Self-Assembly of Discrete Porphyrin/Calix[4]tube Complexes Promoted by Potassium Ion Encapsulation
Abstract
1. Introduction
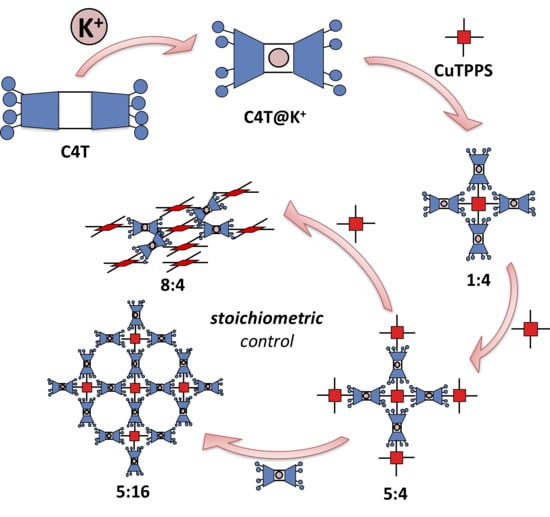
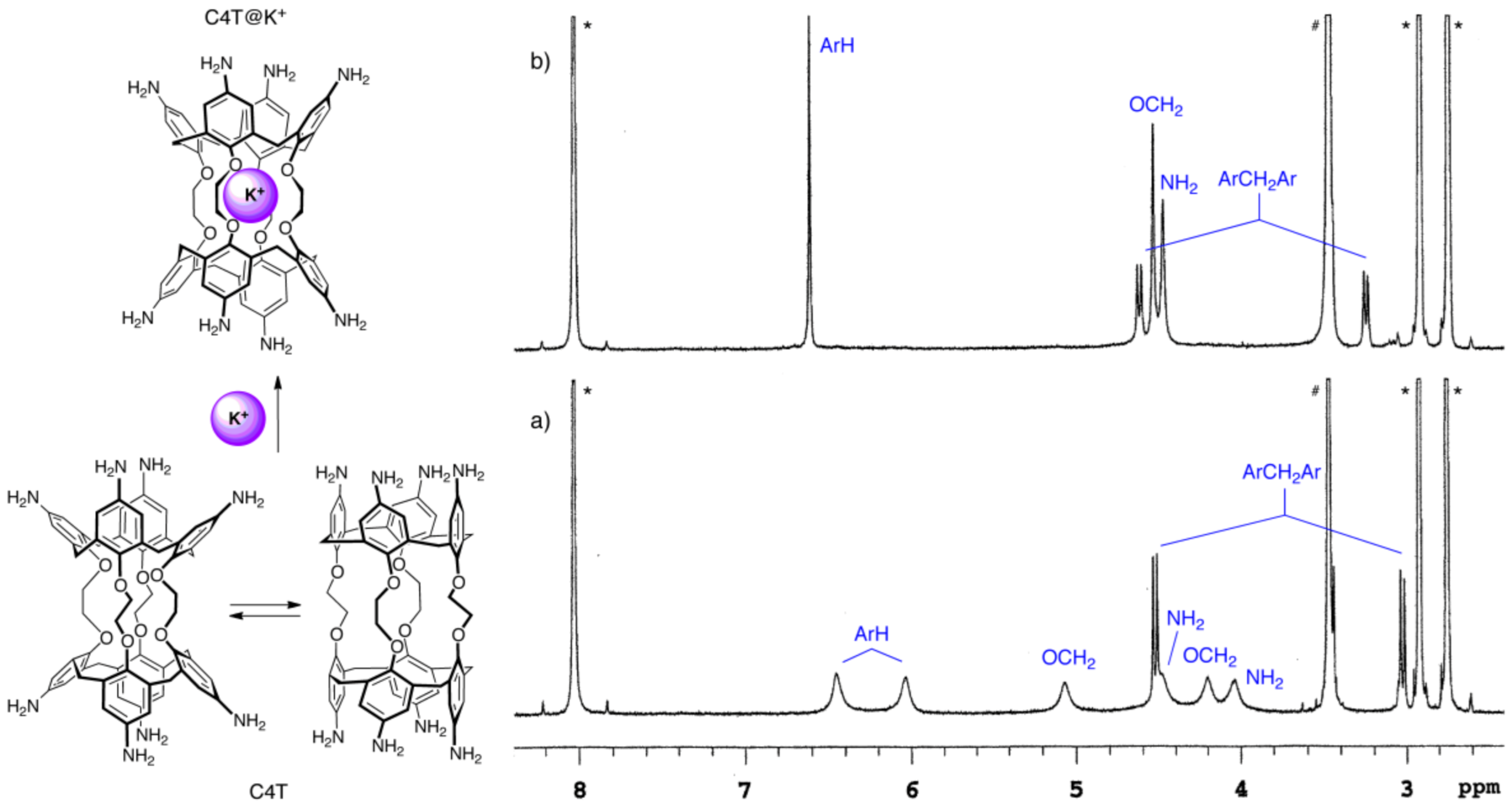
2. Results and Discussion
3. Materials and Methods
3.1. General
3.2. Syntheses of Calix[4]tube C4T and the Potassium Inclusion Complex C4T@K+
3.2.1. Octa-Nitro Calix[4]tube 2
3.2.2. Octa-Amino Calix[4]tube C4T
3.2.3. Formation of the C4T@K+ Complex
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Sample Availability
References and Notes
- Bhyrappa, P.; Sankar, M.; Varghese, B. Mixed substituted porphyrins: Structural and electrochemical redox properties. Inorg. Chem. 2006, 45, 4136–4149. [Google Scholar] [CrossRef] [PubMed]
- Fonda, H.N.; Gilbert, J.V.; Cormier, R.A.; Sprague, J.R.; Kamioka, K.; Connolly, J.S. Spectroscopic, photophysical, and redox properties of some meso-substituted free-base porphyrins. J. Phys. Chem. 1993, 97, 7024–7033. [Google Scholar] [CrossRef]
- Yella, A.; Lee, H.W.; Tsao, H.N.; Yi, C.; Chandiran, A.K.; Nazeeruddin, M.K.; Diau, E.W.G.; Yeh, C.Y.; Zakeeruddin, S.M.; Grätzel, M. Porphyrin-sensitized solar cells with cobalt (II/III)-based redox electrolyte exceed 12 percent efficiency. Science 2011, 334, 629–634. [Google Scholar] [CrossRef]
- Jurow, M.; Schuckman, A.E.; Batteas, J.D.; Drain, C.M. Porphyrins as molecular electronic components of functional devices. Coord. Chem. Rev. 2010, 254, 2297–2310. [Google Scholar] [CrossRef]
- Scandola, F.; Chiorboli, C.; Prodi, A.; Iengo, E.; Alessio, E. Photophysical properties of metal-mediated assemblies of porphyrins. Coord. Chem. Rev. 2006, 250, 1471–1496. [Google Scholar] [CrossRef]
- Hiroto, S.; Miyake, Y.; Shinokubo, H. Synthesis and functionalization of porphyrins through organometallic methodologies. Chem. Rev. 2017, 117, 2910–3043. [Google Scholar] [CrossRef]
- Beletskaya, I.; Tyurin, V.S.; Tsivadze, A.Y.; Guilard, R.; Stern, C. Supramolecular chemistry of metalloporphyrins. Chem. Rev. 2009, 109, 1659–1713. [Google Scholar]
- Drain, C.M.; Varotto, A.; Radivojevic, I. Self-organized porphyrinic materials. Chem. Rev. 2009, 109, 1630–1658. [Google Scholar]
- Medforth, C.J.; Wang, Z.; Martin, E.M.; Song, Y.; Jacobsen, J.L.; Shelnutt, J.A. Self-assembled porphyrin nanostructures. Chem. Commun. 2009, 47, 7261–7277. [Google Scholar] [CrossRef]
- Otsuki, J. Supramolecular approach towards light-harvesting materials based on porphyrins and chlorophylls. J. Mater. Chem. A 2018, 6, 6710–6753. [Google Scholar] [CrossRef]
- Balaban, T.S. Tailoring porphyrins and chlorins for self-assembly in biomimetic artificial antenna systems. Acc. Chem. Res. 2005, 38, 612–623. [Google Scholar] [CrossRef] [PubMed]
- Paolesse, R.; Nardis, S.; Monti, D.; Stefanelli, M.; Di Natale, C. Porphyrinoids for chemical sensor applications. Chem. Rev. 2017, 117, 2517–2583. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Song, Y.; Medforth, C.J.; Shelnutt, J.A. Interfacial synthesis of dendritic platinum nanoshells templated on benzene nanodroplets stabilized in water by a photocatalytic lipoporphyrin. J. Am. Chem. Soc. 2006, 128, 9284–9285. [Google Scholar] [CrossRef] [PubMed]
- Rabiee, N.; Yaraki, M.T.; Garakani, S.M.; Garakani, S.M.; Ahmadi, S.; Lajevardi, A.; Bagherzadeh, M.; Rabiee, M.; Tayebi, L.; Tahriri, M.; et al. Recent advances in porphyrin-based nanocomposites for effective targeted imaging and therapy. Biomaterials 2020, 232, 119707. [Google Scholar]
- Tian, J.; Huang, B.; Nawaz, M.H.; Zhang, W. Recent advances of multi-dimensional porphyrin-based functional materials in photodynamic therapy. Coord. Chem. Rev. 2020, 420, 213410. [Google Scholar] [CrossRef]
- Doan, S.C.; Shanmugham, S.; Aston, D.E.; McHale, J.L. Counterion Dependent Dye Aggregates: Nanorods and nanorings of tetra(p-carboxyphenyl)porphyrin. J. Am. Chem. Soc. 2005, 127, 5885–5892. [Google Scholar] [CrossRef]
- Schwab, A.D.; Smith, D.E.; Rich, C.S.; Young, E.R.; Smith, W.F.; de Paula, J.C. Porphyrin nanorods. J. Phys. Chem. B 2003, 107, 11339–11345. [Google Scholar] [CrossRef]
- Guo, P.; Chen, P.; Liu, M. One-dimensional porphyrin nanoassemblies assisted via graphene oxide: Sheetlike functional surfactant and enhanced photocatalytic behaviors. ACS Appl. Mater. Interfaces 2013, 5, 5336–5345. [Google Scholar] [CrossRef]
- Koepf, M.; Conradt, J.; Szmytkowski, J.; Wytko, J.A.; Allouche, L.; Kalt, H.; Balaban, T.S.; Weiss, J. Highly linear self-assembled porphyrin wires. Inorg. Chem. 2011, 50, 6073–6082. [Google Scholar] [CrossRef]
- Fathalla, M.; Neuberger, A.; Li, S.-C.; Schmehl, R.; Diebold, U.; Jayawickramarajah, J. Straightforward self-assembly of porphyrin nanowires in water: Harnessing adamantane/cyclodextrin interactions. J. Am. Chem. Soc. 2010, 132, 9966–9967. [Google Scholar] [CrossRef]
- Lee, S.J.; Hupp, J.T.; Nguyen, S.T. Growth of narrowly dispersed porphyrin nanowires and their hierarchical assembly into macroscopic columns. J. Am. Chem. Soc. 2008, 130, 9632–9633. [Google Scholar] [CrossRef]
- Wang, Z.; Medforth, C.J.; Shelnutt, J.A. Porphyrin nanotubes by ionic self-assembly. J. Am. Chem. Soc. 2004, 126, 15954–15955. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Medforth, C.J.; Shelnutt, J.A. Self-assembly and self-metallization of porphyrin nanosheets. J. Am. Chem. Soc. 2007, 129, 2440–2441. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, B.; Zhu, M.; Grayson, S.M.; Schmehl, R.; Jayawickramarajah, J. Water-soluble porphyrin nanospheres: Enhanced photo-physical properties achieved via cyclodextrin driven double self-inclusion. Chem. Commun. 2014, 50, 4853–4855. [Google Scholar] [CrossRef] [PubMed]
- Aratani, N.; Kim, D.; Osuka, A. Discrete cyclic porphyrin arrays as artificial light-harvesting antenna. Acc. Chem. Res. 2009, 42, 1922–1934. [Google Scholar] [CrossRef] [PubMed]
- Imamura, T.; Fukushima, K. Self-assembly of metallopyridylporphyrin oligomers. Coord. Chem. Rev. 2000, 198, 133–156. [Google Scholar] [CrossRef]
- Frühbeißer, S.; Gröhn, F. Catalytic activity of macroion–porphyrin nanoassemblies. J. Am. Chem. Soc. 2012, 134, 14267–14270. [Google Scholar] [CrossRef]
- Onouchi, H.; Miyagawa, T.; Morino, K.; Yashima, E. Assisted formation of chiral porphyrin homoaggregates by an induced helical poly(phenylacetylene) template and their chiral memory. Angew. Chem. 2006, 45, 2381–2384. [Google Scholar] [CrossRef]
- Liu, K.; Xing, R.; Chen, C.; Shen, G.; Yan, L.; Zou, Q.; Ma, G.; Mçhwald, H.; Yan, X. Peptide-induced hierarchical long-range order and photocatalytic activity of porphyrin assemblies. Angew. Chem. 2015, 54, 500–505. [Google Scholar] [CrossRef]
- Oliveras-González, C.; Di Meo, F.; González-Campo, A.; Beljonne, D.; Norman, P.; Simón-Sorbed, M.; Linares, M.; Amabilino, D.B. Bottom-up hierarchical self-assembly of chiral porphyrins through coordination and hydrogen bonds. J. Am. Chem. Soc. 2015, 137, 15795–15808. [Google Scholar] [CrossRef]
- Boccalon, M.; Iengo, E.; Tecilla, P. Metal-organic transmembrane anopores. J. Am. Chem. Soc. 2012, 134, 20310–20313. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Yoshizawa, M.; Kato, T.; Watanabe, K.; Fujita, M. Porphine dimeric assemblies in organic-pillared coordination cages. Angew. Chem. 2007, 46, 1803–1806. [Google Scholar] [CrossRef] [PubMed]
- Fujita, N.; Biradha, K.; Fujita, M.; Sakamoto, S.; Yamaguchi, K. A Porphyrin prism: Structural switching triggered by guest inclusion. Angew. Chem. 2001, 40, 1718–1721. [Google Scholar] [CrossRef]
- Sasaki, K.; Nakagawa, H.; Zhang, X.; Sakurai, S.; Kano, K.; Kuroda, Y. Construction of porphyrin–cyclodextrin self-assembly with molecular wedge. Chem. Commun. 2004, 4, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Kano, K.; Nishiyabu, R.; Asada, T.; Kuroda, Y. Static and dynamic behavior of 2:1 inclusion complexes of cyclodextrins and charged porphyrins in aqueous organic media. J. Am. Chem. Soc. 2002, 124, 9937–9944. [Google Scholar] [CrossRef]
- Feiters, M.C.; Fyfe, M.C.T.; Martínez-Díaz, M.-V.; Menzer, S.; Nolte, R.J.M.; Stoddart, J.F.; van Ken, P.J.M.; Williams, D.J. A supramolecular analog of the photosynthetic special pair. J. Am. Chem. Soc. 1997, 119, 8119–8120. [Google Scholar] [CrossRef]
- Tian, H.-W.; Liu, Y.-C.; Guo, D.-S. Assembling features of calixarene-based amphiphiles and supra-amphiphiles. Mater. Chem. Front. 2020, 4, 46–98. [Google Scholar] [CrossRef]
- Pisagatti, I.; Barbera, L.; Gattuso, G.; Parisi, M.F.; Geremia, S.; Hickey, N.; Notti, A. Guest-length driven high fidelity self-sorting in supramolecular capsule formation of calix[5]arenes in water. Org. Chem. Front. 2019, 6, 3804–3809. [Google Scholar] [CrossRef]
- Giuliani, M.; Morbioli, I.; Sansone, F.; Casnati, A. Moulding calixarenes for biomacromolecule targeting. Chem. Commun. 2015, 51, 14140–14159. [Google Scholar] [CrossRef]
- Arena, G.; Pappalardo, A.; Pappalardo, S.; Gattuso, G.; Notti, A.; Parisi, M.F.; Pisagatti, I.; Sgarlata, C. Complexation of biologically active amines by a water-soluble calix[5]arene. J. Therm. Anal. Calorim. 2015, 121, 1073–1079. [Google Scholar]
- Pisagatti, I.; Barbera, L.; Gattuso, G.; Villari, V.; Micali, N.; Fazio, E.; Neri, F.; Parisi, M.F.; Notti, A. Tuning the aggregation of an amphiphilic anionic calix[5]arene by selective host-guest interactions with bola-type dications. New J. Chem. 2019, 43, 7936–7940. [Google Scholar] [CrossRef]
- Manganaro, N.; Lando, G.; Gargiulli, C.; Pisagatti, I.; Notti, A.; Pappalardo, S.; Parisi, M.F.; Gattuso, G. Unique binding behavior of water-soluble polycationic oxacalix[4]arene tweezers towards the paraquat dication. Chem. Commun. 2015, 51, 12657–12660. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.-S.; Liu, Y. Supramolecular chemistry of p-sulfonatocalix[n]arenes and its biological applications. Acc. Chem. Res. 2014, 47, 1925–1934. [Google Scholar] [CrossRef]
- Gattuso, G.; Notti, A.; Pappalardo, S.; Parisi, M.F.; Pisagatti, I.; Patanè, S. Encapsulation of monoamine neurotransmitters and trace amines by amphiphilic anionic calix[5]arene micelles. New J. Chem. 2014, 38, 5983–5990. [Google Scholar] [CrossRef]
- D’Urso, A.; Fragalà, M.E.; Purrello, R. From self-assembly to noncovalent synthesis of programmable porphyrins’ arrays in aqueous solution. Chem. Commun. 2012, 48, 8165–8176. [Google Scholar] [CrossRef]
- Guo, D.-S.; Chen, K.; Zhang, H.-Q.; Liu, Y. Nano-supramolecular assemblies constructed from water-soluble bis(calix[5]arenes) with porphyrins and their photoinduced electron transfer properties. Chem. Asian J. 2009, 4, 436–445. [Google Scholar] [CrossRef]
- Alex, J.M.; McArdle, P.; Crowley, P.B. Supramolecular stacking in a high Z′ calix[8]arene-porphyrin assembly. CrystEngComm 2020, 22, 14–17. [Google Scholar] [CrossRef]
- Brancatelli, G.; De Zorzi, R.; Hickey, N.; Siega, P.; Zingone, G.; Geremia, S. New multicomponent porous architecture of self-assembled porphyrins/calixarenes driven by nickel ions. Cryst. Growth Des. 2012, 12, 5111–5117. [Google Scholar] [CrossRef]
- De Zorzi, R.; Guidolin, N.; Randaccio, L.; Geremia, S. A bifunctionalized porous material containing discrete assemblies of copper-porphyrins and calixarenes metallated by ion diffusion. CrystEngComm 2010, 12, 4056–4058. [Google Scholar] [CrossRef]
- De Zorzi, R.; Guidolin, N.; Randaccio, L.; Purrello, R.; Geremia, S. Nanoporous crystals of calixarene/porphyrin supramolecular complex functionalized by diffusion and coordination of metal ions. J. Am. Chem. Soc. 2009, 131, 2487–2489. [Google Scholar] [CrossRef]
- Di Costanzo, L.; Geremia, S.; Randaccio, L.; Purrello, R.; Lauceri, R.; Sciotto, D.; Gulino, F.G.; Pavone, V. Calixarene-porphyrin supramolecular complexes: pH-tuning of the complex stoichiometry. Angew. Chem. 2001, 40, 4245–4247. [Google Scholar] [CrossRef]
- D’Urso, A.; Nicotra, P.F.; Centonze, G.; Fragalà, M.E.; Gattuso, G.; Notti, A.; Pappalardo, A.; Pappalardo, S.; Parisi, M.F.; Purrello, R. Induction of chirality in porphyrin-(bis)calixarene assemblies: A mixed covalent-non-covalent vs a fully non-covalent approach. Chem. Commun. 2012, 48, 4046–4048. [Google Scholar] [CrossRef] [PubMed]
- D’Urso, A.; Cristaldi, D.A.; Fragalà, M.E.; Gattuso, G.; Pappalardo, A.; Villari, V.; Micali, N.; Pappalardo, S.; Parisi, M.F.; Purrello, R. Sequence, stoichiometry, and dimensionality control in porphyrin/biscalix[4]arene self-assemblies in aqueous. Chem. Eur. J. 2010, 16, 10439–10446. [Google Scholar] [PubMed]
- D’Urso, A.; Marino, N.; Gaeta, M.; Rizzo, M.S.; Cristaldi, D.A.; Fragalà, M.E.; Pappalardo, S.; Gattuso, G.; Notti, A.; Parisi, M.F.; et al. Porphyrin stacks as an efficient molecular glue to induce chirality in hetero-component calixarene-porphyrin assemblies. New J. Chem. 2017, 41, 8078–8083. [Google Scholar] [CrossRef]
- Gaeta, M.; Sortino, G.; Randazzo, R.; Pisagatti, I.; Notti, A.; Fragalà, M.E.; Parisi, M.F.; D’Urso, A.; Purrello, R. Long-range chiral induction by a fully noncovalent approach in supramolecular porphyrin–calixarene assemblies. Chem. Eur. J. 2020, 26, 3515–3518. [Google Scholar] [CrossRef]
- Gulino, F.G.; Lauceri, R.; Frish, L.; Evan-Salem, T.; Cohen, Y.; De Zorzi, R.; Geremia, S.; Di Costanzo, L.; Randaccio, L.; Sciotto, D.; et al. Noncovalent synthesis in aqueous solution and spectroscopic characterization of multi-porphyrin complexes. Chem. Eur. J. 2006, 12, 2722–2729. [Google Scholar] [CrossRef]
- Schmitt, P.; Beer, P.D.; Drew, M.G.B.; Sheen, P.D. Calix[4]tube: A tubular receptor with remarkable potassium ion selectivity. Angew. Chem. 1997, 36, 1840–1842. [Google Scholar] [CrossRef]
- Matthews, S.E.; Schmitt, P.; Felix, V.; Drew, M.G.B.; Beer, P.D. Calix[4]tubes: A new class of potassium-selective ionophore. J. Am. Chem. Soc. 2002, 124, 1341–1353. [Google Scholar] [CrossRef]
- Buhdka, J.; Lhotak, P.; Stibor, I.; Michlova, V.J.; Sykova, J.; Cisarova, I. A biscalix[4]arene-based ditopic hard/soft receptor for K+/Ag+complexation. Tetrahedron Lett. 2002, 43, 2857–2861. [Google Scholar] [CrossRef]
- Matthews, S.E.; Felix, V.; Drew, M.G.B.; Beer, P.D. Halo-derivatised calix[4]tubes. Org. Biomol. Chem. 2003, 1, 1232–1239. [Google Scholar] [CrossRef]
- Puchnin, K.; Zaikin, P.; Cheshkov, D.; Vatsouro, I.; Kovalev, V. Calix[4]tubes: An approach to functionalization. Chem. Eur. J. 2012, 18, 10954–10968. [Google Scholar] [CrossRef] [PubMed]
- Puchnin, K.; Cheshkov, D.; Zaikin, P.; Vatsouro, I.; Kovalev, V. Tuning conformations of calix[4]tubes by weak intramolecular interactions. New J. Chem. 2013, 37, 416–424. [Google Scholar] [CrossRef]
- The pH of the solution was adjusted to 3.0, by dropwise addition of an aqueous 0.5 M HCl solution, to ensure protonation of the eight amino groups of C4T or C4T@K+ and ultimately solubility in water.
- Moschetto, G.; Lauceri, R.; Gulino, F.G.; Sciotto, D.; Purrello, R. Non-Covalent synthesis in aqueous solution of discrete multi-porphyrin aggregates with programmable stoichiometry and sequence. J. Am. Chem. Soc. 2002, 124, 14536–14537. [Google Scholar] [PubMed]
- Herrmann, O.; Mehdi, S.H.; Corsini, A. Heterogeneous metal-insertion: A novel reaction with porphyrins. Can. J. Chem. 2006, 56, 1084–1087. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaeta, M.; Rodolico, E.; Fragalà, M.E.; Pappalardo, A.; Pisagatti, I.; Gattuso, G.; Notti, A.; Parisi, M.F.; Purrello, R.; D’Urso, A. Self-Assembly of Discrete Porphyrin/Calix[4]tube Complexes Promoted by Potassium Ion Encapsulation. Molecules 2021, 26, 704. https://doi.org/10.3390/molecules26030704
Gaeta M, Rodolico E, Fragalà ME, Pappalardo A, Pisagatti I, Gattuso G, Notti A, Parisi MF, Purrello R, D’Urso A. Self-Assembly of Discrete Porphyrin/Calix[4]tube Complexes Promoted by Potassium Ion Encapsulation. Molecules. 2021; 26(3):704. https://doi.org/10.3390/molecules26030704
Chicago/Turabian StyleGaeta, Massimiliano, Elisabetta Rodolico, Maria E. Fragalà, Andrea Pappalardo, Ilenia Pisagatti, Giuseppe Gattuso, Anna Notti, Melchiorre F. Parisi, Roberto Purrello, and Alessandro D’Urso. 2021. "Self-Assembly of Discrete Porphyrin/Calix[4]tube Complexes Promoted by Potassium Ion Encapsulation" Molecules 26, no. 3: 704. https://doi.org/10.3390/molecules26030704
APA StyleGaeta, M., Rodolico, E., Fragalà, M. E., Pappalardo, A., Pisagatti, I., Gattuso, G., Notti, A., Parisi, M. F., Purrello, R., & D’Urso, A. (2021). Self-Assembly of Discrete Porphyrin/Calix[4]tube Complexes Promoted by Potassium Ion Encapsulation. Molecules, 26(3), 704. https://doi.org/10.3390/molecules26030704