Comparison of the Phenolic Compound Profile and Antioxidant Potential of Achillea atrata L. and Achillea millefolium L.
Abstract
1. Introduction
2. Results
2.1. Phytochemical Comparison of A. atrata and A. millefolium
2.2. Antioxidant Capacity and Contents of Phenolics and Volatile Compounds
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Plant Material
5.2. Chemicals
5.3. Extraction and Fractionation of the Plant Material
5.4. LC-MSn Analyses for Phenolic Compound Characterization
5.5. Gas Chromatography-Mass Spectrometry (GC-MS) Analyses for Volatile Compound Assessment
5.6. 2,2-Diphenyl-Picryl Hydrazyl (DPPH) Spectrophotometric Assay for Assessing Radical Scavenging Capacity
5.7. Folin–Ciocalteu Method for Total Phenolics Quantitation
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Naikoo, M.I.; Dar, M.I.; Raghib, F.; Jaleel, H.; Ahmad, B.; Raina, A.; Naushin, F. Role and regulation of plants phenolics in abiotic stress tolerance: An overview. In Plant Signaling Molecules: Role and Regulation under Stressful Environments, 1st ed.; Khan, M.I.R., Reddy, P.S., Ferrante, A., Khan, N.A., Eds.; Woodhead Publishing: Cambridge, UK, 2019; Volume 1, pp. 157–168. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A.; Bhardwaj, R.; Landi, M.; Zheng, B. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Fernandez-Panchon, M.S.; Villano, D.; Troncoso, A.M.; Garcia-Parrilla, M.C. Antioxidant activity of phenolic compounds: From in vitro results to in vivo evidence. Crit. Rev. Food Sci. Nutr. 2008, 48, 649–671. [Google Scholar] [CrossRef]
- Conn, E.E. The Shikimic Acid Pathway, 1st ed.; Springer Nature: Basingstoke, UK, 1986; pp. 13–33. [Google Scholar]
- de la Rosa, L.A.; Moreno-Escamilla, J.O.; Rodrigo-García, J.; Alvarez-Parrilla, E. Phenolic compounds. In Postharvest Physiology and Biochemistry of Fruits and Vegetables, 1st ed.; Yahia, E., Carrillo-Lopez, A., Eds.; Woodhead Publishing: Santiago de Querétaro, Mexico, 2018; pp. 253–271. [Google Scholar]
- Ncube, B.; Finnie, J.F.; Van Staden, J. Quality from the field: The impact of environmental factors as quality determinants in medicinal plants. S. Afr. J. Bot. 2012, 82, 11–20. [Google Scholar] [CrossRef]
- Granot, E.; Kohen, R. Oxidative stress in childhood—In health and disease states. Clin. Nutr. 2004, 23, 3–11. [Google Scholar] [CrossRef]
- Dhalla, N.S.; Temsah, R.M.; Netticadan, T. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 2000, 18, 655–673. [Google Scholar] [CrossRef] [PubMed]
- Giorgi, A.; Bombelli, R.; Luini, A.; Speranza, G.; Cosentino, M.; Lecchini, S.; Cocucci, M. Antioxidant and cytoprotective properties of infusions from leaves and inflorescences of Achillea collina Becker ex Rchb. Phytother. Res. 2009, 23, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, S.; Tabatabaei, B.E.S.; Saeidi, G.; Goli, S.A.H.; Talebi, M. Total phenolic content and antioxidant activity of three Iranian endemic Achillea species. Ind. Crop. Prod. 2013, 50, 154–158. [Google Scholar] [CrossRef]
- Nemeth, E.; Bernath, J. Biological activities of yarrow species (Achillea spp.). Curr. Pharm. Des. 2008, 14, 3151–3167. [Google Scholar] [CrossRef]
- Vitalini, S.; Beretta, G.; Iriti, M.; Orsenigo, S.; Basilico, N.; Dall’Acqua, S.; Fico, G. Phenolic compounds from Achillea millefolium L. and their bioactivity. Acta Biochim. Pol. 2011, 58, 203–209. [Google Scholar] [CrossRef]
- Vitalini, S.; Madeo, M.; Tava, A.; Iriti, M.; Vallone, L.; Avato, P.; Argentieri, M.P. Chemical Profile, Antioxidant and Antibacterial Activities of Achillea moschata Wulfen, an Endemic Species from the Alps. Molecules 2016, 21, 830. [Google Scholar] [CrossRef]
- Benedec, D.; Vlase, L.; Oniga, I.; Mot, A.C.; Damian, G.; Hanganu, D.; Duma, M.; Silaghi-Dumitrescu, R. Polyphenolic Composition, Antioxidant and Antibacterial Activities for Two Romanian Subspecies of Achillea distans Waldst. et Kit. ex Willd. Molecules 2013, 18, 8725–8739. [Google Scholar] [CrossRef]
- Barros, L.; Pereira, E.; Calhelha, R.C.; Dueñas, M.; Carvalho, A.M.; Santos-Buelga, C.; Ferreira, I.C. Bioactivity and chemical characterization in hydrophilic and lipophilic compounds of Chenopodium ambrosioides L. J. Funct. Foods 2013, 5, 1732–1740. [Google Scholar] [CrossRef]
- Spínola, V.; Castilho, P.C. Evaluation of Asteraceae herbal extracts in the management of diabetes and obesity. Contribution of caffeoylquinic acids on the inhibition of digestive enzymes activity and formation of advanced glycation end-products (in vitro). Phytochemistry 2017, 143, 29–35. [Google Scholar] [CrossRef]
- Benetis, R.; Radusiene, J.; Janulis, V. Variability of phenolic compounds in flowers of Achillea millefolium wild populations in Lithuania. Medicina 2008, 44, 775–781. [Google Scholar] [CrossRef]
- Dias, M.I.; Barros, L.; Dueñas, M.; Pereira, E.; Carvalho, A.M.; Alves, R.C.; Oliveira, M.B.P.; Santos-Buelga, C.; Ferreira, I.C. Chemical composition of wild and commercial Achillea millefolium L. and bioactivity of the methanolic extract, infusion and decoction. Food Chem. 2013, 141, 4152–4160. [Google Scholar] [CrossRef]
- Valant-Vetschera, K.M.; Wollenweber, E. Leaf flavonoids of the Achillea millefolium group part II: Distribution patterns of free aglycones in leaf exudates. Biochem. Syst. Ecol. 1988, 16, 605–614. [Google Scholar] [CrossRef]
- Valant-Vetschera, K.M. Flavonoid diversification in Achillea ptarmica and allied taxa. Biochem. Syst. Ecol. 1985, 13, 15–21. [Google Scholar] [CrossRef]
- Manhita, A.; Balcaen, L.; Vanhaecke, F.; Ferreira, T.; Candeias, A.; Dias, C.B. Unveiling the colour palette of Arraiolos carpets: Material study of carpets from the 17th to 19th century period by HPLC-DAD-MS and ICP-MS. J. Cult. Herit. 2014, 15, 292–299. [Google Scholar] [CrossRef]
- Karľová, K. Accumulation of flavonoid compounds in flowering shoots of Achillea collina Becker ex. Rchb. Alba during flower development. Hortic. Sci. 2011, 33, 158–162. [Google Scholar] [CrossRef]
- Csepregi, R.; Temesfői, V.; Das, S.; Alberti, Á.; Tóth, C.A.; Herczeg, R.; Kőszegi, T. Cytotoxic, antimicrobial, antioxidant properties and effects on cell migration of phenolic compounds of selected Transylvanian medicinal plants. Antioxidants 2020, 9, 166. [Google Scholar] [CrossRef]
- Sasidharan, S.; Chen, Y.; Saravanan, D.; Sundram, K.M.; Latha, L.Y. Extraction, isolation and characterization of bioactive compounds from plants’ extracts. AJTCAM. 2011, 8, 1–10. [Google Scholar] [CrossRef]
- Gouveia, S.C.; Castilho, P.C. Characterization of phenolic compounds in Helichrysum melaleucum by high-performance liquid chromatography with on-line ultraviolet and mass spectrometry detection. Rapid Commun. Mass Spectrom. 2010, 24, 1851–1868. [Google Scholar] [CrossRef]
- Jaiswal, R.; Kiprotich, J.; Kuhnert, N. Determination of the hydroxycinnamate profile of 12 members of the Asteraceae family. Phytochemistry 2011, 72, 781–790. [Google Scholar] [CrossRef]
- Parejo, I.; Jauregui, O.; Viladomat, F.; Bastida, J.; Codina, C. Characterization of acylated flavonoid-O-glycosides and methoxylated flavonoids from Tagetes maxima by liquid chromatography coupled to electrospray ionization tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2004, 18, 2801–2810. [Google Scholar] [CrossRef]
- Bariş, Ö.; Güllüce, M.; Şahin, F.; Özer, H.; Kiliç, H.; Özkan, H.; Sökmen, M.; Özbek, T. Biological activities of the essential oil and methanol extract of Achillea biebersteinii Afan. (Asteraceae). Turk. J. Biol. 2006, 30, 65–73. [Google Scholar]
- Ola, S.S.; Catia, G.; Marzia, I.; Francesco, V.F.; Afolabi, A.A.; Nadia, M. HPLC/DAD/MS characterisation and analysis of flavonoids and cynnamoil derivatives in four Nigerian green-leafy vegetables. Food Chem. 2009, 115, 1568–1574. [Google Scholar] [CrossRef]
- Trendafilova, A.; Todorova, M.; Mikhova, B.; Duddeck, H. Flavonoids in flower heads of three Achillea species belonging to Achillea millefolium group. Chem. Nat. Compd. 2007, 43, 212–213. [Google Scholar] [CrossRef]
- Sarker, S.D.; Latif, Z.; Gray, A.I. Natural Products Isolation, 2nd ed.; Humana Press, Methods in Biotechnology: Totowa, NJ, USA, 2006; pp. 1–26. [Google Scholar]
- Aljanĉić, I.; Vajs, V.; Menković, N.; Karadźić, I.; Juranić, N.; Milosavljević, S.; Macura, S. Flavones and sesquiterpene lactones from Achillea atrata subsp. multifida: Antimicrobial activity. J. Nat. Prod. 1999, 62, 909–911. [Google Scholar] [CrossRef] [PubMed]
- Ristić, M.; Soković, M.; Grubišić, D.; Kovacević, N. Chemical analysis and antifungal activity of the essential oil of Achillea atrata L. J. Essent. Oil Res. 2004, 16, 75–78. [Google Scholar] [CrossRef]
- Apel, L.; Lorenz, P.; Urban, S.; Sauer, S.; Spring, O.; Stintzing, F.C.; Kammerer, D.R. Phytochemical characterization of different yarrow species (Achillea sp.) and investigations into their antimicrobial activity. Z. Naturforsch. C 2020, 76, 55–65. [Google Scholar] [CrossRef]
- Barchan, A.; Bakkali, M.; Arakrak, A.; Pagán, R.; Laglaoui, A. The effects of solvents polarity on the phenolic contents and antioxidant activity of three Mentha species extracts. Int. J. Curr. Microbiol. Appl. Sci. 2014, 3, 399–412. [Google Scholar]
- Wildi, B.; Lutz, C. Antioxidant composition of selected high alpine plant species from different altitudes. Plant Cell Environ. 1996, 19, 138–146. [Google Scholar] [CrossRef]
- Brahmachari, G. Nevadensin: Isolation, chemistry and bioactivity. Int. J. Green Pharm. 2010, 4, 213. [Google Scholar] [CrossRef]
- Hirano, R.; Sasamoto, W.; Matsumoto, A.; Itakura, H.; Igarashi, O.; Kondo, K. Antioxidant ability of various flavonoids against DPPH radicals and LDL oxidation. J. Nutr. Sci. Vitaminol. 2001, 47, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Pérez, G.R.M.; Vargas, S.R.; Martinez, M.F.J.; Cordova, R.I. Antioxidant and free radical scavenging activities of 5,7,3′-trihydroxy-3,6,4′-trimethoxyflavone from Brickellia veronicaefolia. Phytother. Res. 2004, 18, 428–430. [Google Scholar] [CrossRef]
- Argentieri, M.P.; Madeo, M.; Avato, P.; Iriti, M.; Vitalini, S. Polyphenol content and bioactivity of Achillea moschata from the Italian and Swiss Alps. Z. Naturforsch. C 2020, 75, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Lorenz, P.; Hradecky, M.; Berger, M.; Bertrams, J.; Meyer, U.; Stintzing, F.C. Lipophilic constituents from aerial and root parts of Mercurialis perennis L. Phytochem. Anal. 2010, 21, 234–245. [Google Scholar] [CrossRef] [PubMed]
- Heinrich, M.; Lorenz, P.; Daniels, R.; Stintzing, F.C.; Kammerer, D.R. Lipid and Phenolic Constituents from Seeds of Hypericum perforatum L. and Hypericum tetrapterum Fr. and their Antioxidant Activity. Chem. Biodivers. 2017, 14, e1700100. [Google Scholar] [CrossRef] [PubMed]
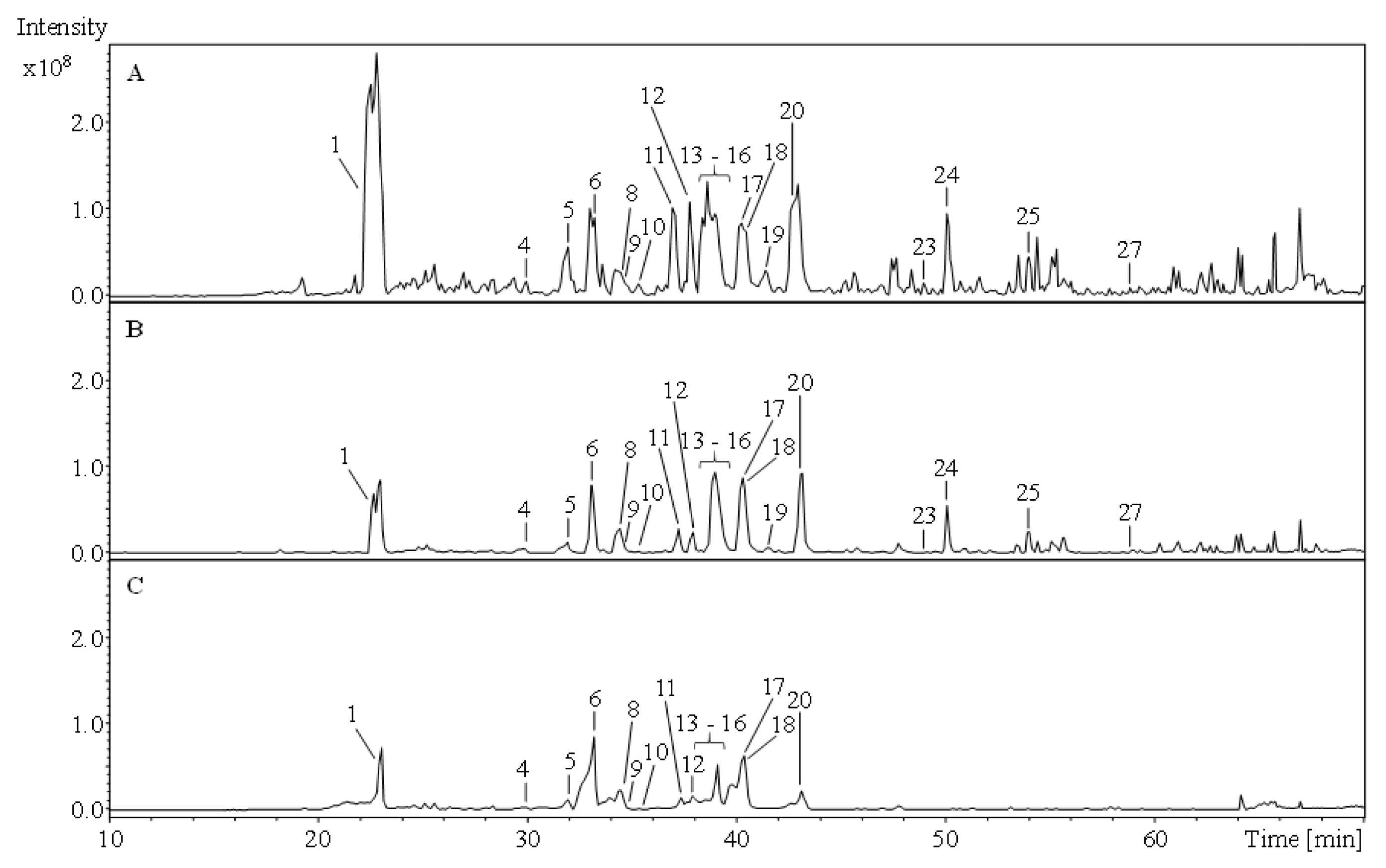
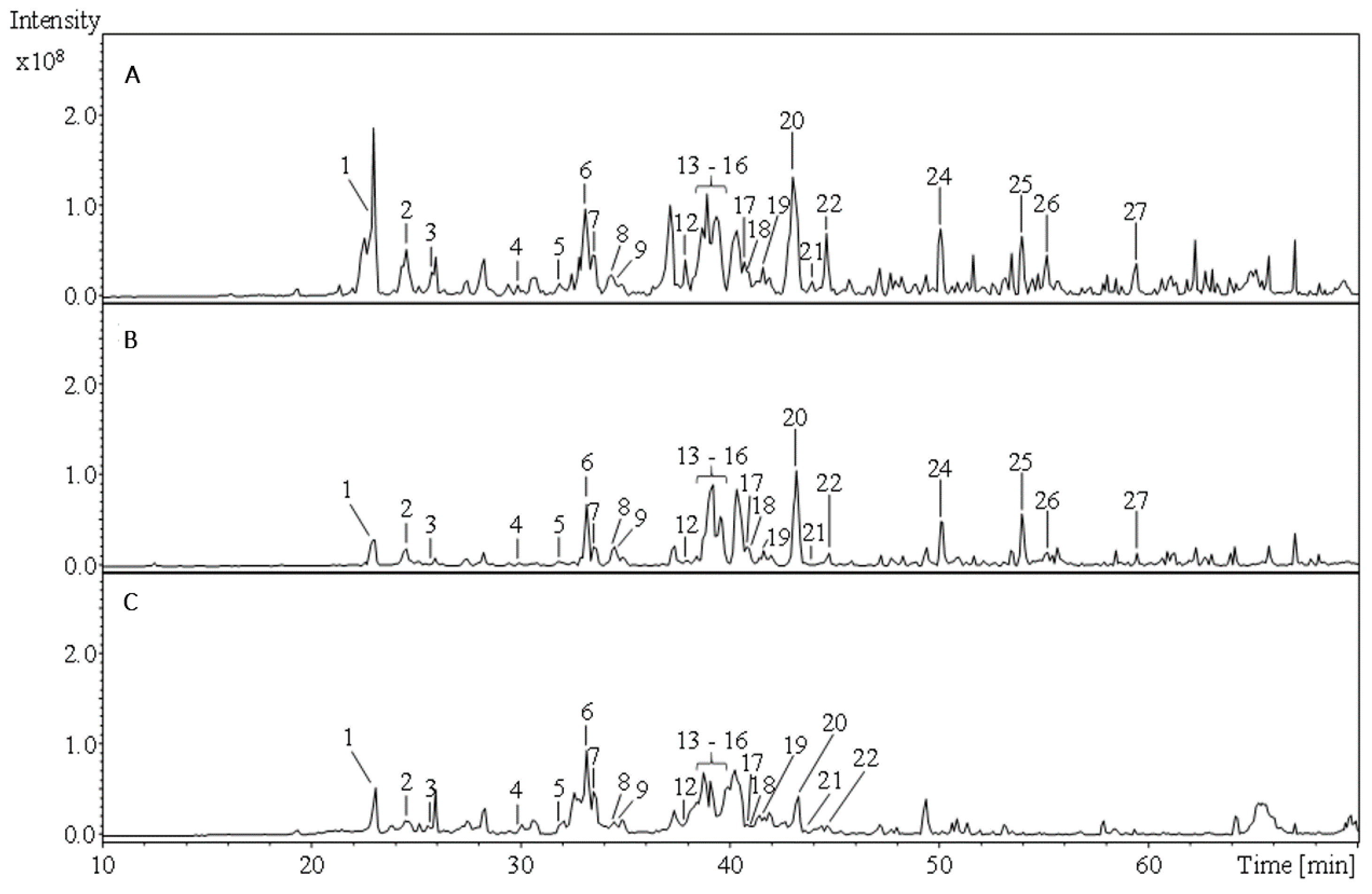
| Peak No. | Rt [min] | Peak Assignment | UV λmax [nm] | MSn Data [m/z] | A. atrata | A. millefolium | Ref. | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS 1 | MS 2 | MS 3 | aw | EtOAc | n-but | aw | EtOAc | n-but | |||||
| 1 | 22.6 23.0 | Chlorogenic acid | 216, 326 | 353 | 191 | 173 | + | + | + | RS*1/[14]*2 | |||
| + | + | + | |||||||||||
| 2 | 24.5 | p-Coumaroyl acid derivative | 326 | 387 | 207 | 163 | - | - | - | + | + | + | [15] *1 |
| 3 | 25.5 | Apigenin-6,8-di-C-hexoside | 194, 280 | 593 | 473 | 353 | - | - | - | + | + | + | [16] *1/[17] *2 |
| 4 | 30.0 | Quercetin-O-hexoside I | 282, 342 | 463 | 301 | 283 | + | + | + | + | + | + | [15] *1/[18] *2 |
| 5 | 31.8 32.1 | 4-Methyl-3-methoxy-9a- hydroxyligballinol-O-glucoside | 202, 278 202, 276 | 565 | 339 | 324 | + | + | + | [16] *1 | |||
| + | + | + | |||||||||||
| 6 | 33.0 | Quercetin-3-O-rutinoside | 200, 374 | 609 | 301 | 179 | + | + | + | + | + | + | RS *1/[19] *2 |
| 7 | 33.4 | Kaempferol-3-O-rutinoside I | 202, 342 | 593 | 285 | 255 | - | - | - | + | + | + | [16] *1/[20] *2 |
| 8 | 34.4 | Luteolin-hexoside | 266, 348 | 447 | 285 | 255 | + | + | + | + | + | + | [21] *1/[22] *2 |
| 9 | 34.4 34.7 | Quercetin-O-hexoside II | 204, 328 | 463 | 301 | 151 | + | + | + | [15] *1/[18] *2 | |||
| + | + | + | |||||||||||
| 10 | 35.3 | Mearnsetin-hexoside | 198, 336 | 493 | 331 | 316 | + | + | - | - | - | - | [23] *1/[24] *2 |
| 11 | 36.5 | Isorhamnetin-O-hexoside I | 204, 328 | 477 | 315 | 300 | + | + | + | - | - | - | [16] *1/[18] *2 |
| 12 | 37.9 38.1 | Kaempferol-3-O-rutinoside II | 266, 342 | 593 | 285 | 255 | + | + | + | [16] *1/[20] *2 | |||
| + | + | ||||||||||||
| 13 | 38.1 | Dicaffeoylquinic acid I | 204, 324 | 515 | 353 | 191 | + | + | + | + | + | + | [16] *1/[12] *2 |
| 14 | 38.4 | Dicaffeoylquinic acid II | 204, 324 | 515 | 353 | 191 | + | + | + | + | + | + | [16] *1/[12] *2 |
| 15 | 38.6 | Dicaffeoylquinic acid III | 204, 324 | 515 | 353 | 191 | + | + | + | + | + | + | [16] *1/[12] *2 |
| 16 | 39.3 39.9 | Dicaffeoylquinic acid IV | 204, 324 | 515 | 353 | 191 | + | + | + | [16] *1/[12] *2 | |||
| + | + | + | |||||||||||
| 17 | 40.2 | Apigenin-7-O-glucoside | 268, 338 | 431 | 269 | 225 | + | + | + | + | + | + | RS *1/[17] *2 |
| 18 | 40.6 | Isorhamnetin-O-hexoside II | 200, 332 | 447 | 315 | 300 | + | + | + | - | - | - | [16] *1/[18] *2 |
| 19 | 41.4 | Dicaffeoylquinic acid V | 194, 324 | 515 | 353 | 191 | + | + | - | + | + | + | [16] *1/[12] *2 |
| 20 | 43.0 | Dicaffeoylquinic acid VI | 216, 326 | 515 | 353 | 191 | + | + | + | + | + | + | [16] *1/[12] *2 |
| 21 | 43.8 | Dicaffeoylquinic acid VII | 216, 326 | 515 | 353 | 191 | - | - | - | + | + | + | [16] *1/[12] *2 |
| 22 | 44.6 | Cinnamic acid derivative | - | 549 | 387 | 369 | - | - | - | + | + | + | [23] *1 |
| 23 | 48.4 | Caffeoyl-feruloylquinic acid | 196, 326 | 529 | 367 | 191 | + | + | - | - | - | - | [25] *1/[26] *2 |
| 24 | 50.1 | Luteolin | 252, 346 | 285 | 241 | 217 | + | + | - | + | + | - | RS *1/[19] *2 |
| 25 | 54.0 | Apigenin | 286, 332 | 269 | 225 | - | + | + | - | + | + | - | RS *1/[17] *2 |
| 26 | 55.9 | Centaureidin | 234, 314 | 359 | 344 | 329 | - | - | - | + | + | - | [27] *1/[28] *2 |
| 27 | 57.9 | Nevadensin | 332 | 343 | 328 | 313 | + | + | - | + | + | - | [29] *1/[30] *2 |
| Peak No. | Peak Assignment | Rt [min] | Calc. Mr [Da] (tms) | Characteristic Fragments, m/z (BPI [%]) | A. atrata | A. millefolium |
|---|---|---|---|---|---|---|
| 1 | α-Thujene | 6.29 | 136.15 | 93 (100), 92 (42), 91 (50), 79 (20), 77 (24), 63 (9) | + | + |
| 2 | Bornylene | 6.57 | 136.15 | 93 (100), 89 (29), 84 (32), 79 (25), 72 (88), 63 (29) | - | + |
| 3 | β-Thujene | 6.81 | 136.15 | 93 (100), 91 (43), 79 (26), 77 (36), 69 (9) | - | + |
| 4 | Sabinene | 6.98 | 136.15 | 93 (100), 91 (32), 79 (19), 77 (17), 69 (21), 67 (8) | - | + |
| 5 | γ-Terpinene | 7.34 | 136.15 | 93 (100), 92 (22), 91 (50), 77 (29), 57 (4) | + | - |
| 6 | Eucalyptol | 7.86 | 154.14 | 111 (36), 108 (59), 84 (89), 81 (100), 71 (68), 67 (32), 55 (33) | - | + |
| 7 | β-Terpineol | 8.53 | 154.14 | 93 (70), 92 (33), 84 (25), 71 (100), 64 (16), 55 (49) | - | + |
| 8 | α-Thujone | 9.37 | 152.12 | 110 (58), 109 (27), 95 (41), 81 (100), 79 (18), 69 (49), 68 (57) | - | + |
| 9 | Borneol | 9.61 | 154.14 | 95 (100), 77 (94), 74 (30), 72 (51), 69 (25), 65 (30), 57 (56) | - | + |
| 10 | Camphor | 10.47 | 152.12 | 95 (100), 83 (23), 81 (63), 69 (27), 67 (17), 55 (20) | - | + |
| 11 | (+)-Borneol | 11.03 | 154.14 | 110 (18), 95 (100), 67 (9) | - | + |
| 12 | α-Terpineol | 11.48 | 154.14 | 136 (54), 121 (52), 93 (100), 89 (20), 81 (36), 77 (24), 59 (95) | - | + |
| 13 | β-Bisabolol | 12.84 | 222.20 | 82 (100), 78 (26), 73 (31), 65 (19), 58 (18), 55 (21), 53 (36) | - | + |
| 14 | Isoborneol | 13.92 | 154.14 | 95 (100), 89 (20), 79 (22), 77 (15), 70 (29), 68 (26), 64 (18) | - | + |
| 15 | Caryophyllene | 17.14 | 204.19 | 105 (46), 93 (100), 91 (94), 81 (23), 79 (61), 77 (33), 55 (52) | + | + |
| 16 | (+)-Nerolidol | 21.83 | 222.20 | 107 (39), 93 (100), 81 (39), 79 (22), 71 (43), 67 (36), 55 (31) | - | + |
| 17 | Caryophyllene oxide | 22.95 | 220.18 | 107 (38), 106 (33), 95 (48), 93 (68), 91 (55), 79 (100), 69 (33) | + | + |
| 18 | α-Eudesmol | 24.00 | 222.20 | 204 (96), 161 (100), 149 (40), 108 (32), 93 (48), 79 (27), 59 (79) | + | + |
| 19 | Alkane I | 40.19 | - | 113 (7), 99 (90), 85 (83), 71 (94), 57 (100), 55 (22) | + | + |
| 20 | Alkane II | 44.27 | - | 113 (10), 99 (20), 85 (76), 71 (87), 57 (100), 55 (19) | + | + |
| 21 | Alkane III | 48.05 | - | 113 (12), 99 (30), 85 (84), 71 (97), 69 (19), 57 (100), 55 (22) | + | + |
| 22 | Alkane IV | 51.57 | - | 207 (4), 99 (32), 97 (20), 85 (85), 71 (99), 57 (100), 55 (16) | + | + |
| 23 | Alkane V | 54.88 | - | 209(6), 99 (28), 85 (83), 83 (25), 71 (99), 57 (100), 55 (16) | + | + |
| Samples | Regression Equation | R2 | IC50 [µg/mL] ± SD |
|---|---|---|---|
| Trolox | y = 6.5233x + 1.3342 | 0.9975 | 7.5 ± 0.1 |
| A. atrata | |||
| ethyl acetate fraction | y = 4.0888x − 0.1440 | 0.9983 | 12.2 ± 0.3 |
| n-butanol fraction | y = 0.6263x + 2.3083 | 0.9984 | 76.15 ± 0.3 |
| dichloromethane fraction | - | - | - |
| A. millefolium | |||
| ethyl acetate fraction | y = 3.0799x − 2.2077 | 0.9964 | 17.0 ± 0.3 |
| n-butanol fraction | y = 0.6131x − 0.4695 | 0.9988 | 82.3 ± 0.8 |
| dichloromethane fraction | - | - | - |
| Samples | Phenolic Content (mg GAE/g DW ± SD) |
|---|---|
| A. atrata | |
| ethyl acetate fraction | 250 ± 2.5 |
| n-butanol fraction | 70 ± 1.0 |
| dichloromethane fraction | - |
| A. millefolium | |
| ethyl acetate fraction | 175 ± 1.0 |
| n-butanol fraction | 80 ± 1.5 |
| dichloromethane fraction | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomon, L.; Lorenz, P.; Bunse, M.; Spring, O.; Stintzing, F.C.; Kammerer, D.R. Comparison of the Phenolic Compound Profile and Antioxidant Potential of Achillea atrata L. and Achillea millefolium L. Molecules 2021, 26, 1530. https://doi.org/10.3390/molecules26061530
Salomon L, Lorenz P, Bunse M, Spring O, Stintzing FC, Kammerer DR. Comparison of the Phenolic Compound Profile and Antioxidant Potential of Achillea atrata L. and Achillea millefolium L. Molecules. 2021; 26(6):1530. https://doi.org/10.3390/molecules26061530
Chicago/Turabian StyleSalomon, Lysanne, Peter Lorenz, Marek Bunse, Otmar Spring, Florian C. Stintzing, and Dietmar R. Kammerer. 2021. "Comparison of the Phenolic Compound Profile and Antioxidant Potential of Achillea atrata L. and Achillea millefolium L." Molecules 26, no. 6: 1530. https://doi.org/10.3390/molecules26061530
APA StyleSalomon, L., Lorenz, P., Bunse, M., Spring, O., Stintzing, F. C., & Kammerer, D. R. (2021). Comparison of the Phenolic Compound Profile and Antioxidant Potential of Achillea atrata L. and Achillea millefolium L. Molecules, 26(6), 1530. https://doi.org/10.3390/molecules26061530






