Chemical Characterization and Anti-HIV-1 Activity Assessment of Iridoids and Flavonols from Scrophularia trifoliata
Abstract
:1. Introduction
2. Results
2.1. Anti-HIV Activity of Methanolic Crude Extract
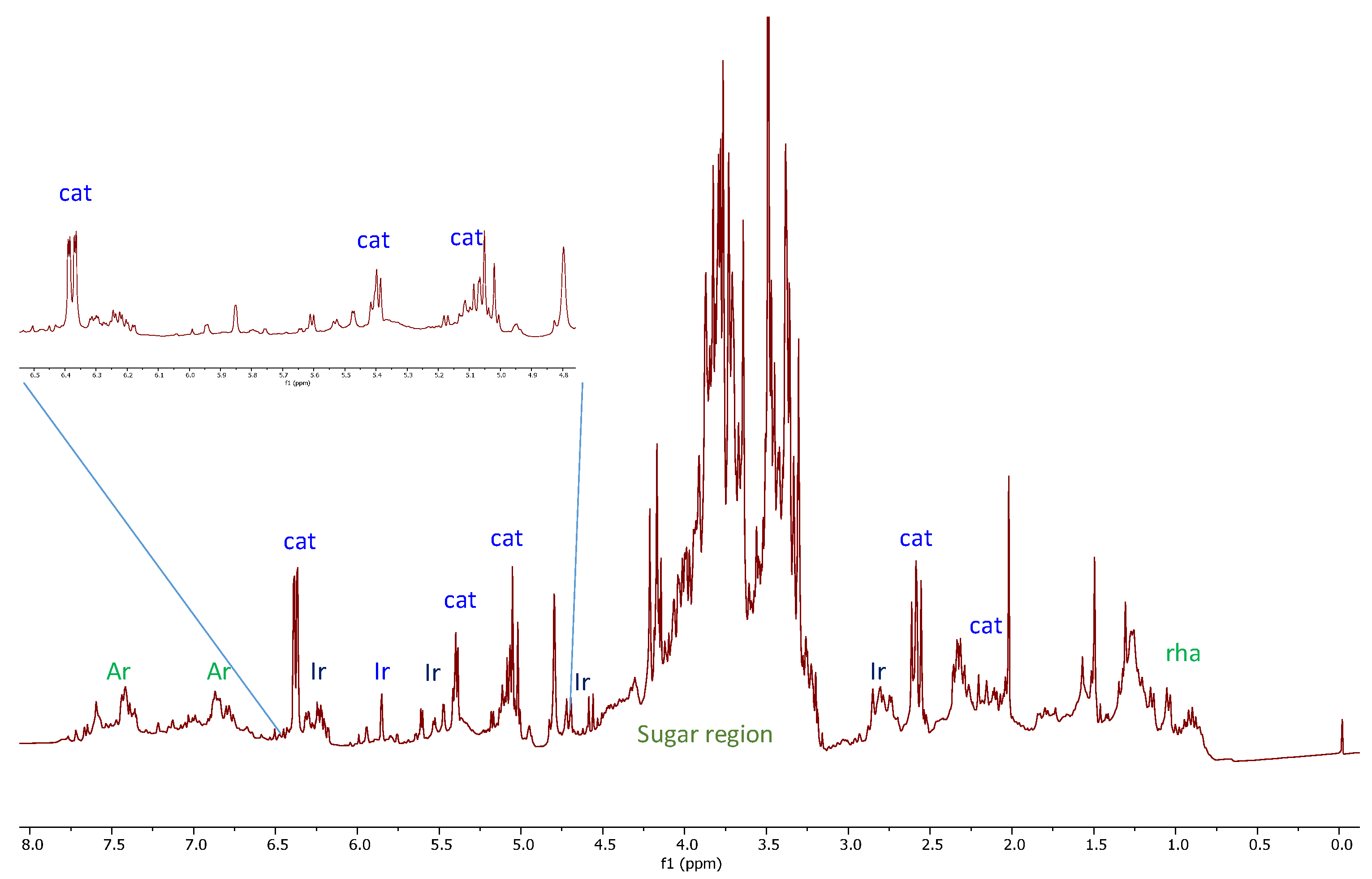
2.2. 2D-NMR Investigation of Crude Extract
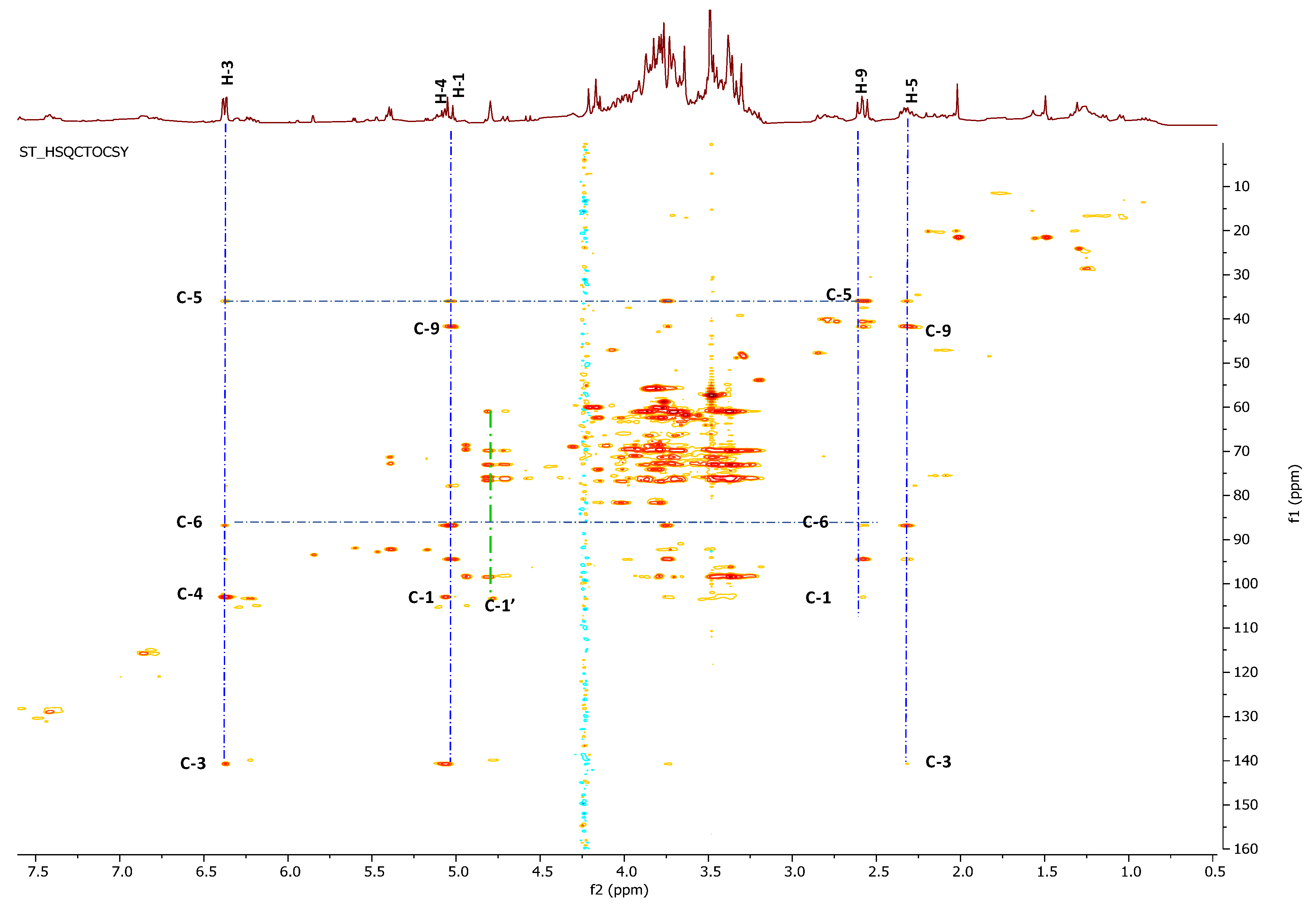
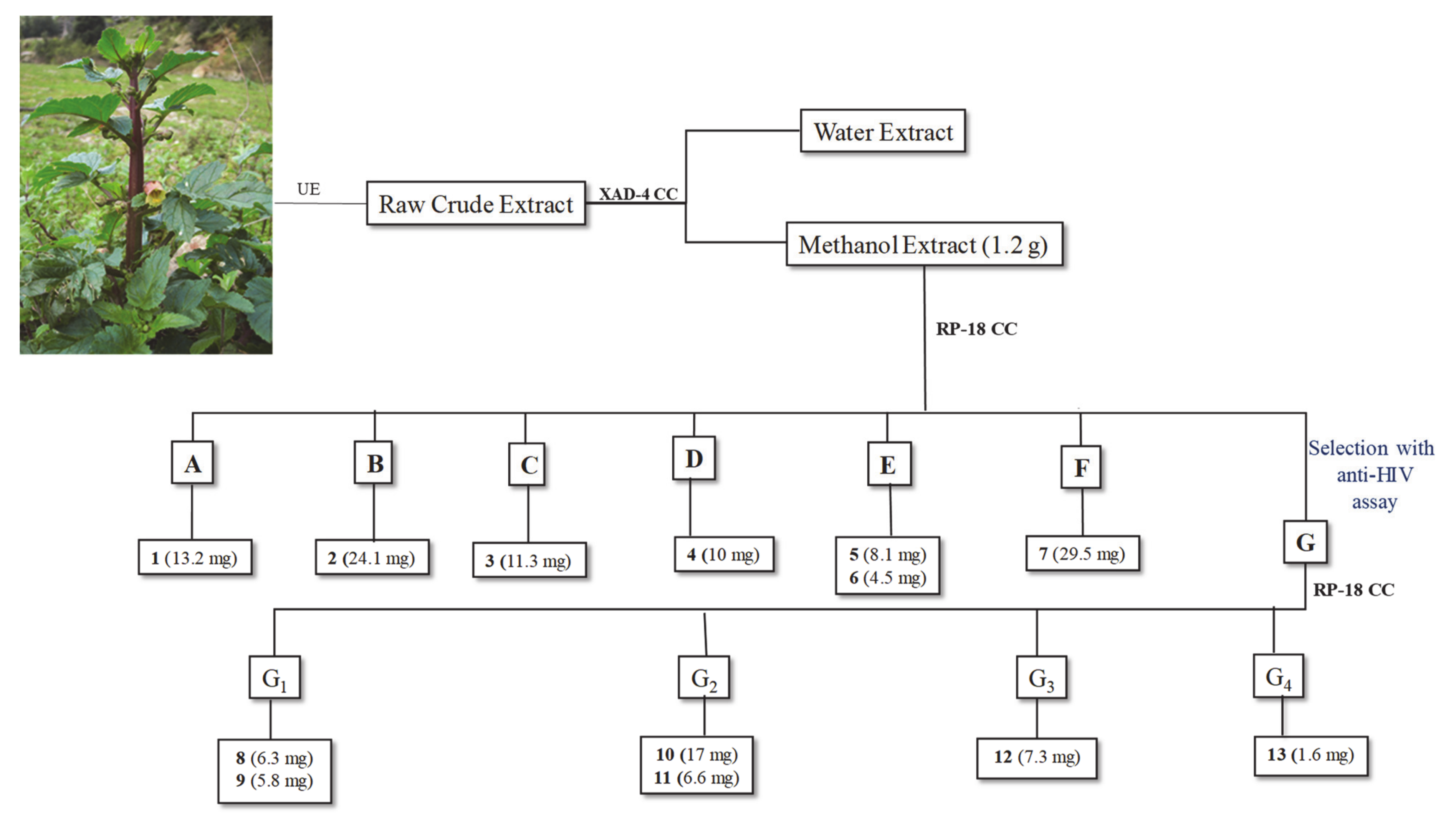
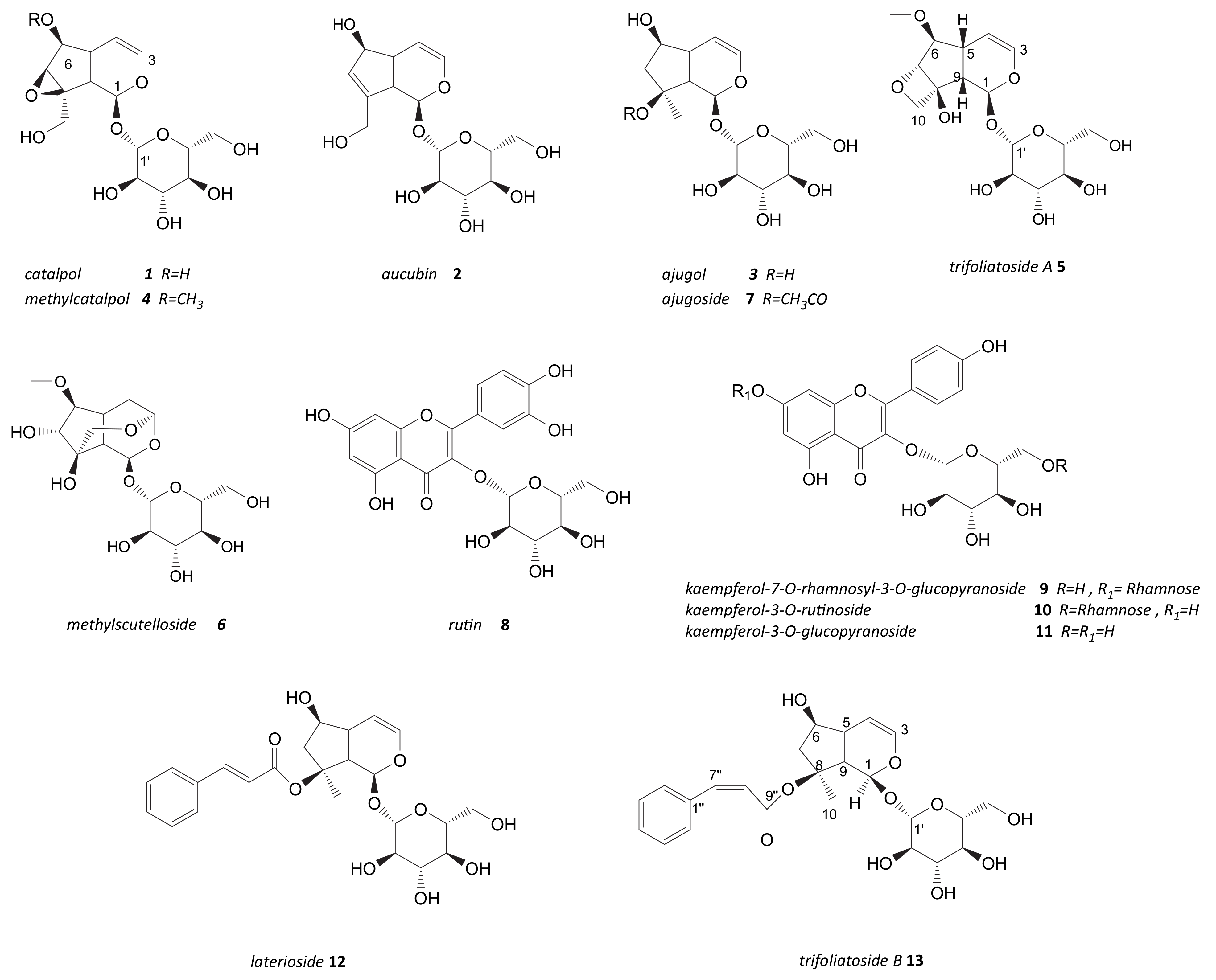
2.3. Phytochemical Study of Scrophularia trifoliata
2.4. Anti-HIV Activity of Pure Compounds and Fraction G
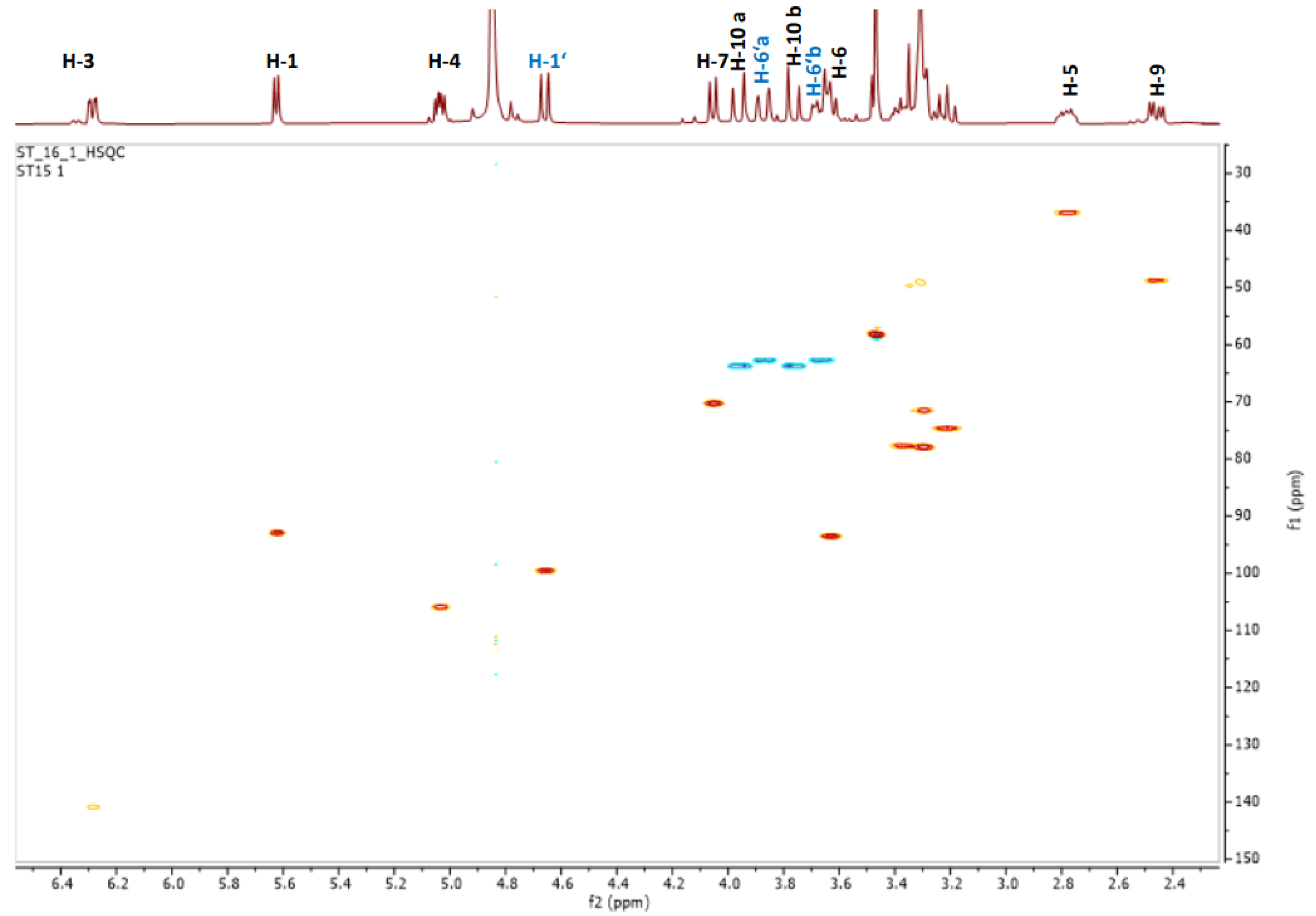
2.5. Phytochemical Investigation of Fraction Active G: Structural Characterization of Flavonols and Acylated Iridoid Glicosides
2.6. Anti-HIV Activity of Pure Compounds Isolated from Fraction G
3. Discussion
4. Materials and Methods
4.1. Plant Material
4.2. Preparation of Crude Extract for Bioassay
4.3. Extraction Procedure for NMR Analysis
4.4. General Chromatographic Procedures
4.5. NMR Experiments
4.6. MALDI-TOF MS Analyses
4.7. Hydroalcoholic Extraction of S. trifoliata Leaves and Compounds Purification
4.8. Anti-HIV Enzymatic Assays
4.8.1. Expression and Purification of Recombinant HIV-1RT
4.8.2. Expression and Purification of Recombinant HIV-1 IN and LEDGF
4.8.3. RNase H Polymerase-Independent Cleavage Assay
4.8.4. Homogeneous Time Resolved Fluorescence (HTRF) LEDGF Dependent Assay
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Sample Availability
References
- Valsecchi, F. Observations sur quelques especes du genre «Scrophularia» L.en Sardaigne. Webbia 1979, 34, 265–288. [Google Scholar] [CrossRef]
- Ortega-Olivencia, A.; Rodrıguez-Riano, T.; Perez-Bote, J.L.; Lopez, J.; Mayo, C.; Valtuena, F.J.; Navarro-Perez, M.L. Insects, birds and lizards as pollinators of the largest-flowered Scrophularia of Europe and Macaronesia. Ann. Bot. 2012, 109, 153–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Navarro-Pèrez, M.L.; Lòpez, J.; Rodrìguez-Riano, T.; Bacchetta, G.; de Miguel Gordillo, C.; Ortega-Olivencia, A. Confirmed mixed-bird-insect pollination system of Scrophularia trifoliata L., a Tyrrhenina species with corolla spots. Plant Biol. 2017, 9, 460–468. [Google Scholar] [CrossRef] [PubMed]
- Pasdaran, A.; Hamedi, A. The genus Scrophularia: A source of iridoids and terpenoids with a diverse biological activity. Pharm. Bio. 2017, 55, 2211–2233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trampettia, F.; Pereira, C.; Rodriguesa, M.J.; Celaj, O.; D’Abrosca, B.; Zenginc, G.; Mollica, A.; Stefanucci, A.; Custódio, L. Exploring the halophyte Cistanche phelypaea (L.) Cout as a source of health promoting products: In vitro antioxidant and enzyme inhibitory properties, metabolomic profile and computational studies. J. Pharm. Biomed. Anal. 2019, 165, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhu, T.; Qian, F.; Xu, J.; Dorje, G.; Zhao, Z.; Guo, F.; Li, Y. Iridoid glycosides isolated from Scrophularia dentata Royle ex Benth. and their anti-inflammatory activity. Fitoterapia 2014, 98, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.R.; Sung, S.H.; Jang, Y.P.; Markelonis, G.J.; Oh, T.H.; Kim, Y.C. E-p-methoxycinnamic acid protects cultured neuronal cells against neurotoxicity induced by glutamate. Br. J. Pharmacol. 2002, 135, 1281–1291. [Google Scholar] [CrossRef] [Green Version]
- Stavri, M.; Mathew, K.; Gibbons, S. Antimicrobial constituents of Scrophularia deserti. Phytochemistry 2006, 67, 1530–1533. [Google Scholar] [CrossRef]
- Monsef-Esfahani, H.R.; Hajiaghaee, R.; Shahverdi, A.R.; Khorramizadeh, M.R.; Amini, M. Flavonoids, cinnamic acid and phenyl propanoid from aerial parts of Scrophularia striata. Pharm. Biol. 2010, 48, 333–336. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.; Ahn, M.; Lee, S. Isolation and identification of phytochemical constituents from Scrophularia takesimensis. J. Med. Plant Res. 2012, 6, 3923–3930. [Google Scholar]
- Akhmedov, S.; Litvinenko, V. Scrophulein—A new flavonoid from Scrophularia grossheimii. Chem. Nat. Compd. 1969, 5, 47–48. [Google Scholar] [CrossRef]
- Mahboubi, M.; Kazempour, N.; Nazar, A.R.B. Total phenolic, total flavonoids, antioxidant and antimicrobial activities of Scrophularia striata Boiss extracts. Jundishapur J. Nat. Pharm. Prod. 2013, 8, 15–19. [Google Scholar] [CrossRef] [Green Version]
- Nasri, S.; Cheraghi, J.; Soltanbaygi, S. Antinociceptive and anti-inflammatory effect of alcoholic extract of root and stem of Scrophularia striata Boiss. in male mice. IJMAP 2013, 29, 74–83. [Google Scholar]
- Bruni, A.; Ballero, M.; Poli, F. Quantitative ethnopharmacological study of the Campidano Valley and Urzulei district, Sardinia, Italy. J. Ethnopharmacol. 1997, 57, 97–124. [Google Scholar] [CrossRef]
- Sanna, C.; Ballero, M.; Maxia, A. Le piante medicinali utilizzate contro le patologie epidermiche in Ogliastra (Sardegna centro-orientale). Atti. Soc. Tosc. Sci. Nat. Mem. 2006, 113, 73–82. [Google Scholar]
- Sanna, C.; Maxia, A.; Fenu, G.; Loi, M.C. So Uncommon and so Singular, but Underexplored: An Updated Overview on Ethnobotanical Uses, Biological Properties and Phytoconstituents of Sardinian Endemic Plants. Plants 2020, 9, 958. [Google Scholar] [CrossRef]
- Ramunno, A.; Serrilli, A.; Piccioni, F.; Serafini, M.; Ballero, M. Taxonomical markers in two endemic plants of Sardinia: Verbascum conocarpum and Scrophularia trifoliata. Nat. Prod. Res. 2006, 20, 511–516. [Google Scholar] [CrossRef]
- Bianco, A.; Serrilli, A.; Venditti, A.; Petitto, V.; Serafini, M. Chem. Endemic Plants of Italy and Their Peculiar Molecular Pattern. Stud. Nat. Prod. 2016, 50, 215–247. [Google Scholar]
- Sanna, C.; Scognamiglio, M.; Fiorentino, A.; Corona, A.; Graziani, V.; Caredda, A.; Cortis, P.; Montisci, M.; Ceresola, E.R.; Canducci, F.; et al. Prenylated phloroglucinols from Hypericum scruglii, an endemic species of Sardinia (Italy), as new dual HIV-1 inhibitors effective on HIV-1 replication. PLoS ONE 2018, 13, e0195168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Esposito, F.; Sanna, C.; Del Vecchio, C.; Cannas, V.; Venditti, A.; Corona, A.; Bianco, A.; Serrilli, A.M.; Guarcini, L.; Parolin, C.; et al. Hypericum hircinum L. components as new single-molecule inhibitors of both HIV-1 reverse transcriptase-associated DNA polymerase and ribonuclease H activities. Pathog. Dis. 2013, 68, 116–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanna, C.; Rigano, D.; Corona, A.; Piano, D.; Formisano, C.; Farci, D.; Franzini, G.; Ballero, M.; Chianese, G.; Tramontano, E.; et al. Dual HIV-1 reverse transcriptase and integrase inhibitors from Limonium morisianum Arrigoni, an endemic species of Sardinia (Italy). Nat. Prod. Res. 2019, 33, 1798–1803. [Google Scholar] [CrossRef] [PubMed]
- Bai, C.I.; Wang, Q.H.; Xu, Y.H.; Han, J.S.; Bao, Y.P. The isolation and structural elucidation of a new iridoid glycoside from Cymbaria dahurica L. Z. Naturforsch 2018, 73, 377–379. [Google Scholar] [CrossRef]
- Saracoglu, I.; Oztunca, F.H.; Nagatsu, A.; Harput, U.S. Iridoid content and biological activities of Veronica cuneifolia subsp. Cuneifolia and V. cymbalaria. Pharm. Bio. 2011, 49, 1150–1157. [Google Scholar] [CrossRef] [Green Version]
- Pacifico, S.; D’Abrosca, B.; Pascarella, M.T.; Letizia, M.; Uzzo, P.; Piscopo, V.; Fiorentino, A. Antioxidant efficacy of iridoid and phenylethanoid glycoside from the medicinal plant Teucrium chamaedris in cell-free systems. Bioorg. Med. Chem. Lett. 2009, 17, 6173–6179. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, R.M.P.; Solis, R.V.; Vaez, E.G.; Martinez, F.M. Effect on Capillary Permeability in Rabbits of Iridoids from Buddleia scordioides. Phytother. Res. 2006, 20, 542–545. [Google Scholar] [CrossRef] [PubMed]
- Tai, B.H.; Nhiem, N.X.; Quang, T.H.; Ngan, N.T.T.; Tung, N.H.; Kim, Y.; Lee, J.J.; Myung, C.S.; Cuong, N.M.; Kim, Y.H. A new iridoid and effect on the rat aortic vascular smooth muscle cell proliferation of isolated compounds from Buddleja officinalis. Bioorg. Med. Chem. Lett. 2011, 21, 3462–3466. [Google Scholar] [CrossRef]
- Erukainure, O.L.; Ebuehi, O.A.T.; Choudhary, I.M.; Adhikari, A.; Hafizur, R.M.; Perveen, S.; Muhammad, A.; Elemo, G.N. Iridoid glycoside from the leaves of Clerodendrum volubile beauv. Shows potent antioxidant activity against oxidative stress in rat brain and hepatic tissues. J. Diet. Suppl. 2014, 11, 19–29. [Google Scholar] [CrossRef]
- Aisyah, L.S.; Yun, Y.F.; Herlina, T.; Julaeha, E.; Zainuddin, A.; Nurfarida, I. Flavonoids Compounds from the Leaves of Kalanchoe prolifera and Their Cytotoxic Activity againist p-388 Murine Leukimia Cells. Nat. Prod. Sci. 2017, 23, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Formisano, C.; Rigano, D.; Sentaore, F.; Bancheva, S.; Maggio, A.; Rosselli, S.; Bruno, M. Flavonoids in Subtribe Centaureinae (Cass.) DUMORT. (Tribe Cardueae, Asteraceae): Distribution and 13 C-NMR Spectral Data. Chem. Biodivers. 2012, 9, 2096–2158. [Google Scholar] [CrossRef]
- de Oliveira, D.M.; Siqueira, P.; Yule, R.; Nunes, F.; Cota, B.B. Flavonoids from leaves of Mauritia flexuosa. Rev. Bras. Farmacogn. 2013, 23, 614–620. [Google Scholar] [CrossRef]
- Youn, I.S.; Han, A.R.; Roh, M.S.; Seo, E.K. Constituents of the leaves of Verbascum blattaria. Nat. Prod. Comm. 2014, 10, 445–446. [Google Scholar] [CrossRef] [Green Version]
- Park, J.G.; Park, J.C.; Hyr, J.M.; Park, S.J.; Choi, D.R.; Shin, D.Y.; Park, K.Y.; Cho, H.W.; Kim, M.S. Phenolic compounds from Orostachys japonicus having anti-HIV-1 protease activity. Nat. Prod. Sci. 2000, 6, 117–121. [Google Scholar]
- Bermejo, P.; Abad, M.J.; Díaz, A.M.; Fernández, L.; De Santos, J.; Sanchez, S.; Villaescusa, L.; Carrasco, L.; Irurzun, A. Antiviral activity of seven iridoids, three saikosaponins and one phenylpropanoid glycoside extracted from Bupleurum rigidum and Scrophularia scorodonia. Planta Med. 2002, 68, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.-R.; Wang, R.-F.; Shang, M.-Y.; Cai, S.-Q. A new iridoid glycoside from Scrophularia ningpoensis. Nat. Prod. Res. 2009, 23, 1181–1188. [Google Scholar] [CrossRef] [PubMed]
- Chebaki, R.; Haba, H.; Long, C.; Marcourt, L.; Benkhaled, M. Acylated iridoid glycosides from Scrophularia saharae Batt. & Trab. Biochem. Syst Ecol. 2011, 39, 902–905. [Google Scholar]
- Venditti, A.; Frezza, C.; Riccardelli, M.; Foddai, S.; Nicoletti, M.; Serafini, M.; Bianco, A. Secondary metabolites from Scrophularia canina L. Nat. Prod. Res. 2015, 30, 1665–1669. [Google Scholar] [CrossRef]
- Nicoletti, M.; Serafini, M.; Garbarino, J.A.; Gambaro, V. A chemosystematic study of Scrophulariaceae: Iridoid glycosides. Plant Biosyst. 1988, 122, 13–24. [Google Scholar] [CrossRef]
- Behbahani, M. Evaluation of anti-HIV-1-activity of a new iridoid glycoside isolated from Avicenna marina, in vitro. Int. Immunopharmacol. 2014, 23, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Esposito, F.; Tramontano, E. Past and future. Current drugs targeting HIV-1 integrase and reverse transcriptase-associated ribonuclease H activity: Single and dual active site inhibitors. Antivir. Chem. Chemother. 2014, 23, 129–144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corona, A.; Masaoka, T.; Tocco, G.; Tramontano, E.; Le Grice, S.F. Active site and allosteric inhibitors of the ribonuclease H activity of HIV reverse transcriptase. Future Med. Chem. 2013, 18, 2127–2139. [Google Scholar] [CrossRef]
- Tramontano, E.; Corona, A.; Menendez-Arias, L. Ribonuclease H, an unexploited target for antiviral intervention against HIV and hepatitis B virus. Antivir. Res. 2019, 21, 104613. [Google Scholar] [CrossRef] [PubMed]
- Cherepanov, P.; Maertens, G.; Proost, P.; Devreese, B.; Van Beeumen, J.; Engelborghs, Y.; De Clercq, E.; Debyser, Z. HIV-1 integrase forms stable tetramers and associates with LEDGF/p75 protein in human cells. J. Biol. Chem. 2003, 278, 372–381. [Google Scholar] [CrossRef] [Green Version]
- Carcelli, M.; Rogolino, D.; Gatti, A.; Pala, N.; Corona, A.; Caredda, A.; Tramontano, E.; Pannecoque, C.; Naesens, L.; Esposito, F. Chelation motifs affecting metal-dependent viral enzymes: N’-Acylhydrazone ligands as dual target inhibitors of HIV-1 integrase and reverse transcriptase ribonuclease H domain. Front. Microbiol. 2017, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Li, B.Q.; Fu, T.; Yan, Y.D.; Baylor, N.W.; Ruscetti, F.W.; Kung, H.F. Inhibition of HIV infection by baicalin-a flavonoid compound purified from Chinese herbal medicine. Cell Mol. Biol. 1993, 39, 119–124. [Google Scholar]
- Byung, S.M.; Hyeong, K.L.; Sang, M.L.; Young, H.K.; Ki, H.B.; Toru, O.; Norio, N.; Masao, H. Anti-human immunodeficiency virus-type 1 activity of constituents from Junglas mandshurica. Arc. Pharmacal Res. 2002, 25, 441–445. [Google Scholar]
- D’Abrosca, B.; Ciaramella, V.; Graziani, V.; Papaccio, F.; Della Corte, C.M.; Potenza, N.; Fiorentino, A.; Ciardiello, F.; Morgillo, F. Urtica dioica, L. inhibits proliferation and enhances cisplatin cytotoxicity in NSCLC cells via Endoplasmic Reticulum-stress mediated apoptosis. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Fois, B.; Corona, A.; Tramontano, E.; Distinto, S.; Maccioni, E.; Meleddu, R.; Caboni, P.; Floris, C.; Cottiglia, F. Flavonoids and Acid-Hydrolysis Derivatives of Neo-Clerodane Diterpenes from Teucrium flavum subsp. glaucum as Inhibitors of the HIV-1 Reverse Transcriptase–Associated RNase H Function. J. Enzyme. Inhib. Med. Chem. 2021, 36, 749–757. [Google Scholar] [CrossRef] [PubMed]
- Tintori, C.; Esposito, F.; Morreale, F.; Martini, R.; Tramontano, E.; Botta, M. Investigation on the sucrose binding pocket of HIV-1 Integrase by molecular dynamics and synergy experiments. Bioorg. Med. Chem. Lett. 2015, 25, 3013–3016. [Google Scholar] [CrossRef]
- Corona, A.; Meleddu, R.; Esposito, F.; Distinto, S.; Bianco, G.; Masaoka, T.; Maccioni, E.; Menéndez-Arias, L.; Alcaro, S.; Le Grice, S.F.J.; et al. Ribonuclease H/DNA polymerase HIV-1 reverse transcriptase dual inhibitor: Mechanistic studies on the allosteric mode of action of isatin-based compound RMNC6. PLoS ONE 2006, 11, e0147225. [Google Scholar] [CrossRef] [Green Version]
- Pisano, M.B.; Cosentino, S.; Viale, S.; Spanò, D.; Corona, A.; Esposito, F.; Tramontano, E.; Montoro, P.; Tuberoso, C.I.; Medda, R.; et al. Biological Activities of Aerial Parts Extracts of Euphorbia characias. Biomed. Res. Int. 2016, 2016, 1538703. [Google Scholar] [CrossRef] [Green Version]
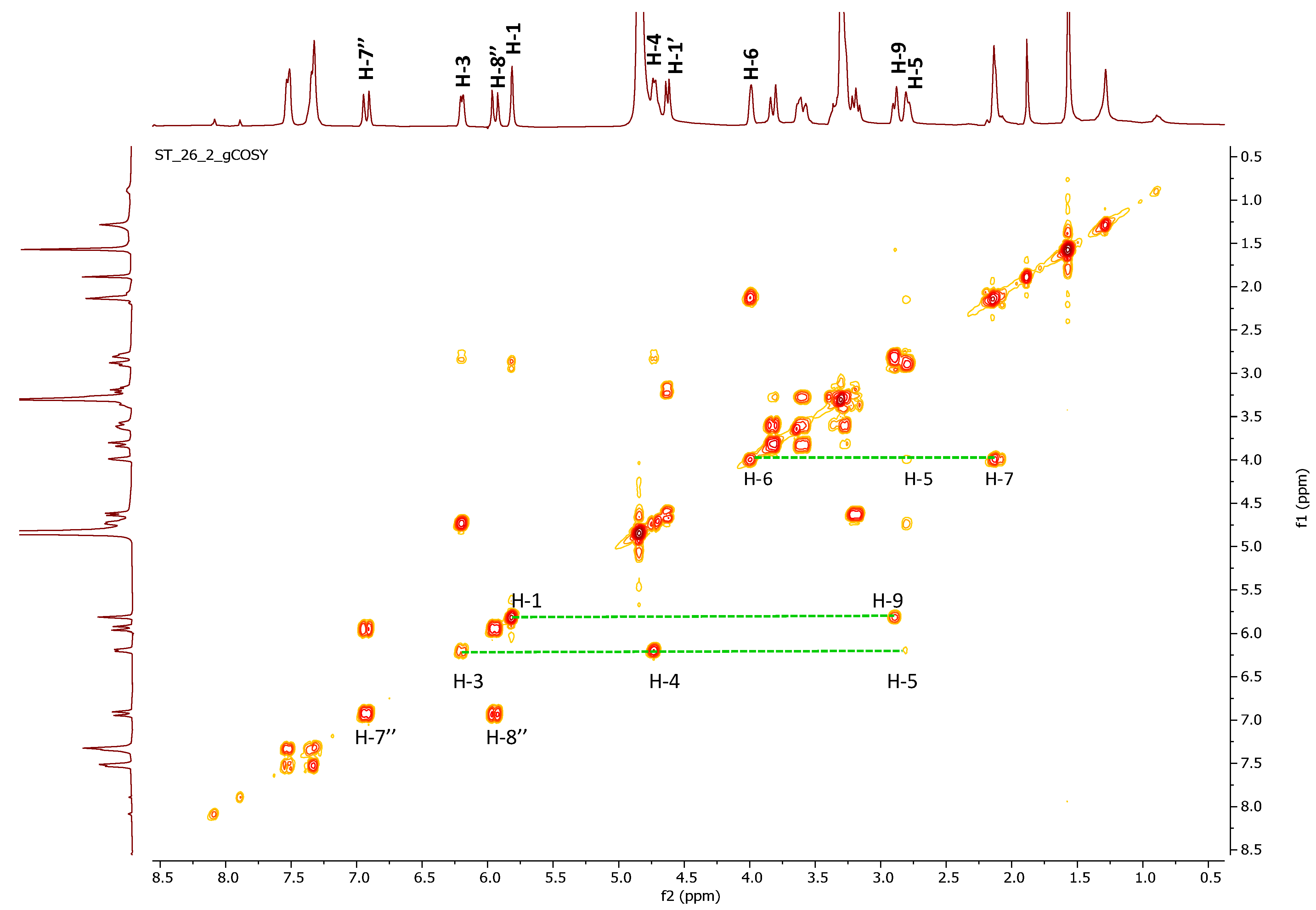
| Position | δC (Type) | δH (J in Hz) | HMBC (H→C) |
|---|---|---|---|
| 1 | 93.0 (CH) | 5.62 (d, J = 4.2 Hz) | C-3, C-5, C-1′ |
| 3 | 140.3 (CH) | 6.28 (dd, J = 6.3, 1.8 Hz) | C-1, C-4 |
| 4 | 105.9 (CH) | 5.03 (dd, J = 6.3, 3.6 Hz) | C-1, C-3, C-5, C-9 |
| 5 | 37.1 (CH) | 2.77 (m) | C-6, C-8, C-9 |
| 6 | 93.5 (CH) | 3.62 (d, J = 6 Hz) | C-5, C-7, C-OCH3 |
| 7 | 70.2 (CH) | 4.04 (d, J = 7 Hz) | C-6, C-8,C-10 |
| 8 | 81.5 (C) | ||
| 9 | 48.9 (CH) | 2.45 (dd, J = 4.2 Hz; 10.2 Hz) | C-4, C-5, C-10 |
| 10 | 63.7 (CH2) | 3.95/ 3.75 (d, J = 12.0 Hz) | C-7, C-8, C-9 |
| 1′ | 99.6 (CH) | 4.65 (d, J = 7.8 Hz) | C-1 |
| 2′ | 74.8 (CH) | 3.20 (t, J = 7.8 Hz) | C-3′, C-1′ |
| 3′ | 77.8 (CH) | 3.29 (ov) | C-4′ |
| 4′ | 71.6 (CH) | 3.37 (ov) | C-3′, C-5′ |
| 5′ | 77.9 (CH) | 3.30 (ov) | C-4′ |
| 6′ | 63.7 (CH2) | 3.87(d, J = 13.8 Hz) 3.66 (ov) | C-5′ |
| -OCH3 | 58.1 (CH3) | 3.46 (s) | C-6 |
| a HIV-1 RNase H IC50 (µM) | b HIV-1 IN LEDGF-Dependent IC50 (µM) | |
|---|---|---|
| Crude extract | 9.9 ± 0.93 c | 2.5 ± 0.4 |
| 1 | >100 (100%) d | >100 (100%) d |
| 2 | >100 (100%) d | >100 (100%) d |
| 3 | >100 (100%) d | >100 (77%) d |
| 4 | >100 (100%) d | >100 (80%) d |
| 5 | >100 (93%) d | 95.5 ± 4.5 |
| 6 | >100 (100%) d | 100.0 ± 0.5 |
| 7 | >100 (100%) d | 74.0 ± 3.5 |
| Fraction G | 26.6 ± 1.3 c | 6.1 ± 0.92 c |
| RDS 1759 e | 7.3 ± 0.10 | |
| Raltegravir e | 0.058 ± 0.01 |
| Position | δC (Type) | δH (J in Hz) |
|---|---|---|
| 1 | 94.1 (CH) | 5.82 (brs) |
| 3 | 141.5 (CH) | 6.20 (dd, J = 6.3, 2.7 Hz) |
| 4 | 103.2 (CH) | 4.73 (m) |
| 5 | 41.3 (CH) | 2.80 (m) |
| 6 | 76.8 (CH) | 4.00 (m) |
| 7 | 48.5 (CH2) | 2.14 m |
| 8 | 90.3 (C) | |
| 9 | 49.4 (CH) | 2.89 (m) |
| 10 | 22.4 (CH3) | 1.58 (s) |
| 1′ | 99.8 (CH) | 4.63 (d, J = 7.8 Hz) |
| 2′ | 74.6 (CH) | 3.20 (t, J = 7.8 Hz) |
| 3′ | 77.8 (CH) | 3.28 ov |
| 4′ | 71.4 (CH) | 3.28 ov |
| 5′ | 77.9 (CH) | 3.36 ov |
| 6′ | 62.7 (CH2) | 3.83 (d, J = 12.0 Hz) 3.60 m |
| 1″ | 135.9 (C) | |
| 2′ | 126.8 (CH) | 7.39 ov |
| 3″ | 121.9 (CH) | 7.33 ov |
| 4″ | 131.8 (CH) | 7.58 ov |
| 5″ | 126.8 (CH) | 7.39 ov |
| 6″ | 121.9 (CH) | 7.39 ov |
| 7″ | 142.4 (CH) | 6.93 (d, J = 12.6 Hz) |
| 8″ | 122.3 (CH) | 5.95 (d, J = 12.6 Hz) |
| 9″ | 168.2 (C) |
| Compounds | a HIV-1 RNase H (µM) | b HIV-1 IN LEDGF-Dependent (µM) |
|---|---|---|
| 8 | >100 (100%) c | 0.33 ± 0.04 |
| 9 | >100 (100%) c | 0.11 ± 0.005 |
| 10 | >100 (100%) c | 1.76 ± 0.017 |
| 11 | >100 (100%) c | 0.024 ± 0.001 |
| 12 | >100 (100%) c | >100 (80%) c |
| 13 | >100 (92%) c | 5.96 ± 0.10 |
| Raltegravir d | 0.058 ± 0.010 | |
| RDS1759 d | 7.3 ± 0.10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guzzo, F.; Russo, R.; Sanna, C.; Celaj, O.; Caredda, A.; Corona, A.; Tramontano, E.; Fiorentino, A.; Esposito, F.; D’Abrosca, B. Chemical Characterization and Anti-HIV-1 Activity Assessment of Iridoids and Flavonols from Scrophularia trifoliata. Molecules 2021, 26, 4777. https://doi.org/10.3390/molecules26164777
Guzzo F, Russo R, Sanna C, Celaj O, Caredda A, Corona A, Tramontano E, Fiorentino A, Esposito F, D’Abrosca B. Chemical Characterization and Anti-HIV-1 Activity Assessment of Iridoids and Flavonols from Scrophularia trifoliata. Molecules. 2021; 26(16):4777. https://doi.org/10.3390/molecules26164777
Chicago/Turabian StyleGuzzo, Francesca, Rosita Russo, Cinzia Sanna, Odeta Celaj, Alessia Caredda, Angela Corona, Enzo Tramontano, Antonio Fiorentino, Francesca Esposito, and Brigida D’Abrosca. 2021. "Chemical Characterization and Anti-HIV-1 Activity Assessment of Iridoids and Flavonols from Scrophularia trifoliata" Molecules 26, no. 16: 4777. https://doi.org/10.3390/molecules26164777
APA StyleGuzzo, F., Russo, R., Sanna, C., Celaj, O., Caredda, A., Corona, A., Tramontano, E., Fiorentino, A., Esposito, F., & D’Abrosca, B. (2021). Chemical Characterization and Anti-HIV-1 Activity Assessment of Iridoids and Flavonols from Scrophularia trifoliata. Molecules, 26(16), 4777. https://doi.org/10.3390/molecules26164777











