Expanding the Averievite Family, (MX)Cu5O2(T5+O4)2 (T5+ = P, V; M = K, Rb, Cs, Cu; X = Cl, Br): Synthesis and Single-Crystal X-ray Diffraction Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis
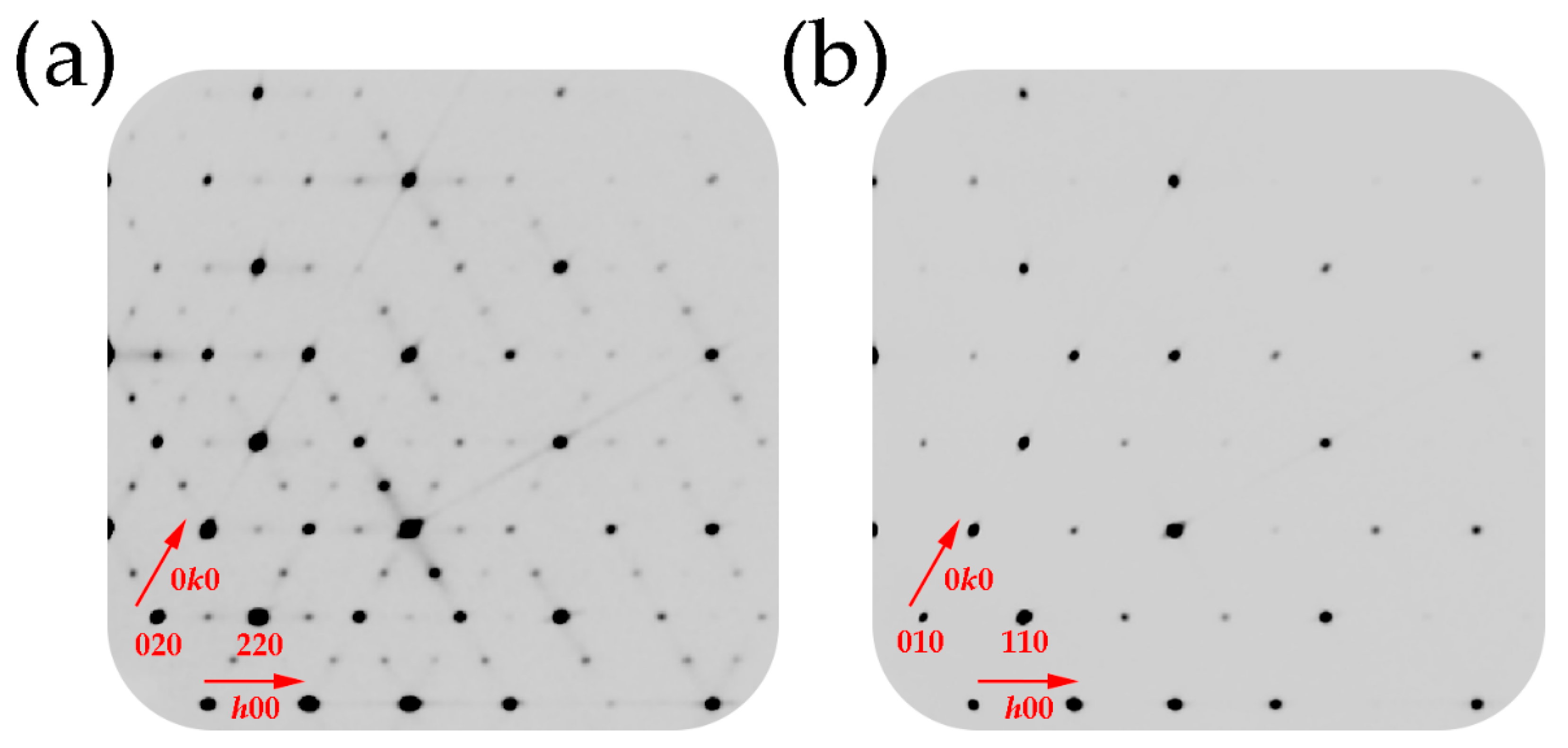
2.2. Single-Crystal X-ray Diffraction Study
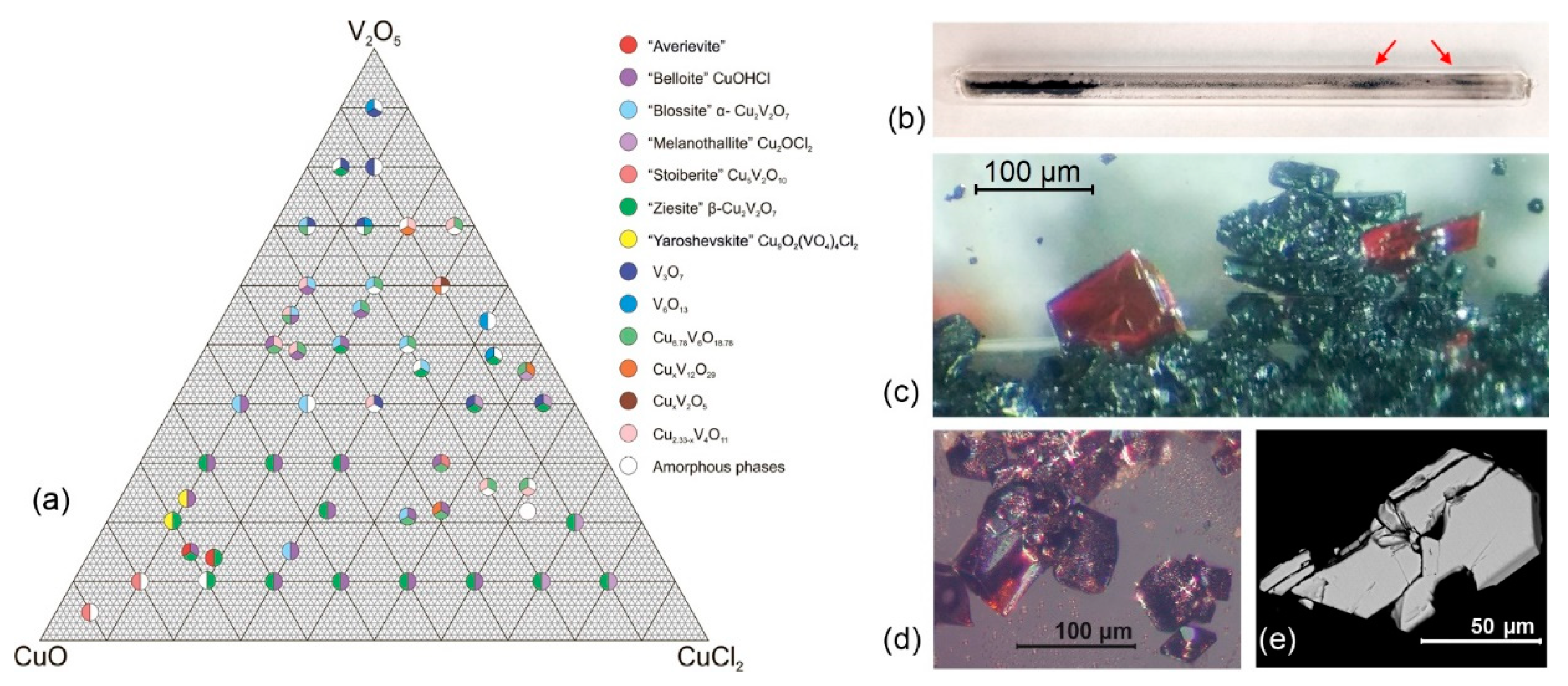
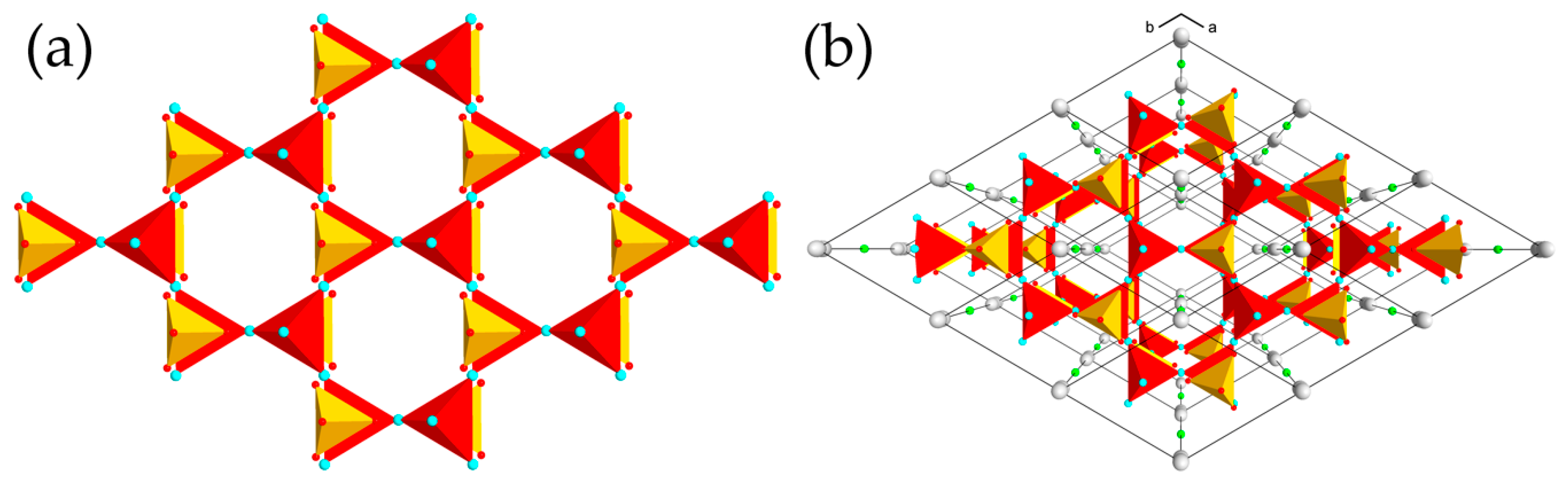
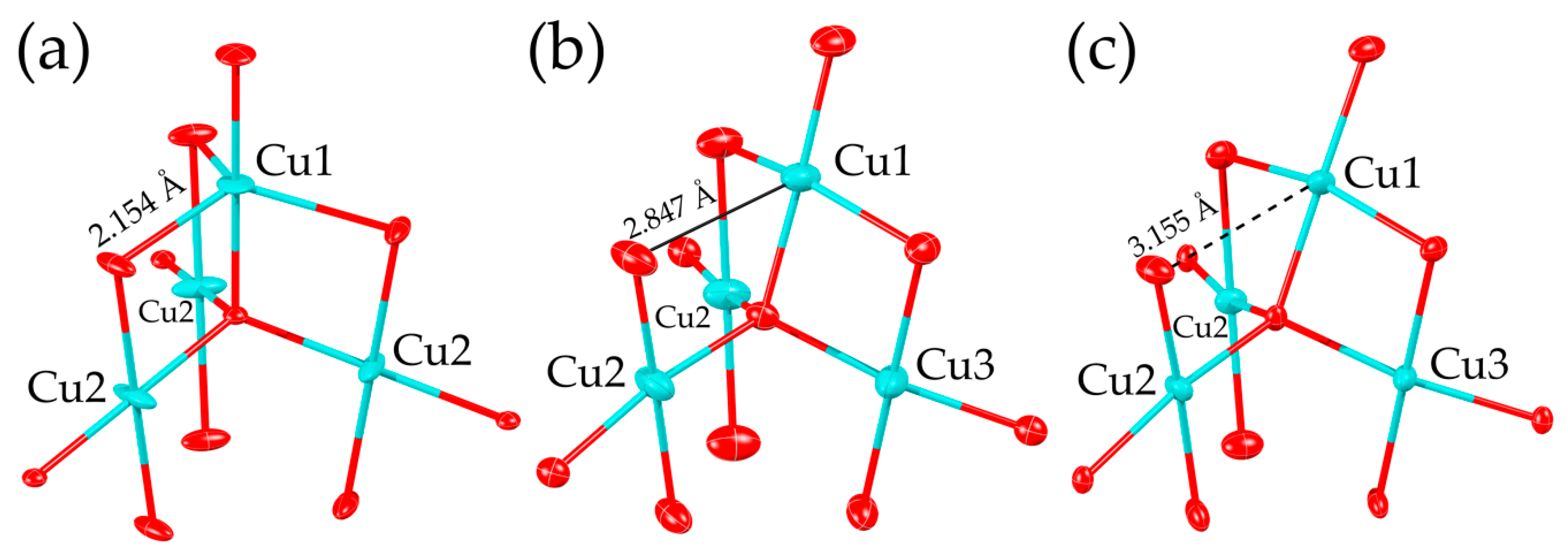
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
Appendix A
| Compound 1 | |||
| Bond distances, Å | |||
| Cu1—Cu2 | 2.9637 (5) | Cu2—O3 | 1.8545 (9) |
| Cu1—O1 | 1.840 (4) | P1—O1 | 1.505 (4) |
| Cu1—O2 | 2.154 (2) | P1—O2 | 1.541 (2) |
| Cu1—O3 | 1.863 (3) | P1—O2 | 1.541 (2) |
| Cu2—O2 | 2.0251 (19) | P1—O2 | 1.541 (2) |
| Angles, ° | |||
| Cu2—O3—Cu1 | 105.72 (10) (×3) | Cu2—O3—Cu2 | 112.95 (8) (×3) |
| Compound 2 | |||
| Bond distances, Å | |||
| Cu1—O3 | 2.066 (3) | Cu4—O3 | 2.005 (3) |
| Cu1—O5 | 1.874 (5) | Cu4—O6 | 2.003 (3) |
| Cu1—O7 | 1.911 (5) | Cu4—O7 | 1.8747 (10) |
| Cu2—O1 | 2.069 (3) | Cu4—O8 | 1.874 (2) |
| Cu2—O2 | 2.061 (3) | V1—O1 | 1.720 (3) |
| Cu2—O4 | 1.876 (3) | V1—O2 | 1.731 (3) |
| Cu2—O6 | 2.066 (3) | V1—O3 | 1.726 (3) |
| Cu2—O8 | 1.906 (3) | V1—O4 | 1.667 (3) |
| Cu3—O1v | 2.004 (3) | V2—O5 | 1.668 (5) |
| Cu3—O2 | 2.001 (3) | V2—O6 | 1.727 (3) |
| Cu3—O8 | 1.875 (2) | V2—O6 | 1.727 (3) |
| Cu3—O8 | 1.871 (2) | V2—O6 | 1.727 (3) |
| Angles, ° | |||
| Cu4—O7—Cu1v | 101.10 (13) (×3) | Cu4—O7—Cu4 | 116.38 (9) (×3) |
| Cu3—O8—Cu2 | 101.34 (12) | Cu4—O8—Cu2 | 101.20 (12) |
| Cu3—O8—Cu3v | 116.28 (13) | Cu4—O8—Cu3v | 115.98 (13) |
| Cu3—O8—Cu4 | 116.64 (13) | ||
| Compound 3 | |||
| Bond distances, Å | |||
| Cu1—O1 | 1.992 (4) | Cu3—O1 | 1.989 (3) |
| Cu1—O2 | 1.974 (4) | Cu3—O1 | 1.989 (3) |
| Cu1—O4 | 1.855 (3) | Cu3—O5 | 1.875 (3) |
| Cu1—O5 | 1.884 (3) | Cu3—O5 | 1.875 (3) |
| Cu2—O2 | 2.036 (3) | P1—O1 | 1.556 (4) |
| Cu2—O3v | 1.981 (4) | P1—O2 | 1.545 (4) |
| Cu2—O5 | 1.869 (3) | P1—O3 | 1.517 (4) |
| Cu2—O5 | 1.900 (3) | P1—O4 | 1.501 (4) |
| Angles, ° | |||
| Cu1—O5—Cu2v | 99.56 (14) | Cu2—O5—Cu3 | 109.90 (16) |
| Cu2—O5—Cu1 | 119.56 (17) | Cu3—O5—Cu1 | 98.30 (14) |
| Cu2—O5—Cu2v | 111.65 (16) | Cu3—O5—Cu2v | 117.36 (17) |
| Compound 4 | |||
| Bond distances, Å | |||
| Cu1—O1 | 1.984 (7) | Cu2—O5 | 1.932 (6) |
| Cu1—O2 | 1.953 (7) | Cu3—O1 | 1.971 (6) |
| Cu1—O4 | 1.865 (6) | Cu3—O5 | 1.895 (6) |
| Cu1—O5 | 1.889 (6) | P1—O1 | 1.554 (7) |
| Cu2—O2 | 2.037 (6) | P1—O2 | 1.551 (7) |
| Cu2—O3 | 1.969 (7) | P1—O3 | 1.522 (8) |
| Cu2—O5 | 1.894 (6) | P1—O4 | 1.510 (6) |
| Angles, ° | |||
| Cu1—O5—Cu2 | 96.7 (3) | Cu2—O5—Cu2 | 112.1 (3) |
| Cu1—O5—Cu2 | 126.3 (3) | Cu2—O5—Cu3 | 108.0 (3) |
| Cu1—O5—Cu3 | 95.2 (3) | Cu3—O5—Cu2v | 118.4 (3) |
| Compound 5 | |||
| Bond distances, Å | |||
| Cu1—O1 | 1.982 (6) | Cu2—O5 | 1.884 (5) |
| Cu1—O2 | 1.956 (6) | Cu3—O1 | 1.982 (6) |
| Cu1—O4 | 1.866 (5) | Cu3—O5 | 1.888 (5) |
| Cu1—O5 | 1.900 (5) | P1—O1 | 1.559 (6) |
| Cu2—O2 | 2.044 (6) | P1—O2 | 1.556 (6) |
| Cu2—O3 | 1.980 (6) | P1—O3 | 1.514 (6) |
| Cu2—O5 | 1.923 (5) | P1—O4 | 1.516 (5) |
| Angles, ° | |||
| Cu1—O5—Cu2 | 97.2 (2) | Cu2—O5—Cu2 | 111.2 (3) |
| Cu1—O5—Cu2 | 124.6 (3) | Cu2—O5—Cu3 | 108.7 (3) |
| Cu1—O5—Cu3 | 96.1 (2) | Cu3—O5—Cu2 | 119.0 (3) |
| Compound 6 | |||
| Bond distances, Å | |||
| Cu1—O1 | 1.889 (1) ×2 | Cu2A—O2 | 1.888 (2) |
| Cu1—O3 | 1.979 (3) ×2 | Cu2A—O1 | 1.928 (1) |
| Cu2—Cu2A | 0.25 (5) | Cu2A—O3 | 1.98 (2) ×2 |
| Cu2—O2 | 1.852 (2) | Cu2A—O3 | 2.35 (5) |
| Cu2—O1 | 1.930 (2) | V1—O2 | 1.655 (4) |
| Cu2—O3 | 2.098 (4) ×3 | V1—O3 | 1.742 (3) ×3 |
References
- Krivovichev, S.V. (Ed.) Minerals as Advanced Materials I; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Krivovichev, S.V. (Ed.) Minerals as Advanced Materials II; Springer: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Bindi, L.; Nespolo, M.; Krivovichev, S.V.; Chapuis, G.; Biagioni, C. Producing highly complicated materials. Nature does it better. Rep. Progr. Phys. 2020, 83, 106501. [Google Scholar] [CrossRef]
- Han, T.-H.; Singleton, J.; Schlueter, J.A. Barlowite: A spin-1212 antiferromagnet with a geometrically perfect kagome motif. Phys. Rev. Lett. 2014, 113, 227203. [Google Scholar] [CrossRef] [PubMed]
- Norman, M.R. Herbertsmithite and the search for the quantum spin liquid. Rev. Mod. Phys. 2016, 88, 041002. [Google Scholar] [CrossRef]
- Malcherek, T.; Mihailova, B.; Welch, M.D. Structural phase transitions of clinoatacamite and the dynamic Jahn-Teller effect. Phys. Chem. Miner. 2017, 44, 307–321. [Google Scholar] [CrossRef]
- Malcherek, T.; Welch, M.D.; Williams, P.A. The atacamite family of minerals—A testbed for quantum spin liquids. Acta Crystallogr. 2018, 74, 519–526. [Google Scholar] [CrossRef]
- Siidra, O.; Nazarchuk, E.; Agakhanov, A.; Polekhovsky, Y. Aleutite [Cu5O2](AsO4)(VO4)·(Cu0.5□0.5)Cl, a new complex salt-inclusion mineral with Cu2+ substructure derived from a Kagome-net. Mineral. Mag. 2019, 83, 847–853. [Google Scholar] [CrossRef]
- Smaha, R.W.; He, W.; Jiang, J.M. Materializing rival ground states in the barlowite family of kagome magnets: Quantum spin liquid, spin ordered, and valence bond crystal states. NPJ Quantum Mater. 2020, 5, 23. [Google Scholar] [CrossRef]
- Hiroi, Z.; Ishikawa, H.; Yoshida, H.; Yamaura, J.; Okamoto, Y. Orbital transitions and frustrated magnetism in the kagome-type copper mineral volborthite. Inorg. Chem. 2019, 58, 11949–11960. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Zolotarev, A.A.; Yapaskurt, V.O.; Krivovichev, S.V.; Belakovskiy, D.I.; Lykova, I.; Vigasina, M.F.; Kasatkin, A.V.; Sidorov, E.G.; et al. Dioskouriite, CaCu4Cl6(OH)4·4H2O: A New Mineral Description, Crystal Chemistry and Polytypism. Minerals 2021, 11, 90. [Google Scholar] [CrossRef]
- Vergasova, L.P.; Filatov, S.K. A study of volcanogenic exhalation mineralization. J. Volcanol. Seismol. 2016, 10, 71–85. [Google Scholar] [CrossRef]
- Pekov, I.V.; Agakhanov, A.A.; Zubkova, N.V.; Koshlyakova, N.V.; Shchipalkina, N.V.; Sandalov, F.D.; Yapaskurt, V.O.; Turchkova, A.G.; Sidorov, E.G. Oxidizing-type fumaroles of the Tolbachik Volcano, a mineralogical and geochemical unique. Russ. Geol. Geophys. 2020, 61, 675–688. [Google Scholar] [CrossRef]
- Pekov, I.V.; Zubkova, N.V.; Pushcharovsky, D.Y. Copper minerals from volcanic exhalations—A unique family of natural compounds: Crystal chemical review. Acta Crystallogr. 2018, B74, 502–518. [Google Scholar] [CrossRef]
- Siidra, O.I.; Nazarchuk, E.V.; Zaitsev, A.N.; Polekhovsky, Y.S.; Wenzel, T.; Spratt, J. Dokuchaevite, Cu8O2(VO4)3Cl3, a new mineral with remarkably diverse Cu2+ mixed-ligand coordination environments. Mineral. Mag. 2019, 83, 749–755. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Mentré, O.; Siidra, O.I.; Colmont, M.; Filatov, S.K. Anion-centered tetrahedra in inorganic compounds. Chem. Rev. 2013, 113, 6459–6535. [Google Scholar] [CrossRef] [PubMed]
- Vergasova, L.P.; Starova, G.L.; Filatov, S.K.; Ananiev, V.V. Averievite Cu5(VO4)2O2·nMX—A new mineral of volcanic exhalations. Dokl. Akad. Nauk 1998, 359, 804–807. (In Russian) [Google Scholar]
- Starova, G.L.; Krivovichev, S.V.; Fundamensky, V.S.; Filatov, S.K. The crystal structure of averievite, Cu5O2(VO4)2·nMX: Comparison with related compounds. Min. Mag. 1997, 61, 441–446. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K.; Vergasova, L.P. Crystal structure of averievite Cu5O2(VO4)·nMClx (M = Cu, Cs, Rb, K). Zap. Ross. Mineral. Obchsh. 2015, 144, 101–109. (In Russian) [Google Scholar]
- Botana, A.S.; Zheng, H.; Lapidus, S.H.; Mitchell, J.F.; Norman, M.R. Averievite: A copper oxide kagome antiferromagnet. Phys. Rev. B 2018, 98, 054421. [Google Scholar] [CrossRef]
- Volkova, L.M.; Marinin, D.V. Antiferromagnetic spin-frustrated layers of corner-sharing Cu4 tetrahedra on the kagome lattice in volcanic minerals Cu5O2(VO4)2(CuCl), NaCu5O2(SeO3)2Cl3, and K2Cu5Cl8(OH)4·2H2O. J. Phys. Condens. Matter 2018, 30, 425801. [Google Scholar] [CrossRef]
- Winiarski, M.J.; Tran, T.T.; Chamorro, J.R.; McQueen, T.M. (CsX)Cu5O2(PO4)2 (X = Cl, Br, I): A Family of Cu2+ S = ½ Compounds with Capped-Kagomé Networks Composed of °Cu4 Units. Inorg. Chem. 2019, 58, 4328–4336. [Google Scholar] [CrossRef] [PubMed]
- Dey, D.; Botana, A.S. Role of chemical pressure on the electronic and magnetic properties of the spin-1/2 kagome mineral averievite. Phys. Rev. B 2020, 102, 125106. [Google Scholar] [CrossRef]
- Kovrugin, V.M.; Siidra, O.I.; Colmont, M.; Mentré, O.; Krivovichev, S.V. Emulating exhalative chemistry: Synthesis and structural characterization of ilinskite, Na[Cu5O2](SeO3)2Cl3, and its K-analogue. Miner. Petrol. 2015, 109, 421–430. [Google Scholar] [CrossRef]
- Siidra, O.I.; Vladimirova, V.A.; Tsirlin, A.A.; Chukanov, N.V.; Ugolkov, V.L. Cu9O2(VO4)4Cl2, the first copper oxychloride vanadate: Mineralogically inspired synthesis and magnetic behavior. Inorg. Chem. 2020, 59, 2136–2143. [Google Scholar] [CrossRef]
- Queen, W.L.; West, J.P.; Hwu, S.J.; VanDerveer, D.G.; Zarzyczny, M.C.; Pavlick, R.A. The versatile chemistry and noncentrosymmetric crystal structures of salt-inclusion vanadate hybrids. Angew. Chem. Int. Ed. 2008, 47, 3791–3794. [Google Scholar] [CrossRef] [PubMed]
- Pekov, I.V.; Zubkova, N.V.; Zelenski, M.E.; Yapaskurt, V.O.; Polekhovsky, Y.S.; Fadeeva, O.A.; Pushcharovsky, D.Y. Yaroshevskite, Cu9O2(VO4)4Cl2, a new mineral from the Tolbachik volcano, Kamchatka, Russia. Mineral. Mag. 2013, 77, 107–116. [Google Scholar] [CrossRef]
- Shannon, R.D.; Calvo, C. Crystal structure of Cu5V2O10. Acta Cryst. 1973, 29, 1338–1345. [Google Scholar] [CrossRef]
- CrysAlisPro Software System, version 1.171.38.46; Rigaku Oxford Diffraction: Oxford, UK, 2015.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal structure determination. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Sheldrick, G.M. Crystal structure refinement with SHELXL. Acta Crystallogr. 2015, 71, 3–8. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A complete structure solution, refinement and analysis program. J. Appl. Cryst. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Spek, A.L. Single-crystal structure validation with the program PLATON. J. Appl. Crystallogr. 2003, 36, 7–13. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Starova, G.L.; Filatov, S.K. “Face-to-face” relationships between oxocentered tetrahedra and cation-centered tetrahedral oxyanions in crystal structures of minerals and inorganic compounds. Mineral. Mag. 1999, 63, 263–266. [Google Scholar] [CrossRef]
- Krivovichev, S.V.; Filatov, S.K. Structural principles for minerals and inorganic compounds containing anion-centered tetrahedra. Amer. Mineral. 1999, 84, 1099–1106. [Google Scholar] [CrossRef]
- Kimura, T.; Sekio, Y.; Nakamura, H.; Siegrist, T.; Ramirez, A.P. Cupric Oxide as an Induced-Multiferroic with High-TC. Nat. Mater. 2008, 7, 291–294. [Google Scholar] [CrossRef] [PubMed]
- Shannon, R.D. Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. Acta Crystallogr. 1976, 32, 751–767. [Google Scholar] [CrossRef]
- Rocquefelte, X.; Schwarz, K.; Blaha, P. Theoretical Investigation of the Magnetic Exchange Interactions in Copper(II) Oxides under Chemical and Physical Pressures. Sci. Rep. 2012, 2, 759. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Structural complexity of minerals: Information storage and processing in the mineral world. Miner. Mag. 2013, 77, 275–326. [Google Scholar] [CrossRef]
- Krivovichev, S.V. Which inorganic structures are the most complex? Angew. Chem. Int. Ed. 2014, 53, 654–661. [Google Scholar] [CrossRef] [PubMed]
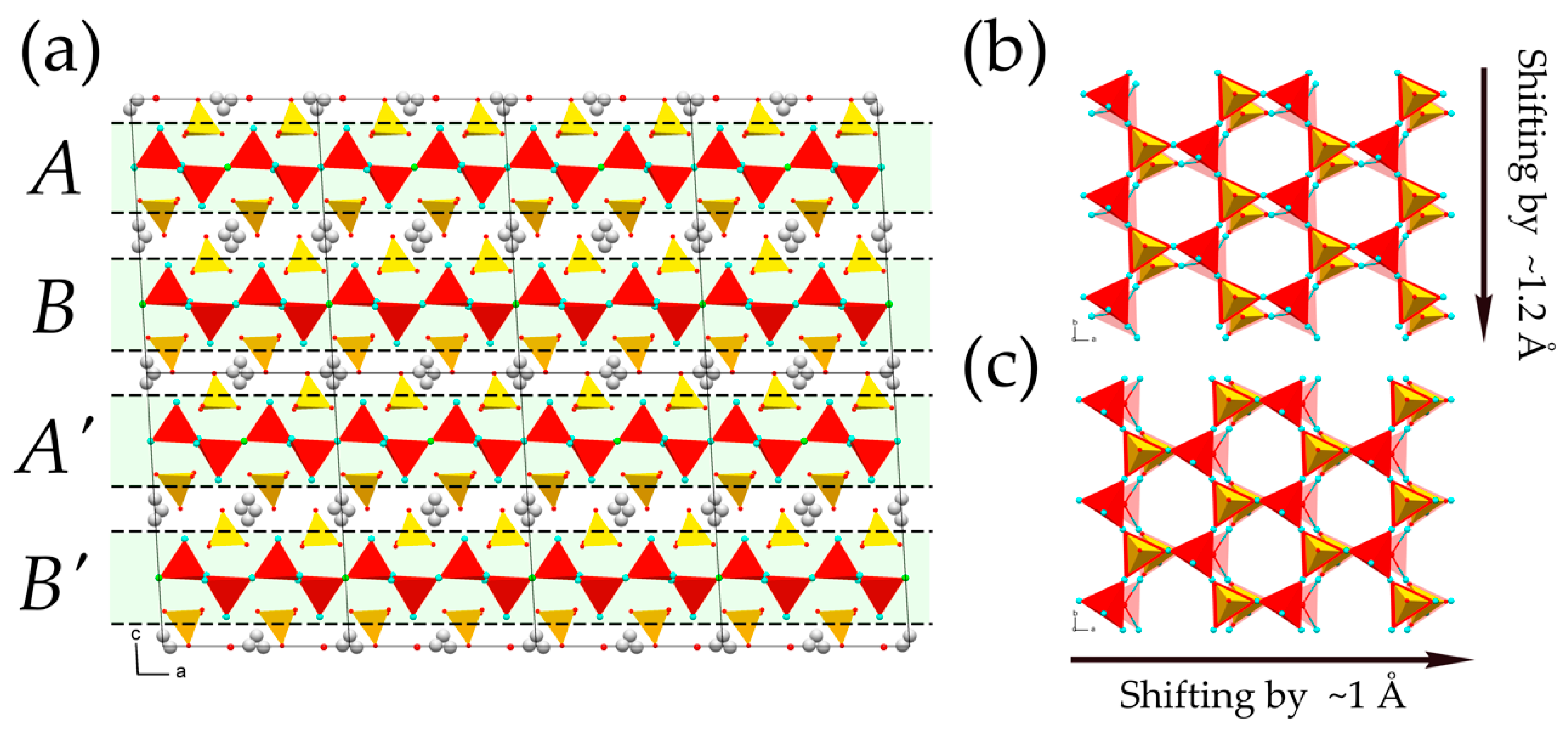
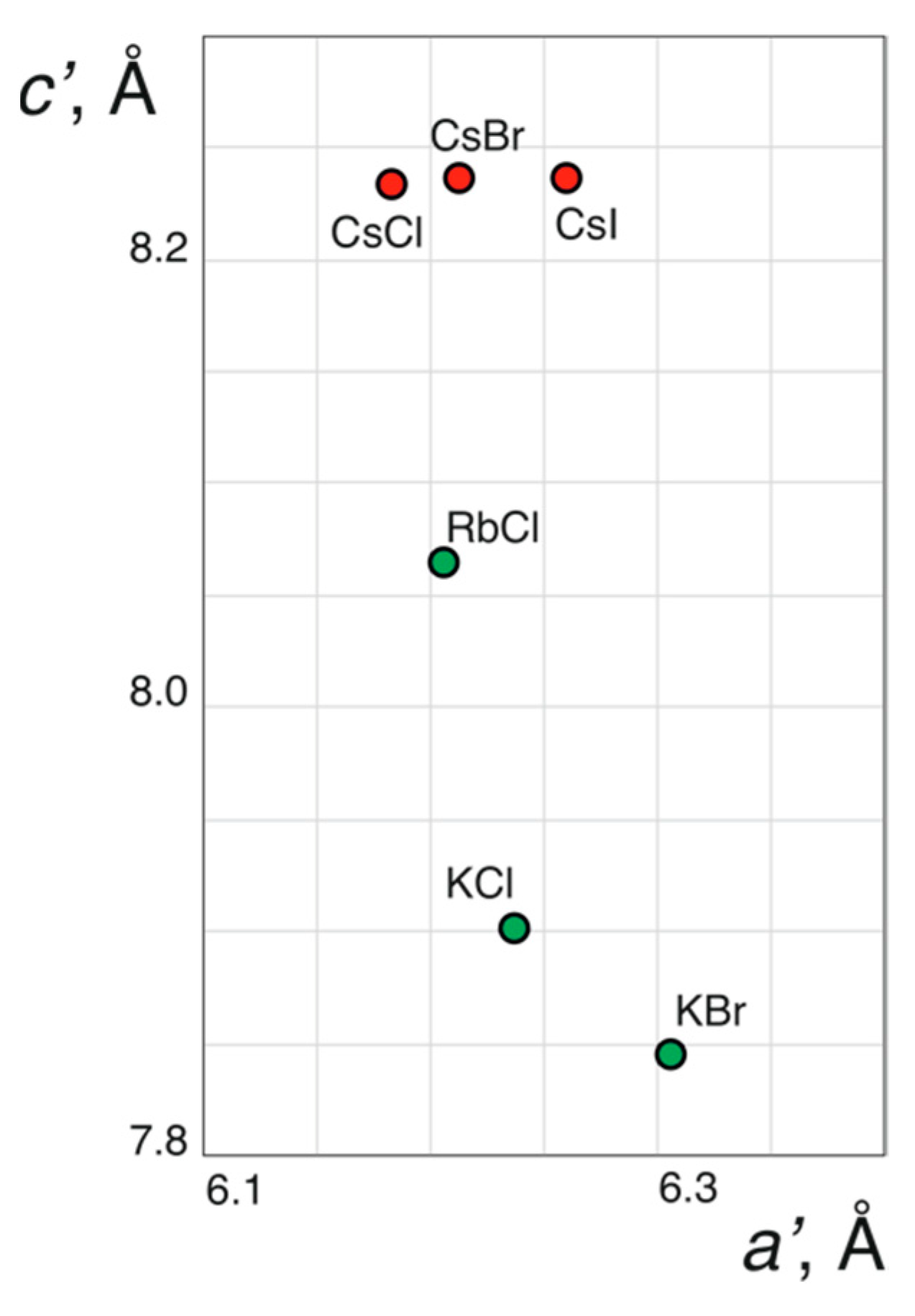

| Compound | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Formula | (CsCl)Cu5O2(PO4)2 | (CsCl)Cu5O2(VO4)2 | (RbCl)Cu5O2(PO4)2 | (KBr)Cu5O2(PO4)2 | (KCl)Cu5O2(PO4)2 | (CuCl)Cu5O2(VO4)2 |
| Space Group | Pm1 | P | C2/c | C2/c | C2/c | Pm1 |
| a, Å | 6.1842(1) | 12.7249(4) | 10.8869(4) | 10.9738(5) | 10.9808(10) | 6.406(4) |
| b, Å | 6.1842(1) | 12.7249(4) | 6.2074(3) | 6.3067(3) | 6.2384(5) | 6.406(4) |
| c, Å | 8.2333(2) | 8.3767(2) | 16.1562(7) | 15.7610(5) | 15.8684(14) | 8.403(5) |
| β, ° | 90 | 90 | 93.377(4) | 95.424(4) | 95.227(8) | 90 |
| V, Å3 | 272.691(11) | 1174.66(8) | 1089.93(8) | 1085.91(8) | 1082.51(16) | 298.6(4) |
| μ, mm−1 | 13.460 | 13.733 | 14.615 | 14.032 | 10.632 | 14.178 |
| Z | 1 | 4 | 4 | 4 | 4 | 1 |
| Dcalc, g/cm3 | 4.311 | 4.233 | 4.026 | 4.029 | 3.769 | 4.324 |
| Color | Light yellowish green | Black | Dark green | Dark green | Dark green | Black |
| Total reflections | 2219 | 6203 | 11646 | 3738 | 5375 | 7232 |
| Unique reflections | 269 | 1810 | 2632 | 3738 | 1218 | 643 |
| Reflection with |Fo| ≥ 4σF | 267 | 1371 | 2214 | 3572 | 1077 | 578 |
| Angle range 2θ, °, MoKα | 7.61 to 54.998 | 3.696 to 54.934 | 7.5 to 74.606 | 5.192 to 59.992 | 7.454 to 54.994 | 4.85 to 75.332 |
| Rint, Rσ | 0.0086, 0.0032 | 0.0223, 0.0135 | 0.0527, 0.0307 | Merged 1, 0.0091 | 0.0802, 0.0506 | 0.0315, 0.0145 |
| R1, wR2 (|Fo| ≥ 4σF) | 0.0153, 0.0365 | 0.0332, 0.0944 | 0.0562, 0.1467 | 0.0552, 0.1395 | 0.0626, 0.1539 | 0.0326, 0.0784 |
| R1, wR2 (all data) | 0.0154, 0.0365 | 0.0419, 0.1011 | 0.0676, 0.1534 | 0.0581, 0.1427 | 0.0707, 0.1596 | 0.0369, 0.0815 |
| GOOF | 1.155 | 1.106 | 1.198 | 1.126 | 1.126 | 1.108 |
| ρmin, ρmax, e/Å3 | 0.72/−1.04 | 1.29/−0.91 | 1.73/−1.25 | 2.22/−1.53 | 1.88/−1.42 | 2.31/−4.60 |
| CSD | 2048047 | 2048048 | 2048049 | 2048046 | 2064850 | 2063823 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kornyakov, I.V.; Vladimirova, V.A.; Siidra, O.I.; Krivovichev, S.V. Expanding the Averievite Family, (MX)Cu5O2(T5+O4)2 (T5+ = P, V; M = K, Rb, Cs, Cu; X = Cl, Br): Synthesis and Single-Crystal X-ray Diffraction Study. Molecules 2021, 26, 1833. https://doi.org/10.3390/molecules26071833
Kornyakov IV, Vladimirova VA, Siidra OI, Krivovichev SV. Expanding the Averievite Family, (MX)Cu5O2(T5+O4)2 (T5+ = P, V; M = K, Rb, Cs, Cu; X = Cl, Br): Synthesis and Single-Crystal X-ray Diffraction Study. Molecules. 2021; 26(7):1833. https://doi.org/10.3390/molecules26071833
Chicago/Turabian StyleKornyakov, Ilya V., Victoria A. Vladimirova, Oleg I. Siidra, and Sergey V. Krivovichev. 2021. "Expanding the Averievite Family, (MX)Cu5O2(T5+O4)2 (T5+ = P, V; M = K, Rb, Cs, Cu; X = Cl, Br): Synthesis and Single-Crystal X-ray Diffraction Study" Molecules 26, no. 7: 1833. https://doi.org/10.3390/molecules26071833
APA StyleKornyakov, I. V., Vladimirova, V. A., Siidra, O. I., & Krivovichev, S. V. (2021). Expanding the Averievite Family, (MX)Cu5O2(T5+O4)2 (T5+ = P, V; M = K, Rb, Cs, Cu; X = Cl, Br): Synthesis and Single-Crystal X-ray Diffraction Study. Molecules, 26(7), 1833. https://doi.org/10.3390/molecules26071833








