Comparison of the Phytochemical Properties, Antioxidant Activity and Cytotoxic Effect on HepG2 Cells in Mongolian and Taiwanese Rhubarb Species
Abstract
1. Introduction
2. Results
2.1. Total Phenolic, Anthraquinone, and Flavonoid Contents
2.2. Determination and Quantification of Bioactive Constituents of Each Extract through High-Performance Liquid Chromatography (HPLC) Assay
2.3. Antioxidant Activities of Each Extract
2.4. Cytotoxicity of RU and RC Extracts on Liver Cancer Cell Lines
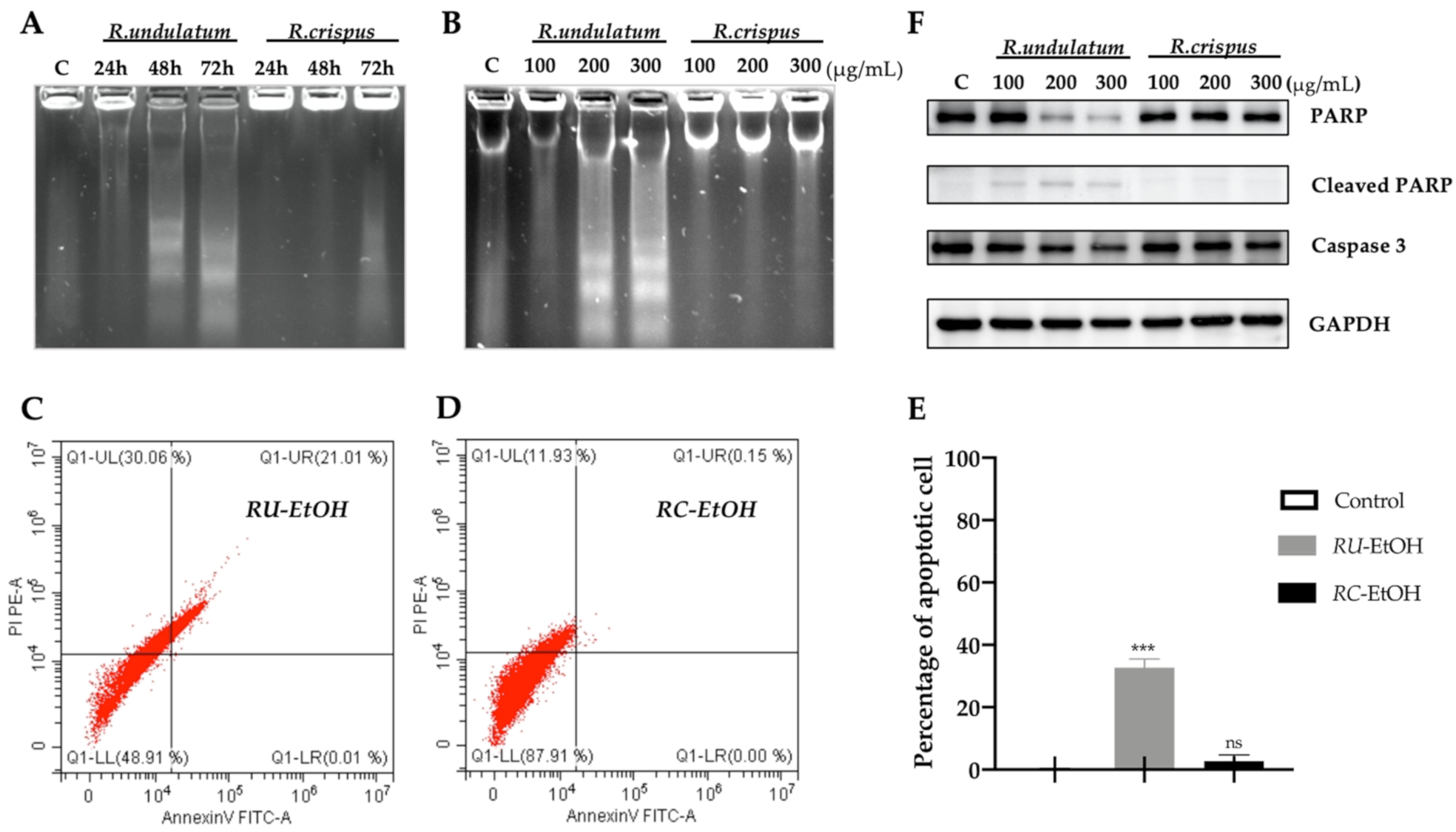
2.5. Elucidation of Liver Cancer Cell Death
3. Discussion
4. Materials and Methods
4.1. Plant Material Collection
4.2. Plant Extraction
4.3. Quantitative Phytochemical Analysis of Extracts
4.3.1. Total Phenolic Content
4.3.2. Total Anthraquinone Content
4.3.3. Total Flavonoid Content
4.4. Standardization and Quantitative Analysis of Anthraquinone Derivatives from Extract of RU and RC through the Use of High-Performance Liquid Chromatography (HPLC)
4.5. Analysis of Antioxidant and Free Radical Scavenging Activities
4.5.1. ABTS Scavenging Activity
4.5.2. DPPH Scavenging Activity
4.5.3. Ferric Reducing Antioxidant Power Assay (FRAP)
4.6. In Vitro Assays
4.6.1. Cell Culture
4.6.2. Cell Viability Assay: (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide Assay (MTT Assay)
4.7. DNA Fragmentation Assay
4.7.1. Gel Preparation
4.7.2. DNA Loading Procedure
4.8. Western Blot
4.9. Annexin V-Fluorescein Isothiocyanate/Propidium Iodide Analysis
4.10. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Zhuang, T.; Gu, X.; Zhou, N.; Ding, L.; Yang, L.; Zhou, M. Hepatoprotection and hepatotoxicity of Chinese herb Rhubarb (Dahuang): How to properly control the “General (Jiang Jun)” in Chinese medical herb. Biomed. Pharmacother. 2020, 127, 110224. [Google Scholar] [CrossRef] [PubMed]
- Tan, Z.-B.; Fan, H.-J.; Wu, Y.-T.; Xie, L.-P.; Bi, Y.-M.; Xu, H.-L.; Chen, H.-M.; Li, J.; Liu, B.; Zhou, Y.-C. Rheum palmatum extract exerts anti-hepatocellular carcinoma effects by inhibiting signal transducer and activator of transcription 3 signaling. J. Ethnopharmacol. 2019, 232, 62–72. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.W.; Hwang, B.S.; Kim, M.-H.; Park, C.-S.; Lee, W.S.; Oh, H.-M.; Rho, M.-C. Inhibition of LFA-1/ICAM-1-mediated cell adhesion by stilbene derivatives from Rheum undulatum. Arch. Pharmacal Res. 2012, 35, 1763–1770. [Google Scholar] [CrossRef]
- Choi, E.-S.; Cho, S.-D.; Jeon, J.-G.; Cho, N.-P. The Apoptotic Effect of the Hexane Extract of Rheum undulatum L. in Oral Cancer Cells through the Down-regulation of Specificity Protein 1 and Survivin. Lab. Anim. Res. 2011, 27, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.J.; Hong, N.R.; Park, H.S.; Ahn, T.S.; Jung, M.H. Association of a Methanol Extract of Rheum undulatum L. Mediated Cell Death in AGS Cells with an Intrinsic Apoptotic Pathway. J. Pharmacopunct. 2015, 18, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.-J.; Yoon, G.; Hwang, Y.R.; Kim, Y.K.; Kim, S.-N. Anti-obesity and hypolipidemic effects of Rheum undulatum in high-fat diet-fed C57BL/6 mice through protein tyrosine phosphatase 1B inhibition. BMB Rep. 2012, 45, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Moon, M.K.; Kang, D.G.; Lee, J.K.; Kim, J.S.; Lee, H.S. Vasodilatory and anti-inflammatory effects of the aqueous extract of rhubarb via a NO-cGMP pathway. Life Sci. 2006, 78, 1550–1557. [Google Scholar] [CrossRef] [PubMed]
- Cavers, P.B.; Harper, J.L. Rumex obtusifolius L. and R. crispus L. J. Ecol. 1964, 52, 737–766. [Google Scholar] [CrossRef]
- Vasas, A.; Orbán-Gyapai, O.; Hohmann, J. The Genus Rumex: Review of traditional uses, phytochemistry and pharmacology. J. Ethnopharmacol. 2015, 175, 198–228. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.-S.; Lee, B.; Ma, J.Y. Water extract of Rumex crispus prevents bone loss by inhibiting osteoclastogenesis and inducing osteoblast mineralization. BMC Complement. Altern. Med. 2017, 17, 483. [Google Scholar] [CrossRef] [PubMed]
- Feduraev, P.; Chupakhina, G.; Maslennikov, P.; Tacenko, N.; Skrypnik, L. Variation in Phenolic Compounds Content and Anti-oxidant Activity of Different Plant Organs from Rumex crispus L. and Rumex obtusifolius L. at Different Growth Stages. Antioxidants 2019, 8, 237. [Google Scholar]
- El–Serag, H.B.; Rudolph, K.L. Hepatocellular Carcinoma: Epidemiology and Molecular Carcinogenesis. Gastroenterology 2007, 132, 2557–2576. [Google Scholar] [CrossRef]
- Baatarkhuu, O.; Gerelchimeg, T.; Munkh-Orshikh, D.; Batsukh, B.; Sarangua, G.; Amarsanaa, J. Epide-miology, Genotype Distribution, Prognosis, Control and Management of Viral Hepatitis B, C, D, and Hepatocellular Carcinoma in Mongolia. Euroasian J. Hepato-Gastroenterol. 2018, 8, 57. [Google Scholar] [CrossRef]
- Ferlay, J.; Ervik, M.; Lam, F.; Colombet, M.; Mery, L.; Pineros, M.; Znaor, A.; Soerjotaram, I.; Bray, F. Global Cancer Observatory: Cancer Today. International Agency for Research on Cancer Lyon. 2018. Available online: https://gco.iarc.fr/today (accessed on 20 February 2021).
- Park, J.W.; Chen, M.; Colombo, M.; Roberts, L.R.; Schwartz, M.; Chen, P.J.; Sherman, M. Global patterns of hepatocellular carcinoma man-agement from diagnosis to death: The BRIDGE Study. Liver Int. 2015, 35, 2155–2166. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Hamid, N.M.; Abass, S.A.; Mohamed, A.A.; Hamid, D.M. Herbal management of hepatocellular carcinoma through cutting the pathways of the common risk factors. Biomed. Pharmacother. 2018, 107, 1246–1258. [Google Scholar] [CrossRef]
- Muraina, I.; Adaudi, A.; Mamman, M.; Kazeem, H.; Eloff, J. Effects of geographical location on the yield and bioactivity of Anoigeissus leiocarpus. J. Pharm. Bioresour. 2010, 5, 68–72. [Google Scholar] [CrossRef]
- Zhao, B.; Li, J.; Yuan, R.; Mao, S. Adaptive evolution of the rbcL gene in the genus Rheum (Polygonaceae). Biotechnol. Biotechnol. Equip. 2017, 31, 493–498. [Google Scholar] [CrossRef]
- Zhang, L.; He, D.; Li, K.; Liu, H.; Wang, B.; Zheng, L.; Li, J. Emodin targets mitochondrial cyclophilin D to induce apoptosis in HepG2 cells. Biomed. Pharmacother. 2017, 90, 222–228. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.H.; Chen, P.Y.; Lu, H.F.; Yang, J.S.; Huang, H.Y.; Wu, S.H.; Chung, J.G. Chrysophanol-induced necrotic-like cell death through an im-paired mitochondrial ATP synthesis in Hep3B human liver cancer cells. Arch. Pharm. Res. 2012, 35, 887–895. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.P.; Wang, C.; Li, Y.; Huang, L.H. Physcion induces apoptosis through triggering endoplasmic reticulum stress in hepato-cellular carcinoma. Biomed. Pharmacother. 2018, 99, 894–903. [Google Scholar] [CrossRef]
- Cheung, Y.; Meenu, M.; Yu, X.; Xu, B. Phenolic acids and flavonoids profiles of commercial honey from different floral sources and geographic sources. Int. J. Food Prop. 2019, 22, 290–308. [Google Scholar] [CrossRef]
- Trinh, T.A.; Lee, D.; Park, S.; Kim, S.H.; Park, J.G.; Kim, J.H.; Kang, K.S. Stilbenes contribute to the anticancer effects of Rheum undulatum L. through activation of apoptosis. Oncol. Lett. 2019, 17, 2953–2959. [Google Scholar] [CrossRef]
- Eom, T.; Kim, E.; Kim, J.S. In Vitro Antioxidant, Antiinflammation, and Anticancer Activities and Anthraquinone Content from Rumex crispus Root Extract and Fractions. Antioxidants 2020, 9, 726. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.-J.; Choi, J.-H.; Koo, B.-S.; Ryu, S.-Y.; Han, Y.-H.; Lee, S.-I.; Lee, D.-U. Antimutagenicity and Cytotoxicity of the Constituents from the Aerial Parts of Rumex acetosa. Biol. Pharm. Bull. 2005, 28, 2158–2161. [Google Scholar] [CrossRef] [PubMed]
- Freeman, B.L.; Eggett, D.L.; Parker, T.L. Synergistic and Antagonistic Interactions of Phenolic Compounds Found in Navel Oranges. J. Food Sci. 2010, 75, C570–C576. [Google Scholar] [CrossRef] [PubMed]
- Mercado-Mercado, G.; De La Rosa, L.A.; Alvarez-Parrilla, E.; De La Rosa-Carrillo, L.A. Effect of pectin on the interactions among phenolic compounds determined by antioxidant capacity. J. Mol. Struct. 2020, 1199, 126967. [Google Scholar] [CrossRef]
- Hsu, C.-M.; Hsu, Y.-A.; Tsai, Y.; Shieh, F.-K.; Huang, S.-H.; Wan, L.; Tsai, F.-J. Emodin inhibits the growth of hepatoma cells: Finding the common anti-cancer pathway using Huh7, Hep3B, and HepG2 cells. Biochem. Biophys. Res. Commun. 2010, 392, 473–478. [Google Scholar] [CrossRef]
- Lu, G.D.; Shen, H.-M.; Ong, C.N.; Chung, M.C.M. Anticancer effects of aloe-emodin on HepG2 cells: Cellular and proteomic studies. Proteom. Clin. Appl. 2007, 1, 410–419. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, C.M.; Singh, A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018, 19, 448. [Google Scholar] [CrossRef]
- Gaur, K.; Vázquez-Salgado, A.M.; Duran-Camacho, G.; Dominguez-Martinez, I.; Benjamín-Rivera, J.A.; Fernández-Vega, L.; Sarabia, L.C.; García, A.C.; Pérez-Deliz, F.; Román, J.A.M.; et al. Iron and Copper Intracellular Chelation as an Anticancer Drug Strategy. Inorganics 2018, 6, 126. [Google Scholar] [CrossRef]
- Kontoghiorghes, G.J. Advances on Chelation and Chelator Metal Complexes in Medicine. Int. J. Mol. Sci. 2020, 21, 2499. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Wang, C.; Zhang, T. Retracted: Physcion Synergistically Enhances the Cytotoxicity of Sorafenib in Hepatocellular Carcinoma. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2019, 302, 2171–2177. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.-C.; Yang, J.-S.; Huang, A.-C.; Hsia, T.-C.; Chou, S.-T.; Kuo, C.-L.; Lu, H.-F.; Lee, T.-H.; Wood, W.G.; Chung, J.-G. Chrysophanol induces necrosis through the production of ROS and alteration of ATP levels in J5 human liver cancer cells. Mol. Nutr. Food Res. 2010, 54, 967–976. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antiox-idants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Sakulpanich, A.; Gritsanapan, W. Extraction method for high content of anthraquinones from cassia fistula pods. J. Health Res. 2008, 22, 167–172. [Google Scholar]
- Ghosh, S.; Derle, A.; Ahire, M.; More, P.; Jagtap, S.; Phadatare, S.D.; Patil, A.B.; Jabgunde, A.M.; Sharma, G.K.; Shinde, V.S.; et al. Phytochemical Analysis and Free Radical Scavenging Activity of Medicinal Plants Gnidia glauca and Dioscorea bulbifera. PLoS ONE 2013, 8, e82529. [Google Scholar] [CrossRef]
- Zheleva-Dimitrova, D.; Nedialkov, P.; Kitanov, G. Radical scavenging and antioxidant activities of methanolic extracts from Hypericum species growing in Bulgaria. Pharmacogn Mag. 2010, 6, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH antioxidant assay revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Faria, A.; Oliveira, J.; Neves, P.; Gameiro, P.; Santos-Buelga, C.; De Freitas, V.; Mateus, N. Antioxidant Properties of Prepared Blueberry (Vaccinium myrtillus) Extracts. J. Agric. Food Chem. 2005, 53, 6896–6902. [Google Scholar] [CrossRef]
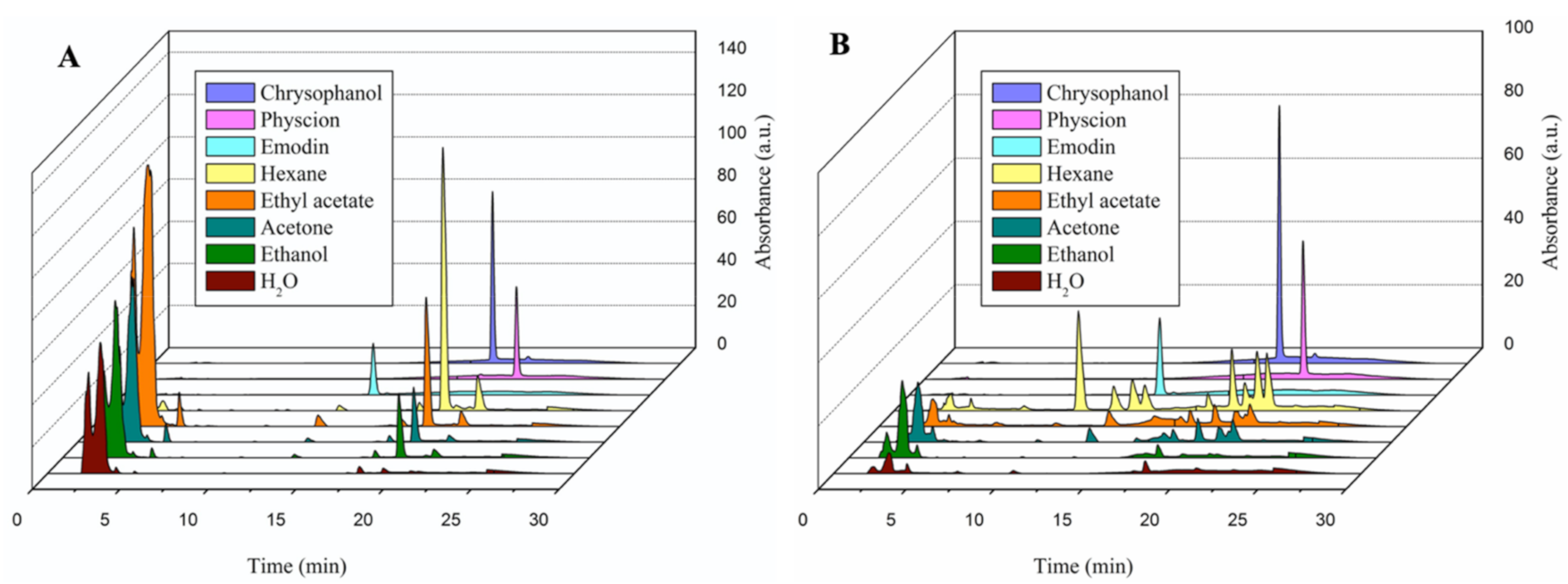
| Total Phenols (mg GAE/g DW) | Total Anthraquinones (mg EE/g DW) | Total Flavonoids (mg QE/g DW) | ||||
|---|---|---|---|---|---|---|
| RU | RC | RU | RC | RU | RC | |
| n-Hex | 2.44 ± 0.69 | 4.23 ± 0.36 ns | 94.10 ± 4.41 b | 73.65 ± 11.30 | 47.47 ± 1.64 | 192.60 ± 0.47 a |
| EtOAc | 11.16 ± 1.41 a | 2.89 ± 0.29 | 33.68 ± 2.28 | 34.14 ± 5.47 ns | 50.98 ± 1.79 | 70.99 ± 3.72 b |
| Ac | 8.45 ± 0.61 b | 5.01 ± 0.08 | 15.14 ± 2.47 | 43.54 ± 3.82 a | 37.92 ± 12.44 | 121.63 ± 10.87 a |
| EtOH | 9.57 ± 1.03 a | 2.96 ± 0.17 | 25.15 ± 2.69 b | 11.99 ± 2.90 | 50.70 ± 2.96 ns | 42.66 ± 1.21 |
| H2O | 8.39 ± 1.83 a | 3.5 ± 0.44 | 9.43 ± 3.31 | 12.73 ± 2.27 ns | 49.63 ± 10.53 ns | 39.51 ± 4.96 |
| RU | RC | |||||
|---|---|---|---|---|---|---|
| Chrysophanol | Physcion | Emodin | Chrysophanol | Physcion | Emodin | |
| n-Hex | 120.06 ± 13.82 a | 19.02 ± 2.58 ns | 3.33 ± 0.54 | 17.25 ± 5.23 | 20.34 ± 3.69 | 10.59 ± 2.47 a |
| EtOAc | 58.83 ± 6.29 a | 8.07 ± 0.82 ns | 6.51 ± 1.67 ns | 5.01 ± 0.27 | 6.48 ± 1.02 | 5.19 ± 0.89 |
| Ac | 24.15 ± 2.69 a | 3.5 ± 0.58 | 3.12 ± 0.21 | 6.47 ± 0.59 | 7.05 ± 1.98 a | 5.42 ± 1.02 a |
| EtOH | 28.81 ± 2.08 a | 4.53 ± 0.34 a | 2.39 ± 0.27 a | 0.09 ± 0.02 | 0.98 ± 0.19 | 0.74 ± 0.08 |
| H2O | 1.29 ± 0.2 a | 0.79 ± 0.14 a | 0.26 ± 0.08 a | 0 ± 0.00 | 0.40 ± 0.06 | 0 ± 0.01 |
| IC50 (µg/mL) | FRAP (mMFe2+/g) | |||||
|---|---|---|---|---|---|---|
| ABTS | DPPH | |||||
| GA | 3.16 ± 0.15 | 1.25 ± 0.20 | nt | |||
| Trolox | 13.53 ± 0.40 | 6.28 ± 1.58 | nt | |||
| RU | RC | RU | RC | RU | RC | |
| n-Hex | 93.23 ± 4.18 | 75.12 ± 8.98 a | 1800.87 ± 151.03 | 74.39 ± 0.01 a | 0.99 ± 0.30 | 1.79 ± 0.13 b |
| EtOAc | 5.67 ± 0.23 a | 207.17 ± 2.50 | 41.37 ± 1.98 b | 246.76 ± 20.02 | 6.42 ± 0.24 a | 1.26 ± 0.15 |
| Ac | 10.25 ± 0.51 a | 52.05 ± 2.64 | 53.49 ± 4.93 ns | 70.01 ± 0.01 | 5.17 ± 0.19 a | 2.65 ± 0.13 |
| EtOH | 14.08 ± 0.13 a | 77.95 ± 3.31 | 52.12 ± 3.29 ns | 146.72 ± 1.46 | 4.66 ± 0.04 a | 1.98 ± 0.17 |
| H2O | 14.09 ± 1.88 a | 70.55 ± 1.81 | 46.15 ± 4.66 ns | 137.40 ± 1.82 | 4.86 ± 0.26 a | 2.34 ± 0.22 |
| Solvents | RU (IC50 µg/mL) | RC (IC50 µg/mL) | ||||
|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 24 h | 48 h | 72 h | |
| n-Hex | 625.22 ± 30.73 | 419.77 ± 13.20 | 427.55 ± 21.54 | nt | 1973.14 ± 257.15 | 146.09 ± 24.99 |
| EtOAc | 449.82 ± 51.68 | 208.46 ± 30.63 | 191.63 ± 26.31 | nt | 925.30 ± 126.58 | 468.95 ± 7.59 |
| Ac | 403.69 ± 52.45 | 187.18 ± 20.59 | 170.16 ± 28.03 | nt | 606.56 ± 41.88 | 456.74 ± 20.10 |
| EtOH | 262.28 ± 13.99 | 171.94 ± 6.56 | 167.09 ± 8.99 | 2792.33 ± 396.28 | 1753.02 ± 77.84 | 1617.32 ± 31.49 |
| H2O | 880.33 ± 64.16 | 555.84 ± 2.09 | 635.09 ± 22.58 | 2465.74 ± 317.40 | 1397.90 ± 30.76 | 1176.79 ± 16.36 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jargalsaikhan, G.; Wu, J.-Y.; Chen, Y.-C.; Yang, L.-L.; Wu, M.-S. Comparison of the Phytochemical Properties, Antioxidant Activity and Cytotoxic Effect on HepG2 Cells in Mongolian and Taiwanese Rhubarb Species. Molecules 2021, 26, 1217. https://doi.org/10.3390/molecules26051217
Jargalsaikhan G, Wu J-Y, Chen Y-C, Yang L-L, Wu M-S. Comparison of the Phytochemical Properties, Antioxidant Activity and Cytotoxic Effect on HepG2 Cells in Mongolian and Taiwanese Rhubarb Species. Molecules. 2021; 26(5):1217. https://doi.org/10.3390/molecules26051217
Chicago/Turabian StyleJargalsaikhan, Ganbolor, Jin-Yi Wu, Yen-Chou Chen, Ling-Ling Yang, and Ming-Shun Wu. 2021. "Comparison of the Phytochemical Properties, Antioxidant Activity and Cytotoxic Effect on HepG2 Cells in Mongolian and Taiwanese Rhubarb Species" Molecules 26, no. 5: 1217. https://doi.org/10.3390/molecules26051217
APA StyleJargalsaikhan, G., Wu, J.-Y., Chen, Y.-C., Yang, L.-L., & Wu, M.-S. (2021). Comparison of the Phytochemical Properties, Antioxidant Activity and Cytotoxic Effect on HepG2 Cells in Mongolian and Taiwanese Rhubarb Species. Molecules, 26(5), 1217. https://doi.org/10.3390/molecules26051217





