The Antioxidant Effect of Curcumin and Rutin on Oxidative Stress Biomarkers in Experimentally Induced Periodontitis in Hyperglycemic Wistar Rats
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Chemical/Drug Used
4.2. Experimental Animals
4.3. Induction of Diabetes Mellitus
4.4. Induction of Periodontitis
4.5. Group Allocation and Experimental Design
4.6. Blood and Tissue Sample Collection and Analyses
4.7. Determination of Oxidative Stress Biomarkers
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References
- World Health Organization (WHO). Global Report on Diabetes. Available online: http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf?ua=1&ua= (accessed on 1 October 2017).
- Bullard, K.M.; Cowie, C.C.; Lessem, S.E.; Saydah, S.H.; Menke, A.; Geiss, L.S.; Orchard, T.J.; Rolka, D.B.; Imperatore, G. Prevalence of Diagnosed Diabetes in Adults by Diabetes Type—United States 2016. Morb. Mortal. Wkly. Rep. 2018, 67, 359–361. [Google Scholar] [CrossRef]
- Thomas, N.J.; Jones, S.E.; Weedon, M.N.; Shields, B.M.; Oram, R.A.; Hattersley, A.T. Frequency and phenotype of type 1 diabetes in the first six decades of life: A cross-sectional, genetically stratified survival analysis from UK Biobank. Lancet Diabetes Endocrinol. 2018, 6, 122–129. [Google Scholar] [CrossRef]
- Litwak, L.; Goh, S.Y.; Hussein, Z.; Malek, R.; Prusty, V.; Khamseh, M.E. Prevalence of diabetes complications in people with type 2 diabetes mellitus and its association with baseline characteristics in the multinational A1chieve study. Diabetol. Metab. Syndr. 2013, 5, 57. [Google Scholar] [CrossRef]
- Genco, R.J.; Borgnakke, W.S. Risk factors for periodontal disease. Periodontology 2013, 62, 59–94. [Google Scholar] [CrossRef]
- Mealey, B.L.; Oates, T.W. Diabetes Mellitus and Periodontal Diseases. J. Periodontol. 2006, 77, 1289–1303. [Google Scholar] [CrossRef] [PubMed]
- Marian, D.; Rusu, D.; Stratul, S.I.; Calniceanu, H.; Sculean, A.; Anghel, A. Association of Vitamin D Receptor Gene Polymor-phisms with Chronic Periodontitis in a Population in Western Romania. Oral Health Prev. Dent. 2019, 17, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef]
- Lee, J.H.; Choi, J.K.; Kim, S.H.; Cho, K.H.; Kim, Y.T.; Choi, S.H.; Jung, U.W. Association between periodontal flap surgery for periodontitis and vasculogenic erectile dysfunction in Koreans. J. Periodontol. Implant Sci. 2017, 47, 96–105. [Google Scholar] [CrossRef]
- Etienne, D. Locally delivered antimicrobials for the treatment of chronic periodontitis. Oral Dis. 2003, 9, 45–50. [Google Scholar] [CrossRef]
- Bartold, P.M.; McCulloch, C.A.; Narayanan, A.S.; Pitaru, S. Tissue engineering: A new paradigm for periodontal regeneration based on molecular and cell biology. Periodontology 2010, 24, 253–269. [Google Scholar] [CrossRef]
- Zhang, Y.; Xing, Y.; Jia, L.; Ji, Y.; Zhao, B.; Wen, Y.; Xu, X. An in vitro comparative study of multisource derived human mesenchymal stem cells for bone tissue engineering. Stem Cells Dev. 2018, 27, 1634–1635. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, W.; Xiong, Y.; Zhang, Y.; Zhang, D.; Xu, X. Effects of rutin on the oxidative stress, proliferation and osteogenic differentiation of periodontal ligament stem cells in LPS-induced inflammatory environment and the underlying mechanism. J. Mol. Histol. 2020, 51, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Caglayan, C.; Kandemir, F.M.; Darendelioğlu, E.; Yıldırım, S.; Kucukler, S.; Dortbudak, M.B. Rutin ameliorates mercuric chlorideinduced hepatotoxicity in rats via interfering with oxidative stress, inflammation and apoptosis. J. Trace Elem. Med. Biol. 2019, 56, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Miao, Q.; Geng, M.; Liu, J.; Hu, Y.; Tian, L.; Pan, J.; Yang, Y. Anti-tumor effect of rutin on human neuroblastoma cell lines through inducing G2/M cell cycle arrest and promoting apoptosis. Sci. World J. 2013, 2013, 269165. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Bankar, R.; Roy, P. Assessment of the role of flavonoids for inducing osteoblast differentiation in isolated mouse bone marrow derived mesenchymal stem cells. Phytomedicine 2013, 20, 683–690. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, B.L.; Ting, H.; Yi, T.; Yang, J.-P.; He, B. Increase of rutin antioxidant activity by generating Maillard reaction products with lysine. Bioorg. Med. Chem. Lett. 2016, 26, 2680. [Google Scholar] [CrossRef] [PubMed]
- Mascaraque, C.; Aranda, C.; Ocon, B.; Monte, M.J.; Suarez, M.D.; Zarzuelo, A.; García Marín, J.J.; Martínez-Augustin, O.; Sánchez de Medina, F. Rutin has intestinal anti-inflammatory effects in the CD4+ CD62L+ T cell transfer model of colitis. Pharmacol. Res. 2014, 90, 48–57. [Google Scholar] [CrossRef]
- Kaatabi, H.; Bamosa, A.O.; Badar, A.; Al-Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Al-Khadra, A.; Al-Almaie, S. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabetes mellitus: Placebo controlled participant Blinded clinical trial. PLoS ONE 2015, 10, e0113486. [Google Scholar] [CrossRef]
- Sattanathan, S.; Dhanapal, C.K.; Umarani, R.; Manavalan, R. Beneficial health effects of rutin supplementation in patients with diabetes mellitus. J. Appl. Pharm. Sci. 2011, 1, 227. [Google Scholar]
- Tian, R.; Yang, W.; Xue, Q.; Gao, L.; Huo, J.; Ren, D.; Chen, X. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur. J. Pharmacol. 2016, 15, 84–92. [Google Scholar] [CrossRef]
- Wang, W.; Wu, Q.-H.; Sui, Y.; Wang, Y.; Qiu, X. Rutin protects endothelial dysfunction by disturbing Nox4 and ROS-sensitive NLRP3 inflammasome. Biomed. Pharmacother. 2017, 86, 32–40. [Google Scholar] [CrossRef]
- Yu, X.-L.; Li, Y.-N.; Zhang, H.; Su, Y.-J.; Zhou, W.-W.; Zhang, Z.-P.; Wang, S.-W.; Xu, P.-X.; Wang, Y.-J.; Liu, R.-T. Rutin inhibits amylin-induced neuro cytotoxicity and oxidative stress. Food Funct. 2015, 6, 3296–3306. [Google Scholar] [CrossRef]
- Aggarwal, B.B.; Sung, B. Pharmacological basis for the role of curcumin in chronic diseases: An age-old spice with modern targets. Trends Pharmacol. Sci. 2009, 30, 85–94. [Google Scholar] [CrossRef]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An Anti-Inflammatory Molecule from a Curry Spice on the Path to Cancer Treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Mali, A.M.; Gilda, S.S.; Paradkar, A.R.; Behal, R. Evaluation of local drug-delivery system containing 2% whole turmeric gel used as an adjunct to scaling and root planing in chronic periodontitis: A clinical and microbiological study. J. Indian Soc. Periodontol. 2011, 15, 35–38. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Chen, N.; Li, Q.; Sun, X.; Song, Y.; Wang, C. Curcumin inhibits inflammatory response and bone loss during experimental periodontitis in rats. Acta Odontol. Scand. 2012, 71, 349–356. [Google Scholar] [CrossRef]
- Guimarães, M.R.; Coimbra, L.S.; De Aquino, S.G.; Spolidorio, L.C.; Kirkwood, K.L.; Rossa, C. Potent anti-inflammatory effects of systemically administered curcumin modulate periodontal disease in vivo. J. Periodontal Res. 2011, 46, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Struillou, X.; Boutigny, H.; Soueidan, A.; Layrolle, P. Experimental Animal Models in Periodontology: A Review. Open Dent. J. 2010, 4, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, A.; Nikai, H.; Niitani, K.; Ijuhin, N. Ultrastructure of the Junctional Epithelium of Germfree Rat Gingiva. J. Periodontol. 1979, 50, 641–648. [Google Scholar] [CrossRef] [PubMed]
- Livada, R.; Shiloah, J.; Tipton, D.A.; Dabbous, M.K. The Potential Role of Curcumin in Periodontal Therapy: A Review of the Literature. J. Int. Acad. Periodontol. 2017, 19, 70. [Google Scholar]
- Giuliani, C.; Napolitano, G.; Bucci, I.; Montani, V.; Monaco, F. NF-κB transcription factor: Role in the pathogenesis of inflammatory, autoimmune, and neoplastic diseases and therapy implications. Clin. Ther. 2001, 152, 249–253. [Google Scholar]
- Hong, J.; Bose, M.; Ju, J.; Ryu, J.-H.; Chen, X.; Sang, S.; Lee, M.-J.; Yang, C.S. Modulation of arachidonic acid metabolism by curcumin and related β-diketone derivatives: Effects on cytosolic phospholipase A2, cyclooxygenases and 5-lipoxygenase. Carcinogenesis 2004, 25, 1671–1679. [Google Scholar] [CrossRef] [PubMed]
- Cohen, A.N.; Veena, M.S.; Srivatsan, E.S.; Wang, M.B. Suppression of Interleukin 6 and 8 Production in Head and Neck Cancer Cells with Curcumin via Inhibition of I?? Kinase. Arch. Otolaryngol. Head Neck Surg. 2009, 135, 190. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, M.; Millhouse, E.; Culshaw, S.; Edwards, C.A.; Ramage, G.; Combet, E. Selected dietary (poly)phenols inhibit periodontal pathogen growth and biofilm formation. Food Funct. 2014, 6, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Hu, P.; Huang, P.; Chen, M.W. Curcumin attenuates cyclooxygenase-2 expression via inhibition of the NF-κB pathway in lipopolysaccharide-stimulated human gingival fi broblasts. Cell Biol. Int. 2013, 37, 443–448. [Google Scholar] [CrossRef]
- Mau, L.P.; Cheng, W.C.; Chen, J.K.; Shieh, Y.S.; Cochran, D.L.; Huang, R.Y. Curcumin ameliorates alveolar bone destruction of experimental periodontitis by modulating osteoclast differentiation, activation and function. J. Funct. Foods 2016, 22, 243–256. [Google Scholar] [CrossRef]
- Bhatia, M. Novel Therapeutic Approach for the Treatment of Periodontitis by Curcumin. J. Clin. Diagn. Res. 2014, 8, ZC65–ZC69. [Google Scholar] [CrossRef]
- Anitha, V.; Rajesh, P.; Shanmugam, M.; Priya, B.M.; Prabhu, S.; Shivakumar, V. Comparative evaluation of natural curcumin and synthetic chlorhexidine in the management of chronic periodontitis as a local drug delivery: A clinical and microbiological study. Indian J. Dent. Res. 2015, 26, 53–56. [Google Scholar] [CrossRef]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef]
- Soskolne, W.A.; Klinger, A. The relationship between periodontal diseases and diabetes: An overview. Ann. Periodontol. 2001, 6, 91–98. [Google Scholar] [CrossRef]
- Tanko, Y.; Salisu, A.I.; Mohammed, K.A.; Musa, S.A.; Jimoh, A.; Yusuf, R. Anti-hyperglycaemic Effects of Rutin on Blood Glu-cose, Oxidative Stress Biomarkers and Lipid Peroxidation in Alloxan-induced Hyperglycaemic Wistar Rats. Niger. J. Physiol. Sci. 2017, 32, 91. [Google Scholar]
- La Casa, C.; Villegas, I.; De La Lastra, C.A.; Motilva, V.; Calero, M.M. Evidence for protective and antioxidant properties of rutin, a natural flavone, against ethanol induced gastric lesions. J. Ethnopharmacol. 2000, 71, 45–53. [Google Scholar] [CrossRef]
- Janbaz, K.H.; Saeed, S.A.; Gilani, A.H. Protective effect of rutin on paracetamol- and CCl4-induced hepatotoxicity in rodents. Fitoterapia 2002, 73, 557–563. [Google Scholar] [CrossRef]
- Fernandes, A.A.H.; Novelli, E.L.B.; Okoshi, K.; Okoshi, M.P.; Di Muzio, B.P.; Guimarães, J.F.C.; Junior, A.F. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed. Pharmacother. 2010, 64, 214–219. [Google Scholar] [CrossRef]
- Do, M.-J.; Kim, K.; Lee, H.; Cha, S.; Seo, T.; Park, H.-J.; Lee, J.-S.; Kim, T.-I. Development of animal experimental periodontitis models. J. Periodontal Implant Sci. 2013, 43, 147–152. [Google Scholar] [CrossRef] [PubMed]
- Klausen, B. Microbiological and Immunological Aspects of Experimental Periodontal Disease in Rats: A Review Article. J. Periodontol. 1991, 62, 59–73. [Google Scholar] [CrossRef]
- Padalkar, R.K.; Shinde, A.V.; Patil, S.M. Lipid profile, serum malondialdehyde, superoxide dismutase in chronic kidney diseases and Type 2 diabetes mellitus. Biomed. Res. 2012, 23, 207–210. [Google Scholar]
- Tomofuji, T.; Ekuni, D.; Irie, K.; Azuma, T.; Endo, Y.; Tamaki, N.; Sanbe, T.; Murakami, J.; Yamamoto, T.; Morita, M. Preventive Effects of a Cocoa-Enriched Diet on Gingival Oxidative Stress in Experimental Periodontitis. J. Periodontol. 2009, 80, 1799–1808. [Google Scholar] [CrossRef] [PubMed]
- De Araújo, R.F.; Souza, T.O.; De Moura, L.M.; Torres, K.P.; De Souza, L.B.; Alves, M.D.S.C.F.; Rocha, H.O.; De Araújo, A.A. Atorvastatin Decreases Bone Loss, Inflammation and Oxidative Stress in Experimental Periodontitis. PLoS ONE 2013, 8, e75322. [Google Scholar] [CrossRef] [PubMed]
- Garcea, G.; Berry, D.P.; Jones, D.J.; Singh, R.; Dennison, A.R.; Farmer, P.B.; Sharma, R.A.; Steward, W.P.; Gescher, A.J. Consumption of the putative chemopreventive agent curcumin by cancer patients: Assessment of curcumin levels in the colorectum and their pharmacodynamic consequences. Cancer Epidemiol. Biomark. Prev. 2005, 14, 120–125. [Google Scholar]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef]
- Tiyaboonchai, W.; Tungpradit, W.; Plianbangchang, P. Formulation and characterization of curcuminoids loaded solid lipid nanoparticles. Int. J. Pharm. 2007, 337, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Braiteh, F.S.; Kurzrock, R. Liposome-encapsulated curcumin: In vitro and in vivo effects on proliferation, apoptosis, sig-naling, and angiogenesis. Cancer 2005, 104, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Suresh, D.; Srinivasan, K. Studies on the in vitro absorption of spice principles—Curcumin, capsaicin and piperine in rat intestines. Food Chem. Toxicol. 2007, 45, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Lou, H.; Zhao, L.; Fan, P. Validated LC/MS/MS assay for curcumin and tetrahydro curcumin in rat plasma and application to pharmacokinetic study of phospholipid complex of curcumin. J. Pharm. Biomed. Anal. 2006, 40, 720–727. [Google Scholar] [CrossRef]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. Pharmacol. 2015, 70, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Madden, T.E.; Caton, J.G. Animal models for periodontal disease. Methods Enzymol. 1994, 235, 106–119. [Google Scholar]
- Cai, X.; Li, C.; Du, G.; Cao, Z. Protective effects of baicalin on ligature-induced periodontitis in rats. J. Periodontal Res. 2008, 43, 14–21. [Google Scholar] [CrossRef]
- Jeong-Hyon, K.; Bon-Hyuk, G.; Sang-Soo, N.; Yeon-Cheol, P. A review of rat models of periodontitis treated with natural extracts. J. Tradit. Chin. Med. Sci. 2020, 7, 95–103. [Google Scholar] [CrossRef]
- Gutierres, V.O.; Pinheiro, C.M.; Assis, R.P.; Vendramini, R.C.; Pepato, M.T.; Brunetti, I.L. Curcumin-supplemented yoghurt improves physiological and biochemical markers of experimental diabetes. Br. J. Nutr. 2011, 108, 440–448. [Google Scholar] [CrossRef]
- Murugan, P.; Pari, L. Antioxidant effect of tetrahydro curcumin in streptozotocin–nicotinamide induced diabetic rats. Life Sci. 2006, 79, 1720–1728. [Google Scholar] [CrossRef]
- Sharma, S.; Kulkarni, S.K.; Chopra, K. Curcumin, The Active Principle of Turmeric (Curcuma Longa), Ameliorates Diabetic Nephropathy in Rats. Clin. Exp. Pharmacol. Physiol. 2006, 33, 940–945. [Google Scholar] [CrossRef]
- Tikoo, K.; Meena, R.L.; Kabra, D.G.; Gaikwad, A.B. Change in post-translational modifications of histone H3, heat-shock protein-27 and MAP kinase p38 expression by curcumin in streptozotocin-induced type I diabetic nephropathy. Br. J. Pharmacol. 2008, 153, 1225–1231. [Google Scholar] [CrossRef]
- Kamalakkannan, N.; Prince, P.S.M. Antihyperglycaemic and Antioxidant Effect of Rutin, a Polyphenolic Flavonoid, in Streptozotocin-Induced Diabetic Wistar Rats. Basic Clin. Pharmacol. Toxicol. 2006, 98, 97–103. [Google Scholar] [CrossRef]
- Peterson, G.L. A simplification of the protein assay method of Lowry et al. which is more generally applicable. Anal. Biochem. 1977, 83, 346–356. [Google Scholar] [CrossRef]
- Conti, M.; Morand, P.C.; Levillain, P.; Lemonnier, A. Improved fluorometric determination of malonaldehyde. Clin. Chem. 1991, 37, 1273. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.-L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzym. 1994, 233, 380–385. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Pippenger, C.E.; Browne, R.W.; Armstrong, D. Regulatory antioxidant enzymes. Methods Mol. Biol. 1998, 108, 299–313. [Google Scholar] [PubMed]
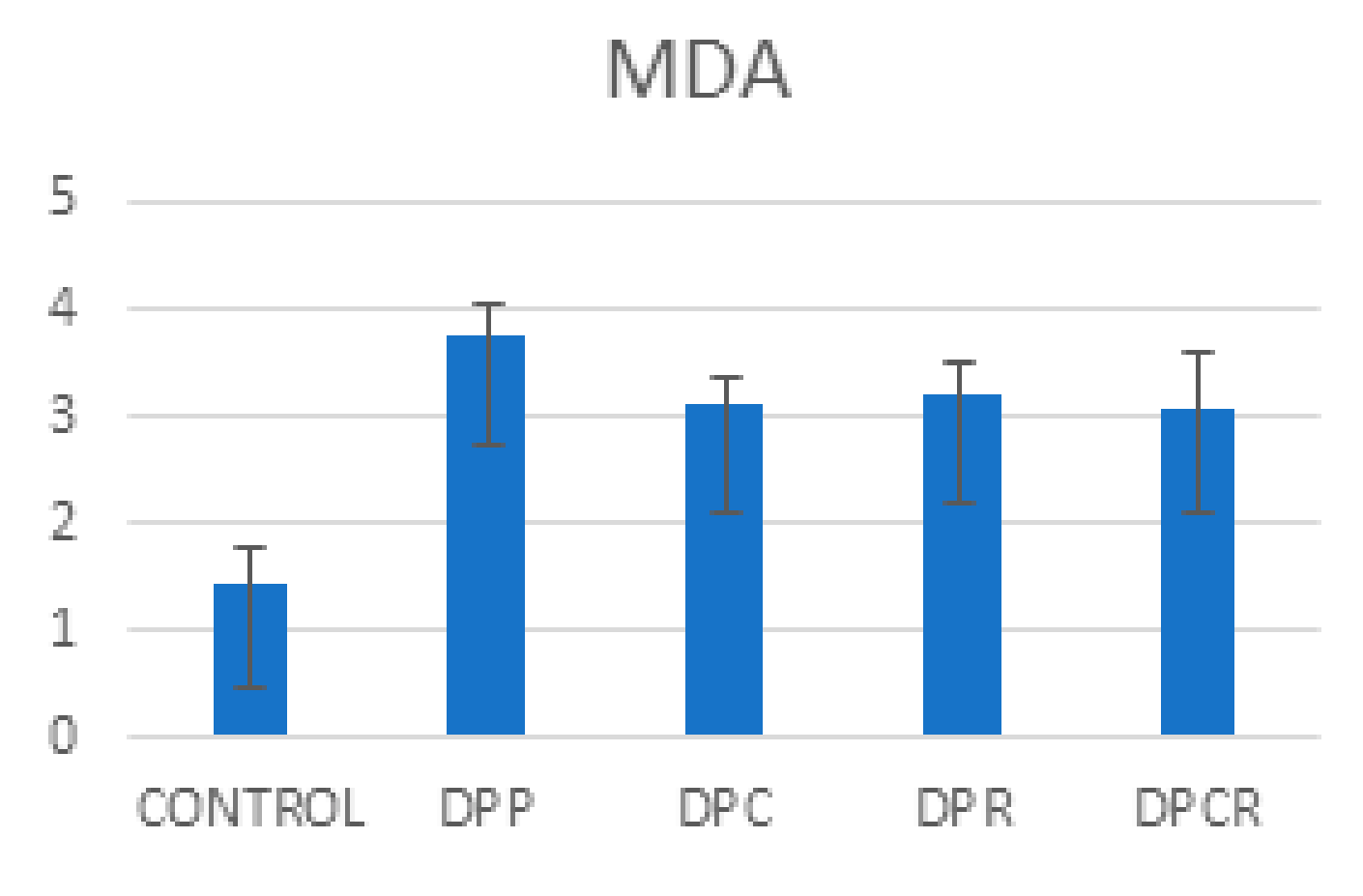



| SS | F | p-Value | |
|---|---|---|---|
| MDA | 34.97 | 52.366 | 0 |
| GSH | 150.29 | 26.08 | 0 |
| GSSG | 5.66 | 22.77 | 0 |
| GSH/GSSG | 882.56 | 55.26 | 0 |
| CAT | 206,138 | 14.1 | 0 |
| MDA | GSH | GSSG | GSH/GSSG | CAT | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | Group | Mean Diff | p-Value | Mean Diff | p-Value | Mean Diff | p-Value | Mean Diff | p-Value | Mean Diff | p-Value |
| 1 | 2 | −2.28 * | 0 | 4.53 * | 0 | −0.87 * | 0 | 11.48 * | 0 | 155.47 * | 0 |
| 3 | −1.65 * | 0 | 2.87 * | 0 | −0.39 * | 0.004 | 8.35 * | 0 | 81.8 * | 0.007 | |
| 4 | −1.73 * | 0 | 3.01 * | 0 | −0.54 * | 0 | 8.69 * | 0 | 87.2 * | 0.003 | |
| 5 | −1.63 * | 0 | 3.04 * | 0 | −0.52 * | 0 | 8.52 * | 0 | 77.4 * | 0.012 | |
| 2 | 1 | −2.28 * | 0 | 4.53 * | 0 | −0.87 * | 0 | 11.48 * | 0 | 155.47 * | 0 |
| 3 | 0.63 * | 0.015 | −1.66 * | 0.02 | 0.48 * | 0 | −3.13 * | 0.015 | −74 * | 0.024 | |
| 4 | 0.54 * | 0.05 | −1.51 * | 0.043 | 0.32 * | 0.026 | −2.78 * | 0.039 | −68.27 * | 0.043 | |
| 5 | 0.65 * | 0.012 | −1.49 * | 0.047 | 0.34 * | 0.016 | −2.95 * | 0.024 | −78.07 * | 0.014 | |
| 3 | 1 | −1.65 * | 0 | 2.87 * | 0 | −0.39 * | 0.004 | 8.35 * | 0 | 81.8 * | 0.007 |
| 2 | 0.63 * | 0.015 | −1.66 * | 0.02 | 0.48 * | 0 | −3.13 * | 0.015 | −74 * | 0.024 | |
| 4 | −0.08 | 0.991 | 0.14 | 0.999 | −0.15 | 0.591 | 0.34 | 0.996 | 5.4 | 0.999 | |
| 5 | 0.013 | 1 | 0.16 | 0.998 | −0.13 | 0.709 | 0.17 | 1 | −4.4 | 1 | |
| 4 | 1 | −1.73 * | 0 | 3.01 * | 0 | −0.54 * | 0 | 8.69 * | 0 | 87.2 * | 0.003 |
| 2 | 0.54 * | 0.05 | −1.51 * | 0.043 | 0.32 * | 0.026 | −2.78 * | 0.039 | −68.27 * | 0.043 | |
| 3 | −0.08 | 0.991 | 0.14 | 0.999 | −0.15 | 0.591 | 0.34 | 0.996 | 5.4 | 0.999 | |
| 5 | 0.1 | 0.984 | 0.02 | 1 | 0.01 | 1 | −0.16 | 1 | −9.8 | 0.993 | |
| SS | F | p-Value | |
|---|---|---|---|
| MDA | 4.28 | 0.19 | 0.825 |
| GSH | 15.64 | 0.14 | 0.862 |
| GSSG | 1.13 | 1.91 | 0.167 |
| GSH/GSSG | 32.25 | 0.25 | 0.778 |
| CAT | 45,341.37 | 0.14 | 0.866 |
| SS | F | p-Value | |
|---|---|---|---|
| MDA | 4.68 | 8.01 | 0 |
| CAT | 9975.88 | 11.6 | 0 |
| MDA | CAT | ||||
|---|---|---|---|---|---|
| Group | Group | Mean Diff | p-Value | Mean Diff | p-Value |
| 1 | 2 | −1.05 * | 0.01 | 46.89 * | 0.0 |
| 3 | −0.63 * | 0.05 | 33.38 * | 0.00 | |
| 4 | −0.67 * | 0.03 | 44.23 * | 0.00 | |
| 5 | −0.64 * | 0.05 | 31.45 * | 0.01 | |
| 2 | 1 | 1.05 * | 0.0 | −46.89 * | 0.00 |
| 3 | 0.41 | 0.33 | −13.5 | 0.56 | |
| 4 | 0.38 | 0.42 | −2.65 | 0.99 | |
| 5 | 0.41 | 0.34 | −15.43 | 0.43 | |
| 3 | 1 | 0.63 * | 0.05 | −33.38 * | 0.00 |
| 2 | −0.41 | 0.33 | 13.5 | 0.56 | |
| 4 | −0.03 | 1 | 10.84 | 0.74 | |
| 5 | −0.003 | 1 | −1.93 | 0.99 | |
| 4 | 1 | 0.67 * | 0.03 | −44.23 * | 0.00 |
| 2 | −0.38 | 0.42 | 2.65 | 0.99 | |
| 3 | 0.03 | 1 | −10.84 | 0.74 | |
| 5 | 0.03 | 1 | −12.78 | 0.61 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iova, G.M.; Calniceanu, H.; Popa, A.; Szuhanek, C.A.; Marcu, O.; Ciavoi, G.; Scrobota, I. The Antioxidant Effect of Curcumin and Rutin on Oxidative Stress Biomarkers in Experimentally Induced Periodontitis in Hyperglycemic Wistar Rats. Molecules 2021, 26, 1332. https://doi.org/10.3390/molecules26051332
Iova GM, Calniceanu H, Popa A, Szuhanek CA, Marcu O, Ciavoi G, Scrobota I. The Antioxidant Effect of Curcumin and Rutin on Oxidative Stress Biomarkers in Experimentally Induced Periodontitis in Hyperglycemic Wistar Rats. Molecules. 2021; 26(5):1332. https://doi.org/10.3390/molecules26051332
Chicago/Turabian StyleIova, Gilda M., Horia Calniceanu, Adelina Popa, Camelia A. Szuhanek, Olivia Marcu, Gabriela Ciavoi, and Ioana Scrobota. 2021. "The Antioxidant Effect of Curcumin and Rutin on Oxidative Stress Biomarkers in Experimentally Induced Periodontitis in Hyperglycemic Wistar Rats" Molecules 26, no. 5: 1332. https://doi.org/10.3390/molecules26051332
APA StyleIova, G. M., Calniceanu, H., Popa, A., Szuhanek, C. A., Marcu, O., Ciavoi, G., & Scrobota, I. (2021). The Antioxidant Effect of Curcumin and Rutin on Oxidative Stress Biomarkers in Experimentally Induced Periodontitis in Hyperglycemic Wistar Rats. Molecules, 26(5), 1332. https://doi.org/10.3390/molecules26051332







