Coumarin-Annulated Ferrocenyl 1,3-Oxazine Derivatives Possessing In Vitro Antimalarial and Antitrypanosomal Potency
Abstract
1. Introduction
2. Results
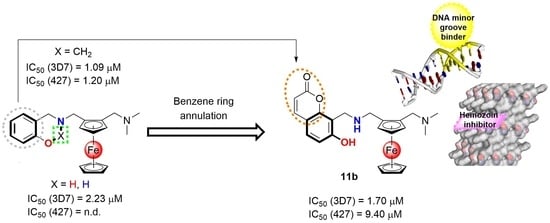
2.1. Chemistry
2.2. Biological Evaluation
2.3. Preliminary Mechanistic Studies
3. Materials and Methods
3.1. General Methods and Instrumentation
3.2. Synthesis of Target Compounds
3.2.1. General Procedure for Synthesis of Acyclic Derivatives 11a,b
3.2.2. General Procedure for Synthesis of Cyclic Coumarin-1,3-oxazin-2-one Derivatives 12a,b
3.2.3. Synthesis of 9-(2-((N,N-Dimethylamino)methyl)ferrocenemethyl)-9,10-dihydrochromeno[8,7-e][1,3]oxazin-2(8H)-one (13)
3.2.4. Synthesis of Acyclic Ferrocenyl Aminocoumarin-4-ols 15a–d
3.2.5. Synthesis of Ferrocenyl Coumarin-1,3-oxazin-2-one Derivatives 16a–d
3.2.6. Synthesis of 7-(2-((N,N-Dimethylamino)methyl)ferrocenemethyl)-7,8-dihydro-6H-[1,3]dioxolo[4’,5’:4,5]benzo[1,2-e][1,3]oxazine (19)
3.3. Biological Evaluation Assays
3.3.1. D7 P. falciparum Antiplasomidal Evaluation
3.3.2. T. b. Brucei 427 Antitrypanosomal Assay
3.3.3. Antiproliferative Assay against the HCC70 Triple-Negative Breast Cancer Cell Line
3.3.4. β-Hematin Binding Assay for Hemozoin Inhibition
3.3.5. UV-Vis DNA Titration Experiment for DNA Binding
3.3.6. Methylene Blue and Hoechst 33342 DNA Binding Assay for Competitive Intercalation or Minor Groove Binding
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Perkins, S.L. Species concepts and malaria parasites: Detecting a cryptic species of Plasmodium. Proc. R. Soc. B 2000, 267, 2345–2350. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Malaria. Available online: https://www.who.int/health-topics/malaria#tab=tab_1 (accessed on 26 November 2020).
- Smith, T.K.; Bringaud, F.; Nolan, D.P.; Figueiredo, L.M. Metabolic reprogramming during the Trypanosoma brucei life cycle. F1000Research 2017, 6. [Google Scholar] [CrossRef] [PubMed]
- Medicines for Malaria Venture. The Pathogen Box. Available online: https://www.mmv.org/mmv-open/pathogen-box (accessed on 20 August 2020).
- Drugs for Neglected Disease Initiative (NDDi). Available online: https://dndi.org/ (accessed on 31 October 2020).
- Nosten, F.; White, N.J. Artemisinin-based combination treatment of falciparum malaria. Am. J. Trop. Med. Hyg. 2007, 77 (Suppl. 6), 181–192. [Google Scholar] [CrossRef]
- World Health Organization. WHO Status Reports on Artemisinin Resistance and ACT Efficacy. Available online: https://www.who.int/malaria/areas/drug_resistance/updates/en/ (accessed on 26 November 2020).
- Patra, M.; Gasser, G. The medicinal chemistry of ferrocene and its derivatives. Nat. Rev. Chem. 2017, 1, 1–12. [Google Scholar] [CrossRef]
- Singh, A.; Lumb, I.; Mehra, V.; Kumar, V. Ferrocene-appended pharmacophores: An exciting approach for modulating the biological potential of organic scaffolds. Dalton Trans. 2019, 48, 2840–2860. [Google Scholar] [CrossRef]
- Xiao, J.; Sun, Z.; Kong, F.; Gao, F. Current scenario of ferrocene-containing hybrids for antimalarial activity. Eur. J. Med. Chem. 2020, 185, 111791. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.H.; Benjelloun-Dakhama, N.; Gola, J.M.; Acree, W.E., Jr.; Cain, W.S.; Cometto-Muniz, J.E. Solvation descriptors for ferrocene, and the estimation of some physicochemical and biochemical properties. New J. Chem. 2000, 24, 825–829. [Google Scholar] [CrossRef]
- Astruc, D. Why is ferrocene so exceptional? Eur. J. Inorg. Chem. 2017, 2017, 6–29. [Google Scholar] [CrossRef]
- Biot, C.; Glorian, G.; Maciejewski, L.A.; Brocard, J.S.; Domarle, O.; Blampain, G.; Millet, P.; Georges, A.J.; Abessolo, H.; Dive, D. Synthesis and antimalarial activity in vitro and in vivo of a new ferrocene− chloroquine analogue. J. Med. Chem. 1997, 40, 3715–3718. [Google Scholar] [CrossRef] [PubMed]
- Top, S.; Tang, J.; Vessières, A.; Carrez, D.; Provot, C.; Jaouen, G. Ferrocenyl hydroxytamoxifen: A prototype for a new range of oestradiol receptor site-directed cytotoxics. Chem. Commun. 1996, 955–956. [Google Scholar] [CrossRef]
- Tan, Y.L.K.; Pigeon, P.; Top, S.; Labbé, E.; Buriez, O.; Hillard, E.A.; Vessières, A.; Amatore, C.; Leong, W.K.; Jaouen, G. Ferrocenyl catechols: Synthesis, oxidation chemistry and anti-proliferative effects on MDA-MB-231 breast cancer cells. Dalton Trans. 2012, 41, 7537–7549. [Google Scholar] [CrossRef] [PubMed]
- Jaouen, G.; Vessières, A.; Top, S. Ferrocifen type anti cancer drugs. Chem. Soc. Rev. 2015, 44, 8802–8817. [Google Scholar] [CrossRef] [PubMed]
- N’Da, D.D.; Smith, P.J. Synthesis, in vitro antiplasmodial and antiproliferative activities of a series of quinoline–ferrocene hybrids. Med. Chem. Res. 2014, 23, 1214–1224. [Google Scholar] [CrossRef]
- Li, Y.; de Kock, C.; Smith, P.J.; Chibale, K.; Smith, G.S. Synthesis and evaluation of a carbosilane congener of ferroquine and its corresponding half-sandwich ruthenium and rhodium complexes for antiplasmodial and β-hematin inhibition activity. Organometallics 2014, 33, 4345–4348. [Google Scholar] [CrossRef]
- Stringer, T.; Wiesner, L.; Smith, G.S. Ferroquine-derived polyamines that target resistant Plasmodium falciparum. Eur. J. Med. Chem. 2019, 179, 78–83. [Google Scholar] [CrossRef]
- Minić, A.; Van de Walle, T.; Van Hecke, K.; Combrinck, J.; Smith, P.J.; Chibale, K.; D’hooghe, M. Design and synthesis of novel ferrocene-quinoline conjugates and evaluation of their electrochemical and antiplasmodium properties. Eur. J. Med. Chem. 2020, 187, 111963. [Google Scholar] [CrossRef]
- Quirante, J.; Dubar, F.; González, A.; Lopez, C.; Cascante, M.; Cortés, R.; Forfar, I.; Pradines, B.; Biot, C. Ferrocene–indole hybrids for cancer and malaria therapy. J. Organomet. Chem. 2011, 696, 1011–1017. [Google Scholar] [CrossRef]
- Radulović, N.S.; Zlatković, D.B.; Mitić, K.V.; Randjelović, P.J.; Stojanović, N.M. Synthesis, spectral characterization, cytotoxicity and enzyme-inhibiting activity of new ferrocene–indole hybrids. Polyhedron 2014, 80, 134–141. [Google Scholar] [CrossRef]
- Muenzner, J.K.; Ahmad, A.; Rothemund, M.; Schrüfer, S.; Padhye, S.; Sarkar, F.H.; Schobert, R.; Biersack, B. Ferrocene-substituted 3, 3′-diindolylmethanes with improved anticancer activity. Appl. Organomet. Chem. 2016, 30, 441–445. [Google Scholar] [CrossRef]
- Toro, P.; Klahn, A.H.; Pradines, B.; Lahoz, F.; Pascual, A.; Biot, C.; Arancibia, R. Organometallic benzimidazoles: Synthesis, characterization and antimalarial activity. Inorg. Chem. Commun. 2013, 35, 126–129. [Google Scholar] [CrossRef]
- Babgi, B.A.; Abdellattif, M.H.; Hussien, M.A.; Eltayeb, N.E. Exploring DNA-Binding and anticancer properties of benzoimidazolyl-ferrocene dye. J. Mol. Struct. 2019, 1198, 126918. [Google Scholar] [CrossRef]
- De Lange, C.; Coertzen, D.; Smit, F.J.; Wentzel, J.F.; Wong, H.N.; Birkholtz, L.-M.; Haynes, R.K.; David, D.D. Synthesis, in vitro antimalarial activities and cytotoxicities of amino-artemisinin-ferrocene derivatives. Bioorg. Med. Chem. Lett. 2018, 28, 289–292. [Google Scholar] [CrossRef] [PubMed]
- De Lange, C.; Coertzen, D.; Smit, F.J.; Wentzel, J.F.; Wong, H.N.; Birkholtz, L.-M.; Haynes, R.K.; David, D.D. Synthesis, antimalarial activities and cytotoxicities of amino-artemisinin-1, 2-disubstituted ferrocene hybrids. Bioorg. Med. Chem. Lett. 2018, 28, 3161–3163. [Google Scholar] [CrossRef]
- Reiter, C.; Fröhlich, T.; Zeino, M.; Marschall, M.; Bahsi, H.; Leidenberger, M.; Friedrich, O.; Kappes, B.; Hampel, F.; Efferth, T. New efficient artemisinin derived agents against human leukemia cells, human cytomegalovirus and Plasmodium falciparum: 2nd generation 1, 2, 4-trioxane-ferrocene hybrids. Eur. J. Med. Chem. 2015, 97, 164–172. [Google Scholar] [CrossRef]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2004, 10, 3797–3811. [Google Scholar] [CrossRef]
- Önder, A. Anticancer activity of natural coumarins for biological targets. Stud. Nat. Prod. Chem. 2020, 64, 85–109. [Google Scholar]
- Sashidhara, K.V.; Kumar, A.; Dodda, R.P.; Krishna, N.N.; Agarwal, P.; Srivastava, K.; Puri, S. Coumarin–trioxane hybrids: Synthesis and evaluation as a new class of antimalarial scaffolds. Bioorg. Med. Chem. Lett. 2012, 22, 3926–3930. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-L.; Gao, C.; Xu, Z.; Liu, M.-L.; Feng, L.-S.; Zhang, G.-D. Recent development of coumarin derivatives as potential antiplasmodial and antimalarial agents. Curr. Top. Med. Chem. 2018, 18, 114–123. [Google Scholar] [CrossRef]
- Pérez-Cruz, F.; Serra, S.; Delogu, G.; Lapier, M.; Maya, J.D.; Olea-Azar, C.; Santana, L.; Uriarte, E. Antitrypanosomal and antioxidant properties of 4-hydroxycoumarins derivatives. Bioorg. Med. Chem. Lett. 2012, 22, 5569–5573. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, G.A.; Cerecetto, H.; Poser, G.; Canto, R.F.S.; Eifler-Lima, V.L. Chagas disease and coumarins: A review of natural and synthetic coumarins as anti-Trypanosoma cruzi agents. Mini-Rev. Med. Chem. 2020. [Google Scholar] [CrossRef]
- Gonçalves, G.A.; Spillere, A.R.; das Neves, G.M.; Kagami, L.P.; von Poser, G.L.; Canto, R.F.S.; Eifler-Lima, V.L. Natural and synthetic coumarins as antileishmanial agents: A review. Eur. J. Med. Chem. 2020, 203, 112514. [Google Scholar] [CrossRef]
- Mandlik, V.; Patil, S.; Bopanna, R.; Basu, S.; Singh, S. Biological activity of coumarin derivatives as anti-leishmanial agents. PLoS ONE 2016, 11, e0164585. [Google Scholar] [CrossRef]
- Keri, R.S.; Sasidhar, B.; Nagaraja, B.M.; Santos, M.A. Recent progress in the drug development of coumarin derivatives as potent antituberculosis agents. Eur. J. Med. Chem. 2015, 100, 257–269. [Google Scholar] [CrossRef]
- Hu, Y.-Q.; Xu, Z.; Zhang, S.; Wu, X.; Ding, J.-W.; Lv, Z.-S.; Feng, L.-S. Recent developments of coumarin-containing derivatives and their anti-tubercular activity. Eur. J. Med. Chem. 2017, 136, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Mbaba, M.; Mabhula, A.N.; Boel, N.; Edkins, A.L.; Isaacs, M.; Hoppe, H.C.; Khanye, S.D. Ferrocenyl and organic novobiocin derivatives: Synthesis and their in vitro biological activity. J. Inorg. Biochem. 2017, 172, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Mbaba, M.; De la Mare, J.-A.; Sterrenberg, J.N.; Kajewole, D.; Maharaj, S.; Edkins, A.L.; Isaacs, M.; Hoppe, H.C.; Khanye, S.D. Novobiocin–ferrocene conjugates possessing anticancer and antiplasmodial activity independent of HSP90 inhibition. J. Biol. Inorg. Chem. 2019, 24, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Biao, L.; Qiuxia, L.; Yuanqing, Z.; Zhaodong, J.; Manyu, Z.; Yan, X.; Maoping, S. Synthesis and Properties of 4-Ferrocenyl-carboxybenzene-coumarin Derivatives. Chin. J. Org. Chem. 2017, 37, 2008–2014. [Google Scholar]
- Wei, J.-N.; Jia, Z.-D.; Zhou, Y.-Q.; Chen, P.-H.; Li, B.; Zhang, N.; Hao, X.-Q.; Xu, Y.; Zhang, B. Synthesis, characterization and antitumor activity of novel ferrocene-coumarin conjugates. J. Organomet. Chem. 2019, 902, 120968. [Google Scholar] [CrossRef]
- Li, W.; Wei, T.; Gao, Y.; Xi, K.; Jia, X. Preparation of novel benzoxazine monomers containing ferrocene moiety and properties of polybenzoxazines. Polymer 2012, 53, 1236–1244. [Google Scholar] [CrossRef]
- Li, W.; Chu, J.; Heng, L.; Wei, T.; Gu, J.; Xi, K.; Jia, X. A novel thermal-resistant copolymer from polysiloxane-based polybenzoxazine precursor and ferrocene-based benzoxazine monomer. Polymer 2013, 54, 4909–4922. [Google Scholar] [CrossRef]
- Kiskan, B. Adapting benzoxazine chemistry for unconventional applications. React. Funct. Polym. 2018, 129, 76–88. [Google Scholar] [CrossRef]
- Mbaba, M.; Dingle, L.M.; Cash, D.; de la Mare, J.-A.; Laming, D.; Taylor, D.; Hoppe, H.C.; Edkins, A.L.; Khanye, S.D. Repurposing a polymer precursor: Synthesis and in vitro medicinal potential of ferrocenyl 1, 3-benzoxazine derivatives. Eur. J. Med. Chem. 2020, 187, 111924. [Google Scholar] [CrossRef] [PubMed]
- Mbaba, M.; Dingle, L.M.; Swart, T.; Cash, D.; Laming, D.; de la Mare, J.A.; Taylor, D.; Hoppe, H.C.; Biot, C.; Edkins, A.L. The in vitro antiplasmodial and antiproliferative activity of new ferrocene-based α-aminocresols targeting hemozoin inhibition and DNA interaction. ChemBioChem 2020, 21, 2643–2658. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, Z.; Urbański, T. 1, 3-Oxazine Derivatives. In Advances in Heterocyclic Chemistry; Elsevier: Amsterdam, The Netherlands, 1979; Volume 23, pp. 1–53. [Google Scholar]
- Urbański, T.; Gürne, D.; Šlopek, S.; Mordarska, H.; Mordarski, M. Anti-neoplastic activity of tetrahydro-1, 3-oxazine derivatives. Nature 1960, 187, 426–427. [Google Scholar] [CrossRef]
- Lohar, T.; Mane, A.; Kamat, S.; Salunkhe, R. A versatile water-stable fluorine containing organometallic lewis acid used in green synthesis of 1, 3-oxazine scaffolds at room temperature. Polycycl. Aromat. Compd. 2018, 1–13. [Google Scholar] [CrossRef]
- Milevskii, B.; Chibisova, T.; Solov’eva, N.; Anisimova, O.; Lebedev, V.; Ivanov, I.; Traven, V. Synthesis and structure of Schiff bases derived from 3-formyl-4-hydroxycoumarin and diamines. Chem. Heterocycl. Compd. 2013, 48, 1781–1792. [Google Scholar] [CrossRef]
- Li, J.-S.; Fu, D.-M.; Xue, Y.; Li, Z.-W.; Li, D.-L.; Da, Y.-D.; Yang, F.; Zhang, L.; Lu, C.-H.; Li, G. One-step synthesis of furocoumarins via oxidative annulation of 4-hydroxycoumarins with DDQ. Tetrahedron 2015, 71, 2748–2752. [Google Scholar] [CrossRef]
- Jung, J.-C.; Jung, Y.-J.; Park, O.-S. A convenient one-pot synthesis of 4-hydroxycoumarin, 4-hydroxythiocoumarin, and 4-hydroxyquinolin-2 (1 H)-one. Synth. Commun. 2001, 31, 1195–1200. [Google Scholar] [CrossRef]
- Rad-Moghadam, K.; Mohseni, M. A route to the synthesis of novel coumarins. Monatsh. Chem. 2004, 135, 817–821. [Google Scholar] [CrossRef]
- Abdel-Magid, A.F.; Carson, K.G.; Harris, B.D.; Maryanoff, C.A.; Shah, R.D. Reductive amination of aldehydes and ketones with sodium triacetoxyborohydride. studies on direct and indirect reductive amination procedures1. J. Org. Chem. 1996, 61, 3849–3862. [Google Scholar] [CrossRef]
- Traven, V.F.; Negrebetsky, V.V.; Vorobjeva, L.I.; Carberry, E.A. Keto–enol tautomerism, NMR spectra, and H–D exchange of 4-hydroxycoumarins. Can. J. Chem. 1997, 75, 377–383. [Google Scholar] [CrossRef]
- Ribeiro, M.; Francisco, W.; Rodrigues-Oliveira, A.F.; Correra, T.C. Benzoxazine formation mechanism evaluation by direct observation of reaction intermediates. J. Phys. Chem. A 2019, 123, 8179–8187. [Google Scholar] [CrossRef] [PubMed]
- D’Addona, D.; Bochet, C.G. Preparation of carbamates from amines and alcohols under mild conditions. Tetrahedron Lett. 2001, 42, 5227–5229. [Google Scholar] [CrossRef]
- Gemma, S.; Camodeca, C.; Brindisi, M.; Brogi, S.; Kukreja, G.; Kunjir, S.; Gabellieri, E.; Lucantoni, L.; Habluetzel, A.; Taramelli, D. Mimicking the intramolecular hydrogen bond: Synthesis, biological evaluation, and molecular modeling of benzoxazines and quinazolines as potential antimalarial agents. J. Med. Chem. 2012, 55, 10387–10404. [Google Scholar] [CrossRef] [PubMed]
- Ncokazi, K.K.; Egan, T.J. A colorimetric high-throughput β-hematin inhibition screening assay for use in the search for antimalarial compounds. Anal. Biochem. 2005, 338, 306–319. [Google Scholar] [CrossRef]
- Lu, W.-J.; Wicht, K.J.; Wang, L.; Imai, K.; Mei, Z.-W.; Kaiser, M.; El Sayed, I.E.T.; Egan, T.J.; Inokuchi, T. Synthesis and antimalarial testing of neocryptolepine analogues: Addition of ester function in SAR study of 2, 11-disubstituted indolo [2, 3-b] quinolines. Eur. J. Med. Chem. 2013, 64, 498–511. [Google Scholar] [CrossRef] [PubMed]
- Sirajuddin, M.; Ali, S.; Badshah, A. Drug–DNA interactions and their study by UV–Visible, fluorescence spectroscopies and cyclic voltametry. J. Photochem. Photobiol. B 2013, 124, 1–19. [Google Scholar] [CrossRef]
- Saito, S.T.; Silva, G.; Pungartnik, C.; Brendel, M. Study of DNA–emodin interaction by FTIR and UV–vis spectroscopy. J. Photochem. Photobiol. B 2012, 111, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Shahabadi, N.; Hadidi, S. Spectroscopic studies on the interaction of calf thymus DNA with the drug levetiracetam. Spectrochim. Acta A 2012, 96, 278–283. [Google Scholar] [CrossRef]
- Mbaba, M. Repurposing a Polymer Precursor Scaffold for Medicinal Application: Synthesis, Characterization and Biological Evaluation of Ferrocenyl 1,3-Benzoxazine Derivatives as Potential Antiprotozoal and Anticancer Agents. Ph.D. Thesis, Rhodes University, Makhanda, South Africa, 2019. [Google Scholar]
- Vardevanyan, P.; Antonyan, A.; Parsadanyan, M.; Shahinyan, M.; Hambardzumyan, L. Mechanisms for binding between methylene blue and DNA. J. Appl. Spectrosc. 2013, 80, 595–599. [Google Scholar] [CrossRef]
- Sandhu, L.C.; Warters, R.L.; Dethlefsen, L.A. Fluorescence studies of Hoechst 33342 with supercoiled and relaxed plasmid pBR322 DNA. Cytometry A 1985, 6, 191–194. [Google Scholar] [CrossRef] [PubMed]
- Bucevičius, J.; Lukinavičius, G.; Gerasimaitė, R. The use of hoechst dyes for DNA staining and beyond. Chemosensors 2018, 6, 18. [Google Scholar] [CrossRef]
- Furniss, B.S.; Hannaford, A.J.; Smith, P.W.G.; Tatchell, A.R. Vogel’s Text Book of Practical Organic Chemistry, 5th ed.; Longmann Scientific & Technical: New York, NY, USA, 1989. [Google Scholar]
- Makler, M.T.; Hinrichs, D.J. Measurement of the lactate dehydrogenase activity of Plasmodium falciparum as an assessment of parasitemia. Am. J. Trop. Med. Hyg. 1993, 48, 205–210. [Google Scholar] [CrossRef] [PubMed]





| Compound | IC50 (µM) | |
|---|---|---|
| 3D7 P. falciparum | T. b. brucei 427 | |
| 11a | 9.1 | n.a. a |
| 11b | 1.7 | 9.4 |
| 12a | n.a. | n.a. |
| 12b | 7.3 | n.a. |
| 13 | 1.0 | n.d. b |
| 15a | n.a. | n.a. |
| 15b | n.a. | n.a. |
| 15c | 8.76 | 19.6 |
| 15d | 5.7 | 5.0 |
| 16a | 13.2 | n.a. |
| 16b | n.a. | n.a. |
| 16c | 12.55 | n.a. |
| 16d | n.a. | n.d. |
| 19 | 4.78 | n.d. |
| 20a c | 3.49 | n.a. |
| 20b c | 4.12 | 0.87 |
| CQ | 0.03 | – |
| Pentamidine | – | 0.014 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mbaba, M.; Dingle, L.M.K.; Zulu, A.I.; Laming, D.; Swart, T.; de la Mare, J.-A.; Hoppe, H.C.; Edkins, A.L.; Khanye, S.D. Coumarin-Annulated Ferrocenyl 1,3-Oxazine Derivatives Possessing In Vitro Antimalarial and Antitrypanosomal Potency. Molecules 2021, 26, 1333. https://doi.org/10.3390/molecules26051333
Mbaba M, Dingle LMK, Zulu AI, Laming D, Swart T, de la Mare J-A, Hoppe HC, Edkins AL, Khanye SD. Coumarin-Annulated Ferrocenyl 1,3-Oxazine Derivatives Possessing In Vitro Antimalarial and Antitrypanosomal Potency. Molecules. 2021; 26(5):1333. https://doi.org/10.3390/molecules26051333
Chicago/Turabian StyleMbaba, Mziyanda, Laura M. K. Dingle, Ayanda I. Zulu, Dustin Laming, Tarryn Swart, Jo-Anne de la Mare, Heinrich C. Hoppe, Adrienne L. Edkins, and Setshaba D. Khanye. 2021. "Coumarin-Annulated Ferrocenyl 1,3-Oxazine Derivatives Possessing In Vitro Antimalarial and Antitrypanosomal Potency" Molecules 26, no. 5: 1333. https://doi.org/10.3390/molecules26051333
APA StyleMbaba, M., Dingle, L. M. K., Zulu, A. I., Laming, D., Swart, T., de la Mare, J.-A., Hoppe, H. C., Edkins, A. L., & Khanye, S. D. (2021). Coumarin-Annulated Ferrocenyl 1,3-Oxazine Derivatives Possessing In Vitro Antimalarial and Antitrypanosomal Potency. Molecules, 26(5), 1333. https://doi.org/10.3390/molecules26051333