Crystals at a Carrefour on the Way through the Phase Space: A Middle Path
Abstract
1. Introduction
2. Results and Discussion
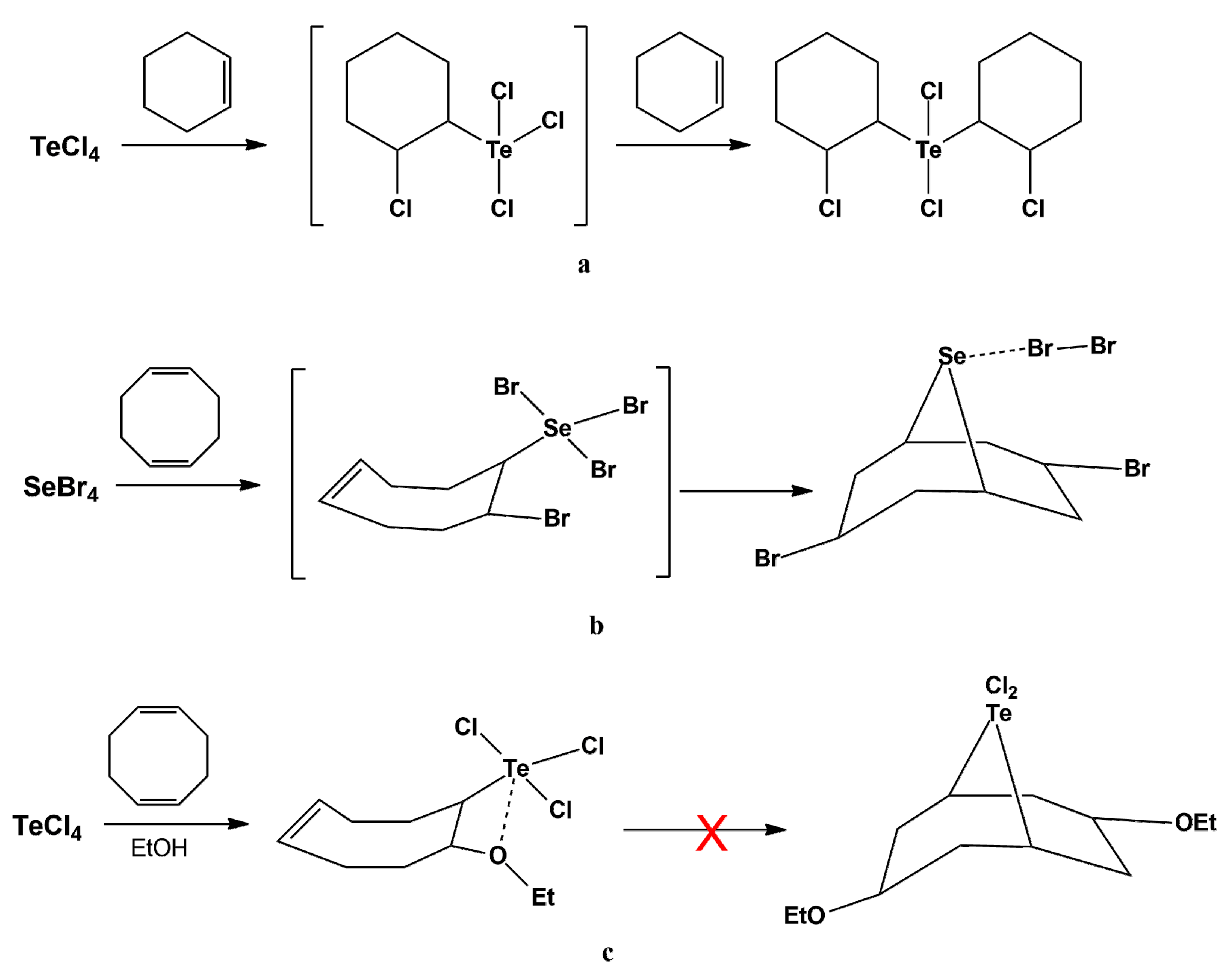
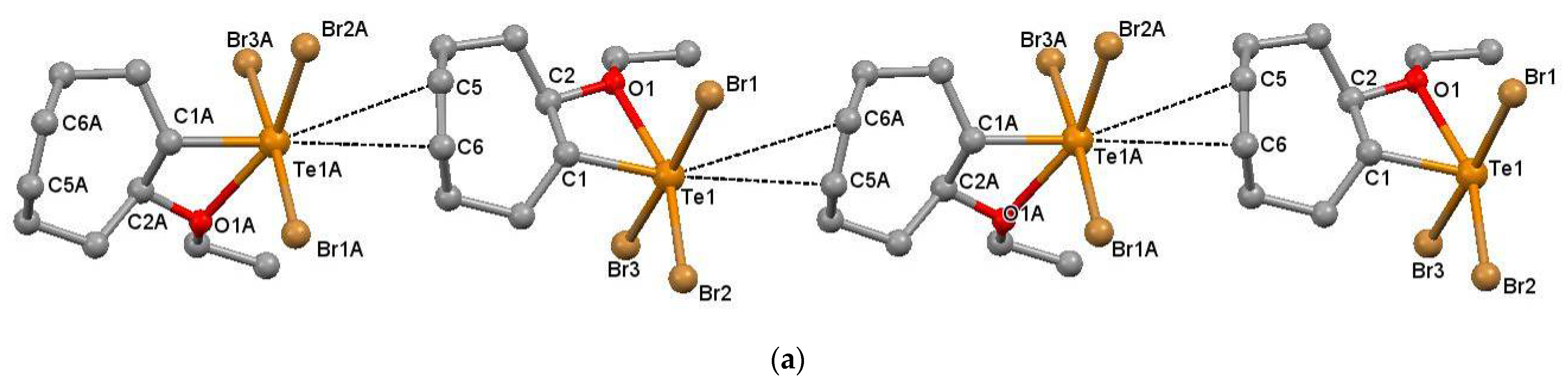
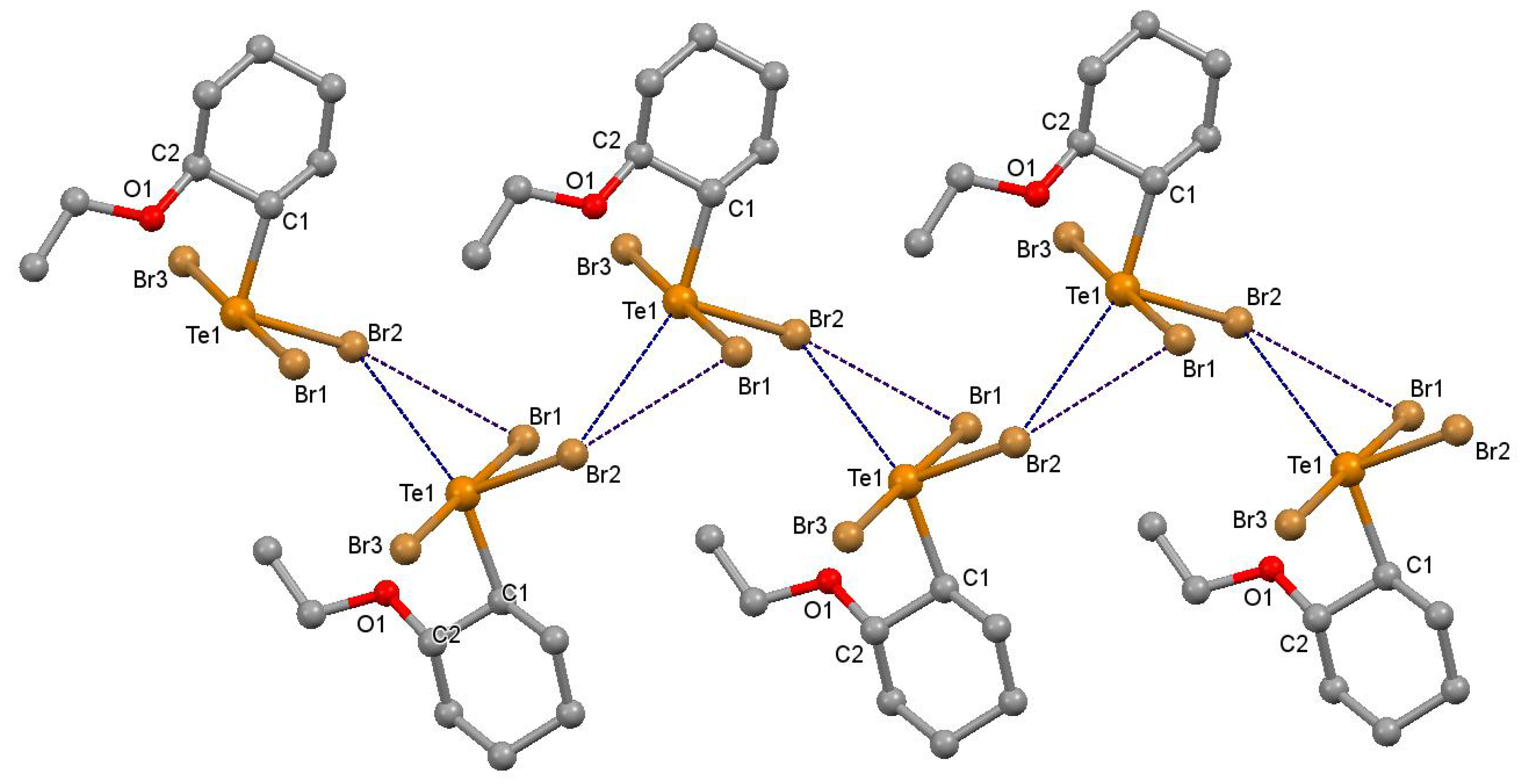
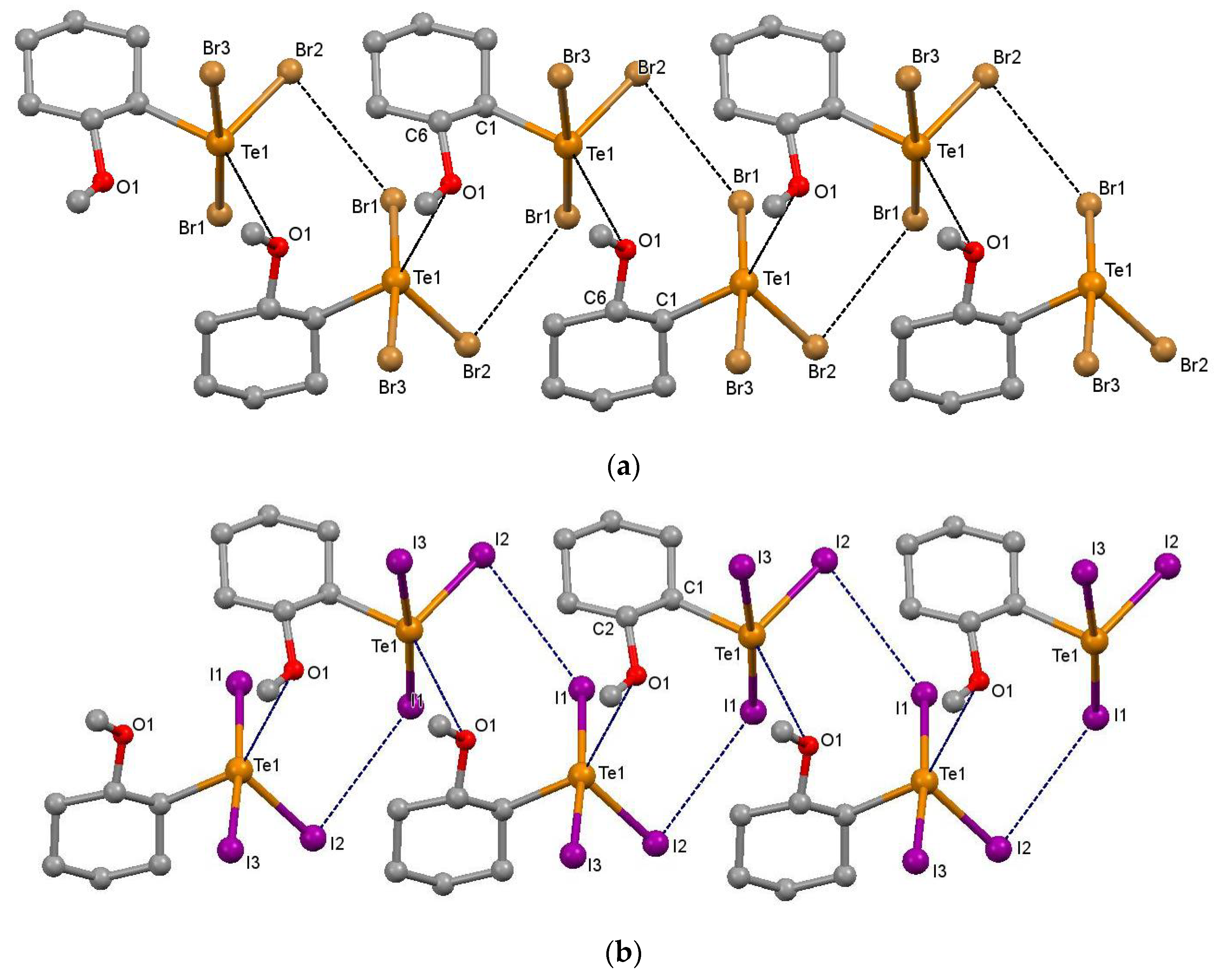
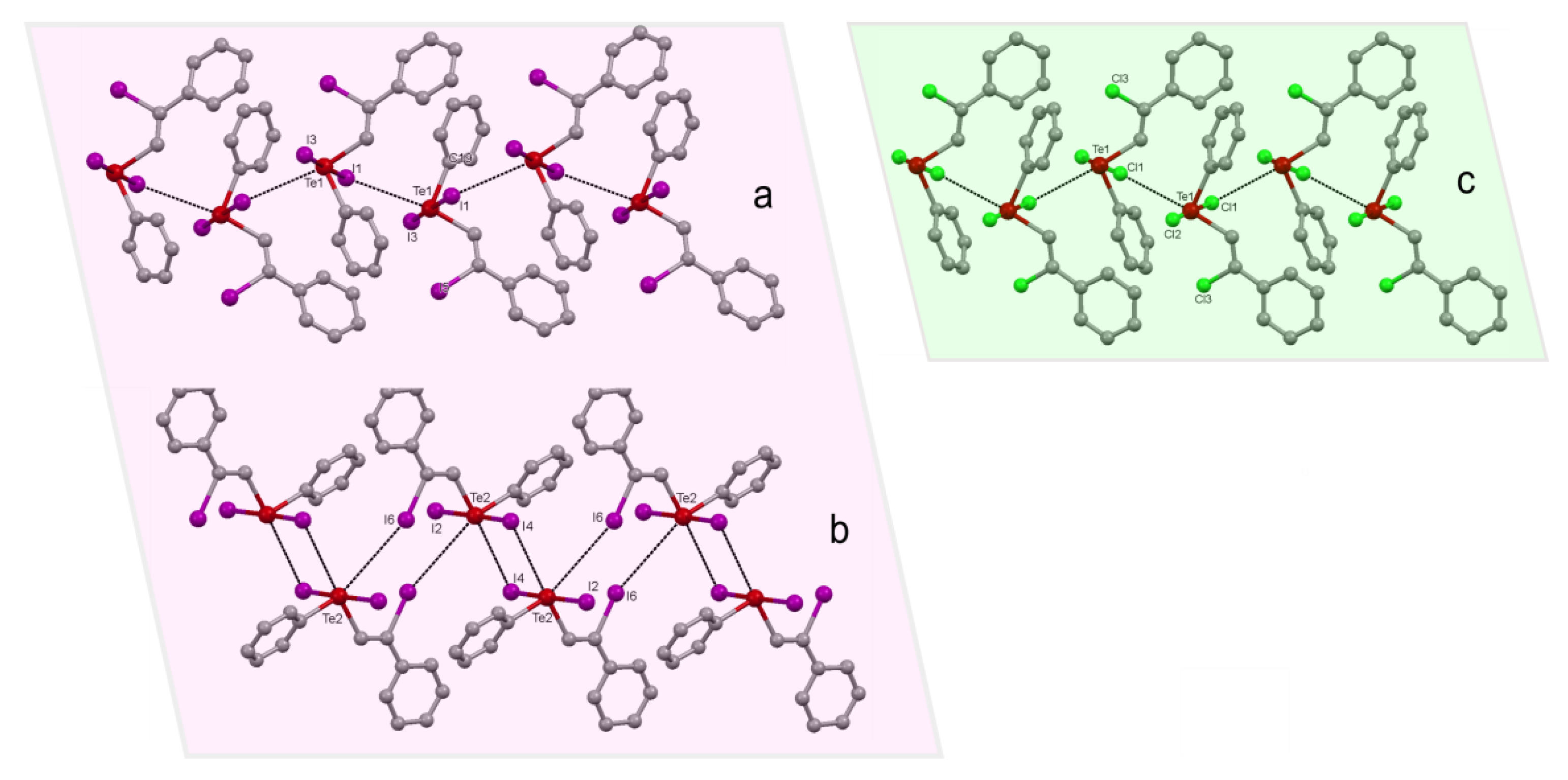
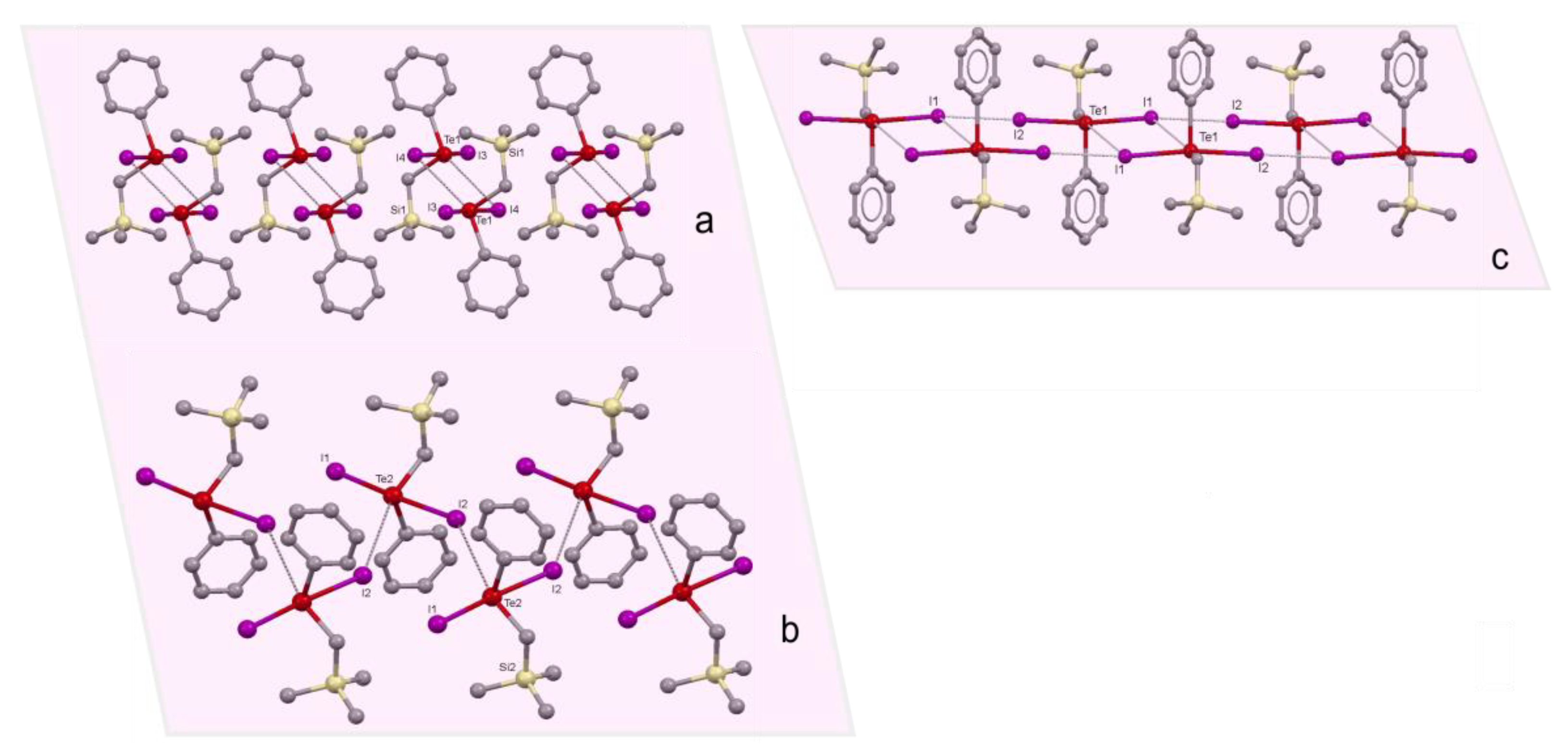
2.1. Supramolecular Architectures of Cyclic Alkoxy-Organotellurium Trihalides
2.2. Polymorphism of Cyclo-C6H10(OEt)TeI3
2.3. Energy Frameworks
3. Materials and Methods
3.1. X-ray Crystallography
3.2. Computational Details
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Hoffmann, R. At First Sight. Cryst. Growth Des. 2001, 1, 3. Available online: https://pubs.acs.org/doi/10.1021/cg0000058 (accessed on 11 March 2021).
- Vogel, L.; Wonner, P.; Huber, S.M. Chalcogen Bonding: An Overview. Angew. Chem. Int. Ed. 2019, 58, 1880–1891. Available online: http://doi.wiley.com/10.1002/anie.201809432 (accessed on 11 March 2021). [CrossRef]
- Raatikainen, K.; Rissanen, K. Hierarchical halogen bonding induces polymorphism. CrystEngComm 2009, 11, 750–752. Available online: http://xlink.rsc.org/?DOI=b821085n (accessed on 11 March 2021). [CrossRef]
- Haddad, S.F.; Willett, R.D.; Twamley, B. The Role of Hydrogen Bonding and Halogen Bonding in the Polymorphic Structures of 3,5-Dibromo-2,6-diaminopyridinium Bromide. J. Chem. Crystallogr. 2010, 40, 902–908. Available online: https://link.springer.com/article/10.1007/s10870-010-9760-4 (accessed on 11 March 2021). [CrossRef]
- Torubaev, Y.; Mathur, P.; Pasynskii, A.A. Regio- and stereo-specific addition of organotellurium trihalides to ferrocenylacetylene: Molecular and crystal structure of (Z)-halovinyl organotellurium dihalides. J. Organomet. Chem. 2010, 695, 1300–1306. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0022328X10001099 (accessed on 11 March 2021). [CrossRef]
- Torubaev, Y.V.; Pasynskii, A.A.; Pavlova, A.V.; Shaikh, M.M. Crystal structures of the products of unusual interactions between organotellurides and iodoacetylenes. Mendeleev Commun. 2017, 27, 141–143. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0959943617300597 (accessed on 11 March 2021). [CrossRef]
- Torubaev, Y.V.; Pavlova, A.V.; Pasynskii, A.A. Metal-metal bond cleavage in [Cp(CO)2Fe-Fe(CO)2Cp] under the action of organotellurium(IV)tribromides. Russ. J. Coord. Chem. 2012, 38, 219–223. Available online: http://link.springer.com/10.1134/S1070328412020091 (accessed on 11 March 2021). [CrossRef]
- Converso, A.; Burow, K.; Marzinzik, A.; Sharpless, K.B.; Finn, M.G. 2,6-Dichloro-9-thiabicyclo [3.3.1]nonane: A Privileged, Bivalent Scaffold for the Display of Nucleophilic Components. J. Org. Chem. 2001, 66, 4386–4392. Available online: https://pubs.acs.org/doi/10.1021/jo015632y (accessed on 11 March 2021). [CrossRef]
- Achampong, A.; Parkins, A.W. Reaction of tellurium tetrachloride with cyclohexene and the crystal structure of racemic bis(trans-2-chlorocyclohexyl)tellurium dichloride. J. Chem. Soc. Dalton Trans. 1997, 4367–4369. Available online: http://xlink.rsc.org/?DOI=a704139j (accessed on 11 March 2021).
- Bergman, J.; Engman, L. Tellurium in organic synthesis. J. Org. Chem. 1979, 181, 335–347. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0022328X00828260 (accessed on 11 March 2021). [CrossRef]
- Torubaev, Y.V.; Pasynskii, A.A.; Pavlova, A.V. Diphenyldichalcogenide complexes of iron, chromium and rhenium carbonyls. Russ. J. Coord. Chem. 2012, 38, 724–732. Available online: http://link.springer.com/10.1134/S1070328412120056 (accessed on 11 March 2021). [CrossRef]
- Alcock, N.W.; Harrison, W.D. Secondary bonding. Part 12. Aryltellurium iodides: Crystal and molecular structures of cis- and trans-phenyltellurium(IV) tri-iodide and two modifications of diphenyltellurium(IV) di-iodide. J. Chem. Soc. Dalton Trans. 1984, 869–875. Available online: http://xlink.rsc.org/?DOI=dt9840000869 (accessed on 11 March 2021).
- Sudha, N.; Singh, H.B. Intramolecular coordination in tellurium chemistry. Coord. Chem. Rev. 1994, 135–136, 469–515. Available online: https://linkinghub.elsevier.com/retrieve/pii/0010854594800758 (accessed on 11 March 2021). [CrossRef]
- Torubaev, Y.V.; Dolgushin, F.M.; Skabitsky, I.V.; Popova, A.E. Isomorphic substitution in molecular crystals and geometry of hypervalent tellurium: Comments inspired by a case study of RMeTeI2 and [RMe2Te]+I− (R = Ph, Fc). N. J. Chem. 2019, 43, 12225–12232. Available online: http://xlink.rsc.org/?DOI=C9NJ02318F (accessed on 11 March 2021). [CrossRef]
- Zukerman-Schpector, J.; Haiduc, I. Tellurium π-aryl interactions: A new bonding motif for supramolecular self-assembly and crystal engineering. CrystEngComm 2002, 4, 178–193. Available online: http://xlink.rsc.org/?DOI=B201969H (accessed on 11 March 2021). [CrossRef]
- Schulte, J.H.; Werz, D.B.; Rominger, F.; Gleiter, R. Syntheses and solid state structures of cyclic diynes with two chalcogen centres ? a competition between weak interactions. Org. Biomol. Chem. 2003, 1, 2788. Available online: http://xlink.rsc.org/?DOI=b303653g (accessed on 11 March 2021). [CrossRef]
- Sabir Ali, M.E.; Azad Malik, M.; Smith, B.C. 2-Alkoxy-trans-cycloalkyltellurium(IV) trihalides. Inorg. Chim. Acta 1989, 162, 157–160. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0020169300831357 (accessed on 11 March 2021). [CrossRef]
- Dakternieks, D.; O’Connell, J.; Tiekink, E.R.T. Synthesis and crystal structures of the monomeric organotellurium(IV) trihalides: Trans-2-ethoxy-cyclohexyl-tellurium(IV) trichloride, trichloro(2-chlorobicyclo[2.2.1]hept-7-yl)-λ4-tellurane, and mesityltellurium(IV) tribromide. J. Org. Chem. 2000, 598, 49–54. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0022328X99006695 (accessed on 11 March 2021). [CrossRef]
- McAdam, C.J.; Cameron, S.A.; Hanton, L.R.; Manning, A.R.; Moratti, S.C.; Simpson, J. Probing CH-π(alkyne) interactions in a series of ethynylferrocenes. CrystEngComm 2012, 14, 4369–4383. [Google Scholar] [CrossRef]
- Torubaev, Y.V.; Pasynskii, A.A.; Mathur, P. (Z)-diiodo(2-iodo-2-phenylvinyl)(phenyl)tellurium PhIC=CHTeI2Ph: Synthesis and complexing properties in a reaction with iron pentacarbonyl. Russ. J. Coord. Chem. 2008, 34, 805–810. [Google Scholar] [CrossRef]
- Zukerman-Schpector, J.; Tiekink, E.R.T. What is a co-crystal? Z. Für Krist. 2007, 9, 833–834. Available online: http://www.degruyter.com/view/j/zkri.2008.223.issue-3/zkri.2008.0021/zkri.2008.0021.xml (accessed on 11 March 2021). [CrossRef]
- Poropudas, M.J.; Vigo, L.; Oilunkaniemi, R.; Laitinen, R.S. Structural trends in TeI2RR′: Crystal structures and NMR spectra of TeI2(CH2SiMe3)2, TeI2Th(CH2SiMe3), TeI2Ph(CH2SiMe3), and TeI2Th2 (Th = 2-Thienyl, C4H3S). Heteroat. Chem. 2011, 22, 348–357, Copyright© 2021 Wiley Periodicals, Inc. Available online: https://onlinelibrary.wiley.com/doi/abs/10.1002/hc.20688 (accessed on 11 March 2021). [CrossRef]
- Aakeröy, C.B.; Panikkattu, S.; Chopade, P.D.; Desper, J. Competing hydrogen-bond and halogen-bond donors in crystal engineering. CrystEngComm 2013, 15, 3125–3136. Available online: http://xlink.rsc.org/?DOI=C2CE26747K (accessed on 11 March 2021). [CrossRef]
- Chauhan, A.K.S.; Anamika; Kumar, A.; Srivastava, R.C.; Butcher, R.J.; Jens, B.; Duthie, A. The interplay of secondary TeN, TeO, TeI and II interactions, Teπ contacts and π-stacking in the supramolecular structures of [{2-(4-nitrobenzylideneamino)-5-methyl}phenyl](4-methoxyphenyl)tellurium dihalides. J. Org. Chem. 2005, 690, 1350–1355. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0022328X04009660 (accessed on 11 March 2021).
- Bacchi, A.; Brillante, A.; Crocco, D.; Chierotti, M.R.; Della Valle, R.G.; Girlando, A.; Masino, M.; Pelagatti, P.; Venuti, E. Exploration of the polymorph landscape for 1,1,4,4-tetraphenyl-1,3-butadiene. CrystEngComm 2014, 16, 8205–8213. [Google Scholar] [CrossRef]
- Turner, M.J.; Thomas, S.P.; Shi, M.W.; Jayatilaka, D.; Spackman, M.A. Energy frameworks: Insights into interaction anisotropy and the mechanical properties of molecular crystals. Chem. Commun. (Camb) 2015, 51, 3735–3738. [Google Scholar] [CrossRef]
- Torubaev, Y.V.; Skabitsky, I.V. Halogen bonding in crystals of free 1,2-diiodo-ethene (C2H2I2) and its π-complex [CpMn(CO)2](π-C2H2I2). Z. Für Kris. Cryst. Mater. 2020, 235, 599–607. Available online: https://www.degruyter.com/document/doi/10.1515/zkri-2020-0064/html (accessed on 11 March 2021). [CrossRef]
- Torubaev, Y.V.; Rai, D.K.; Skabitsky, I.V.; Pakhira, S.; Dmitrienko, A. Energy framework approach to the supramolecular reactions: Interplay of the secondary bonding interaction in Ph2 E2 (E = Se, Te)p-I-C6F4-I co-crystals. N. J. Chem. 2019, 43, 7941–7949. Available online: http://xlink.rsc.org/?DOI=C9NJ00347A (accessed on 11 March 2021). [CrossRef]
- Torubaev, Y.V.; Skabitsky, I.V.; Saratov, G.A.; Barzilovich, P.Y. Halogen vs. ionic bonding: An unusual isomorphism between the neutral (C5Me5)2Fe/C2I2 cocrystal and ionic [(C5Me5)2Fe]Br3 crystal. Mendeleev Commun. 2021, 31, 58–61. Available online: https://linkinghub.elsevier.com/retrieve/pii/S0959943621000171 (accessed on 11 March 2021). [CrossRef]
- Torubaev, Y.V.; Skabitsky, I.V. The energy frameworks of aufbau synthon modules in 4-cyanopyridine co-crystals. CrystEngComm 2019, 21, 7057–7068. Available online: http://xlink.rsc.org/?DOI=C9CE01174A (accessed on 11 March 2021). [CrossRef]
- Torubaev, Y.V.; Skabitsky, I.V. A new supramolecular heterosynthon [C–I---O=C(carboxylate)] at work: Engineering copper acetate cocrystals. CrystEngComm 2020, 22, 6661–6673. Available online: http://xlink.rsc.org/?DOI=D0CE01093F (accessed on 11 March 2021). [CrossRef]
- Desiraju, G.R.; Vittal, J.J.; Ramanan, A. Crystal Engineering: A Textbook; Co-Published with Indian Institute of Science (IISc): Bangalore, India, 2011; ISBN1 978-981-4338-75-2. ISBN2 978-981-4338-76-9. Available online: http://www.worldscientific.com/worldscibooks/10.1142/8060 (accessed on 11 March 2021).
- Kitaigorodskii, A.I. Organic Chemical Crystallography; (Translated from the Russian); Consultants Bureau: New York, NY, USA, 1961. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Crystallogr. 2009, 42, 339–341. Available online: http://scripts.iucr.org/cgi-bin/paper?S0021889808042726 (accessed on 11 March 2021). [CrossRef]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. Available online: http://scripts.iucr.org/cgi-bin/paper?S2053229614024218 (accessed on 11 March 2021). [CrossRef] [PubMed]
- Spackman, M.A.; Jayatilaka, D. Hirshfeld surface analysis. CrystEngComm 2009, 11, 19–32. Available online: http://xlink.rsc.org/?DOI=B818330A (accessed on 11 March 2021). [CrossRef]
- Adamo, C.; Barone, V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999, 110, 6158–6170. [Google Scholar] [CrossRef]
- Riplinger, C.; Pinski, P.; Becker, U.; Valeev, E.F.; Neese, F. Sparse maps—A systematic infrastructure for reduced-scaling electronic structure methods. II. Linear scaling domain based pair natural orbital coupled cluster theory. J. Chem. Phys. 2016, 144, 024109. [Google Scholar] [CrossRef]
- Neese, F. Software update: The ORCA program system, version 4.0. WIREs Comput. Mol. Sci. 2017, 8, e1327. [Google Scholar] [CrossRef]
- Weigend, F.; Ahlrichs, R. Balanced basis sets of split valence, triple zeta valence and quadruple zeta valence quality for H to Rn: Design and assessment of accuracy. Phys. Chem. Chem. Phys. 2005, 7, 3297–3305. [Google Scholar] [CrossRef]
- Hellweg, A.; Hättig, C.; Höfener, S.; Klopper, W. Optimized accurate auxiliary basis sets for RI-MP2 and RI-CC2 calculations for the atoms Rb to Rn. Theor. Chem. Acc. 2007, 117, 587–597. [Google Scholar] [CrossRef]
- Liakos, D.G.; Sparta, M.; Kesharwani, M.K.; Martin, J.M.L.; Neese, F. Exploring the Accuracy Limits of Local Pair Natural Orbital Coupled-Cluster Theory. J. Chem. Theory Comput. 2015, 11, 1525–1539. [Google Scholar] [CrossRef]
- Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104. [Google Scholar] [CrossRef]
- Grimme, S.; Ehrlich, S.; Goerigk, L. Effect of the damping function in dispersion corrected density functional theory. J. Comput. Chem. 2011, 32, 1456–1465. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.M.; Steed, J.W. Comment on “On the presence of multiple molecules in the crystal asymmetric unit (Z′ > 1)” by Gautam, R.; Desiraju. CrystEngComm 2007, 9, 91, 328–330. Available online: http://xlink.rsc.org/?DOI=B701009E (accessed on 11 March 2021). [CrossRef]
- Desiraju, G.R. Crystal engineering: From molecule to crystal. J. Am. Chem. Soc. 2013, 135, 9952–9967. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Cabeza, A.J.; Reutzel-Edens, S.M.; Bernstein, J. Facts and fictions about polymorphism. Chem. Soc. Rev. 2015, 44, 8619–8635. [Google Scholar] [CrossRef] [PubMed]
- Cavallo, G.; Metrangolo, P.; Milani, R.; Pilati, T.; Priimagi, A.; Resnati, G.; Terraneo, G. The Halogen Bond. Chem. Rev. 2016, 116, 2478–2601. Available online: https://pubs.acs.org/doi/10.1021/acs.chemrev.5b00484 (accessed on 11 March 2021). [CrossRef] [PubMed]
- Kolář, M.; Hostaš, J.; Hobza, P. The strength and directionality of a halogen bond are co-determined by the magnitude and size of the σ-hole. Phys. Chem. Chem. Phys. 2014, 16, 9987–9996. Available online: http://xlink.rsc.org/?DOI=C3CP55188A (accessed on 11 March 2021). [CrossRef]
- Wang, W.; Ji, B.; Zhang, Y. Chalcogen Bond: A Sister Noncovalent Bond to Halogen Bond. J. Phys. Chem. A 2009, 113, 8132–8135. Available online: https://pubs.acs.org/doi/10.1021/jp904128b (accessed on 11 March 2021). [CrossRef]
- Das, D.; Banerjee, R.; Mondal, R.; Howard, J.A.K.; Boese, R.; Desiraju, G.R.; Synthon evolution and unit cell evolution during crystallisation. A study of symmetry-independent molecules (Z′ > 1) in crystals of some hydroxy compounds. Chem. Commun. 2006, 555–557. Available online: http://xlink.rsc.org/?DOI=B514076E (accessed on 11 March 2021).
- Desiraju, G.R. On the presence of multiple molecules in the crystal asymmetric unit (Z′ > 1). CrystEngComm 2007, 9, 91–92. Available online: http://xlink.rsc.org/?DOI=B614933B (accessed on 11 March 2021). [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torubaev, Y.V.; Skabitsky, I.V. Crystals at a Carrefour on the Way through the Phase Space: A Middle Path. Molecules 2021, 26, 1583. https://doi.org/10.3390/molecules26061583
Torubaev YV, Skabitsky IV. Crystals at a Carrefour on the Way through the Phase Space: A Middle Path. Molecules. 2021; 26(6):1583. https://doi.org/10.3390/molecules26061583
Chicago/Turabian StyleTorubaev, Yury V., and Ivan V. Skabitsky. 2021. "Crystals at a Carrefour on the Way through the Phase Space: A Middle Path" Molecules 26, no. 6: 1583. https://doi.org/10.3390/molecules26061583
APA StyleTorubaev, Y. V., & Skabitsky, I. V. (2021). Crystals at a Carrefour on the Way through the Phase Space: A Middle Path. Molecules, 26(6), 1583. https://doi.org/10.3390/molecules26061583