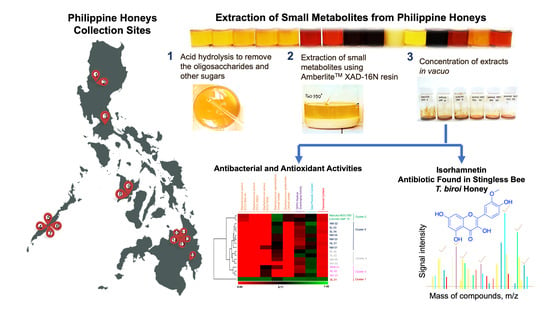
The Isorhamnetin-Containing Fraction of Philippine Honey Produced by the Stingless Bee Tetragonula biroi Is an Antibiotic against Multidrug-Resistant Staphylococcus aureus
Abstract
1. Introduction
2. Results
2.1. Antibacterial Activities
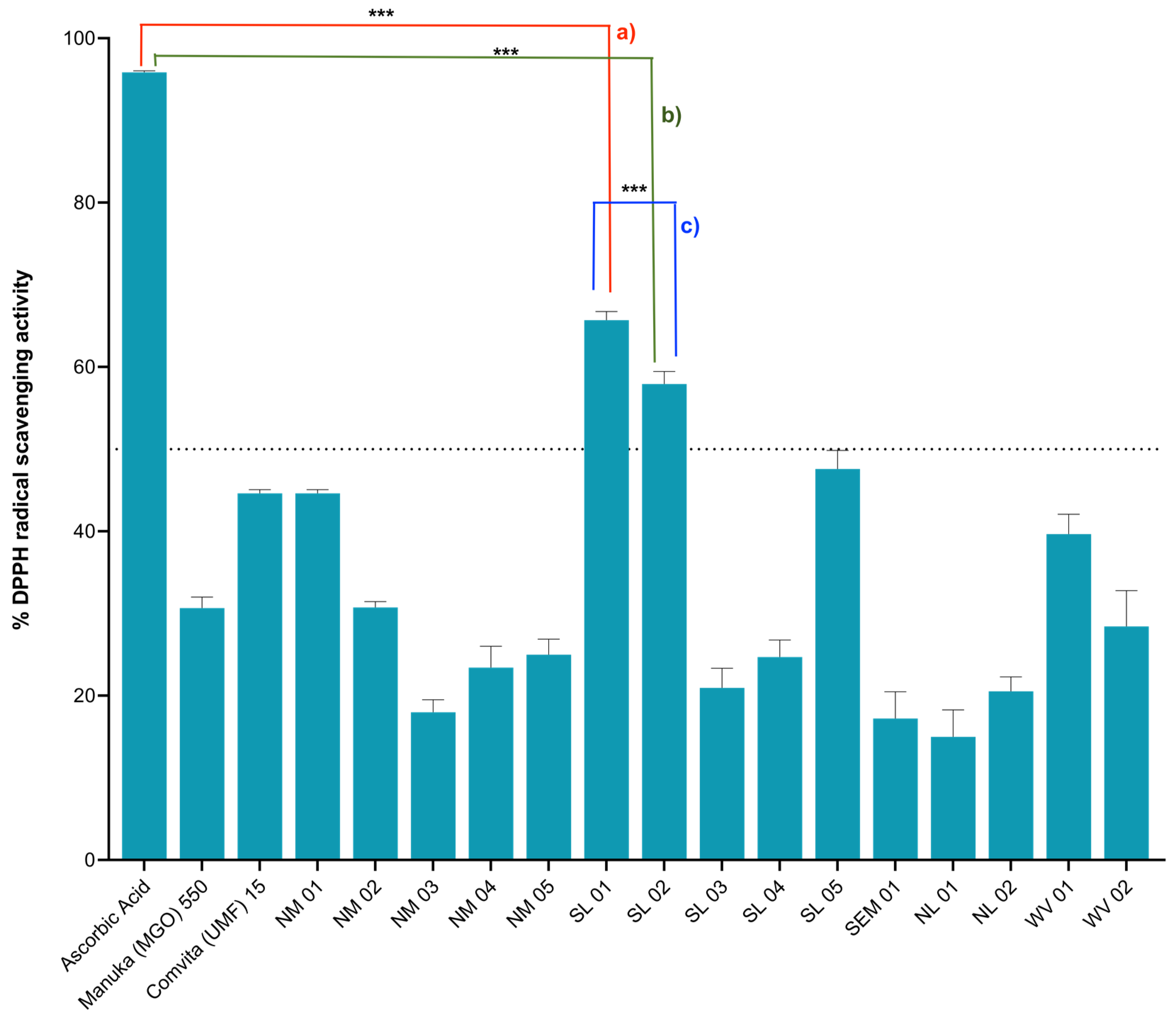
2.2. Antioxidant Activities
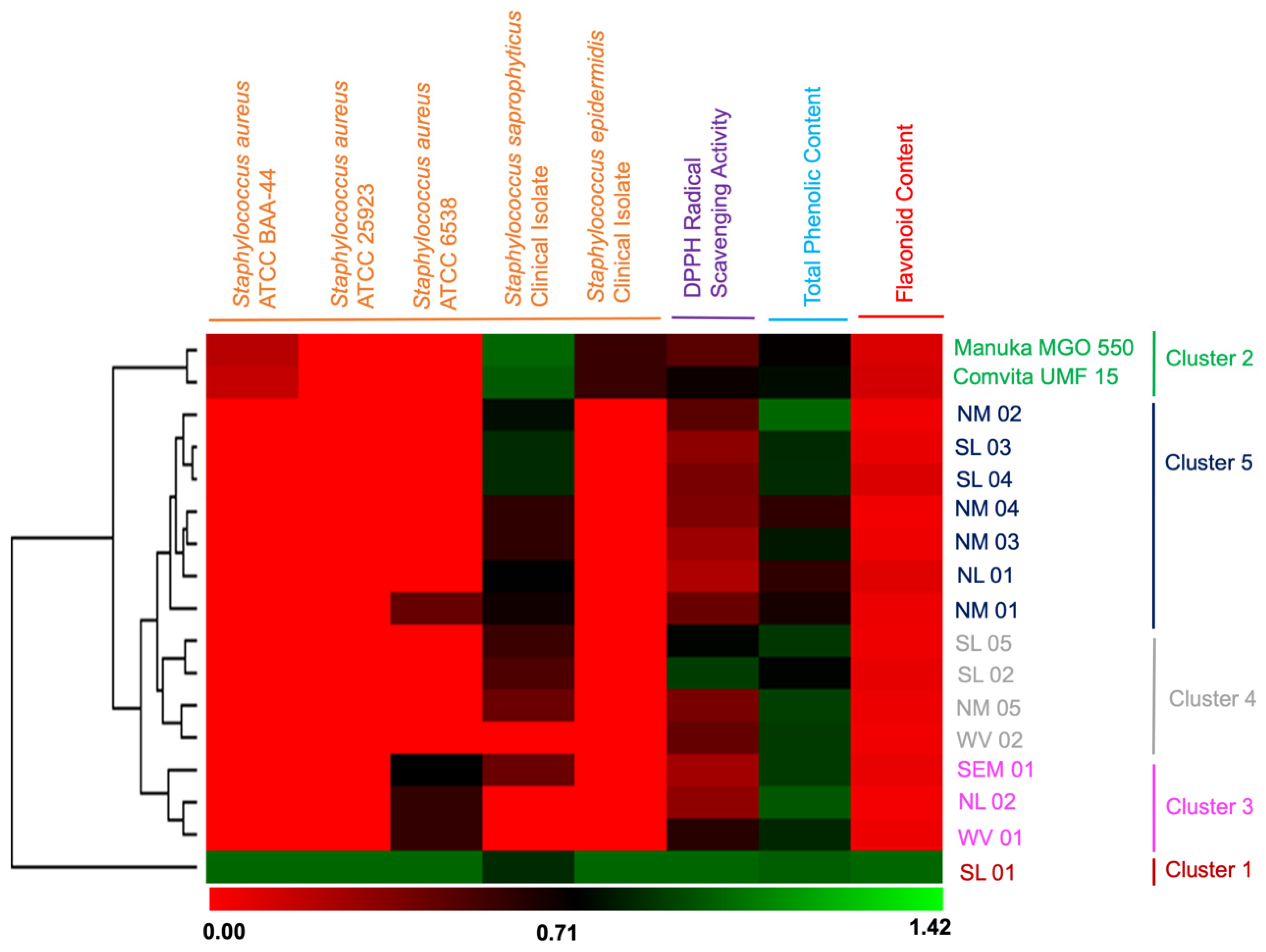
2.3. Flavonoid and Phenolic Contents and their Correlation with Bioactivities
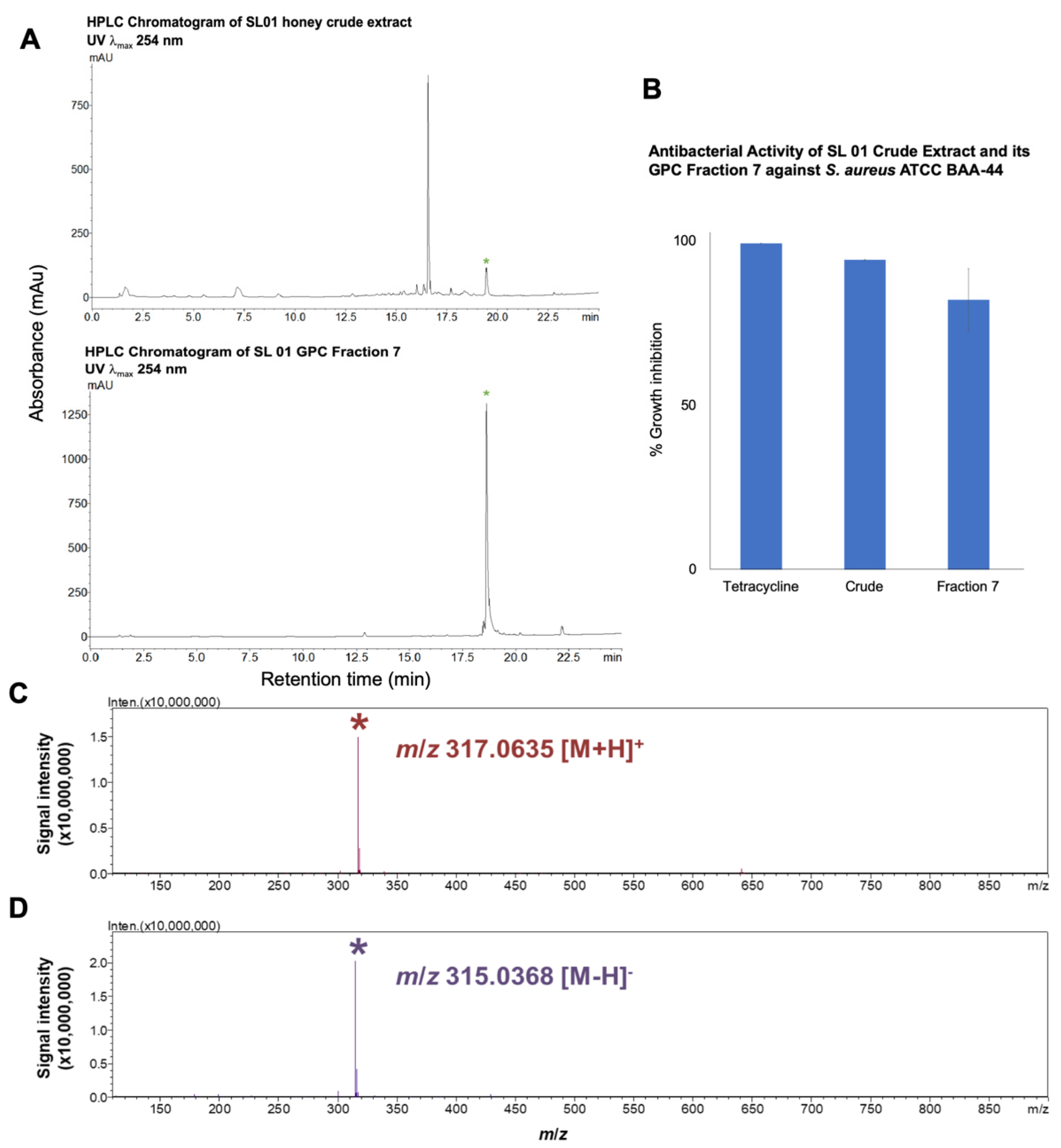
2.4. Purification of the Antibiotic Component from T. biroi Honey
3. Discussion
4. Materials and Methods
4.1. Honey Samples
4.2. Reagents and Standards
4.3. Metabolite Extraction
4.4. Target Organisms for Antibacterial Testing
4.5. Agar Well Diffusion for Antibacterial Assay
4.6. Antioxidant Assay Using 2,2-Diphenyl-1-Picrylhydrazyl (DPPH)
4.7. Quantification of Flavonoids
4.8. Determination of Total Phenolic Content
4.9. Purification and Isolation of Antibiotic Compound from Philippine Honey
4.10. Chemical Profiling of Bioactive Honey GPC Fraction Using High Performance Liquid Chromatography (HPLC)-Diode-Array Detector (DAD)
4.11. Microbroth Susceptibility Assay of GPC Fractions against S. aureus ATCC BAA-44
4.12. Determination of Minimum Inhibitory Concentration (MIC)
4.13. Ultra High Pressure Liquid Chromatography Mass Spectrometry (UPLCMS) and MS/MS Analysis of Bioactive GPC Fraction
4.14. Data Processing and Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Miguel, M.G.; Antunes, M.D.; Faleiro, M.L. Honey as a complementary medicine. Integr. Med. Insights. 2017, 12, 1178633717702869. [Google Scholar] [CrossRef] [PubMed]
- Combarros-Fuertes, P.; Fresno, J.M.; Estevinho, M.M.; Sousa-Pimenta, M.; Tornadijo, M.E.; Estevinho, L.M. Honey: Another alternative in the fight against antibiotic-resistant bacteria? Antibiotics 2020, 9, 774. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.L.; Metters, G.; Hitchings, M.D.; Wilkinson, T.S.; Sousa, L.; Cooper, J.; Dance, H.; Atterbury, R.J.; Jenkins, R. Antibacterial and antivirulence activity of manuka honey against genetically diverse Staphylococcus pseudintermedius Strains. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef] [PubMed]
- Amalia, E.; Diantini, A.; Subarnas, A. Water-soluble propolis and bee pollen of Trigona spp. from South Sulawesi Indonesia induce apoptosis in the human breast cancer MCF-7 cell line. Oncol. Lett. 2020, 20, 274. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Honey, wound repair and regenerative medicine. J. Funct. Biomater. 2018, 9, 34. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Sulaiman, S.A.; Baig, A.A.; Ibrahim, M.; Liaqat, S.; Fatima, S.; Jabeen, S.; Shamim, N.; Othman, N.H. Honey as a potential natural antioxidant medicine: An insight into its molecular mechanisms of action. Oxid. Med. Cell Longev. 2018, 2018, 8367846. [Google Scholar] [CrossRef] [PubMed]
- Bucekova, M.; Jardekova, L.; Juricova, V.; Bugarova, V.; Di Marco, G.; Gismondi, A.; Leonardi, D.; Farkasovska, J.; Godocikova, J.; Laho, M.; et al. Antibacterial activity of different blossom honeys: New findings. Molecules 2019, 24, 1573. [Google Scholar] [CrossRef] [PubMed]
- Barbieri, D.; Gabriele, M.; Summa, M.; Colosimo, R.; Leonardi, D.; Domenici, V.; Pucci, L. Antioxidant, nutraceutical properties, and fluorescence spectral profiles of bee pollen samples from different botanical origins. Antioxidants 2020, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Cridland, J.M.; Tsutsui, N.D.; Ramírez, S.R. The complex demographic history and evolutionary origin of the western honey bee, Apis Mellifera. Genome Biol. Evol. 2017, 9, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Döke, M.A.; McGrady, C.M.; Otieno, M.; Grozinger, C.M.; Frazier, M. Colony size, rather than geographic origin of stocks, predicts overwintering success in honey bees (Hymenoptera: Apidae) in the Northeastern United States. J. Econ. Entomol. 2019, 112, 525–533. [Google Scholar] [CrossRef]
- Homrani, M.; Escuredo, O.; Rodríguez-Flores, M.S.; Fatiha, D.; Mohammed, B.; Homrani, A.; Seijo, M.C. Botanical origin, pollen profile, and physicochemical properties of Algerian honey from different bioclimatic areas. Foods 2020, 9, 938. [Google Scholar] [CrossRef] [PubMed]
- Carabetta, S.; Di Sanzo, R.; Campone, L.; Fuda, S.; Rastrelli, L.; Russo, M. High-Performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) and chemometrics for geographical and floral authentication of honeys from southern Italy (Calabria region). Foods 2020, 9, 1625. [Google Scholar] [CrossRef] [PubMed]
- Zieliński, L.; Deja, S.; Jasicka-Misiak, I.; Kafarski, P. Chemometrics as a tool of origin determination of Polish monofloral and multifloral honeys. J. Agric. Food Chem. 2014, 62, 2973–2981. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Q.X. Chemical composition, characterization, and differentiation of honey botanical and geographical origins. Adv. Food Nutr. Res. 2011, 62, 89–137. [Google Scholar] [PubMed]
- Ciulu, M.; Spano, N.; Pilo, M.I.; Sanna, G. Recent advances in the analysis of phenolic compounds in unifloral honeys. Molecules 2016, 21, 451. [Google Scholar] [CrossRef] [PubMed]
- Sateriale, D.; Facchiano, S.; Colicchio, R.; Pagliuca, C.; Varricchio, E.; Paolucci, M.; Volpe, M.G.; Salvatore, P.; Pagliarulo, C. Synergy of polyphenolic extracts from honey, myrtle and pomegranate against oral pathogens, S. mutans and R. dentocariosa. Front. Microbiol. 2020, 11, 1465. [Google Scholar] [CrossRef]
- Pauliuc, D.; Dranca, F.; Oroian, M. Antioxidant activity, total phenolic content, individual phenolics and physicochemical parameters suitability for Romanian honey authentication. Foods 2020, 9, 306. [Google Scholar] [CrossRef]
- Zothanpuia; Passari, A.K.; Gupta, V.K.; Singh, B.P. Detection of antibiotic-resistant bacteria endowed with antimicrobial activity from a freshwater lake and their phylogenetic affiliation. PeerJ 2016, 4, e2103. [Google Scholar] [CrossRef]
- Kuś, P.M. Honey as source of nitrogen compounds: Aromatic amino acids, free nucleosides and their derivatives. Molecules 2020, 25, 847. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Cheng, N.; Wang, Q.; Zhou, W.; Liu, C.; Liu, X.; Chen, S.; Fan, D.; Cao, W. Effects of honey-extracted polyphenols on serum antioxidant capacity and metabolic phenotype in rats. Food Funct. 2019, 10, 2347–2358. [Google Scholar] [CrossRef] [PubMed]
- Al-Mamary, M.; Al-Meeri, A.; Al-Habori, M. Antioxidant activities and total phenolics of different types of honey. Nutr. Res. 2002, 22, 1041–1047. [Google Scholar] [CrossRef]
- Stephens, J.M.; Schlothauer, R.C.; Morris, B.D.; Yang, D.; Fearnley, L.; Greenwood, D.R.; Loomes, K.M. Phenolic compounds and methylglyoxal in some New Zealand manuka and kanuka honeys. Food Chem. 2010, 120, 78–86. [Google Scholar] [CrossRef]
- Wang, X.; Sankarapandian, K.; Cheng, Y.; Woo, S.O.; Kwon, H.W.; Perumalsamy, H.; Ahn, Y.J. Relationship between total phenolic contents and biological properties of propolis from 20 different regions in South Korea. BMC Complement. Altern. Med. 2016, 16, 65. [Google Scholar] [CrossRef] [PubMed]
- Belina-Aldemita, M.D.; Opper, C.; Schreiner, M.; D’Amico, S. Nutritional composition of pot-pollen produced by stingless bees (Tetragonula biroi Friese) from the Philippines. J. Food Compos. Anal. 2019, 82, 103215. [Google Scholar] [CrossRef]
- Belina-Aldemita, M.D.; Schreiner, M.; D’Amico, S. Characterization of phenolic compounds and antioxidative potential of pot-pollen produced by stingless bees (Tetragonula biroi Friese) from the Philippines. J. Food Biochem. 2020, 44, e13102. [Google Scholar] [CrossRef] [PubMed]
- Cumbao, J.L.T.; Alvarez, P.L.J.; Belina-Aldemita, M.D.; Micor, J.R.L.; Angelia, M.R.N.; Manila-Fajardo, A.C.; Cervancia, C.R. Total phenolics, total flavonoids, antioxidant activity and antibacterial property of propolis produced by the stingless bee, Tetragonula biroi (Friese), from Laguna and Quezon, Philippines. Philipp. Entomol. 2016, 30, 63–74. [Google Scholar]
- Barrera, W.B.J.; Brosas, J.V.; Sacil, M.D. Pollen sources of Tetragonula biroi (Friese, 1898) (Hymenoptera: Apidae, Meliponini) in two agroecosystems in Nagcarlan, Laguna, Philippines. Palynology 2020, 1–9. [Google Scholar] [CrossRef]
- Manila-Fajardo, A.C. Pollen Contents of Apis mellifera Linn. Honey from Davao City, Philippines. J. Nat. Stud. 2012, 11, 96–102. [Google Scholar]
- Manila-Fajardo, A.C.; Cervancia, C.R. Performance of honey bees (Apis mellifera L.) In three ecosystems in Laguna, Philippines. Philipp. Agric. Sci. 2003, 86, 146–157. [Google Scholar]
- Johnston, M.; McBride, M.; Dahiya, D.; Owusu-Apenten, R.; Nigam, P.S. Antibacterial activity of Manuka honey and its components: An overview. AIMS Microbiol. 2018, 4, 655–664. [Google Scholar] [CrossRef]
- Oelschlaegel, S.; Gruner, M.; Wang, P.N.; Boettcher, A.; Koelling-Speer, I.; Speer, K. Classification and characterization of manuka honeys based on phenolic compounds and methylglyoxal. J. Agric. Food Chem. 2012, 60, 7229–7237. [Google Scholar] [CrossRef] [PubMed]
- Montenegro-Burke, J.R.; Guijas, C.; Siuzdak, G. METLIN: A Tandem Mass Spectral Library of Standards. Methods Mol. Biol. 2020, 2104, 149–163. [Google Scholar] [PubMed]
- Zulkhairi Amin, F.A.; Sabri, S.; Mohammad, S.M.; Ismail, M.; Chan, K.W.; Ismail, N.; Norhaizan, M.E.; Zawawi, N. Therapeutic properties of stingless bee honey in comparison with European bee honey. Adv. Pharmacol. Sci. 2018, 2018, 6179596. [Google Scholar] [CrossRef] [PubMed]
- Ewnetu, Y.; Lemma, W.; Birhane, N. Antibacterial effects of Apis mellifera and stingless bees honeys on susceptible and resistant strains of Escherichia coli, Staphylococcus aureus and Klebsiella pneumoniae in Gondar, Northwest Ethiopia. BMC Complement. Altern. Med. 2013, 13, 269. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Othman, N.H. Review of the medicinal effects of tualang honey and a comparison with manuka honey. Malays. J. Med. Sci. 2013, 20, 6–13. [Google Scholar] [PubMed]
- Mirzoeva, O.K.; Grishanin, R.N.; Calder, P.C. Antimicrobial action of propolis and some of its components: The effects on growth, membrane potential and motility of bacteria. Microbiol. Res. 1997, 152, 239–246. [Google Scholar] [CrossRef]
- Jiang, L.; Li, H.; Wang, L.; Song, Z.; Shi, L.; Li, W.; Deng, X.; Wang, J. Isorhamnetin attenuates Staphylococcus aureus-induced lung cell injury by inhibiting alpha-hemolysin expression. J. Microbiol. Biotechnol. 2016, 26, 596–602. [Google Scholar] [CrossRef]
- Tagousop, C.N.; Tamokou, J.D.; Ekom, S.E.; Ngnokam, D.; Voutquenne-Nazabadioko, L. Antimicrobial activities of flavonoid glycosides from Graptophyllum grandulosum and their mechanism of antibacterial action. BMC Complement. Altern. Med. 2018, 18, 252. [Google Scholar] [CrossRef]
- Sabbagh, G.; Berakdar, N. Docking studies of flavonoid compounds as inhibitors of β-ketoacyl acyl carrier protein synthase I (Kas I) of Escherichia coli. J. Mol. Graph Model. 2015, 61, 214–223. [Google Scholar] [CrossRef]
- Mavric, E.; Wittmann, S.; Barth, G.; Henle, T. Identification and quantification of methylglyoxal as the dominant antibacterial constituent of Manuka (Leptospermum scoparium) honeys from New Zealand. Mol. Nutr. Food Res. 2008, 52, 483–489. [Google Scholar] [CrossRef]
- Lane, J.A.; Calonne, J.; Slattery, H.; Hickey, R.M. Oligosaccharides isolated from MGO™ Manuka honey inhibit the adhesion of Pseudomonas aeruginosa, Escherichia coli O157:H7 and Staphylococcus Aureus to human HT-29 cells. Foods 2019, 8, 446. [Google Scholar] [CrossRef]
- Taylor, M.A.; Robertson, A.W.; Biggs, P.J.; Richards, K.K.; Jones, D.F.; Parkar, S.G. The effect of carbohydrate sources: Sucrose, invert sugar and components of mānuka honey, on core bacteria in the digestive tract of adult honey bees (Apis mellifera). PLoS ONE 2019, 14, e0225845. [Google Scholar] [CrossRef] [PubMed]
- Alzahrani, H.A.; Alsabehi, R.; Boukraâ, L.; Abdellah, F.; Bellik, Y.; Bakhotmah, B.A. Antibacterial and antioxidant potency of floral honeys from different botanical and geographical origins. Molecules 2012, 17, 10540–10549. [Google Scholar] [CrossRef] [PubMed]
- Maldonado-Celis, M.E.; Yahia, E.M.; Bedoya, R.; Landázuri, P.; Loango, N.; Aguillón, J.; Restrepo, B.; Guerrero Ospina, J.C. Chemical composition of mango (Mangifera indica L.) fruit: Nutritional and phytochemical compounds. Front. Plant Sci. 2019, 10, 1073. [Google Scholar] [CrossRef] [PubMed]
- Coelho, E.M.; de Souza, M.E.A.O.; Corrêa, L.C.; Viana, A.C.; de Azevêdo, L.C.; Dos Santos Lima, M. Bioactive compounds and antioxidant activity of mango peel liqueurs (Mangifera indica L.) produced by different methods of maceration. Antioxidants 2019, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Ssonko, U.; Muranga, F. Phenolic compounds identification and antioxidant activity in bananas of AAB and ABB genomes grown in Uganda. J. Food Nutr. Popul. Health 2020, 4, 17. [Google Scholar]
- Ranneh, Y.; Ali, F.; Zarei, M.; Akim, A.M.; Hamid, H.A.; Khazaai, H. Malaysian stingless bee and Tualang honeys: A comparative characterization of total antioxidant capacity and phenolic profile using liquid chromatography-mass spectrometry. LWT 2018, 89, 1–9. [Google Scholar] [CrossRef]
- Tran, T.D.; Ogbourne, S.M.; Brooks, P.R.; Sánchez-Cruz, N.; Medina-Franco, J.L.; Quinn, R.J. Lessons from exploring chemical space and chemical diversity of propolis components. Int. J. Mol. Sci. 2020, 21, 4988. [Google Scholar] [CrossRef]
- Popova, M.; Trusheva, B.; Bankova, V. Propolis of stingless bees: A phytochemist’s guide through the jungle of tropical biodiversity. Phytomedicine 2019, 153098. [Google Scholar] [CrossRef]
- de Paula, G.T.; Menezes, C.; Pupo, M.T.; Rosa, C.A. Stingless bees and microbial interactions. Curr. Opin. Insect. Sci. 2020, 44, 41–47. [Google Scholar] [CrossRef]
- Massaro, F.C.; Brooks, P.R.; Wallace, H.M.; Russell, F.D. Cerumen of Australian stingless bees (Tetragonula carbonaria): Gas chromatography-mass spectrometry fingerprints and potential anti-inflammatory properties. Naturwissenschaften 2011, 98, 329–337. [Google Scholar] [CrossRef]
- Al-Hatamleh, M.A.I.; Boer, J.C.; Wilson, K.L.; Plebanski, M.; Mohamud, R.; Mustafa, M.Z. Antioxidant-based medicinal properties of stingless bee products: Recent progress and future directions. Biomolecules 2020, 10, 923. [Google Scholar] [CrossRef] [PubMed]
- Freitas, M.O.; Ponte, F.A.F.; Lima, M.A.S.; Silveira, E.R. Flavonoids and triterpenes from the nest of the stingless bee Trigiona spinipes. J. Braz. Chem. Soc. 2008, 19, 532–535. [Google Scholar] [CrossRef]
- Souza, E.; Menezes, C.; Flach, A. Stingless bee honey (Hymenoptera, Apidae, Meliponini): A review of quality control, chemical profile, and biological potential. Apidologie 2021, 1–20. [Google Scholar] [CrossRef]
- Miyata, R.; Sahlan, M.; Ishikawa, Y.; Hashimoto, H.; Honda, S.; Kumazawa, S. Propolis components from stingless bees collected on South Sulawesi, Indonesia, and their xanthine oxidase inhibitory activity. J. Nat. Prod. 2019, 82, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Kustiawan, P.M.; Phuwapraisirisan, P.; Puthong, S.; Palaga, T.; Arung, E.T.; Chanchao, C. Propolis from the stingless bee Trigona incisa from East Kalimantan, Indonesia, induces in vitro cytotoxicity and apoptosis in cancer cell lines. Asian Pac. J. Cancer Prev. 2015, 16, 6581–6589. [Google Scholar] [CrossRef] [PubMed]
- Massaro, C.F.; Katouli, M.; Grkovic, T.; Vu, H.; Quinn, R.J.; Heard, T.A.; Carvalho, C.; Manley-Harris, M.; Wallace, H.M.; Brooks, P. Anti-staphylococcal activity of C-methyl flavanones from propolis of Australian stingless bees (Tetragonula carbonaria) and fruit resins of Corymbia torelliana (Myrtaceae). Fitoterapia 2014, 95, 247–257. [Google Scholar] [CrossRef] [PubMed]
- Ferreres, F.; Tomáas-Barberáan, F.A.; Gil, M.A.I.; Tomáas-Lorente, F. An HPLC technique for flavonoid analysis in honey. J. Sci. Food. Agric. 1991, 56, 49–56. [Google Scholar] [CrossRef]
- Fitsiou, E.; Mitropoulou, G.; Spyridopoulou, K.; Tiptiri-Kourpeti, A.; Vamvakias, M.; Bardouki, H.; Panayiotidis, M.; Galanis, A.; Kourkoutas, Y.; Chlichlia, K.; et al. Phytochemical profile and evaluation of the biological activities of essential oils derived from the Greek aromatic plant species Ocimum basilicum, Mentha spicata, Pimpinella anisum and Fortunella margarita. Molecules 2016, 21, 1069. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rangel, J.; Benavides, J.; Heredia, J.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D. The Folin-Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5, 5990–5999. [Google Scholar] [CrossRef]
- Kumaravelu, C.; Gopal, A. Detection and quantification of adulteration in honey through near infrared spectroscopy. Int. J. Food Prop. 2015, 18, 1930–1935. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S.; Paduret, S. Honey adulteration detection using raman spectroscopy. Food Anal. Methods 2018, 11, 959–968. [Google Scholar] [CrossRef]
- Rios-Corripio, M.; Rios-Leal, E.; Rojas-López, M.; Delgado-Macuil, R. FTIR characterization of Mexican honey and its adulteration with sugar syrups by using chemometric methods. J. Phys. Conf. Ser. 2011, 274, 012098. [Google Scholar] [CrossRef]
- Lu, X.; Ross, C.F.; Powers, J.R.; Aston, D.E.; Rasco, B.A. Determination of total phenolic content and antioxidant activity of garlic (Allium sativum) and elephant garlic (Allium ampeloprasum) by attenuated total reflectance-Fourier transformed infrared spectroscopy. J. Agric. Food Chem. 2011, 59, 5215–5221. [Google Scholar] [CrossRef] [PubMed]
- Dalisay, D.S.; Williams, D.E.; Wang, X.L.; Centko, R.; Chen, J.; Andersen, R.J. Marine sediment-derived Streptomyces bacteria from British Columbia, Canada are a promising microbiota resource for the discovery of antimicrobial natural products. PLoS ONE 2013, 8, e77078. [Google Scholar] [CrossRef]
- Saldanha, A.J. Java Treeview—Extensible visualization of microarray data. Bioinformatics 2004, 20, 3246–3248. [Google Scholar] [CrossRef]
| Honey Sample (Code) | Floral Source | Geographical Origin | Bee Species |
|---|---|---|---|
| MGO 550 a | Manuka tree | New Zealand | Apis mellifera |
| UMF 15 a | Manuka tree | New Zealand | Apis mellifera |
| NL 01 | Sunflower | NL, Philippines | Apis mellifera |
| NL 02 | Sunflower (mostly), trumpet flowers, squash, calliandra, coffee | NL, Philippines | Apis mellifera |
| NM 01 | Wild flowers, falcata | NM, Philippines | Apis mellifera |
| NM 02 | Cassava, minimal sunflowers | NM, Philippines | Apis mellifera |
| NM 03 | Pineapples | NM, Philippines | Apis mellifera |
| NM 04 | Pineapples | NM, Philippines | Apis mellifera |
| NM 05 | Pineapples, minimal sunflowers | NM, Philippines | Apis mellifera |
| SEM 01 | Banana trees, coconuts, palm trees, Philippine lime | SM, Philippines | Apis mellifera |
| SL 01 | Coconut, bananas, mangoes | SL, Philippines | Tetragonula biroi |
| SL 02 | Coconut, acacia, tamarind, sapodilla fruit, mangrove, kerson fruit | SL, Philippines | Apis mellifera |
| SL 03 | Coconut, mahogany, mangoes, dragon fruit, bamboo, peanut grass, other flowering plants | SL, Philippines | Apis mellifera |
| SL 04 | Acacia, mangoes, coconut, tamarind, avocadoes, coffee | SL, Philippines | Apis mellifera |
| SL 05 | Kerson fruit, mangoes, coconuts, calamansi, mangroves, papaya | SL, Philippines | Apis mellifera |
| WV 01 | Mahogany, Philippine lime, cucumber tree, yellow bell plant | WV, Philippines | Apis mellifera |
| WV 02 | Banana, coconut, cosmos flower, nipa palm and mangoes | WV, Philippines | Apis mellifera |
| Honey Samples a | Antibiotic Activity Zone of Inhibition, mm | ||||
|---|---|---|---|---|---|
| S. aureus ATCC BAA-44 | S. aureus ATCC 25923 | S. aureus ATCC 6538 | S. saprophyticus Clinical Isolate | S. epidermidis Clinical Isolate | |
| Tetracycline (standard) b | 22.0 ± 0.7 | 26.5 ± 1.4 | 24 ± 0.0 | 33 ± 2.8 | 36.5 ± 2.8 |
| DMSO c | (-) | (-) | (-) | (-) | (-) |
| Manuka health MGO 550 d | 2.5 ± 0.7 | (-) | (-) | 6.0 ± 0.0 | 2.5 ± 0.7 |
| Comvita UMF 15 d | 2.0 ± 0.0 | (-) | (-) | 5.8 ± 1.1 | 2.5 ± 0.7 |
| NM 01 | (-) | (-) | 1.5 ± 2.1 | 4.0 ± 1.4 | (-) |
| NM 02 | (-) | (-) | (-) | 4.5 ± 0.7 | (-) |
| NM 03 | (-) | (-) | (-) | 3.5 ± 0.7 | (-) |
| NM 04 | (-) | (-) | (-) | 3.5 ± 2.1 | (-) |
| NM 05 | (-) | (-) | (-) | 2.5 ± 0.7 | (-) |
| SL 01 | 12.5 ± 0.7 | 4.8 ± 0.4 | 3.5 ± 0.7 | 5.0 ± 1.4 | 4.5 ± 0.7 |
| SL 02 | (-) | (-) | (-) | 3.0 ± 1.4 | (-) |
| SL 03 | (-) | (-) | (-) | 5.0 ± 0.0 | (-) |
| SL 04 | (-) | (-) | (-) | 5.0 ± 1.4 | (-) |
| SL 05 | (-) | (-) | (-) | 3.3 ± 0.4 | (-) |
| SEM 01 | (-) | (-) | 2.5 ± 0.7 | 2.5 ± 0.7 | (-) |
| NL 01 | (-) | (-) | (-) | 4.3 ± 0.4 | (-) |
| NL 02 | (-) | (-) | (-) | (-) | (-) |
| WV 01 | (-) | (-) | 2.0 ± 0.0 | (-) | (-) |
| WV 02 | (-) | (-) | (-) | (-) | (-) |
| % Hit rate (Philippine honey n = 15) | 7 | 7 | 27 | 80 | 7 |
| Honey Samples | μg GAE/mg a | μg QE/mg b |
|---|---|---|
| MGO 550 | 184.655 ± 5.226 | 21.857 ± 0.006 |
| UMF 15 | 170.500 ± 15.144 | 26.857 ± 0.009 |
| NL 01 | 204.872 ± 21.868 | 19.000 ± 0.016 |
| NL 02 | 233.246 ± 3.918 | 8.762 ± 0.002 |
| NM 01 | 200.402 ± 3.788 | 10.667 ± 0.003 |
| NM 02 | 236.192 ± 1.371 | 6.857 ± 0.001 |
| NM 03 | 180.955 ± 23.681 | 9.714 ± 0.001 |
| NM 04 | 165.884 ± 0.222 | 7.333 ± 0.002 |
| NM 05 | 235.739 ± 1.278 | 10.905 ± 0.005 |
| SEM 01 | 224.221 ± 13.914 | 13.048 ± 0.001 |
| SL 01 | 227.128 ± 11.064 | 216.143 ± 0.016 |
| SL 02 | 224.809 ± 3.190 | 14.714 ± 0.003 |
| SL 03 | 192.081 ± 5.431 | 12.571 ± 0.004 |
| SL 04 | 229.846 ± 0.222 | 21.619 ± 0.004 |
| SL 05 | 234.569 ± 3.063 | 9.952 ± 0.002 |
| WV 01 | 192.973 ± 15.720 | 11.143 ± 0.002 |
| WV 02 | 218.138 ± 3.909 | 8.048 ± 0.001 |
| Sample/Fraction | Visible Color Profile | Yield from 510.0 mg SL 01 Honey Crude Extract mg | % Growth Inhibition against Multidrug-Resistant S. aureus ATCC BAA-44 |
|---|---|---|---|
| Tetracycline a | - | - | 99.2719 ± 0.03 |
| DMSO b | - | - | no activity |
| Crude extract | - | - | 94.25 ± 0.24 |
| 1 | colorless | 5.1 | no activity |
| 2 | red orange | 23.5 | no activity |
| 3 | yellow orange | 149.5 | no activity |
| 4 | green orange | 219.2 | 72.5 ± 4.38 c |
| 5 | yellow green | 41.6 | no activity |
| 6 | colorless | 39.7 | no activity |
| 7 | light yellow | 24.1 | 82.1 ± 9.50 c |
| 8 | light yellow green | 7.1 | no activity |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suarez, A.F.L.; Tirador, A.D.G.; Villorente, Z.M.; Bagarinao, C.F.; Sollesta, J.V.N.; Dumancas, G.G.; Sun, Z.; Zhan, Z.Q.; Saludes, J.P.; Dalisay, D.S. The Isorhamnetin-Containing Fraction of Philippine Honey Produced by the Stingless Bee Tetragonula biroi Is an Antibiotic against Multidrug-Resistant Staphylococcus aureus. Molecules 2021, 26, 1688. https://doi.org/10.3390/molecules26061688
Suarez AFL, Tirador ADG, Villorente ZM, Bagarinao CF, Sollesta JVN, Dumancas GG, Sun Z, Zhan ZQ, Saludes JP, Dalisay DS. The Isorhamnetin-Containing Fraction of Philippine Honey Produced by the Stingless Bee Tetragonula biroi Is an Antibiotic against Multidrug-Resistant Staphylococcus aureus. Molecules. 2021; 26(6):1688. https://doi.org/10.3390/molecules26061688
Chicago/Turabian StyleSuarez, Angelica Faith L., April Dawn G. Tirador, Zenith M. Villorente, Cathrina F. Bagarinao, Jan Vincent N. Sollesta, Gerard G. Dumancas, Zhe Sun, Zhao Qi Zhan, Jonel P. Saludes, and Doralyn S. Dalisay. 2021. "The Isorhamnetin-Containing Fraction of Philippine Honey Produced by the Stingless Bee Tetragonula biroi Is an Antibiotic against Multidrug-Resistant Staphylococcus aureus" Molecules 26, no. 6: 1688. https://doi.org/10.3390/molecules26061688
APA StyleSuarez, A. F. L., Tirador, A. D. G., Villorente, Z. M., Bagarinao, C. F., Sollesta, J. V. N., Dumancas, G. G., Sun, Z., Zhan, Z. Q., Saludes, J. P., & Dalisay, D. S. (2021). The Isorhamnetin-Containing Fraction of Philippine Honey Produced by the Stingless Bee Tetragonula biroi Is an Antibiotic against Multidrug-Resistant Staphylococcus aureus. Molecules, 26(6), 1688. https://doi.org/10.3390/molecules26061688