; Abreu, O.; Molina Pérez, E.; Yordi, E. Coumarins—An Important Class of Phytochemicals; IntechOpen: London, UK, 2015; pp. 113–140. [Google Scholar]
- Wang, H.-B.; Wang, X.-Y.; Liu, L.-P.; Qin, G.-W.; Kang, T.-G. Tigliane Diterpenoids from the Euphorbiaceae and Thymelaeaceae Families. Chem. Rev. 2015, 115, 2975–3011. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.M.; Zhu, L.; Jiang, J.G.; Yang, L.; Wang, D.Y. Bioactive components and pharmacological action of Wikstroemia indica (L.) C. A. Mey and its clinical application. Curr. Pharm. Biotechnol. 2009, 10, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Durgawale, P.; Patil, M.; Joshi, S.; Korabu, K.; Datkhile, K. Studies on phytoconstituents, in vitro antioxidant, antibacterial, antiparasitic, antimicrobial, and anticancer potential of medicinal plant Lasiosiphon eriocephalus decne (Family: Thymelaeaceae). J. Nat. Sci. Biol. Med. 2019, 10, 38. [Google Scholar]
- Tundis, R.; Loizzo, M.; Boneri, M.; Peruzzi, L.; Efferth, T. Daphne striata Tratt. and D. mezereum L. (Thymelaeaceae): A study of anti-proliferative activity towards human cancer cells and antioxidant properties. Nat. Prod. Res. 2018, 33. [Google Scholar] [CrossRef]
- Huang, S.Z.; Zhang, X.J.; Li, X.Y.; Kong, L.M.; Jiang, H.Z.; Ma, Q.Y.; Liu, Y.Q.; Hu, J.M.; Zheng, Y.T.; Li, Y.; et al. Daphnane-type diterpene esters with cytotoxic and anti-HIV-1 activities from Daphne acutiloba Rehd. Phytochemistry 2012, 75, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Amari, N.O.; Bouzouina, M.; Berkani, A.; Lotmani, B. Phytochemical screening and antioxidant capacity of the aerial parts of Thymelaea hirsuta L. Asian Pac. J. Trop. Dis. 2014, 4, 104–109. [Google Scholar] [CrossRef]
- Sanna, G.; Madeddu, S.; Murgia, G.; Serreli, G.; Begala, M.; Caboni, P.; Incani, A.; Franci, G.; Galdiero, M.; Giliberti, G. Potent and Selective Activity against Human Immunodeficiency Virus 1 (HIV-1) of Thymelaea hirsuta Extracts. Viruses 2020, 12, 664. [Google Scholar] [CrossRef]
- Djeridane, A.; Yousfi, M.; Nadjemi, B.; Boutassouna, D.; Stocker, P.; Vidal, N. Antioxidant activity of some algerian medicinal plants extracts containing phenolic compounds. Food Chem. 2006, 97, 654–660. [Google Scholar] [CrossRef]
- Bnouham, M.; Benalla, W.; Bellahcen, S.; Hakkou, Z.; Ziyyat, A.; Mekhfi, H.; Aziz, M.; Abdelkhaleq, L. Antidiabetic and antihypertensive effect of a polyphenol-rich fraction of Thymelaea hirsuta L. in a model of neonatal streptozotocin-diabetic and NG-nitro-l-arginine methyl ester-hypertensive rats. J. Diabetes 2012, 4, 307–313. [Google Scholar] [CrossRef] [PubMed]
- Badawy, A.; Hassanean, H.; Ibrahim, A.K.; Habib, E.S.; El-Magd, M.A.; Ahmed, S.A. Isolates from thymelaea hirsuta inhibit progression of hepatocellular carcinoma in vitro and in vivo. Nat. Prod. Res. 2019, 1–8. [Google Scholar] [CrossRef]
- Rashed, W.M.; Kandeil, M.A.M.; Mahmoud, M.O.; Ezzat, S. Hepatocellular Carcinoma (HCC) in Egypt: A comprehensive overview. J. Egypt. Natl. Cancer Inst. 2020, 32, 5. [Google Scholar] [CrossRef]
- Elhady, S.S.; Eltamany, E.E.; Shaaban, A.E.; Bagalagel, A.A.; Muhammad, Y.A.; El-Sayed, N.M.; Ayyad, S.N.; Ahmed, A.A.; Elgawish, M.S.; Ahmed, S.A. Jaceidin Flavonoid Isolated from Chiliadenus montanus Attenuates Tumor Progression in Mice via VEGF Inhibition: In Vivo and In Silico Studies. Plants 2020, 9, 1031. [Google Scholar] [CrossRef]
- Vichai, V.; Kirtikara, K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat. Protoc. 2006, 1, 1112–1116. [Google Scholar] [CrossRef]
- Wang, Z.; Qu, Y.; Wang, L.; Zhang, X.; Xiao, H. Ultra-high performance liquid chromatography with linear ion trap-Orbitrap hybrid mass spectrometry combined with a systematic strategy based on fragment ions for the rapid separation and characterization of components in Stellera chamaejasme extracts. J. Sep. Sci. 2016, 39, 1379–1388. [Google Scholar] [CrossRef]
- Feng, B.; Pei, Y.; Hua, H.-M.; Wang, T.; Zhang, Y. Biflavonoids from Stellera chamaejasme. Pharm. Biol. 2008, 41, 59–61. [Google Scholar] [CrossRef]
- Li, J.; Zhao, W.; Hu, J.-L.; Cao, X.; Yang, J.; Li, X.-R. A New C-3/C-3”-Biflavanone from the Roots of Stellera chamaejasme L. Molecules 2011, 16, 6465–6469. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Tatematsu, H.; Kurokawa, M.; Niwa, M.; Hirata, Y. Novel C-3/C-3′’-biflavanones from Stellera chamaejasme L. Chem. Pharm. Bull. 1984, 32, 362–365. [Google Scholar] [CrossRef]
- Yang, G.; Chen, D. Biflavanones, flavonoids, and coumarins from the roots of Stellera chamaejasme and their antiviral effect on hepatitis B virus. Chem. Biodivers. 2008, 5, 1419–1424. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Lu, L.-Y.; Zeng, L.-H.; Zhang, C.; Hu, J.-L.; Li, X.-R. Sikokianin D, A New C-3/C-3 ‘‘-Biflavanone from the Roots of Wikstroemia indica. Molecules 2012, 17, 7792–7797. [Google Scholar] [CrossRef] [PubMed]
- Majumder, P.L.; Sengupta, G.C.; Dinda, B.N.; Chatterjee, A. Edgeworthin, a new bis-coumarin from Edgeworthia gardneri. Phytochemistry 1974, 13, 1929–1931. [Google Scholar] [CrossRef]
- Kabouche, Z.; Benkiki, N.; Seguin, E.; Bruneau, C. A new dicoumarinyl ether and two rare furocoumarins from Ruta montana. Fitoterapia 2003, 74, 194–196. [Google Scholar] [CrossRef]
- Li, J.; Shen, Q.; Bao, C.-H.; Chen, L.-T.; Li, X.-R. A new dicoumarinyl ether from the roots of Stellera chamaejasme L. Molecules 2014, 19, 1603–1607. [Google Scholar] [CrossRef]
- Chakrabarti, R.; Das, B.; Banerji, J. Bis-coumarins from Edgeworthia gardneri. Phytochemistry 1986, 25, 557–558. [Google Scholar] [CrossRef]
- Lin, J.H.; Lin, Y.T.; Huang, Y.J.; Wen, K.C.; Chen, R.-M.; Ueng, T.H.; Liao, C.H. Isolation and cytotoxicity of flavonoids from Daphnis Genkwae Flos. J. Food Drug Anal. 2001, 9, 6–11. [Google Scholar] [CrossRef]
- Fabre, N.; Rustan, I.; de Hoffmann, E.; Quetin-Leclercq, J. Determination of flavone, flavonol, and flavanone aglycones by negative ion liquid chromatography electrospray ion trap mass spectrometry. J. Am. Soc. Mass Spectrom. 2001, 12, 707–715. [Google Scholar] [CrossRef]
- Devi, S.; Kumar, V. Comprehensive structural analysis of cis- and trans-tiliroside and quercetrin from Malvastrum coromandelianum and their antioxidant activities. Arab. J. Chem. 2020, 13, 1720–1730. [Google Scholar] [CrossRef]
- Kizaibek, M.; Cao, P.; Gu, Z.; Bahetjan, D.; Jielile, J. Chemical Constituents of the Stem Bark of Daphne altaica. Chem. Nat. Compd. 2019, 55, 1150–1152. [Google Scholar] [CrossRef]
- Njinga, N.S.; Sule, M.I.; Pateh, U.U.; Hassan, H.S.; Abdullahi, S.T.; Ache, R.N. Isolation and Antimicrobial Activity of β-Sitosterol-3-O-Glucoside from Lannea Kerstingii Engl. & K. Krause (Anacardiacea). Nitte Univ. J. Health Sci. 2016, 06, 4–8. [Google Scholar]
- Maiyo, F.; Moodley, R.; Singh, M. Phytochemistry, Cytotoxicity and apoptosis studies of β-sitosterol-3-oglucoside and β-amyrin from prunus africana. Afr. J. Tradit. Complement. Altern Med. 2016, 13, 105–112. [Google Scholar] [CrossRef]
- Abdelhameed, R.F.A.; Nafie, M.S.; Ibrahim, A.K.; Yamada, K.; Abdel-Kader, M.S.; Ibrahim, A.K.; Ahmed, S.A.; Badr, J.M.; Habib, E.S. Cytotoxic, Apoptosis-Inducing Activities, and Molecular Docking of a New Sterol from Bamboo Shoot Skin Phyllostachys heterocycla var. pubescens. Molecules 2020, 25, 5650. [Google Scholar] [CrossRef]
- Eltamany, E.E.; Elhady, S.S.; Ahmed, H.A.; Badr, J.M.; Noor, A.O.; Ahmed, S.A.; Nafie, M.S. Chemical Profiling, Antioxidant, Cytotoxic Activities and Molecular Docking Simulation of Carrichtera annua DC. (Cruciferae). Antioxidants 2020, 9, 1286. [Google Scholar] [CrossRef]
- Chung, Y.C.; Kim, S.Y.; Hyun, C.G. 8-Methoxycoumarin enhances melanogenesis via the MAPKase signaling pathway. Die Pharm. 2019, 74, 529–535. [Google Scholar]
- Lee, N.; Chung, Y.C.; Kim, Y.B.; Park, S.M.; Kim, B.S.; Hyun, C.G. 7,8-Dimethoxycoumarin stimulates melanogenesis via MAPKs mediated MITF upregulation. Die Pharm. 2020, 75, 107–111. [Google Scholar]
- Lee, N.; Chung, Y.C.; Kang, C.I.; Park, S.-M.; Hyun, C.-G. 7,8-dimethoxycoumarin Attenuates the Expression of IL-6, IL-8, and CCL2/MCP-1 in TNF-α-Treated HaCaT Cells by Potentially Targeting the NF-κB and MAPK Pathways. Cosmetics 2019, 6, 41. [Google Scholar] [CrossRef]
- Moghbelinejad, S.; Nassiri-Asl, M.; Farivar, T.N.; Abbasi, E.; Sheikhi, M.; Taghiloo, M.; Farsad, F.; Samimi, A.; Hajiali, F. Rutin activates the MAPK pathway and BDNF gene expression on beta-amyloid induced neurotoxicity in rats. Toxicol. Lett. 2014, 224, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Delire, B.; Stärkel, P. The Ras/MAPK pathway and hepatocarcinoma: Pathogenesis and therapeutic implications. Eur. J. Clin. Investig. 2015, 45, 609–623. [Google Scholar] [CrossRef] [PubMed]
- Tantawy, E.S.; Amer, A.M.; Mohamed, E.K.; Abd Alla, M.M.; Nafie, M.S. Synthesis, characterization of some pyrazine derivatives as anti-cancer agents: In vitro and in Silico approaches. J. Mol. Struct. 2020, 1210, 128013. [Google Scholar] [CrossRef]
- Nafie, M.S.; Arafa, K.; Sedky, N.K.; Alakhdar, A.A.; Arafa, R.K. Triaryl dicationic DNA minor-groove binders with antioxidant activity display cytotoxicity and induce apoptosis in breast cancer. Chem.-Biol. Interact. 2020, 324, 109087. [Google Scholar] [CrossRef]
- Nafie, M.S.; Amer, A.M.; Mohamed, A.K.; Tantawy, E.S. Discovery of novel pyrazolo[3,4-b]pyridine scaffold-based derivatives as potential PIM-1 kinase inhibitors in breast cancer MCF-7 cells. Bioorg. Med. Chem. 2020, 28, 115828. [Google Scholar] [CrossRef]
- Gad, E.M.; Nafie, M.S.; Eltamany, E.H.; Hammad, M.S.A.G.; Barakat, A.; Boraei, A.T.A. Discovery of New Apoptosis-Inducing Agents for Breast Cancer Based on Ethyl 2-Amino-4,5,6,7-Tetra Hydrobenzo[b]Thiophene-3-Carboxylate: Synthesis, In Vitro, and In Vivo Activity Evaluation. Molecules 2020, 25, 2523. [Google Scholar] [CrossRef]
- Halgren, T.A.; Murphy, R.B.; Friesner, R.A.; Beard, H.S.; Frye, L.L.; Pollard, W.T.; Banks, J.L. Glide: A new approach for rapid, accurate docking and scoring. 2. Enrichment factors in database screening. J. Med. Chem. 2004, 47, 1750–1759. [Google Scholar] [CrossRef]
- Abdelhameed, R.; Elgawish, M.S.; Mira, A.; Ibrahim, A.K.; Ahmed, S.A.; Shimizu, K.; Yamada, K. Anti-choline esterase activity of ceramides from the Red Sea marine sponge Mycale euplectellioides. RSC Adv. 2016, 6, 20422–20430. [Google Scholar] [CrossRef]

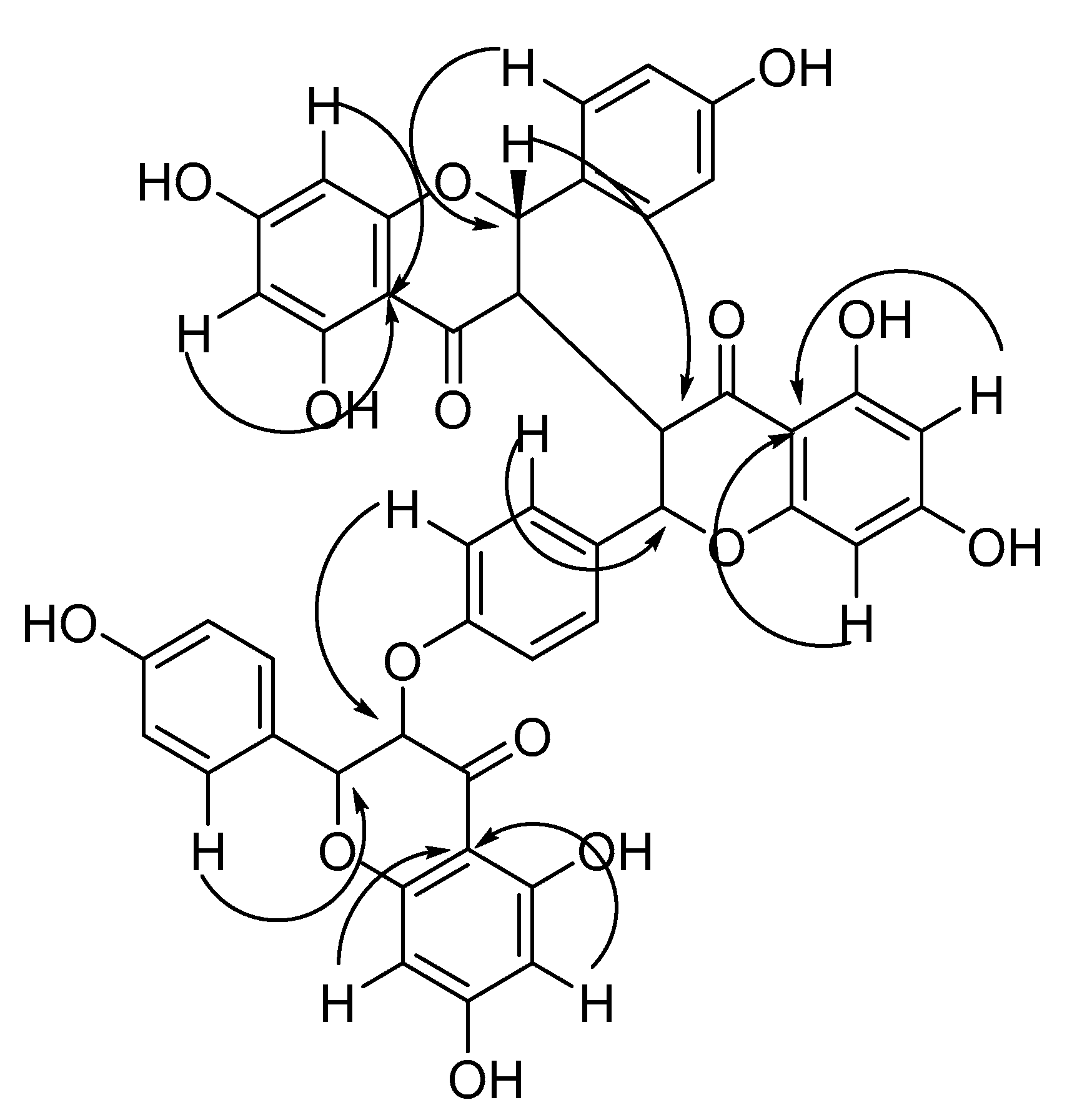

| Position | δH (ppm, m, J Hz) | δC (ppm) | Position | δH (ppm, m, J Hz) | δC (ppm) |
|---|---|---|---|---|---|
| 2 | 5.47 (1H, d, J = 4.5 Hz) | 81.8 | 9`` | 163.7 | |
| 3 | 3.15 (1H, m) | 49.8 | 10`` | 105.5 | |
| 4 | 198.9 | 1``` | 129.1 | ||
| 5 | 165.4 | 2```, 6``` | 6.92 (2H, d, J = 8.4 Hz) | 130.7 | |
| 6 | 5.76 (1H, d, J = 2.1 Hz) | 97.7 | 3```, 5``` | 6.79 (2H, d, J = 8.4 Hz) | 116.9 |
| 7 | 168.6 | 4``` | 158.8 | ||
| 8 | 5.79 (1H, d, J = 2.1 Hz) | 96.5 | 2```` | 4.96 (1H, m) | 82.8 |
| 9 | 164.7 | 3```` | 5.89 (1H, d, J = 2.1 Hz) | 97.8 | |
| 10 | 104.2 | 4```` | 197.3 | ||
| 1` | 129.5 | 5```` | 159.2 | ||
| 2`, 6` | 7.11 (2H, d, J = 8.7 Hz) | 128.9 | 6```` | 5.75 (1H, s) | 96.6 |
| 3`, 5` | 6.64 (2H, d, J = 8.4 Hz) | 116.6 | 7```` | 168.5 | |
| 4` | 159.9 | 8```` | 5.75 (1H, s) | 96.6 | |
| 2`` | 5.14 (1H, d, J = 9.0 Hz) | 83.5 | 9```` | 164.7 | |
| 3`` | 3.32 (1H, m) | 51.2 | 10```` | 103.3 | |
| 4`` | 196.6 | 1````` | 129.1 | ||
| 5`` | 165.7 | 2`````, 6````` | 7.01 (2H, d, J = 8.4 Hz) | 131.3 | |
| 6`` | 5.87 (1H, d, J = 2.1 Hz) | 97.5 | 3`````, 5````` | 6.78 (2H, d, J = 8.7 Hz) | 116.8 |
| 7`` | 168.6 | 4````` | 159.9 | ||
| 8`` | 5.98 (1H, m) | 96.9 |
| Compound | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Cell line | 1 | 2 | 3 | 4 | 5 | 6 | 8 | 9 | Doxo |
| HepG2 | 8.6 | 12.3 | 29.8 | 27.2 | 9.4 | 32.5 | 15.1 | 32.6 | 7.7 |
| Vero | 56.6 | >136.5 | >141.9 | 123.5 | 111.6 | >147.8 | 50.5 | 202.7 | 45.8 |
| Compound | Docking Score (KJ mol−1) | Glide Emodel (Kcal mol−1) |
|---|---|---|
| 2 | −7.82 | −59.02 |
| 3 | −6.24 | −51.75 |
| 4 | −7.37 | −59.42 |
| 5 | −6.82 | −58.55 |
| 6 | −7.14 | −58.90 |
| 7 | −8.55 | −58.78 |
| 8 | −8.34 | −100.70 |
| 9 | −5.49 | −31.27 |
| 10 | −5.29 | −45.43 |
| Rutin | −7.32 | −69.18 |
| Sorafenib | −8.376 | −81.119 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elhady, S.S.; Abdelhameed, R.F.A.; El-Ayouty, M.M.; Ibrahim, A.K.; Habib, E.S.; Elgawish, M.S.; Hassanean, H.A.; Safo, M.K.; Nafie, M.S.; Ahmed, S.A. New Antiproliferative Triflavanone from Thymelaea hirsuta—Isolation, Structure Elucidation and Molecular Docking Studies. Molecules 2021, 26, 739. https://doi.org/10.3390/molecules26030739
Elhady SS, Abdelhameed RFA, El-Ayouty MM, Ibrahim AK, Habib ES, Elgawish MS, Hassanean HA, Safo MK, Nafie MS, Ahmed SA. New Antiproliferative Triflavanone from Thymelaea hirsuta—Isolation, Structure Elucidation and Molecular Docking Studies. Molecules. 2021; 26(3):739. https://doi.org/10.3390/molecules26030739
Chicago/Turabian StyleElhady, Sameh S., Reda F. A. Abdelhameed, Mayada M. El-Ayouty, Amany K. Ibrahim, Eman S. Habib, Mohamed S. Elgawish, Hashim A. Hassanean, Martin K. Safo, Mohamed S. Nafie, and Safwat A. Ahmed. 2021. "New Antiproliferative Triflavanone from Thymelaea hirsuta—Isolation, Structure Elucidation and Molecular Docking Studies" Molecules 26, no. 3: 739. https://doi.org/10.3390/molecules26030739
APA StyleElhady, S. S., Abdelhameed, R. F. A., El-Ayouty, M. M., Ibrahim, A. K., Habib, E. S., Elgawish, M. S., Hassanean, H. A., Safo, M. K., Nafie, M. S., & Ahmed, S. A. (2021). New Antiproliferative Triflavanone from Thymelaea hirsuta—Isolation, Structure Elucidation and Molecular Docking Studies. Molecules, 26(3), 739. https://doi.org/10.3390/molecules26030739