Abstract
Plants have had historical significance in medicine since the beginning of civilization. The oldest medical pharmacopeias of the African, Arabian, and Asian countries solely utilize plants and herbs to treat pain, oral diseases, skin diseases, microbial infections, multiple types of cancers, reproductive disorders among a myriad of other ailments. The World Health Organization (WHO) estimates that over 65% of the world population solely utilize botanical preparations as medicine. Due to the abundance of plants, plant-derived medicines are more readily accessible, affordable, convenient, and have safer side-effect profiles than synthetic drugs. Plant-based decoctions have been a significant part of Jamaican traditional folklore medicine. Jamaica is of particular interest because it has approximately 52% of the established medicinal plants that exist on earth. This makes the island particularly welcoming for rigorous scientific research on the medicinal value of plants and the development of phytomedicine thereof. Viral infections caused by the human immunodeficiency virus types 1 and 2 (HIV-1 and HIV-2), hepatitis virus B and C, influenza A virus, and the severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) present a significant global burden. This is a review of some important Jamaican medicinal plants, with particular reference to their antiviral activity.
1. Introduction
Viral infections like the human immunodeficiency viruses types 1 and 2 (HIV-1 and HIV-2), herpes simplex virus (HSV), respiratory viruses like; rotaviruses, respiratory syncytial viruses (RSV), influenza A virus, the severe acute respiratory syndrome coronavirus 2 (SARS CoV-2) that causes the human coronavirus (COVID19), tuberculosis, and hepatitis B and C viruses, malaria, yellow virus fever (YVF), human papilloma virus (HPV), dengue-virus type 2 (DENV-2) and coxsackie virus all represent a significant global burden. In modern Western medicine, natural preventative and therapeutic alternatives are increasingly gaining attention because of greater accessibility to medicinal plants, and the possibility that they may have fewer and less adverse side-effects, are safer, and have potentially greater therapeutic efficacy than synthetic drugs. These make natural alternatives more desirable as novel drug therapies. Interest in phytoantivirals has also been renewed because of the increasing global burden of viral infections, particularly those that are newly emerging, and increasing antiviral resistance among viral strains against conventional synthetic antivirals drugs like Acyclovir and Ganciclovir.
In many parts of the world, particularly in rural, developing countries, herbalism is the only form of traditional medicine. In 2011, the WHO estimated that between 70 and 95% of the world population use botanical preparations as medicine [1]. Thousands of years of anecdotal evidence support the medicinal claims of these plants, but for the vast majority, rigorous scientific monitoring and research are required to study the mechanisms of action of the bioactive compounds, and their safety and efficacy [2]. It is estimated that some 25% of medicines on the global market are synthesized from natural products [3]. Plants from which popularly used drugs worldwide have been derived include Ephedra sinica Stapf. used to make methamphetamine and pseudoephedrine, Willow bark to make Aspirin, Cannabis sativa L. to make Dronabinol®, Nabilone® and Sativex®, Papaver somniferum L. (opium poppy) to make morphine, codeine and heroin, Taxus brevifolia Nutt. to make taxol, and Vinca rosea L. to make vincristine.
Through ethnobotanical screening, thousands of plants and their biologically active ingredients have been identified. Of the estimated 300,000 plants species that exist worldwide, only around 15% have been evaluated for their pharmacological activity [4]. It is estimated that two-thirds of the world’s plant species have medicinal value [5]. Between 1981 and 2019, the FDA approved more than 1500 drugs either made from unaltered natural products, botanical drug mixtures, derivates of natural products, synthetic drugs with pharmacophores from natural products or mimics of natural products [6,7]. In 2019, the FDA approved 441 small molecule drugs made directly from natural products and their derivates [7]. Refer to Table 1.

Table 1.
Drugs approved by the FDA between 1 January 1981 and 30 September 2019 that were either derived from botanical drugs, unaltered natural products, or synthetic drugs with natural pharmacophore.
Medicinal plants produce primary and secondary metabolites, that, in addition to providing health benefits to humans, may have an original intended use in the plant as biological defenses against herbivores [8]. The plant kingdom (including microbes, lichens, algae, and higher plants) produces an estimated 600,000 to 700,000 phytochemicals, with at least 150,000 to 200,000 being bioactive compounds [9].
Some active compounds in these plants responsible for the therapeutic effects of phytoantivirals include alkaloids, anthraquinones, coumarins, polyphenols (e.g., flavonoids), phenolic acids and their derivatives, lignans, naphthoquinones, peptides, nitrogenated compounds, polysaccharides and terpenes. Lipophilic terpenoids (essentials oils) disrupt the virus envelope’s lipid double layer and may even cause it to lyse [10]. Polyphenols (e.g., flavonoids) and phenolic acids may prevent the virus from docking to the host cell [10]. Phytochemicals like alkaloids (e.g., quinoline), furanocoumarins, aristolochic acid, macrozamin and cyasin can attack and mutilate nucleic acids (DNA and RNA) either by alkylating the DNA molecule or intercalating within the DNA molecule [10]. Other active ingredients that have been used in ancient/traditional medicine include strychnine, quinine, and morphine (European medicine), camptothecin and taxol (Chinese medicine), and Vinca alkaloids vincristine and vinblastine extracts from the Madagascar periwinkle (Catharanthus roseus) [11]. Other important biologically active ingredients that have been discovered from plants include resveratrol and curcumin.
Despite the increasing clinical research on phytoantivirals, there is need for more rigorous screening of more plants to identify new phytochemicals. Further research is required to determine the efficacy, dosage standards, optimum extraction methods/solvents, cytotoxicity/hepatoxicity, pharmacokinetics, molecular mechanisms of action, phytoantiviral screening methods, and drug interactions for many phytoantivirals.
Modern research around the world needs to now focus on the pharmacological activities of the constituent compounds of these plants and mapping their genomes and transcriptomes to produce target drugs. These compounds include alkaloids, anthraquinones, coumarins, polyphenols (flavonoids, tannins and rosmarinic acid), phenolic acids, lignans, naphthoquinones, peptides, polysaccharides and terpenes.
The top five largest orders of medicinal plants include Lamiales, Rosales, Malpighiales, Fabales and Sapindales [12]. The Medicinal Plant Database is a comprehensive multi-omics database of medicinal plants [12]. Using sequencing technology and synthetic biology, this genomic and transcriptomic mapping database will allow for the development of synthetic drugs that will be able to target a certain molecular pathway in a given disease process.
The purpose of this article is to review the state-of-the-art developments in phytomedicines in Jamaica, with particular focus on antivirals. This should provide a valuable reference for further studies on development and clinical translation of these phytomedicines.
2. Jamaican Plants with Medicinal Potential
Medicinally, there is increasing focus on Jamaica because of the wide diversity of medicinal plants. Plant-based decoctions have been a significant part of Jamaican traditional folklore medicine primarily to treat the common cold, flu, headache, nausea, pain, reproductive system disorders and digestive issues, among some of the aforementioned diseases that are a significant global burden. Popular medicinal plants used in traditional Jamaican folk medicine include Momordica charantia L. (Cerasee), Aloe barbadensis Miller (Aloe vera (L.) Burm.f./), Cannabis sativa L. (Ganja), Cola acuminate (P.Beauv.) Schott and Endl. (Bissy), Morinda citrifolia L. (Noni), Pothomorphe umbellata (L.) Miq. (Cowfoot Leaf), Cinnamomum tamala (Buch.-Ham.) T.Nees and Eberm. (Bay leaf), Zingiber officinale Roscoe (Ginger), Bryophyllum pinnatum (Lam.) Oken (Leaf-of-life), Moringa oleifera Lam. (Moringa), Panax ginseng C.A.Mey. (Ginseng), Mikania micrantha Kunth (Quako), Marrubium Vulgare L. (White horehound/”Mint”), Andrographis paniculata (Burm.f.) Nees (Rice bitters), Curcuma Longa L. (turmeric), Petiveria alliacea L. (Guinea Hen Weed), Camellia Sinensis (L.) Kuntze (“Tee tree”), Alysicarpus vagilinis (L.) DC. (Medina) and Allium sativum L. (Garlic). Jamaicans have used a combination of these plants for detoxing and to treat a wide range of ailments including, but not limited to headache, nausea, pain, inflammation, cancer, diabetes, reproductive disorders, viral infections (common cold and “flu”), arthritis and hypertension.
The antiviral properties of these medicinal plants are supported by traditional anecdotal evidence. There is a need for more scientific research to establish efficacy, pharmacological and pharmacokinetic data and standard dosages for medical conditions. Viral infections are the second-most identified health condition for which medicinal plants were used to treat in Jamaica [13].
Popular in the Jamaican ethnomedical heritage is what is known as a “root tonic” or “strong back”. This drink is a decoction of plants including, but not limited to Smilax balbisiana Kunth (“chany root”), Smilax ornata (sarsaparilla), Zingiber officinale Roscoe (“ginger”), Alysicarpus vaginalis (L.) DC. (“medina”), Morinda royoc L. (“redgal”), Desmodium incanum DC. (“tick clover”), Cuphea parsonsia (L.) R.Br. ex Steud. (commonly referred to as “strong back leaf”), Trophis racemosa (L.) Urb. (“ramoon”), and Iresine diffusa Humb. and Bonpl. Ex Willd (“nerve west”) [14]. This decoction is commonly used by males to treat impotence, and increase stamina. However, many of these plants like Z. officinale, C. parsonsia, S. ornata, and A. vaginalis have noted antiviral activity.
Table 2 shows the frequently used medicinal plants to treat the common cold and flu in Jamaica.
A 2011 ethnomedicinal survey by Picking and colleagues investigated popular medicinal plants in Jamaica and confirmed the significance of plants and herbs in primary health care in Jamaica [13]. This survey followed the TRAMIL network ethnomedicine methodology. The TRAMIL network is a Caribbean-based network of collaborators conducting scientific evaluations of medicinal plants in the region. The questionnaire was administered to 407 randomly selected adults, from randomly selected geographical clusters across Jamaica. The survey revealed that respondents used 116 medicinal plants for various ailments. Of these, 94% (107 plants) were distributed across fifty-one plant families. The top five families with the most frequent plant families identified were Fabaceae, Lamiaceae, Asteraceae, Malvaceae, and Piperacerae [13]. Common plants of the Fabaceae family include Legumes, Maranga, Strong Back, Dandelion and Medina. Common herbs of the Lamiaceae family include Basil, Sage, Rosemary, Oregano, Thyme, Mentha and Lavender. The Lamiaceae family is commonly known as the Mint family. Some plants of the Asteraceae family include of Marigold, Spanish Needle, and Quaco Bush. Common plants of the Malvaceae family include Bissy, Sorrel, and Hibiscus.
Of the 107 plants identified by survey respondents, eight are endemic to Jamaica. These are Piper amalago L. (Pepper elder), Rhytidophyllum tomentosum (L.) Mart. (“Search-mi-heart”), Bidens reptans (L.) G.Don (“McKatty Weed”/”Marigold”), Peperomia amplexicaulis (Sw.) A. Dietr (“Jackie’s saddle”), Oryctanthus occidentalis (L.) Eichler (“Godbush”), Pilea microphylla (L.) Liebm. (“Baby puzzle”), Smilax balbisiana Kunth (“Chany Root”), and Boehmeria jamaicaensis Urb. 1907 (“Doctor Johnson”). Of these, P. amalago L. has the most notable medicinal use [13].
The common cold, which may be caused by different viruses, has been among the most prevalent health issues in Jamaica. Leaf of Life (B. pinnatum) and Jack-in-the bush (Euphatorium odoratum L.) have a frequency of use of equal to or greater than 20% for these viral infections in Jamaica [13].
A 2006 study by Mitchell and Ahmad investigated and summarized all the medicinal plant research carried out on Jamaican medicinal plants between 1948 and 2001 at the Faculty of Pure and Applied Science, University of the West Indies (UWI), Mona, Jamaica [15]. Mitchell and Ahmad report that Jamaica has at least 334 medicinal plant species. However, only 193 were tested for their bioactivity—80 plants of which were reported to have reasonable bioactivity. Natural products were also identified from 44 of these plants. Of the plants tested at UWI, only 31 were endemic to Jamaica. Some 23% percent of these 31 plants were tested and found to be bioactive [15].
4. Guinea Hen Weed (Petiveria alliacea L.)
P. alliacea is a herbaceous shrub belonging to Petiveriaceae, the pigeonberry family. It is native to tropical regions like Africa, India, the Caribbean, tropical areas of North and Central America. P. alliacea produces a number of bioactive compounds including polyphenols, alkaloids, tannins, coumarins, steroids, essential oils, dibenzyl trisulfide, and flavonoids [39]. These phytochemicals are responsible for Guinea Hen Weed’s wide therapeutic window as an anxiolytic, anticonvulsant, antinociceptive, neuroprotector, cognitive enhancer, and antidepressant [39].
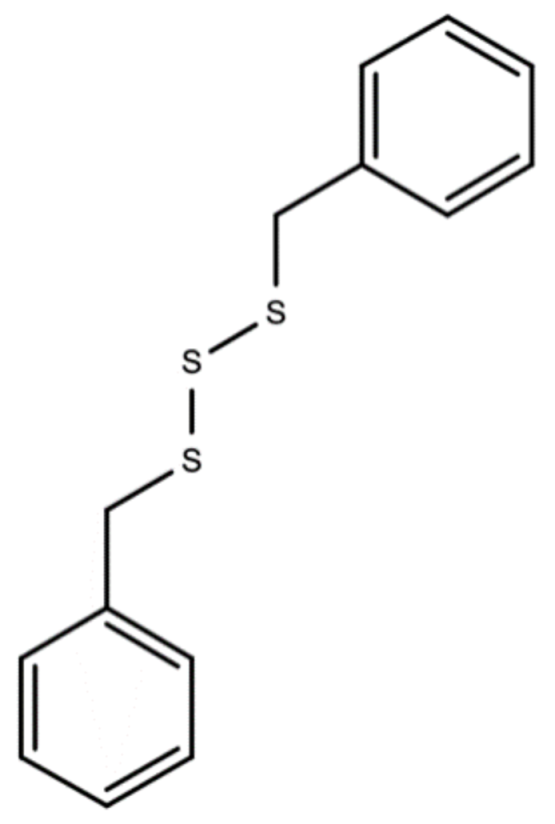
Dibenzyl trisulfide/DTS (C14H14S3) in the Guinea Hen Weed is responsible for its anti-cancer, ant-HIV, and anti-hepatitis C properties [40,41]. Figure 5 below should the chemical structure of dibenzyl trisulfide (DTS) found in Guinea Hen Weed.
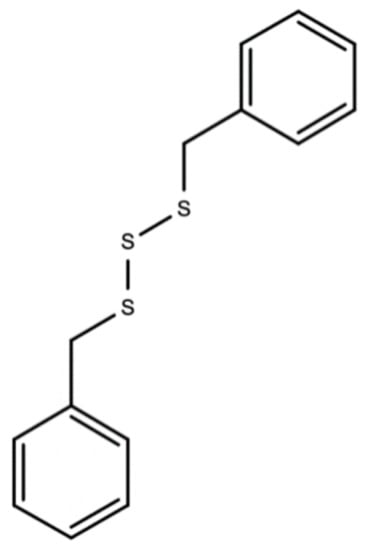
Figure 5.
Chemical structures of dibenzyl trisulfide (DTS) found in Guinea Hen Weed.
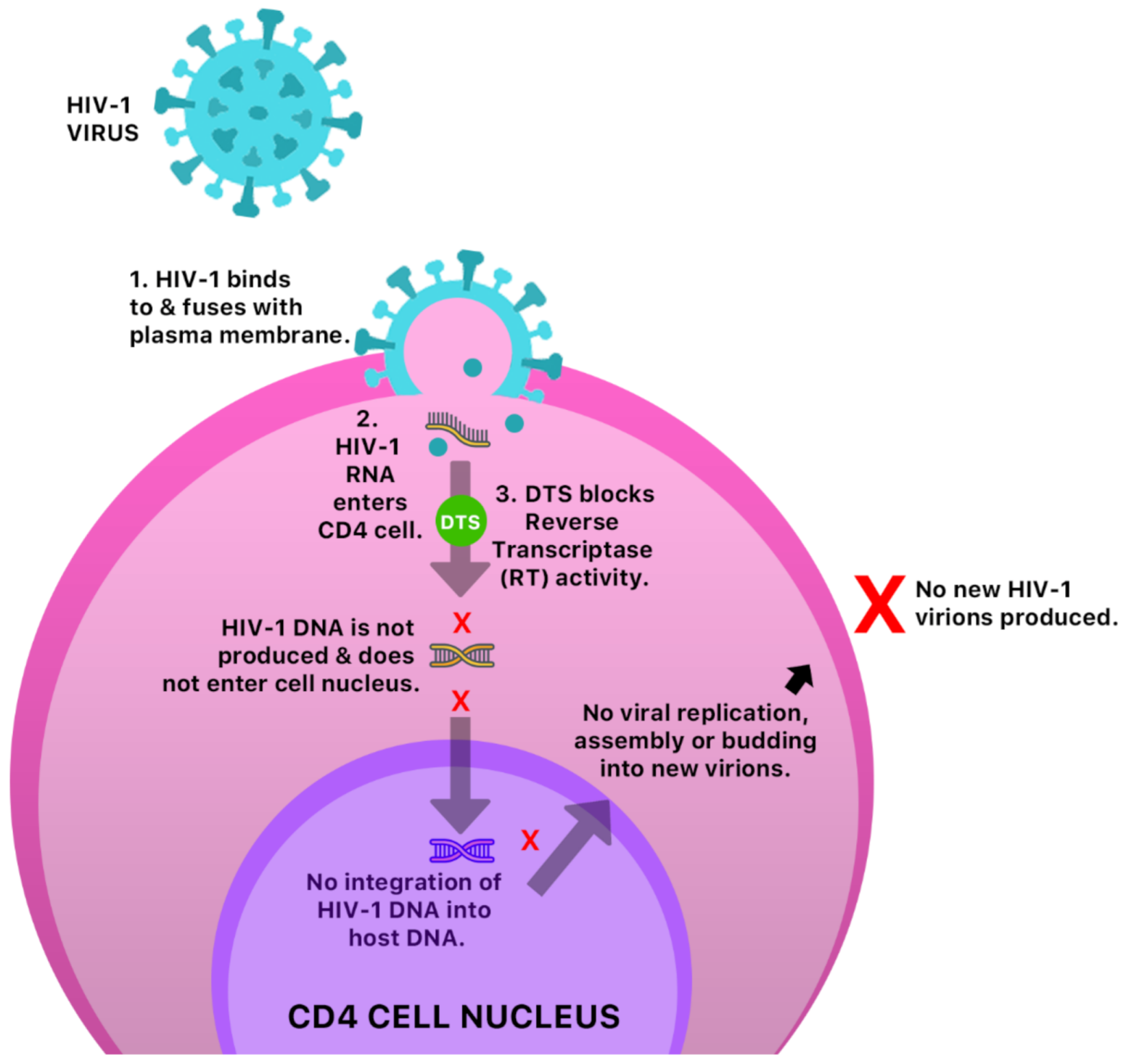
The anti-cancer and antiviral properties of this compound may be due to its interaction with the mitogen-activated protein, extracellular-regulated kinases 1 and 2 (MAP Kinases Erk 1/Erk2) pathway [41] (Figure 6). The mechanism by which DTS inhibits HIV-1 activity is by inhibition of the reverse transcriptase (RT) activity of the HIV-1 virus [40] (Figure 7). Anecdotal evidence also suggests that Guinea Hen Weed may also be used to combat the influenza viruses. DTS has also been reported to demonstrate anti-hepatitis C virus properties responsible for hepatocellular carcinoma [42]. However, the molecular mechanism of action has not yet been elucidated.
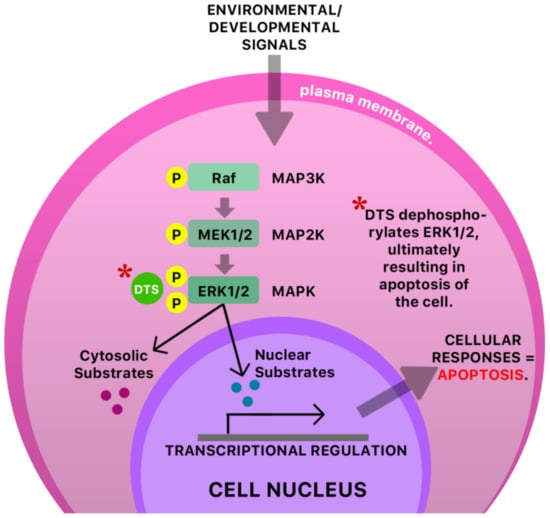
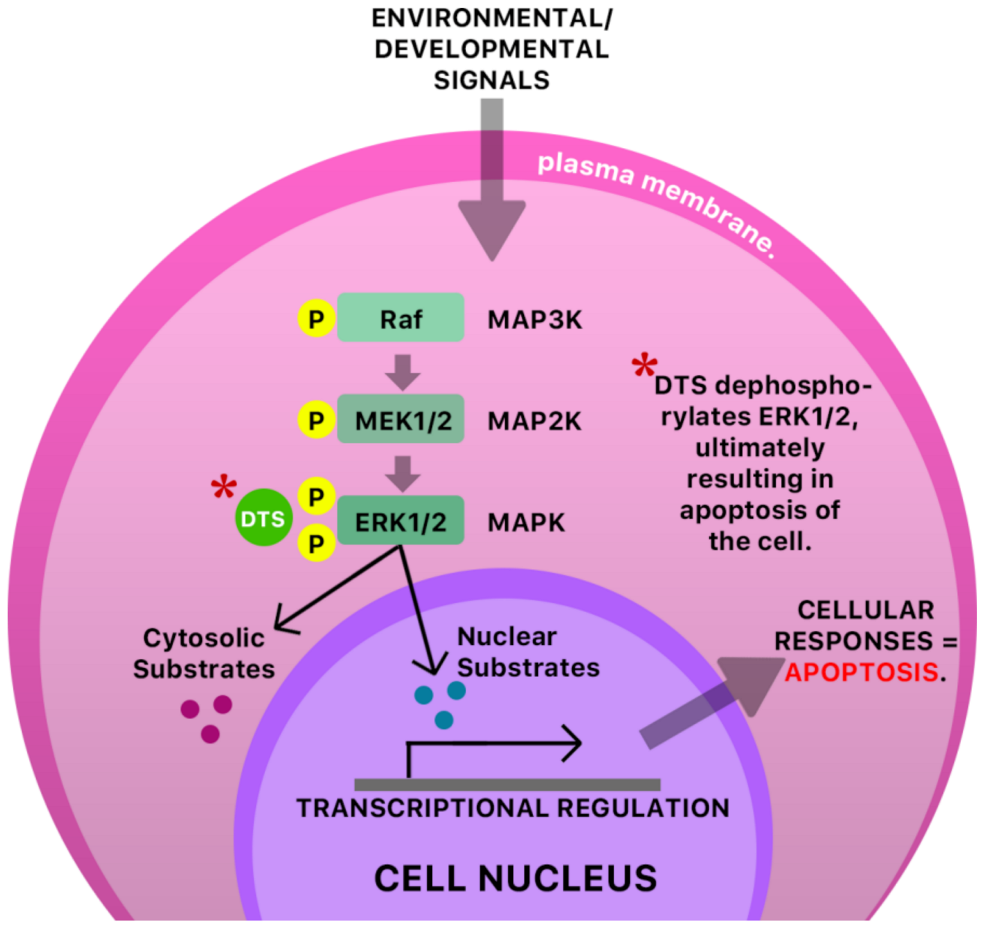
Figure 6.
A possible antiviral mechanism of DTS in an infected cell. The mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK ERK1/ERK2 pathway) is a signaling pathway that is responsible for the transduction of protein components (chemical signals, transcription factors), MAP kinase kinase (MAP2K), and a final kinase, MAP kinase (MAPK), leading to the functioning and regulation of multiple cellular processes like cell proliferation, cell growth, and cell survival. A dysregulation of this pathway or any of its components typically has pathological consequences [43,44]. It has been proposed that some pathological conditions are characterized by increased phosphorylation of kinases [45], possibly resulting in over-proliferation of cells. Dibenzyl trisulfide (DTS) is shown to inhibit the MAPK ERK1/ERK2 pathway in cancer- and (possibly) virally infected cells via dephosphorylation of ERK1/2, ultimately resulting in apoptosis of the cell [45]. MAP—mitogen-activated protein; K—kinase; Raf—rapidly accelerated fibrosarcoma; MEK—mitogen-activated protein kinase.
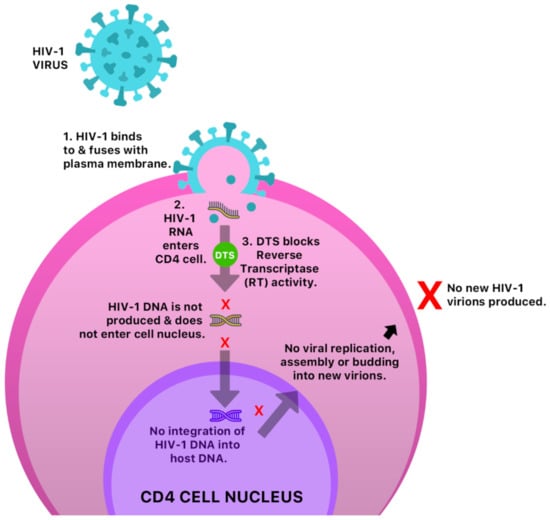
Figure 7.
A possible anti-HIV-1 mechanism of DTS in a CD4 cell.
5. Ginger (Zingiber Officinale Roscoe)
Ginger is frequently used in Jamaica as an antiemetic and antinauseant. In addition to having antioxidant, antimicrobial, anti-inflammatory, and anticoagulant properties, fresh ginger, as opposed to dried, has also been reported to be antiviral, inhibiting human respiratory syncytial virus-induced plaques in human upper (HEp-2) and low (A549) respiratory tract cell lines [46].
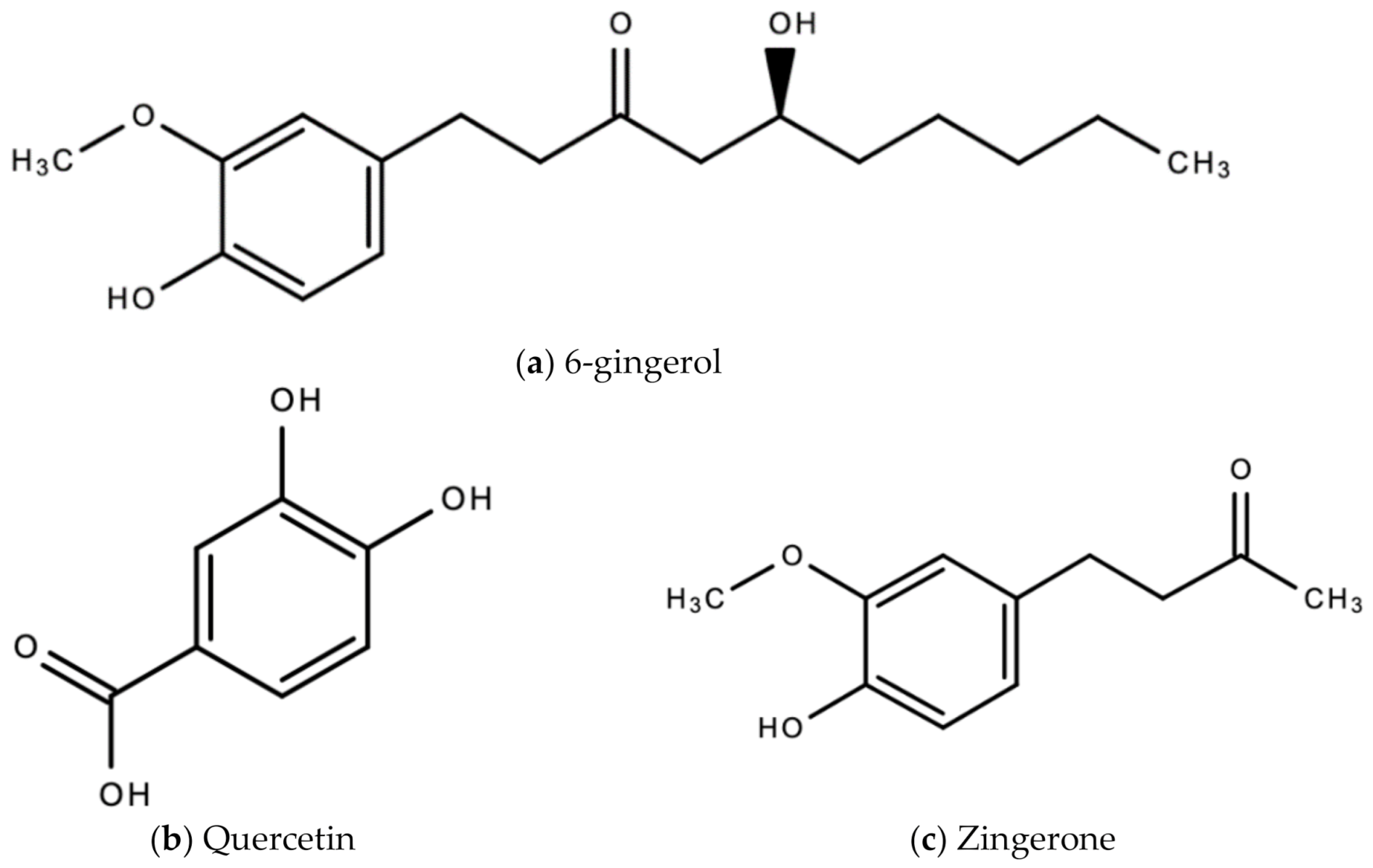
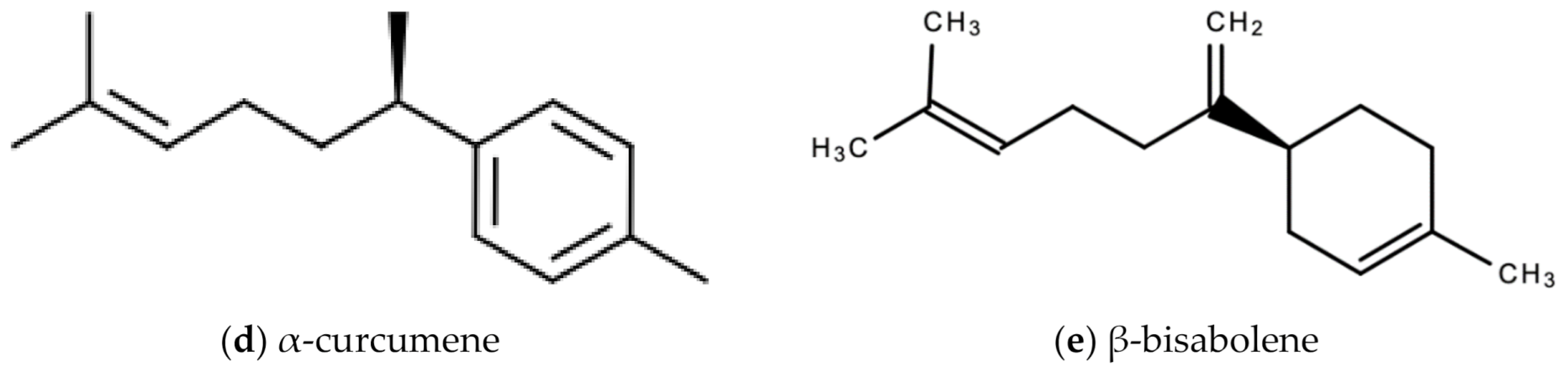
The bioactive compounds in ginger that are responsible for the wide therapeutic window of ginger include gingerols (like shogaols, 6-, 8- and 10- gingerol), paradols, ketones, phenolic compounds (like 6-dehydrogingerdione, zingerone, quercetin, and gingerenone-A), terpenes (like α-curcumene, α-farnesene, β-bisabolene and β-sesquiphellandrene), esters, aldehydes, and alcohols [47]. Figure 8a–e are examples of bioactive molecules found in Zingiber officinale Roscoe and their chemical structures.
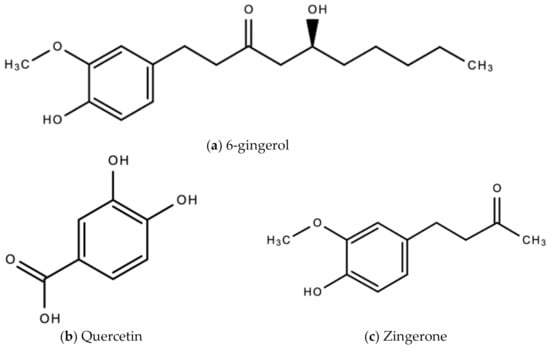
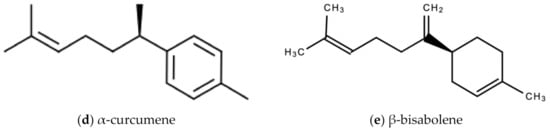
Figure 8.
Chemical structures of some bioactive molecules found in Zingiber officinale Roscoe.
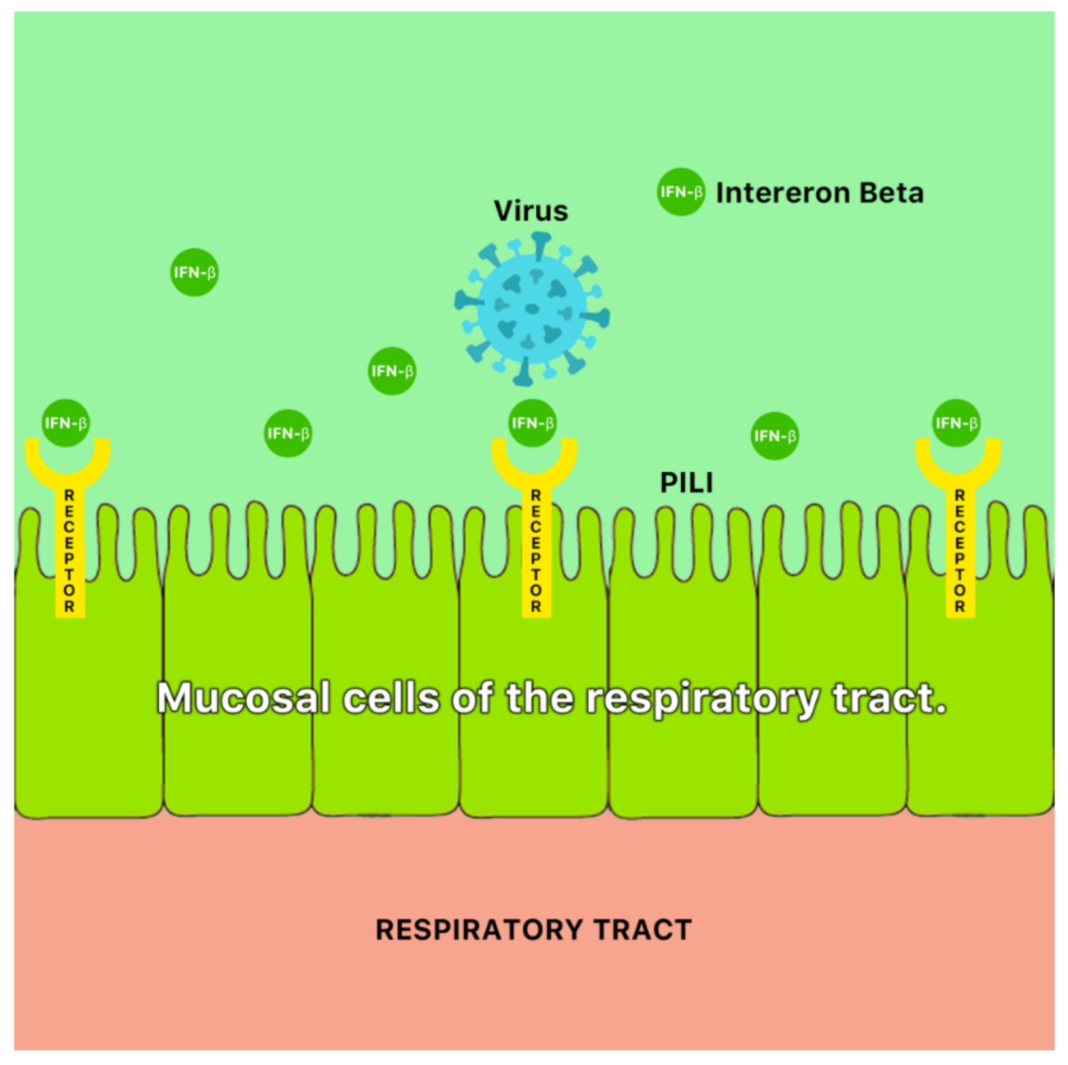
A possible mechanism of antiviral action of ginger is the blocking of viral attachment and internalization via stimulation of mucosal cells of the respiratory tract to secrete interferon- β (IFN- β) [46] (Figure 9). Ginger has also been reported to demonstrate antiviral properties against the hepatitis C virus (HCV) [47].
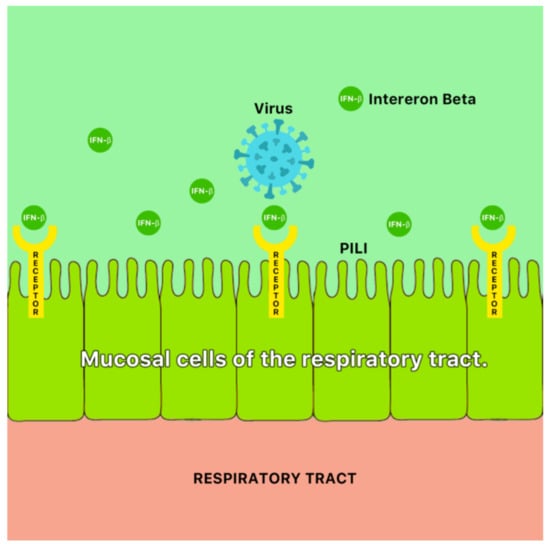
Figure 9.
A possible antiviral mechanism of ginger (Zingiber officinale Roscoe). It is possible that ginger blocks viral attachment and internalization via stimulation of mucosal cells of the respiratory tract to secrete interferon-β (IFN-β) [46].
6. Turmeric (Curcuma Longa L.)
Turmeric (Curcuma longa L.) is the belowground portion (rhizome) of the ginger plant, of the family Zingiberaceae. Turmeric is a widely used spice, food preservative, food coloring and dye but has also been employed for its medicinal value [48]. It has a wide therapeutic window as an antiviral, aseptic, antibacterial, antifungal, anti-cancer, anti-phlegmatic antiprotozoal, antioxidant, anti-inflammatory, antifibrotic, antifertility, antiulcer, antivenom, anticarcinogenic and anticoagulant [48]. In traditional Eastern medicine it is used to treat respiratory ailments, eating disorders, digestive disorders, hepatic disorders and biliary disorders [48].
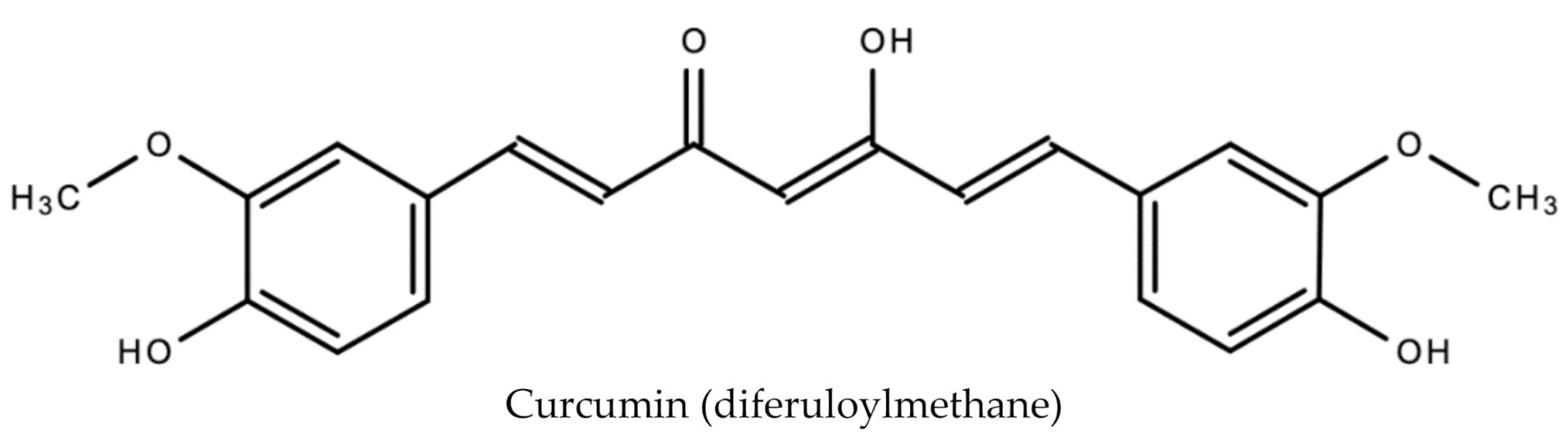
The primary phenolic compounds (curcuminoids) produced by turmeric are curcumin (diferuloylmethane), demothoxycurcumin and bisdemethoxycurcumin [48]. Other bioactive compounds produced include sodium curcuminate, essential oils (e.g., turmerone, zingiberene and sesquiterpines), monoterpenoids, and sesquiterpenoids. In addition to being an antiviral, curcumin is anti-inflammatory, anti-tumorigenic and anti-oxidant [49]. It is also responsible for the orange–yellow color of turmeric. Figure 10 displays the chemical structure of curcumin.
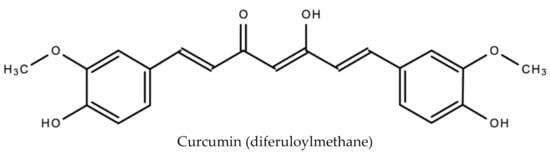
Figure 10.
Chemical structure of curcumin (diferuloylmethane) found in Curcuma Longa L.
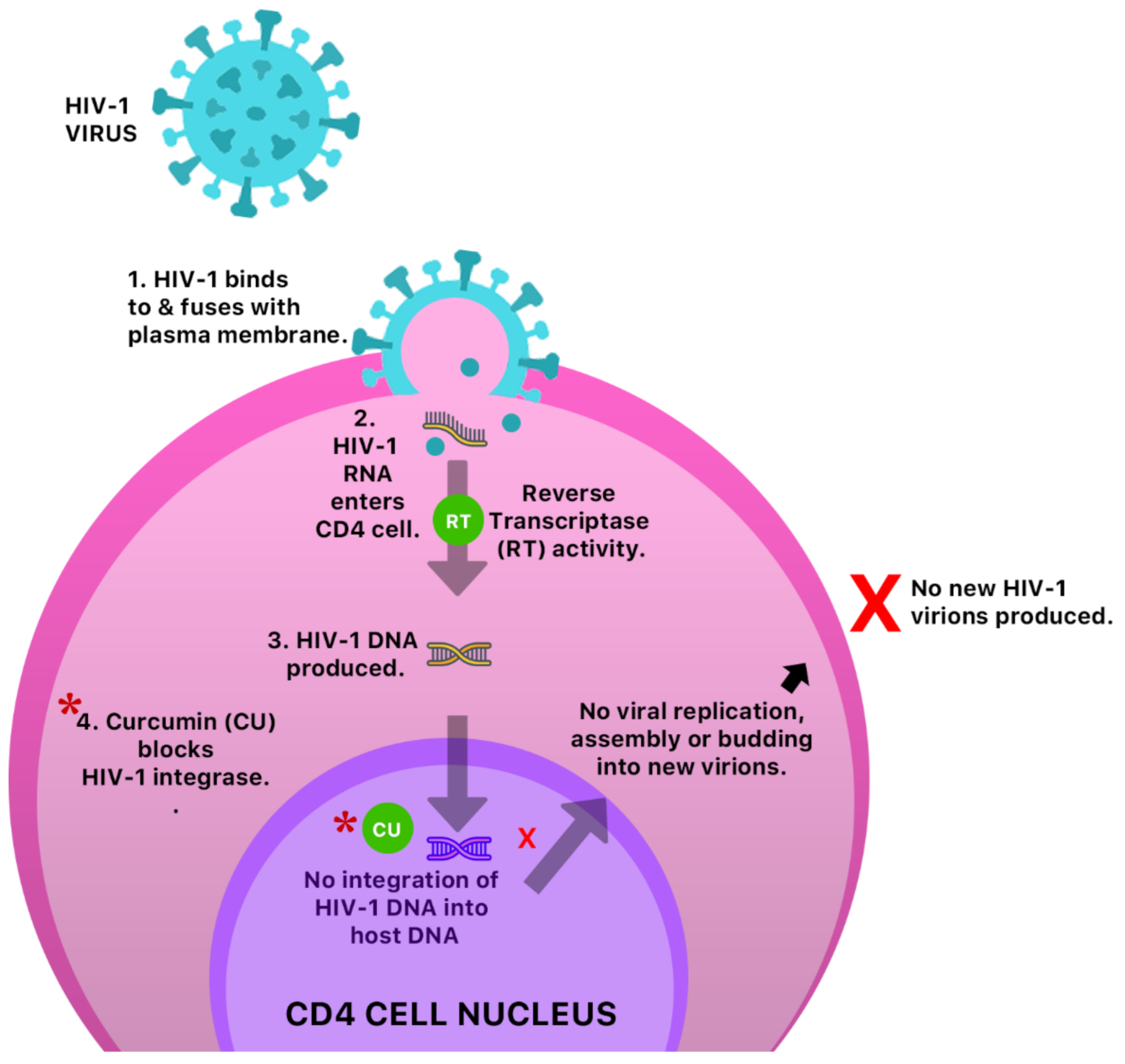
An in vitro study reported the antiviral activity of curcumin against three major molecular pathways responsible for Epstein–Barr virus (EBV) reactivation, and was shown to inhibit the transcription of BamH fragment Z left frame 1 (BZLF1) gene [50] which plays a significant role in lytic EBV DNA replication. The anti-HIV-1 activity of curcumin was also assessed and confirmed via inhibition of the HIV-1 integrase [51] (Figure 11). Turmeric was also shown to display slight antiviral activity against respiratory viruses.
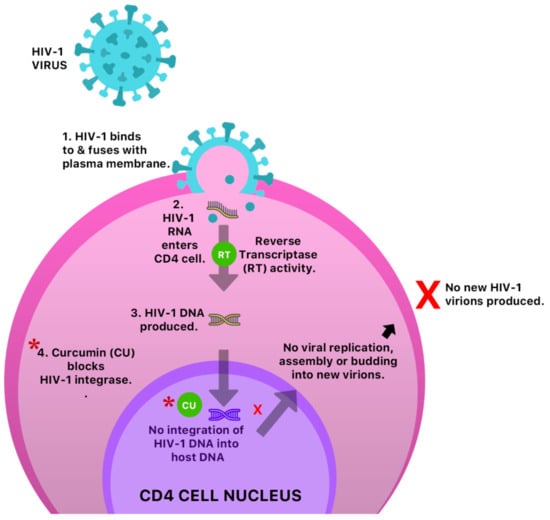
Figure 11.
A possible anti-HIV-1 mechanism of curcumin by inhibition of HIV-1 integrase. This prevents integration of HIV-1 DNA into host cell DNA and ultimately inhibition of viral replication, assembly, budding and infection of new cells.
A 2007 study by Huang and colleagues demonstrated activity against the influenza viruses, parainfluenza viruses 1, 2 and 3, adenovirus, and respiratory syncytial virus [52]. Curcumin was also reported to inhibit H1N1, H6N6, herpes virus, coxsackievirus B3 (CVB3), human T-cell leukemia virus type 1, hepatitis C virus, high risk human papillomaviruses 16 and 18 (HPV-16 and HPV-18) [53].
7. Moringa (Moringa oleifera Lam.)
M. oleifera is popular tree native to India where it is widely eaten as a vegetable and utilized in traditional medicine. It is also used in the Western hemisphere for the same purposes. Moringa has a wide window of benefits, due primarily to bioactive compounds like phenolic compounds, saponins, tannins, amino acids, proteins, phytates, tocopherols (γ and α), carbohydrates, unsaturated fatty acids, oils, antioxidant compounds, and glucosinolates [54]. Other bioactive compounds include vitamins A, B1, B2, B3, B7, C, D, E, K, calcium, potassium, iron, magnesium, phosphorus and zinc [55]. The pharmacological compounds found in the moringa plant have medicinal properties including antimicrobial activity against viruses, bacterial, fungi and parasites. It is also generally known to boost the immune system.
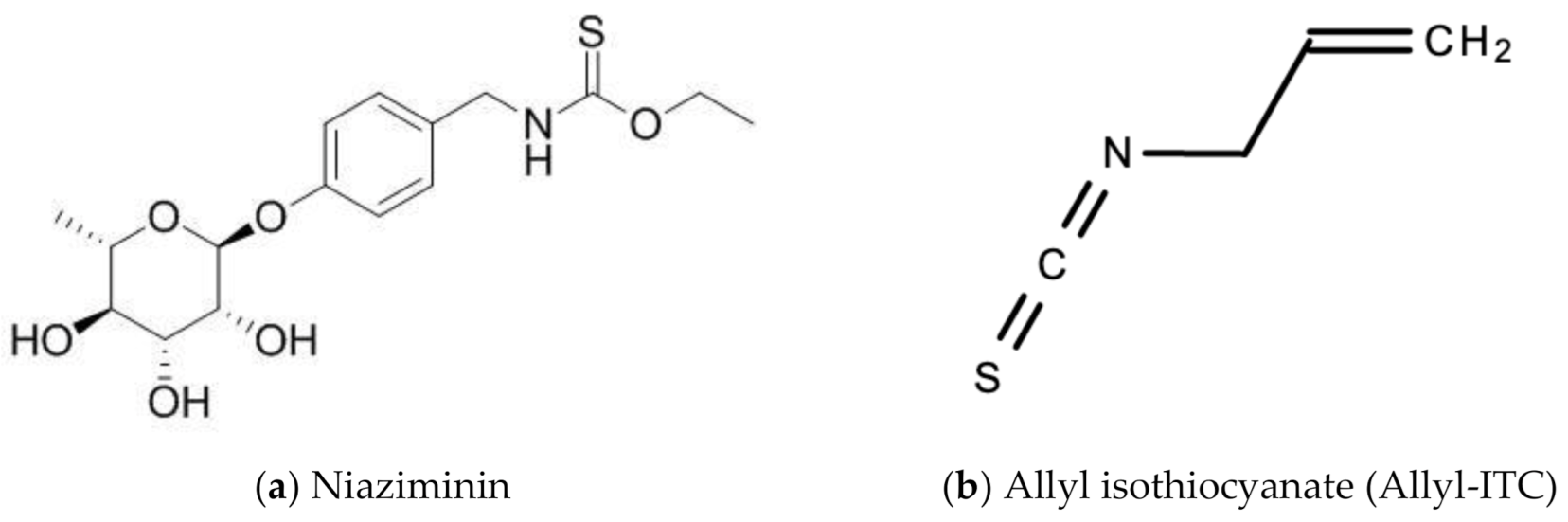
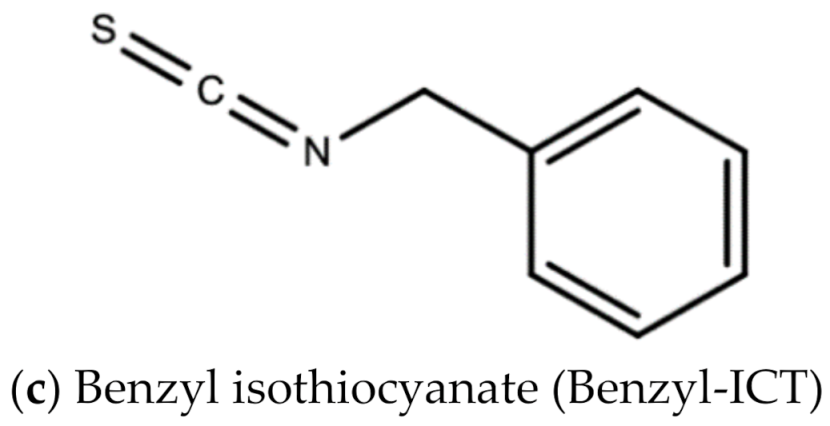
Leaf extracts from Moringa oleifera showed anti-herpetic activity and were reported to successfully inhibit the growth of HSV-1 and 2 in Vero cells by 43.2 and 21.4%, respectively [56]. Ethanolic leaf extracts of Moringa oleifera were also reported to have anti-influenza activity in vitro [57]. A 1998 study by Murakami and colleagues reported that niaziminin, a thiocarbamate, 4-[(4′-O-acetyl-alpha-L-rhamnosyloxy)benzyl] isothyanate (ITC) and allyl- and benzyl-ITC (two isothyanate-related compounds), were all able to inhibit Epstein–Barr virus (EBV) in vitro [58]. Figure 12 shows the chemical structures of niaziminin, allyl-ICT, and benzyl-ICT.
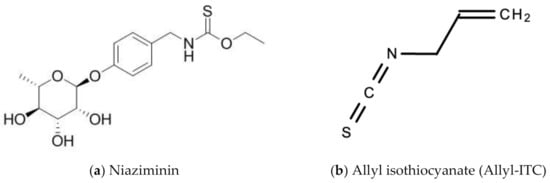
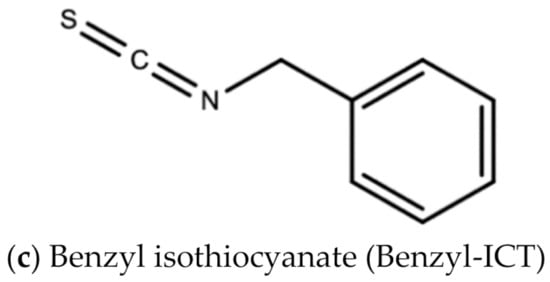
Figure 12.
Chemical structures of some bioactive molecules found in Moringa oleifera Lam.
In another in vitro study, the effects of an aqueous extract of M. oleifera (leaves) were able to decrease the expression of hepatitis B virus genotypes C and H in Huh7 cells [59].
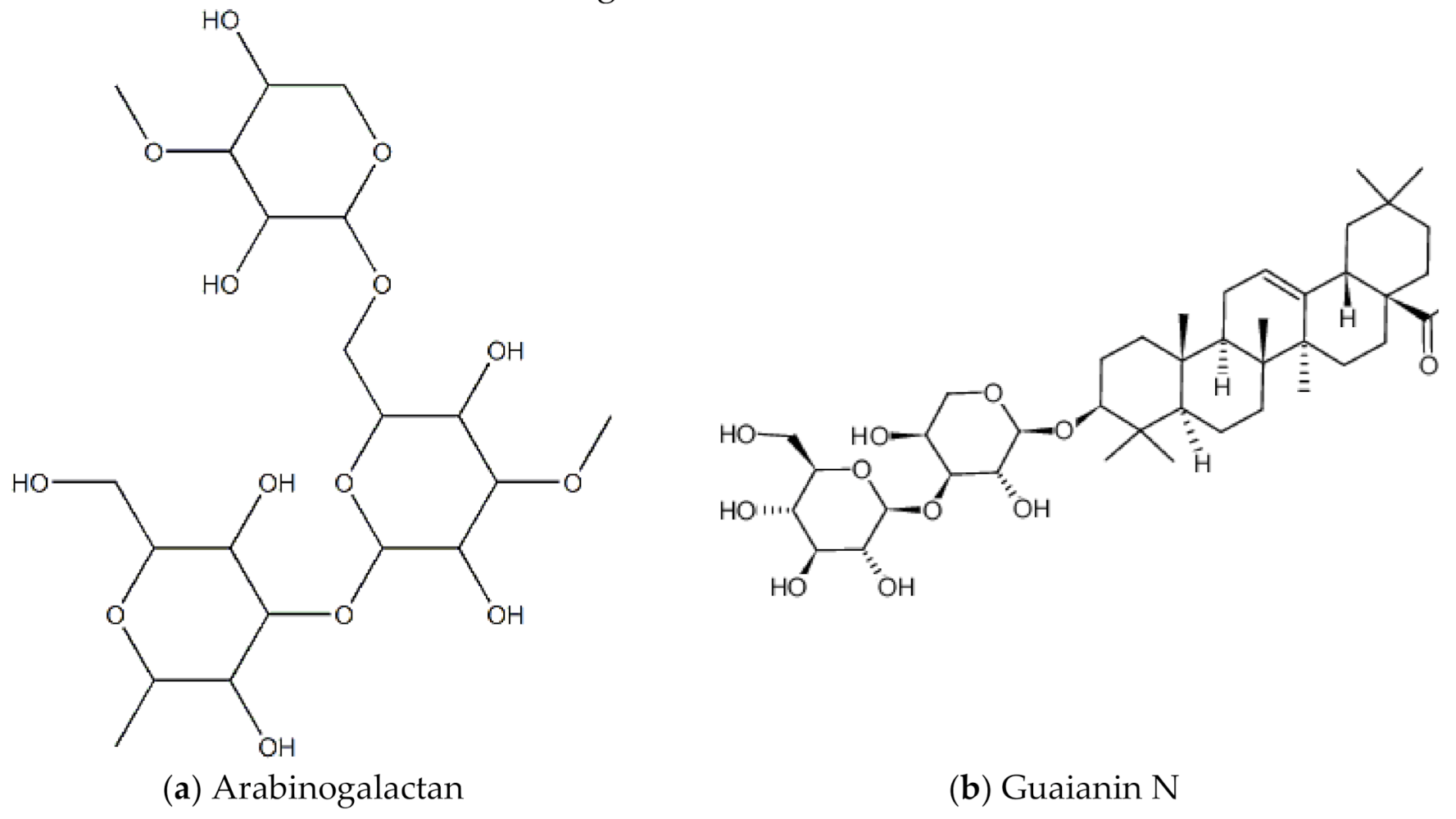
8. Lignum Vitae (Guaiacum officinale L.)
The Lignum vitae (which translates to “wood of life”) is native to the Caribbean and South America. The wood has many industrial purposes, for example to make furniture. The flower is the national flower of Jamaica. In tradition Caribbean medicine, it is used as a stimulant, antiseptic, and to treat syphilis [60]. In Trinidad and Tobago, it is used to control fertility [60]. A 2014 study by Lowe and colleagues screened and investigated the anti-HIV properties of leaf, seed and twig extracts of the lignum vitae plant biomass against HIV-1 (strain HIV-1 BaL) infected peripheral blood mononuclear cells (PBMCs) [61]. The main pharmacological compounds in this plant are saponins, including guaianin A, guaianin B and guaianin N [61]. All the types of extracts/compounds tested inhibited HIV-1 replication in the infected PBM cells. Figure 13 shows chemical structures of some bioactive molecules found in lignum vitae.
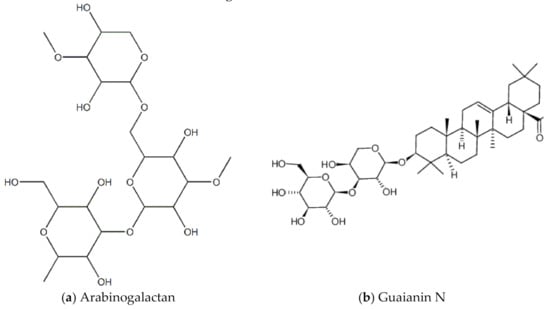
Figure 13.
Chemical structures of some bioactive molecules found in Lignum Vitae.
9. Garlic (Allium sativum L.)
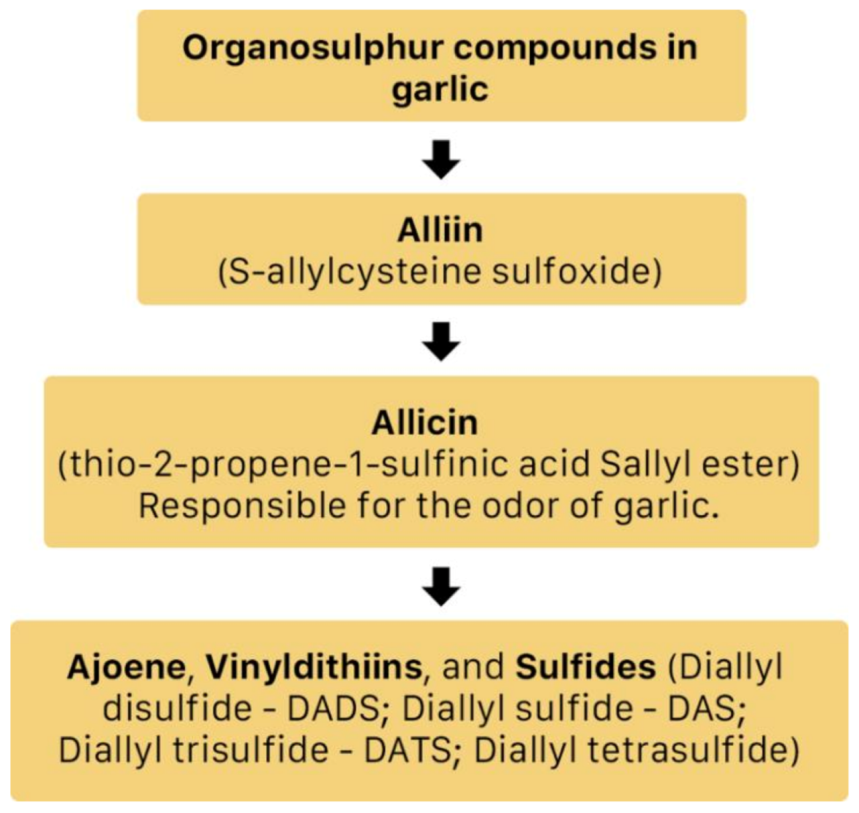
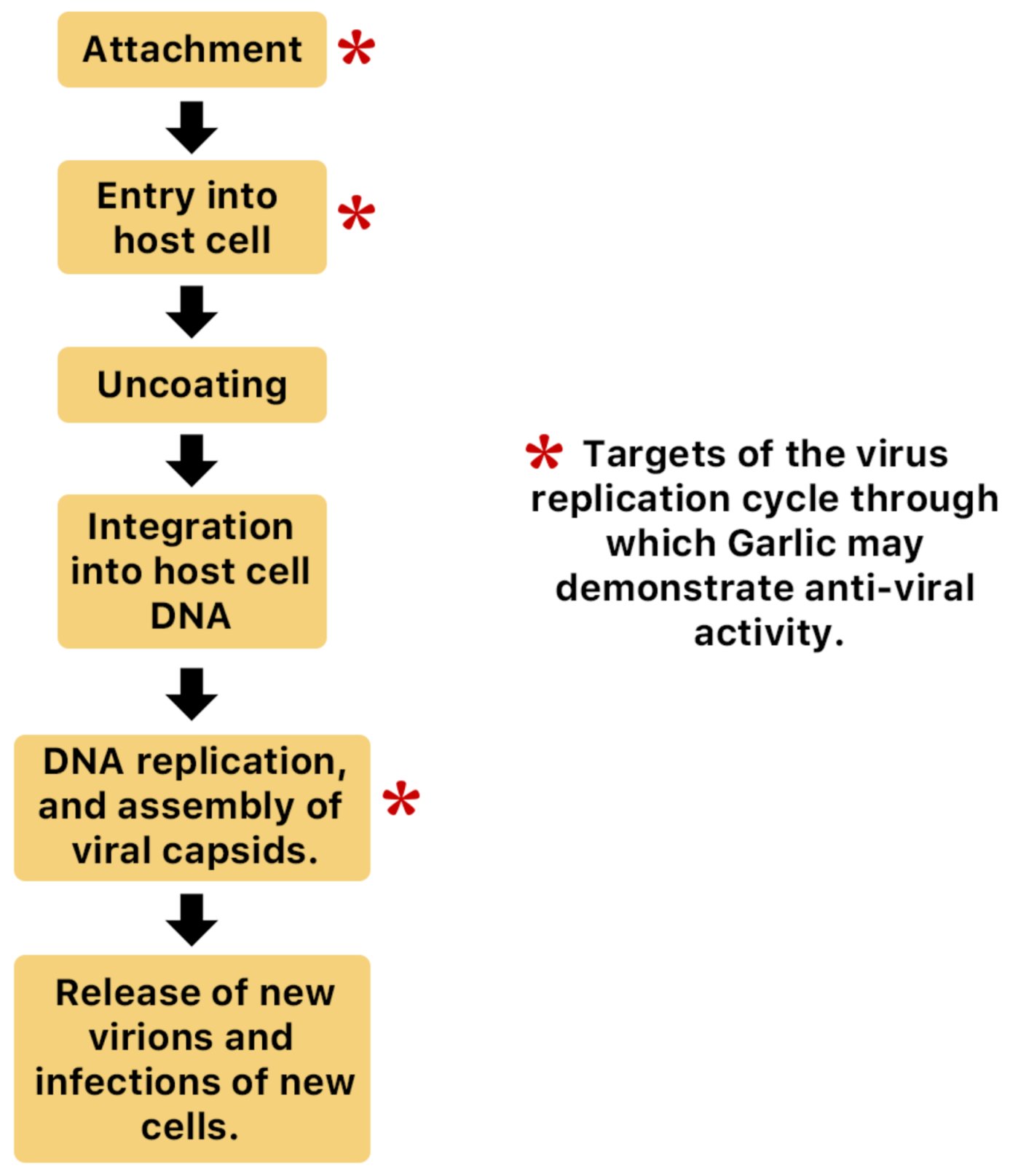
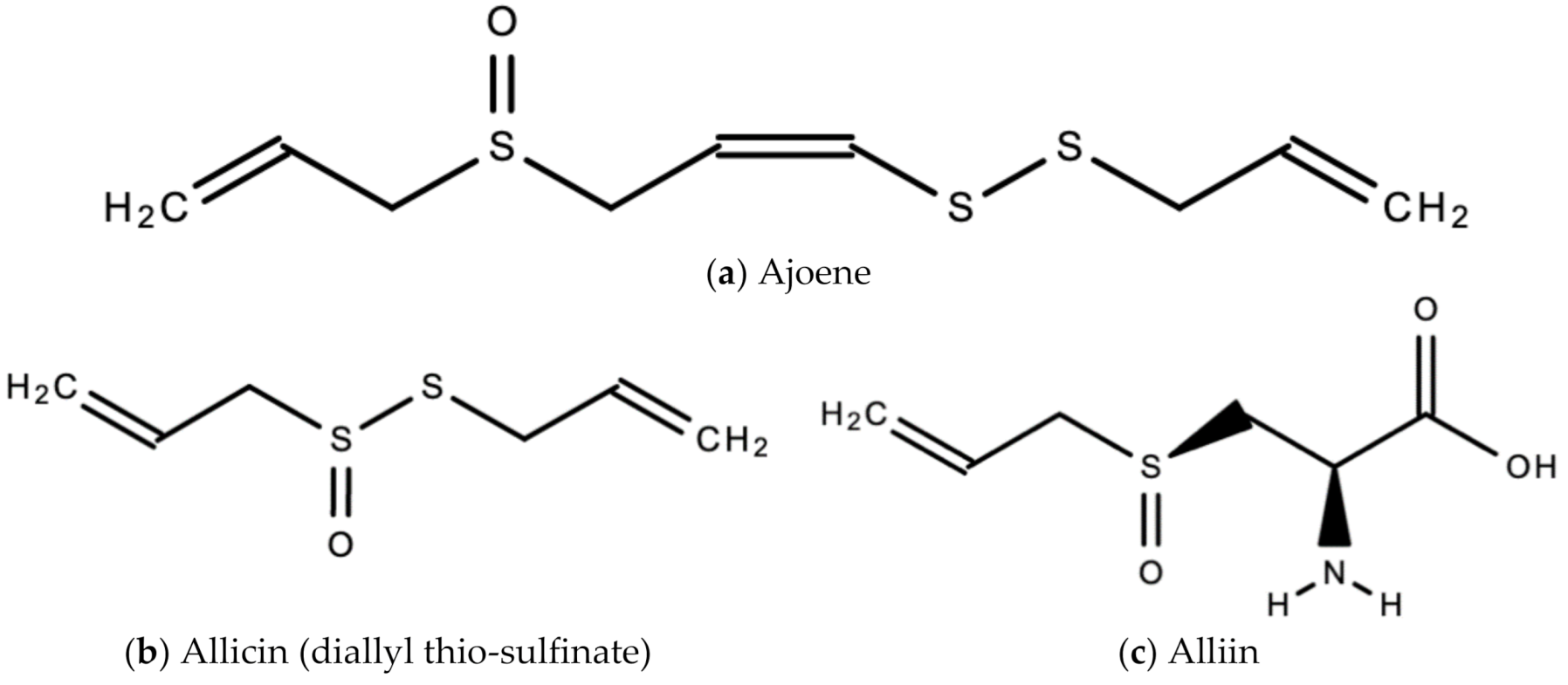
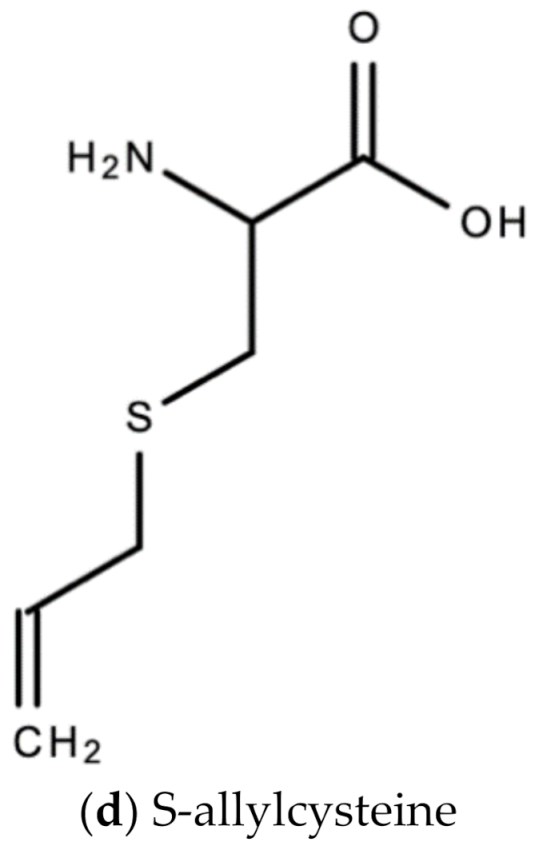
A. sativum is a member of the onion genus, Allium. It is another spice that is traditionally used in fundamental dishes around the world, but has also been used in traditional medicine for thousands of years. It has a wide therapeutic window as an antiviral, antioxidant and antibacterial against both Gram-negative and Gram-positive bacteria, antifungal activity against Candida albicans, and is antiprotozoal against Giardia lamblia and Entamoeba histolytica [62]. Anecdotal and scientific data also confirm garlic’s therapeutic efficacy against diabetes, cancer, free-radical damage, atherosclerosis, heavy metal intoxication and hyperlipidemia. Garlic produces many bioactive compounds including flavonoids, enzymes, fructo-oligosaccharides, Maillard reaction products, and organosulfur compounds like S-allylcysteine, diallyl polysulfides, alliin, vinyldithins, allicin (diallyl thio-sulfinate), allyl methyl thiosulfinate, and ajoene [63]. These are responsible for garlic’s wide therapeutic window, including as an antiviral against influenza A and B, HSV-1 and HSV-2, common cold, and viral pneumonia among other respiratory viruses [64]. Figure 14 is a graphical representation of the conversion of organosulfur compounds in garlic. Organosulfur compounds demonstrate antiviral activity via inhibition of various stages of the general virus life cycle including viral attachment, entry and multiplication [64] (Figure 15). Figure 16a–d show the chemical structures of some bioactive molecules found in garlic.
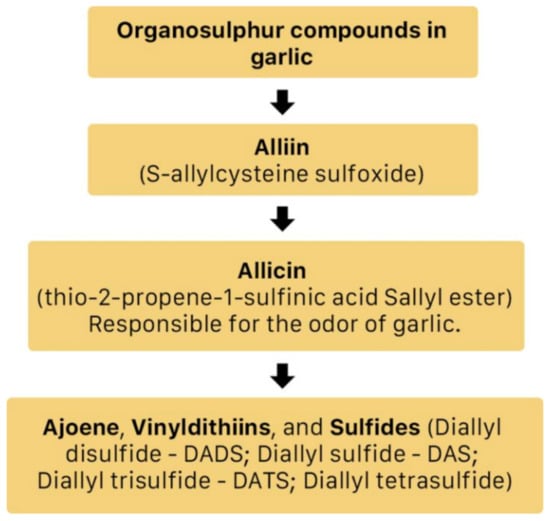
Figure 14.
The conversion of organosulfur compounds in garlic [64].
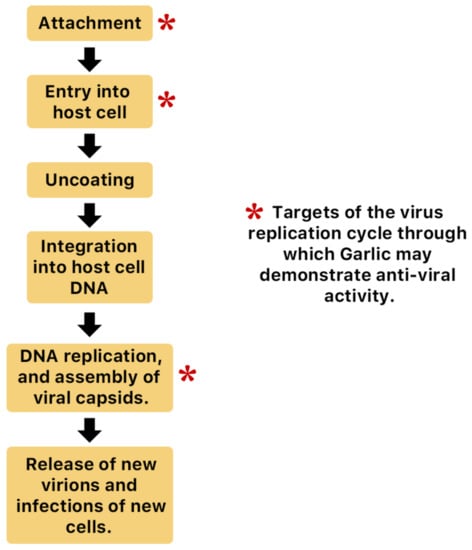
Figure 15.
Possible anti-influenza mechanisms of garlic. Organosulfur compounds produced by garlic may inhibit various stages of the general virus life cycle including viral attachment, entry and multiplication [65]. Another possible mechanism of action is via inhibition of components of viral signaling pathways [65].
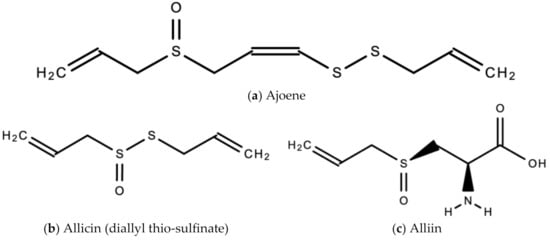
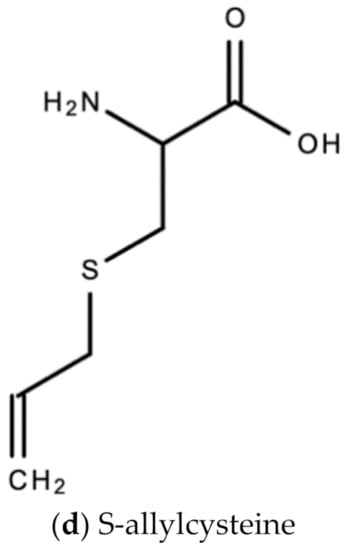
Figure 16.
Chemical structures of some bioactive molecules found in garlic.
These compounds are released and may be extracted from the cells of fresh, crushed garlic bulbs, and are responsible for the characteristic garlic smell. This is why it is usually eaten raw. Allicin is the main bioactive compound found in garlic [62]. The bioactivity of allicin and other organosulfur compounds may be attributed to their chemical interaction with thiol groups of other molecules [65].
A 2009 study by Mehrbod and colleagues reported garlic’s antiviral activity against the influenza virus in vitro [65], further confirming the anecdotal evidence for its frequency of use in Jamaica against the flu and to “boost the immune system”. Garlic is frequently used in Jamaica to treat the common cold, too. A 2001 study by Josling evaluated an allicin-containing supplement against the common cold and confirmed this bioactivity [66]. In another study, an aged garlic extract supplement was reported to boost the immune system by inducing NK cell function and proliferation of γδ-T cells [67]. In vitro studies of garlic extracts were also shown to produce anti-influenza B and anti-herpetic activity (anti-HSV-1) [68]. This bioactivity was dose-dependent.
Further studies are required to assess the antiviral activity of garlic in human and animals. Garlic is also reported to be good for the cardiovascular system, having the ability to lower systolic blood pressure and ultimately treat uncontrolled hypertension [69]. A 2013 study by Ashraf and colleagues also confirm garlic’s dose-dependent and duration-dependent ability to significantly lower both systolic blood pressure and diastolic blood pressure [70]. Garlic is further reported to be good for lowering cholesterol and thus may be used to treat hypercholesterolemia (a form of hyperlipidemia) [71].
10. Sorrel (Hibiscus sabdariffa L.)
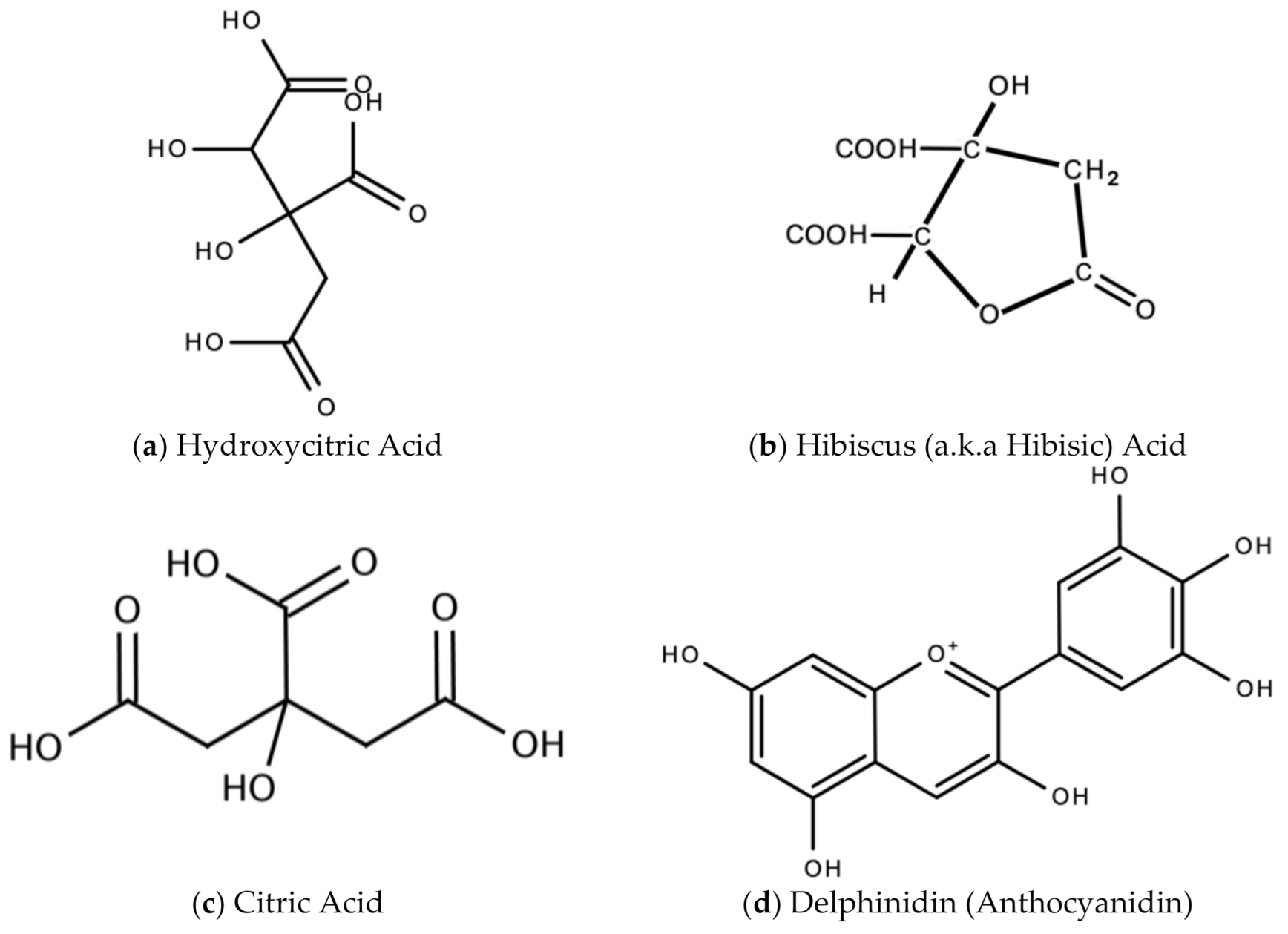
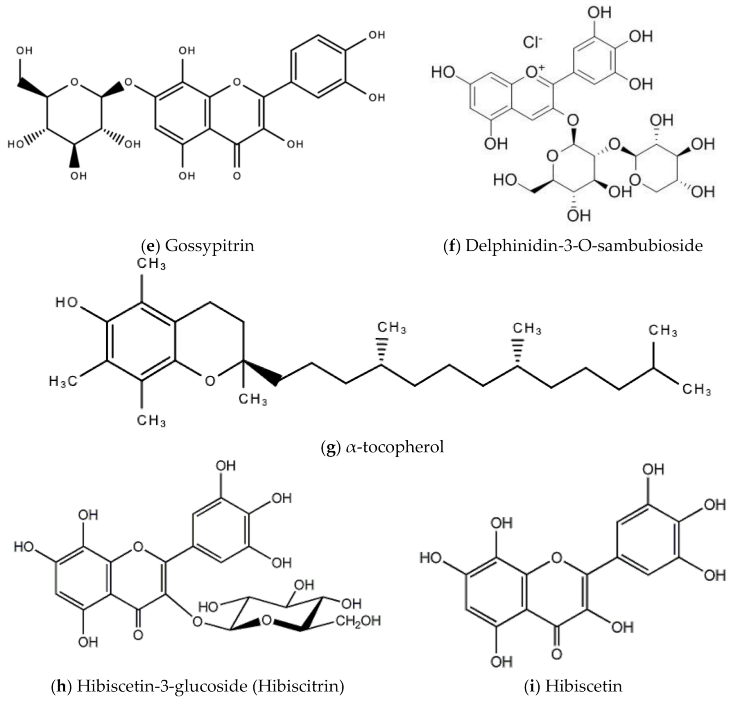
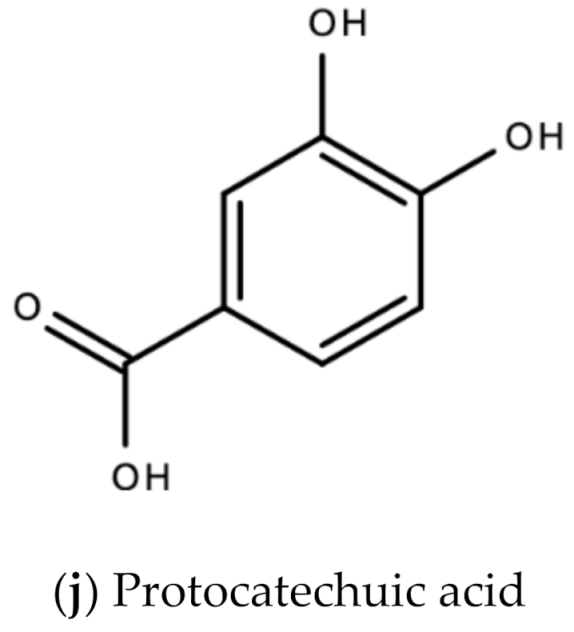
In Jamaica the fresh calyx of the flower of sorrel is commonly made into a drink and eaten in salads. However, it possesses significant medicinal value and may have applicability as a diuretic, sedative, emollient, demulcent antiscorbutic, analgesic, purgative, antipyretic, cholagogue, antiseptic, anti-tumorigenic, anti-cancer, aphrodisiac, and may be used to treat dyspepsia, disorders of the heart, hypertension, biliary disorders and abscesses [72]. Sorrel is also known for antimicrobial, antioxidant, and anti-inflammatory properties. The antiviral activity of sorrel was also assessed and shown to inhibit HSV-2 in vitro with safe cytotoxicity [73]. Sorrel was also reported to have anti-human influenza A virus activity in vitro [74]. This bioactivity attributed to sorrel may be attributed to a number of secondary metabolites including saponins, flavonoids (e.g., quercetin, gossypitrin and hibiscetin-3-glucoside, organic acids (e.g., protocatechuic, malic acid, hydroxycitric acid, and hibiscus acid), anthocyanins (e.g., anthocyanidin and delphinidin-3-O-sambubioside), glycosides, alkaloids, phenolic acids and phenolic compounds (e.g., α-tocopherol) [75,76]. Some of these secondary metabolites and their chemical structures are shown in Figure 17 below.
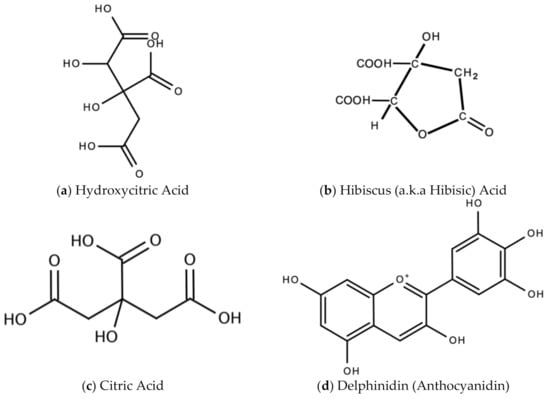
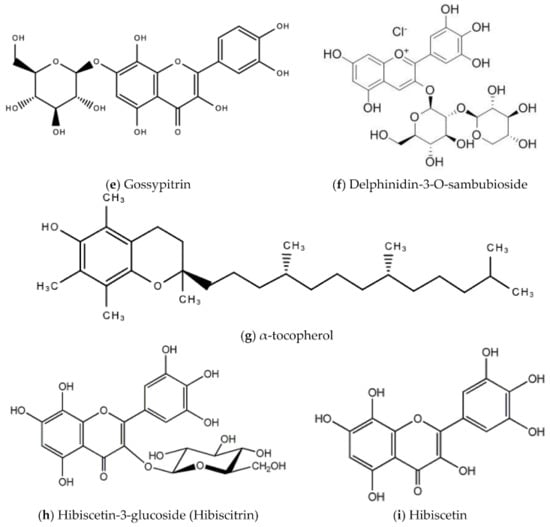
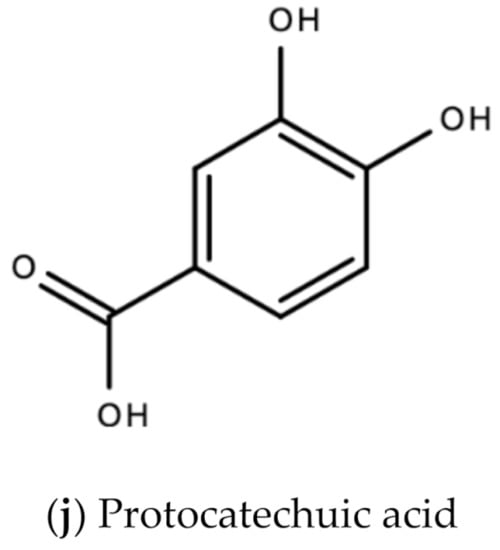
Figure 17.
Chemical structures of some bioactive molecules found in sorrel.

Table 2.
Summary of frequently used medicinal plants in Jamaica.
Table 2.
Summary of frequently used medicinal plants in Jamaica.
| Plant | Origin | Anti-Viral Window | Part of Plant Used to Make Preparation | Method of Preparation/Admin | Proposed Antiviral Mechanism (s) | |
|---|---|---|---|---|---|---|
| 1. | Ginger (Zingiber officinale Roscoe) | Native to Asia. Cultivated widely in Jamaica. | Anti-viral activity against the respiratory syncytial virus (RSV) [46], hepatitis C virus (HCV) [47], common cold (anecdotal), and the human influenza viruses (anecdotal). | Root Nodule/Rhizome | Decoction made from 1 tsp or ~1 inch of fresh root nodule brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. This is to treat the common cold and flu. | Inhibition of viral attachment and internalization via stimulation of mucosal cells of the respiratory tract to secrete interferon-β (IFN-β) [46]. |
| 2. | Turmeric (Curcuma longa L.) | Cultivated (Not indigenous to Jamaica) | Anti-viral activity againsthepatitis C virus (HCV), Epstein–Barr virus (EBV) [50], HIV-1 [51], human influenza viruses- H1N1, H6N6 [53], parainfluenza viruses 1, 2 and 3 [53], vesicular stomatitis virus (VSV) [53] and respiratory syncytial virus [53]. | Root Nodule/Rhizome | Decoction made from 1 tsp or ~1 inch of fresh root nodule brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. This is to treat the common cold and flu. | Inhibition of Epstein–Barr virus BZLF1 transcription in Raji DR-LUC cells [50]. Inhibition of HIV-1 integrase [51]. |
| 3. | Ball Moss/ “Old Man’s Beard” (Tillandsia recurvata L.) | Indigenous to Jamaica | Anti-viral activity against HIV [17,18,19], common cold (anecdotal),and flu (anecdotal) | Leaf (Fresh or dried) | Decoction made from 1 tsp or ~1 inch of fresh root nodule brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. This is to treat the common cold and flu. | Mechanism of antiviral activity unknown. |
| 4. | A. vera (Aloe barbadensis Miller) | Cultivated (Not indigenous to Jamaica) | Anti-viral activity against the human influenza viruses [21,22], varicella-zoster virus (VZV) [22], cytomegalovirus (CMV) [22], pseudorabies virus [22], herpes simplex virus types 1 and 2 (HSV-1 and -2) [24,25], and common cold (anecdotal) | Inner-leaf, gel-like pulp. | Juice made of inner gel-like pulp. This is to treat the common cold and flu. | Partial destruction of the virus’ envelope (herpes simplex virus types 1 and 2, varicella-zoster virus, pseudorabies virus, and the influenza virus) [22]. Inhibition of HSV-2 attachment and post-attachment processes, and entry into Vero cells [25]. |
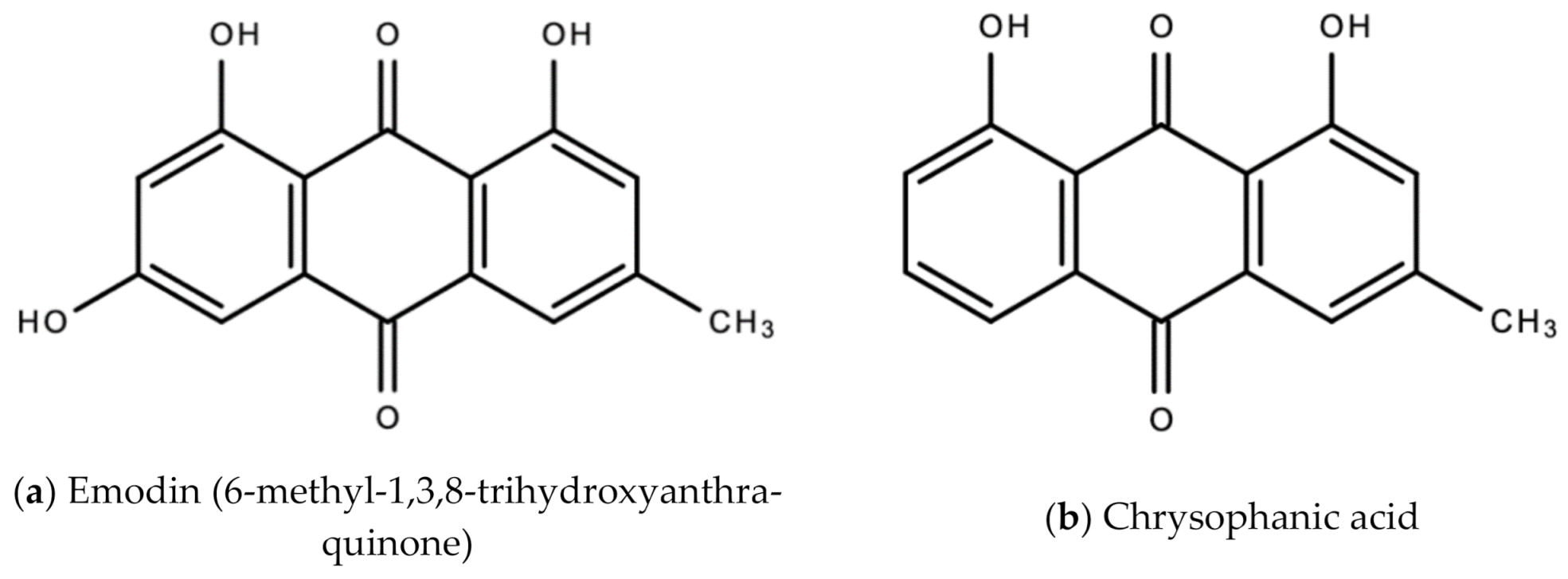
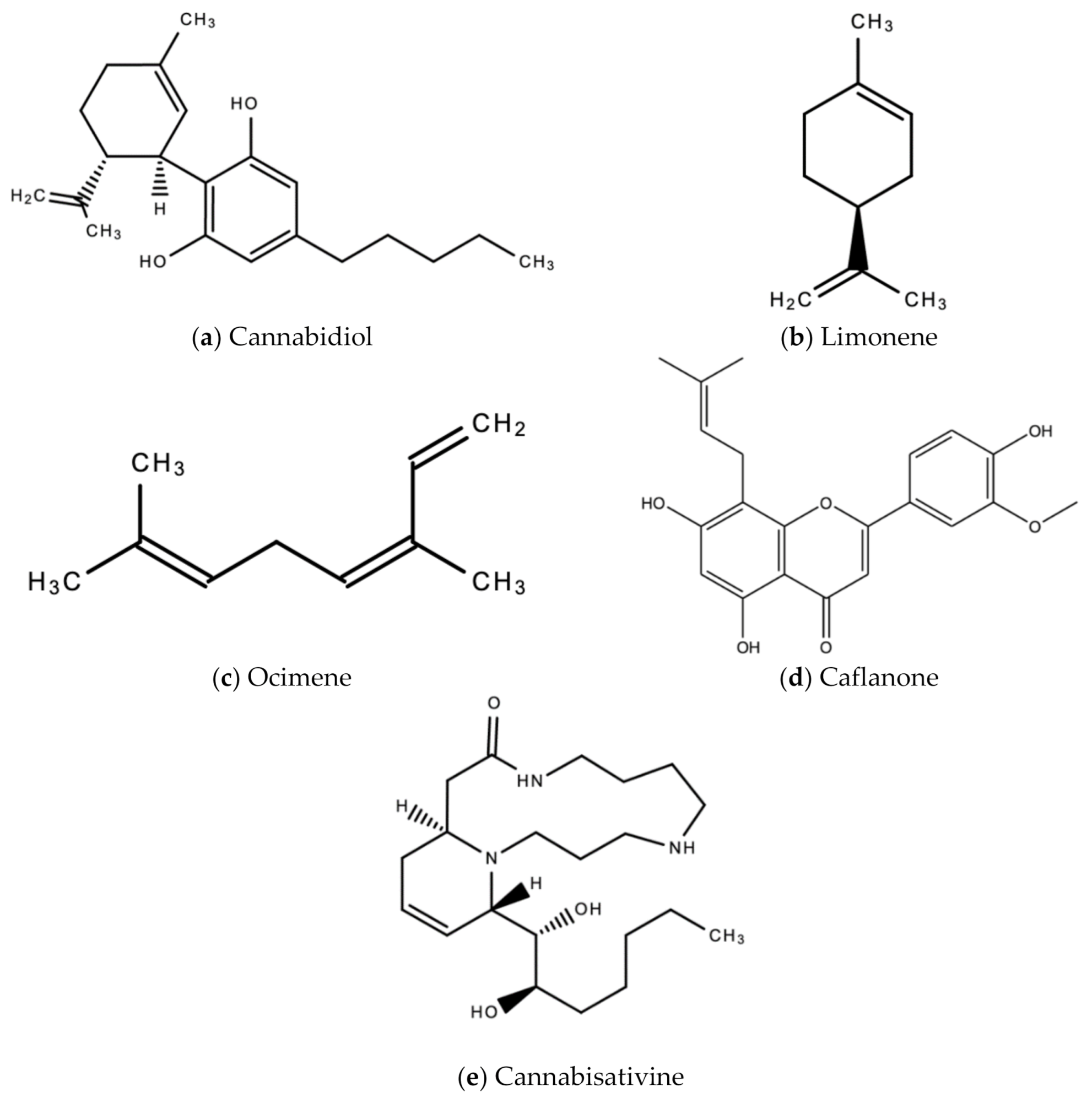
| 5. | Ganja (Cannabis sativa L.) | Cultivated (Not indigenous to Jamaica. Indigenous to Central Asia) | Anti-viral activity against HIV/AIDS wasting syndrome [33], hepatitis viruses [35], human coronavirus [34], and common cold (anecdotal). | Leaf (Fresh or dried) | Decoction made of 1–2 tsp of dried leaves brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. This is to treat the common cold and flu. | Cannabidiol (CBD) inhibits replication of hepatitis B and C viruses [35]. CBD also inhibits the pathogenesis of autoimmune hepatitis by inducing the apoptosis of thymocytes and splenocytes [35]. Caflanone inhibits the human coronavirus (hCov-OC43) via inhibition of the Angiotensin-converting enzyme 2 (ACE2) receptor found in the lung and respiratory tract [31]. |
| 6. | Guinea Hen Weed (Petiveria alliacea L.) | Cultivated (Not indigenous to Jamaica. Native to Central and South America) | Anti-viral activity against HIV [40], common cold (anecdotal), human influenza viruses (anecdotal), and hepatitis C virus (HCV) [42]. | Whole plant (roots stem, and leaves (fresh or dried) | Decoction made of 1–2 tsp of dried leaves brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. This is for the common cold and flu. | DTS inhibits HIV-1 activity is by inhibition of the reverse transcript-tase (RT) activity of the HIV-1 virus [40]. |
| 7. | Moringa (Moringa oleifera Lam.) | Cultivated (Not indigenous to Jamaica. Native to South Asia) | Anti-viral activity against the human influenza viruses (anecdotal), HSV-1 and 2 [56], Epstein–Barr Virus (EBV) [58], and hepatitis B virus (HBV) [59]. | Leaf (primarily dried) | Decoction made of 1–2 tsp of dried leaves brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. This is for the common cold and flu. | The antiviral mechanism of action is unknown. |
| 8. | Lignum Vitae (Guaiacum officinale L.) | Cultivated (Native to the Caribbean) | Anti-viral activity against HIV [61], common cold (anecdotal), and flu (anecdotal). | Flowers, Leaves (fresh or dried), Powdered bark, | Decoction made of 1–2 tsp of dried leaves brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. This is for the common cold and flu. | The antiviral mechanism of action is unknown. |
| 9. | Garlic (Allium sativum L.) | Cultivated (Native to Asia) | Anti-viral activity against the human influenza viruses (anecdotal), parainfluenza virus type 3, HSV-1 and -2 [64], and common cold (anecdotal). | - Raw bulb (most effective), Cooked, Supplement, or garlic extract. | Eat or cook raw bulb (2–3) to combat the common cold and flu viruses. | The antiviral mechanism of action is unknown. |
| 10. | Sorrel (Hibiscus sabdariffa L.) | Cultivated (Not native to Jamaica) | Anti-viral activity against HSV-2 virus [73], and human influenza viruses [74], Common cold (anecdotal), | Calyx | Juice made from calyx. Calyx is left to brew overnight in boiled water, cooled and strained, then sweetened to taste. | The antiviral mechanism of action is unknown. |
| 11. | “Search-mi-heart” (Rhytidophyllum tomentosum (L.) Mart. | Cultivated. Native to South America and the Caribbean | Common cold and flu (anecdotal) | Leaf (primarily dried) | Decoction made of 1–2 tsp of dried leaves brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
| 12. | Pepper elder (Piper amalago L.) | Cultivated (Not native to Jamaica) | Common cold and flu (anecdotal) | Fresh Leaves | Decoction made of 1–2 tsp of dried leaves brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
| 13. | McKatty Weed/Marigold (Bidens reptans (L.) G.Don | Cultivated (Not native to Jamaica) | Common cold and flu (anecdotal) | Flowers and/or Leaves (primarily dried) | Decoction made of 1 tbsp of dried leaves brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
| 14. | Chany Root a.k.a. Jamaican Sasparilla (Smilax balbisiana Kunth) | Native to Jamaica | Common cold and flu (anecdotal) | Root | Decoction or tonic made of pre-soaked roots brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
| 15. | Lemongrass/Fevergrass(Cymbopogen citratus L.) | Cultivated (Not native to Jamaica) | Common cold and flu (anecdotal) | Leaf (Fresh or dried) | Decoction made from a handful of fever grass leaves and stems in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
| 16. | Cerasee (Momordica charantia L.) | Cultivated (Not native to Jamaica. Native to Africa and Middle East) | Common cold and flu (anecdotal) | Leaf (Fresh or dried) and stems | Decoction made from a handful of cerasee leaves and stems in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
| 17. | Soursop (Annona muricata L.) | Cultivated (Not native to Jamaica. Native to South America) | Common cold and flu (anecdotal) | Leaf (Fresh or dried) | Decoction made from 2–3 leaves in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
| 18. | Leaf of life (Bryophyllum pinnatum (Lam.) Oken | Cultivated (Not native to Jamaica. Native to Africa) | Common cold and flu (anecdotal) | Leaf (Fresh or dried) | Decoction made from 2–3 leaves in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
| 19. | Dogblood (Rivina humilis L.) | Cultivated (Not native to Jamaica) | Common cold and flu (anecdotal) | Whole plant, but primarily leaf (Fresh or dried) and stem | Decoction made of 1–2 tsp of dried leaves brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
| 20. | Lime Leaf Tea (Citrus aurantiifolia (Christm.) Swingle | Cultivated (Not native to Jamaica. Native to Asia) | Common cold and flu (anecdotal) | Leaf (Fresh or dried) | Decoction made 5–6 leaves in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
| 21. | Jack-in-the-bush (Eupatorium odoratum L.) | Cultivated (Not native to Jamaica. | Common cold (anecdotal) | Leaf (Fresh or dried) and stem | Decoction made of 1–2 tsp of dried leaves brewed in 1 cup of water. Steep for ~10 min. Sweeten as desired. | The antiviral mechanism of action is unknown. |
The effective dosage of each medicinal plant preparation is currently unknown. There is not enough rigid scientific research to recommend a standard dosage for any medical condition. Anecdotal evidence recommends one to two cups of oral administration of these decoctions per day, until symptom relief is experienced.
11. Toxic Compounds and Adverse Side-Effects
A limitation to homemade decoctions and infusions is due to the fact that, water, regarded as a universal solvent, will, in addition to dissolving plant material, also dissolve unwanted material such as contaminants and toxic compounds. These may have adverse side-effects if ingested. On this tangent, it is of importance that herbs used to make decoctions are grown in a suitable environment. Table 3 below lists some toxic compounds and adverse side-effects associated with frequently used medicinal plants in Jamaica.

Table 3.
Some toxic compounds and adverse side-effects associated with frequently used medicinal plants in Jamaica.
13. Conclusions and Future Prospects
As estimated by the World Health Organization (WHO), over 65% of the world population utilize botanical preparations as medicine [1]. Natural alternatives are increasingly gaining attention because of greater accessibility to medicinal plants, and the possibility that they may have fewer and less adverse side-effects than synthetic drugs. This makes natural alternatives more desirable as novel drug therapies. Thousands of plant species still remain to be screened for their bioactivity. Only approximately 15% of some 250,000 species of higher plants have been studied for their pharmaceutical potential [4]. Only 5% of these plants had one or more forms of biological activity [101]. This means that there is an enormous, untapped potential for the development of a myriad of plant-based therapeutics and pharmaceutical-grade proteins likes vaccines, hormones, antibodies and cytokines [102].
Jamaica is home to many established medicinal plants such as ginger, garlic, medina, ganja, cerasee, ball moss and fever grass [103]. This makes the island particularly welcoming for rigorous scientific research on the medicinal value of plants and the development of phytomedicine thereof. This could have great economic and medicinal implications, not only for Jamaica, but for the region. Our traditional use of these medicinal plants in Jamaica can be attributed to the passing of knowledge from traditional Asian and African medicine.
Jamaica’s enormous “ethnopharmacopoeia” in addition to rigorous community-driven scientific data sharing through globalization could potentially expedite natural-product development, lower the cost to purchase natural-products and the cost for commercialization of natural-based drugs, and increase the availability of natural-based pharmaceuticals. Despite the increasing scientific evidence supporting the medicinal value of plants native to and cultivated in Jamaica, commercialization of natural-products derived from these efforts is limited by factors including inadequate testing systems, uncertain regulatory landscapes in natural-product biomedical research, manufacturing practices, commercialization, licensing and intellectual property. As a result, the lack of a rational approach to a creating a sustainable natural-product pharmaceutical industry has been limited.
Modern research around the world should now focus on the pharmacological activities of the phytochemicals and mapping their genomes and transcriptomes to produce target drugs. There is a need for more systematic botanical, physicochemical and chemical analyses of thousands of potential medicinal plants. Further research is required to determine the efficacy, dosage standards, optimum extraction methods/solvents, cytotoxicity/hepatoxicity, pharmacokinetics, molecular mechanisms of action, phytoantiviral screening methods, and drug interactions for many phytoantivirals.
Creating and using universal sets of primers, databases and standards to catalogue species by research groups all around the world, would increase the level of reliability and the number of species available for study (which has reached the greatest level ever achieved by the scientific community) while also making it possible to identify an ever-growing number of species [104]. In addition, a greater awareness of efficacies, toxicities and drug-to-drug interactions of traditional herbal medicines is required. Most importantly, before these plant-based drugs can be incorporated into conventional medicine, more randomized, double-blind, placebo-controlled clinical trials are need on a larger scale. Data generated from such experiments should also be logged in a universal, open-access database. Countries dependent on traditional, synthetic medicine, need to re-shift focus to programs aimed at systematically studying the bioactive compounds from plants, and synthesizing new drugs from compounds.
Author Contributions
Conceptualization: H.L., and B.S.; writing, review and editing: H.L., B.S., J.B., N.T., E.F. and W.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable. This study did not involve humans or animals.
Informed Consent Statement
Not applicable. This study did not involve humans or animals.
Data Availability Statement
Data sharing is not applicable to this article. No new data were created or analyzed in this study.
Conflicts of Interest
Authors H.L. and N.T. are employees of Vilotos and Flavocure and both companies have commercial interest in Caflanone. The other authors declare no conflict of interest. Neither Vilotos nor Flavocure took part in the study or experience.
References
- Robinson, M.M.; Zhang, X. The World Medicines Situation 2011: Traditional Medicines: Global Situation, Issues and Challenges, 3rd ed.; World Health Organization (WHO): Geneva, Switzerland, 2011. [Google Scholar]
- Ekor, M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2013, 4, 177. [Google Scholar] [CrossRef] [PubMed]
- Barnes, J.; Anderson, L.A.; Phillipson, J.D. Herbal medicines: A Guide for Health-Care Professionals, 3rd ed.; Pharmaceutical Press: London, UK, 2007. [Google Scholar]
- De Luca, V.; Salim, V.; Atsumi, S.M.; Yu, F. Mining the biodiversity of plants: A revolution in the making. Science 2012, 336, 1658–1661. [Google Scholar] [CrossRef] [PubMed]
- Krishnaiah, D.; Sarbatly, R.; Nithyanandam, R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011, 89, 217–233. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs from 1981 to 2014. J. Nat. Prod. 2016, 79, 629–661. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Goyal, S.; Lambert, C.; Cluzet, S.; Mérillon, J.M.; Ramawat, K.G. Secondary Metabolites and Plant Defence. In Plant Defence: Biological Control. Progress in Biological Control; Mérillon, J., Ramawat, K., Eds.; Springer: Dordrecht, The Netherlands, 2012; Volume 12. [Google Scholar] [CrossRef]
- Bérdy, J. Bioactive Microbial Metabolites. J. Antibiot. 2005, 58, 1–26. [Google Scholar] [CrossRef]
- Wink, M. Potential of DNA Intercalating Alkaloids and Other Plant Secondary Metabolites against SARS-CoV-2 Causing COVID-19. Diversity 2020, 12, 175. [Google Scholar] [CrossRef]
- Moudi, M.; Go, R.; Yien, C.Y.S.; Nazre, M. Vinca Alkaloids. Int. J. Prev. Med. 2013, 4, 1231–1235. [Google Scholar]
- China National Gene Bank: Database of 10,000 Medicinal Plants. Available online: https://db.cngb.org/10kmp/ (accessed on 13 December 2020).
- Picking, D.; Delgoda, R.; Younger, N.; Germosén-Robineau, L.; Boulogne, I.; Mitchell, S. TRAMIL ethnomedicinal survey in Jamaica. J. Ethnopharmacol. 2015, 169, 314–327. [Google Scholar] [CrossRef]
- Mitchell, S.A. Plants used in Jamaican folk medicine against the common cold, flu and diarrhea. J. Antivir. Antiretrovir. 2011, 3. [Google Scholar] [CrossRef]
- Mitchell, S.; Ahmad, M.H. A review of medicinal plant research at the University of the West Indies, Jamaica, 1948-2001. West Indian Med J. 2006, 55. [Google Scholar] [CrossRef] [PubMed]
- Estrella, P.; Edgar, A.; María, F.-C.; Blancas-Flores, G.; Koch, S.; Alarcon, F. Review The Tillandsia genus: History, uses, chemistry, and biological activity. Chemistry and biological activity of the Tillandsia L. Genus 2019, 18, 239–264. [Google Scholar]
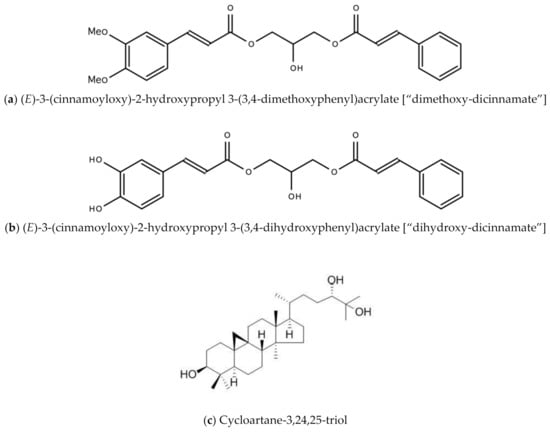
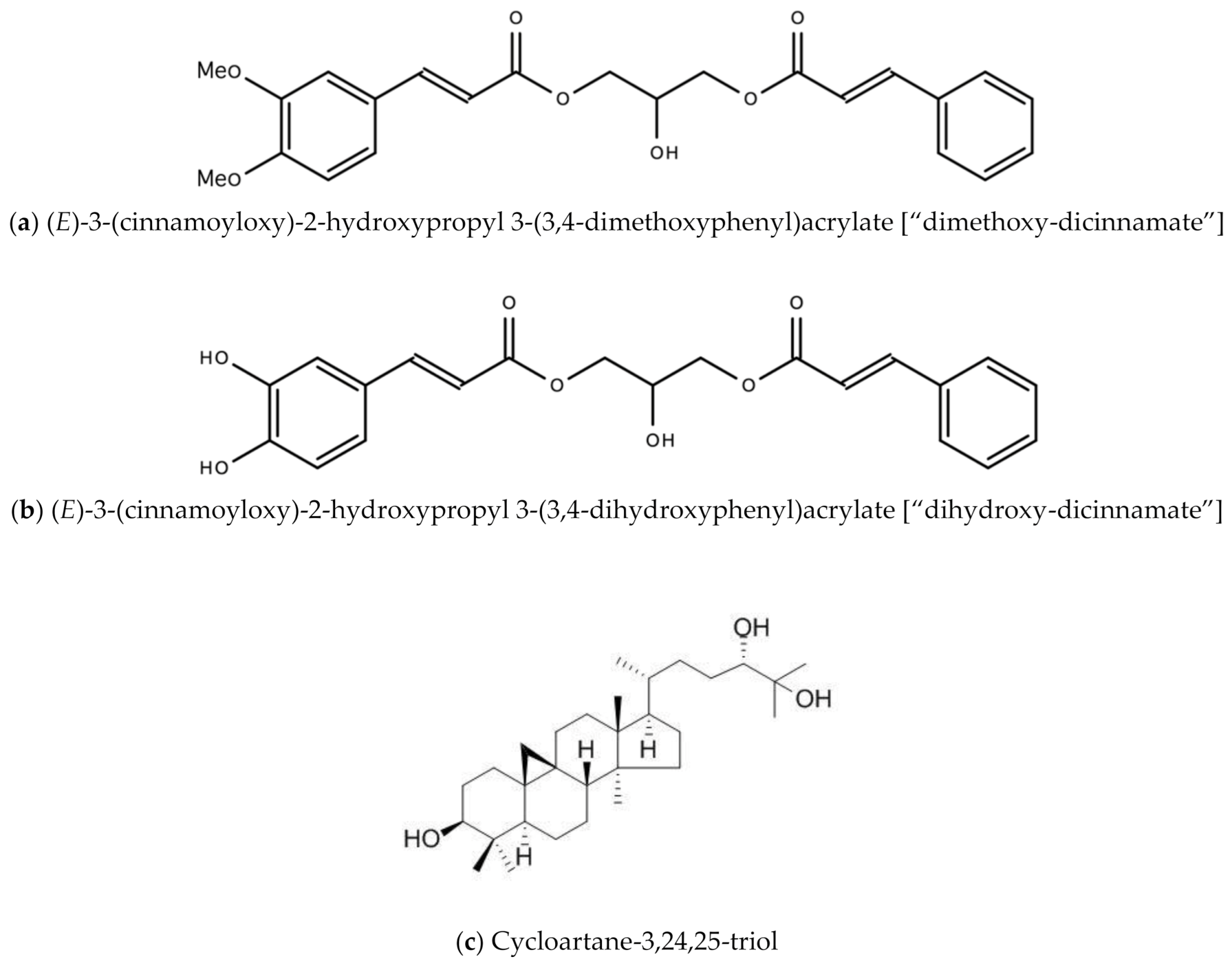
- Lowe, H.; Bryant, J.L. Anti-Tumor and Anti-inflammatory Dicinnamoyl-Glycerol Esters and Their Analogues. U.S. Patent 8907117B2, 5 November 2010. [Google Scholar]
- Lowe, H.I.; Toyang, N.J.; Watson, C.T.; Ayeah, K.N.; Bryant, J. Antileukemic activity of Tillandsia recurvata and some of its cycloartanes. Anticancer. Res. 2014, 34, 3505–3510. [Google Scholar] [PubMed]
- Gao, W.; Dong, X.; Wei, T.; Xing, W. The Chemical Structure and Bioactivity of Cycloartane-type Compounds. Curr. Org. Chem. 2020, 23, 2848–2872. [Google Scholar] [CrossRef]
- Joseph, B.; Raj, S. An Overview: Pharmacognostic Properties of Phyllanthus amarus Linn. Int. J. Pharmacol. 2010, 7, 40–45. [Google Scholar] [CrossRef]
- Gansukh, E.; Gopal, J.; Paul, D.; Muthu, M.; Kim, D.-H.; Oh, J.-W.; Chun, S. Ultrasound mediated accelerated Anti-influenza activity of Aloe vera. Sci. Rep. 2018, 8, 17782. [Google Scholar] [CrossRef]
- Sydiskis, R.J.; Owen, D.G.; Lohr, J.L.; Rosler, K.H.; Blomster, R.N. Inactivation of enveloped viruses by anthraquinones extracted from plants. Antimicrob. Agents Chemother. 1991, 35, 2463–2466. [Google Scholar] [CrossRef]
- Saoo, K.; Miki, H.; Ohmori, M.; Winters, W.D. Antiviral Activity of Aloe Extracts against Cytomegalovirus. Phytotherapy Res. 1996, 10, 348–350. [Google Scholar] [CrossRef]
- Rezazadeh, F.; Moshaverinia, M.; Motamedifar, M.; Alyaseri, M. Assessment of Anti HSV-1 Activity of Aloe Vera Gel Extract: An In Vitro Study. J. Dent. (Shiraz, Iran) 2016, 17, 49–54. [Google Scholar]
- Zandi, K.; Zadeh, M.A.; Sartavi, K.; Rastian, Z. Antiviral activity of Aloe vera against herpes simplex virus type 2: An in vitro study. Afr. J. Biotechnol. 2007, 6, 1770–1773. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309–3314. [Google Scholar] [CrossRef] [PubMed]
- Pollastro, F.; Minassi, A.; Fresu, L. Cannabis Phenolics and their Bioactivities. Curr. Med. Chem. 2018, 25, 1160–1185. [Google Scholar] [CrossRef] [PubMed]
- Mechoulam, R. Chapter 2 Alkaloids in Cannabis Sativa L. Alkaloids Chem. Pharmacol. 1989, 34, 77–93. [Google Scholar] [CrossRef]
- Johnson, J.; Theisen, E. What Are Terpenes? Available online: https://www.medicalnewstoday.com/articles/what-are-terpenes (accessed on 28 July 2020).
- Loizzo, M.R.; Saab, A.M.; Tundis, R.; Statti, G.A.; Menichini, F.; Lampronti, I.; Gambari, R.; Cinatl, J.; Doerr, H.W. Phytochemical Analysis andin vitro Antiviral Activities of the Essential Oils of Seven Lebanon Species. Chem. Biodivers. 2008, 5, 461–470. [Google Scholar] [CrossRef] [PubMed]
- Ngwa, W.; Kumar, R.; Thompson, D.; Lyerly, W.; Moore, R.M.; Reid, T.-E.; Lowe, H.; Toyang, N. Potential of Flavonoid-Inspired Phytomedicines against COVID-19. Molecules 2020, 25, 2707. [Google Scholar] [CrossRef]
- Śledziński, P.; Nowak, A.; Zeyland, J.; Słomski, R. Endocannabinoid system and anticancer properties of cannabinoids. Folia Biol. Oecologica 2016, 12, 11–25. [Google Scholar] [CrossRef][Green Version]
- E Lutge, E.; Gray, A.; Siegfried, N. The medical use of cannabis for reducing morbidity and mortality in patients with HIV/AIDS. Cochrane Database Syst. Rev. 2013, CD005175. [Google Scholar] [CrossRef]
- Naga Swetha Samji, M. Viral Hepatitis. Available online: https://emedicine.medscape.com/article/775507-overview (accessed on 20 August 2020).
- Toyang, N.; Lowe, H.I.C.; McLaughlin, W. Potential of cannabidiol for the treatment of viral hepatitis. Pharmacogn. Res. 2017, 9, 116–118. [Google Scholar] [CrossRef]
- Hegde, V.L.; Nagarkatti, P.S.; Nagarkatti, M. Role of Myeloid-Derived Suppressor Cells in Amelioration of Experimental Autoimmune Hepatitis Following Activation of TRPV1 Receptors by Cannabidiol. PLoS ONE 2011, 6, e18281. [Google Scholar] [CrossRef]
- Price, S. Could Cannabis Terpene Formulation Treat COVID-19? Available online: https://www.healtheuropa.eu/cannabis-terpene-formulation-treat-covid-19/99586/ (accessed on 20 August 2020).
- Shaghaghi, Neda (2020): Molecular Docking Study of Novel COVID-19 Protease with Low Risk Terpenoides Compounds of Plants. ChemRxiv. Preprint. Available online: https://doi.org/10.26434/chemrxiv.11935722.v1 (accessed on 10 December 2020).
- Luz, D.A.; Pinheiro, A.M.; Silva, M.L.; Monteiro, M.C.; Prediger, R.D.; Maia, C.S.F.; Fontes-Júnior, E.A. Ethnobotany, phytochemistry and neuropharmacological effects of Petiveria alliacea L. (Phytolaccaceae): A review. J. Ethnopharmacol. 2016, 185, 182–201. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Heredia, A.; Ayeah, K.; Watson, C.; Bryant, J. Petiveria alliacea L (Guinea Hen Weed) and Its Major Metabolite Dibenzyl Trisulfide Demonstrate HIV-1 Reverse Transcriptase Inhibitory Activity. Eur. J. Med. Plants 2015, 5, 88–94. [Google Scholar] [CrossRef]
- Williams, L.A.D.; Rösner, H.; Kraus, W. Molecules with Potential for Cancer Therapy in the Developing World: Dibenzyl Trisulfide (DTS). Adv. Microb. Ecol. 2012, 273–278. [Google Scholar] [CrossRef]
- Lowe, H.; Toyang, N.; Roy, S.; Watson, C.; Bryant, J. Inhibition of the Human Hepatitis C Virus by Dibenzyl Trisulfide from Petiveria alliacea L (Guinea Hen Weed). Br. Microbiol. Res. J. 2016, 12, 1–6. [Google Scholar] [CrossRef]
- Meister, M.; Tomasovic, A.; Banning, A.; Tikkanen, R. Mitogen-Activated Protein (MAP) Kinase Scaffolding Proteins: A Recount. Int. J. Mol. Sci. 2013, 14, 4854–4884. [Google Scholar] [CrossRef]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef]
- Williams, L.A.D.; Rosner, H.; Levy, H.G.; Barton, E.N. A critical review of the therapeutic potential of dibenzyl trisulphide isolated from Petiveria alliacea L (guinea hen weed, anamu). West Indian Med. J. 2007, 56, 17–21. [Google Scholar] [CrossRef]
- Chang, J.S.; Wang, K.C.; Yeh, C.F.; Shieh, D.E.; Chiang, L.C. Fresh ginger (Zingiber officinale) has anti-viral activity against human respiratory syncytial virus in human respiratory tract cell lines. J. Ethnopharmacol. 2013, 145, 146–151. [Google Scholar] [CrossRef]
- Mao, Q.-Q.; Xu, X.-Y.; Cao, S.-Y.; Gan, R.-Y.; Corke, H.; Beta, T.; Li, H.-B. Bioactive Compounds and Bioactivities of Ginger (Zingiber officinale Roscoe). Foods 2019, 8, 185. [Google Scholar] [CrossRef]
- Chattopadhyay, I.; Biswas, K.; Bandyopadhyay, U.; Banerjee, R.K. Turmeric and curcumin: Biological actions and medicinal applications. Curr. Sci. 2004, 87, 44–53. [Google Scholar]
- Mathew, D.; Hsu, W.-L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Hergenhahn, M.; Soto, U.; Weninger, A.; Polack, A.; Hsu, C.-H.; Cheng, A.-L.; Rösl, F. The chemopreventive compound curcumin is an efficient inhibitor of Epstein-Barr virus BZLF1 transcription in Raji DR-LUC cells. Mol. Carcinog. 2002, 33, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, A.; Raghavan, K.; Weinstein, J.; Kohn, K.W.; Pommier, Y. Inhibition of human immunodeficiency virus type-1 integrase by curcumin. Biochem. Pharmacol. 1995, 49, 1165–1170. [Google Scholar] [CrossRef]
- Huang, Y.; Xiang, Q.; Yao, C.-S.; Zhang, F.-X.; Zhang, H.; Li, X. Study on the preparation of zedoary turmeric oil spray and its anti-virus effects. J. Chin. Med. Mater. 2007, 30, 342–345. [Google Scholar]
- Moghadamtousi, S.Z.; Kadir, H.A.; Hassandarvish, P.; Tajik, H.; Abubakar, S.; Zandi, K. A Review on Antibacterial, Antiviral, and Antifungal Activity of Curcumin. BioMed Res. Int. 2014, 2014, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, P.M.P.; Farias, D.F.; Oliveira, J.T.D.A.; Carvalho, A.F.U. Moringa oleifera: Bioactive compounds and nutritional potential. Revista de Nutrição 2008, 21, 431–437. [Google Scholar] [CrossRef]
- Mahmood, K.T.; Mugal, T.; Ul Haq, I. Moringa oleifera: A natural gift-A review. J. Pharm. Sci. Res. 2010, 2, 775–781. [Google Scholar]
- Nasr-Eldin, M.A.; Abdelhamid, A.; Baraka, D. Antibiofilm and Antiviral Potential of Leaf Extracts from Moringa oleifera and Rosemary (Rosmarinus officinalis Lam.). Egypt. J. Microbiol. 2018, 129–139. [Google Scholar] [CrossRef]
- Ashraf, M.; Shahzad, S.S.; Fatima, M.; Altaf, I.; Khan, F.; Afzal, A. Comparative anti-influenza potential of moringa oleifera leaves and amantadine invitro. Pak. Postgrad. Med. J. 2017, 28, 127–131. [Google Scholar]
- Murakami, A.; Kitazono, Y.; Jiwajinda, S.; Koshimizu, K.; Ohigashi, H. Niaziminin, a Thiocarbamate from the Leaves of Moringa oleifera, Holds a Strict Structural Requirement for Inhibition of Tumor-Promoter-Induced Epstein-Barr Virus Activation. Planta Medica 1998, 64, 319–323. [Google Scholar] [CrossRef]
- Feustel, S.; Ayón-Pérez, F.; Sandoval-Rodriguez, A.; Rodriguez-Echevarria, R.; Contreras-Salinas, H.; Armendariz-Borunda, J.; Sanchez-Orozco, L. Protective Effects of Moringa oleifera on HBV Genotypes C and H Transiently Transfected Huh7 Cells. J. Immunol. Res. 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- A Lans, C. Ethnomedicines used in Trinidad and Tobago for reproductive problems. J. Ethnobiol. Ethnomedicine 2007, 3, 13. [Google Scholar] [CrossRef] [PubMed]
- Lowe, H.I.C.; Toyang, N.J.; Heredia, A.; Watson, C.T.; Bryant, J. Anti HIV-1 Activity of the Crude Extracts of Guaiacum officinale L. (Zygophyllaceae). Eur. J. Med. Plants 2014, 4, 483–489. [Google Scholar] [CrossRef][Green Version]
- Ankri, S.; Mirelman, D. Antimicrobial properties of allicin from garlic. Microbes Infect. 1999, 1, 125–129. [Google Scholar] [CrossRef]
- Weber, N.D.; Andersen, D.O.; North, J.A.; Murray, B.K.; Lawson, L.D.; Hughes, B.G. In Vitro Virucidal Effects ofAllium sativum(Garlic) Extract and Compounds. Planta Medica 1992, 58, 417–423. [Google Scholar] [CrossRef]
- Sharma, N. Efficacy of Garlic and Onion against virus. Int. J. Res. Pharm. Sci. 2019, 10, 3578–3586. [Google Scholar] [CrossRef]
- Mehrbod, P.; Amini, E.; Tavassoti-Kheiri, M. Pasteur Institute of IRAN (Influenza Unit) Antiviral activity of garlic extract on Influenza virus. Iran. J. Virol. 2009, 3, 19–23. [Google Scholar] [CrossRef]
- Josling, P. Preventing the common cold with a garlic supplement: A double-blind, placebo-controlled survey. Adv. Ther. 2001, 18, 189–193. [Google Scholar] [CrossRef]
- Nantz, M.P.; Rowe, C.A.; Muller, C.E.; Creasy, R.A.; Stanilka, J.M.; Percival, S.S. Supplementation with aged garlic extract improves both NK and γδ-T cell function and reduces the severity of cold and flu symptoms: A randomized, double-blind, placebo-controlled nutrition intervention. Clin. Nutr. 2012, 31, 337–344. [Google Scholar] [CrossRef]
- Tsai, Y.; Cole, L.; Davis, L.; Lockwood, S.; Simmons, V.; Wild, G. Antiviral Properties of Garlic:In vitroEffects on Influenza B, Herpes Simplex and Coxsackie Viruses. Planta Medica 1985, 51, 460–461. [Google Scholar] [CrossRef]
- Ried, K.; Frank, O.R.; Stocks, N.P. Aged garlic extract lowers blood pressure in patients with treated but uncontrolled hypertension: A randomised controlled trial. Maturitas 2010, 67, 144–150. [Google Scholar] [CrossRef]
- Ashraf, R.; Alam Khan, R.; Ashraf, I.; Qureshi, A. Effects of Allium sativum (garlic) on systolic and diastolic blood pressure in patients with essential hypertension. Pak. J. Pharm. Sci. 2013, 26, 859–863. [Google Scholar] [PubMed]
- Sobenin, I.; Andrianova, I.V.; Demidova, O.N.; Gorchakova, T.; Orekhov, A.N. Lipid-Lowering Effects of Time-Released Garlic Powder Tablets in Double-Blinded Placebo-Controlled Randomized Study. J. Atheroscler. Thromb. 2008, 15, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Mungole, A.; Chaturvedi, A. Hibiscus sabdariffal a rich source ofsecondary metabolites. Int. J. Pharm. Sci. Rev. Res. 2011, 6, 83–87. [Google Scholar]
- Hassan, S.T.S.; Švajdlenka, E.; Bímová, K.B. Hibiscus sabdariffa L. and Its Bioactive Constituents Exhibit Antiviral Activity against HSV-2 and Anti-enzymatic Properties against Urease by an ESI-MS Based Assay. Molecules 2017, 22, 722. [Google Scholar] [CrossRef] [PubMed]
- Takeda, Y.; Okuyama, Y.; Nakano, H.; Yaoita, Y.; Machida, K.; Ogawa, H.; Imai, K. Antiviral Activities of Hibiscus sabdariffa L. Tea Extract Against Human Influenza A Virus Rely Largely on Acidic pH but Partially on a Low-pH-Independent Mechanism. Food Environ. Virol. 2019, 12, 9–19. [Google Scholar] [CrossRef]
- Jabeur, I.; Pereira, E.; Barros, L.; Calhelha, R.C.; Soković, M.; Oliveira, M.B.P.; Ferreira, I. Hibiscus sabdariffa L. as a source of nutrients, bioactive compounds and colouring agents. Food Res. Int. 2017, 100, 717–723. [Google Scholar] [CrossRef]
- Ojulari, O.V.; Lee, S.G.; Nam, J.-O. Beneficial Effects of Natural Bioactive Compounds from Hibiscus sabdariffa L. on Obesity. Molecules 2019, 24, 210. [Google Scholar] [CrossRef]
- Elhag, M.; Al-Ghamdi, A.A.M.; Galal, H.K.; Dahlan, A. Evaluation of aloe vera l. As phytoremediator of heavy metals contaminated soils in arid environments. Appl. Ecol. Environ. Res. 2018, 16, 6033–6045. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Aloe vera: A review of toxicity and adverse clinical effects. J. Environ. Sci. Heal. Part C 2016, 34, 77–96. [Google Scholar] [CrossRef]
- 8 Side Effects of Aloe Vera: Here’s Why Anything In Excess Is Bad. Available online: https://food.ndtv.com/health/side-effects-of-aloe-vera-heres-why-anything-in-excess-is-bad-1882205 (accessed on 6 December 2020).
- Seltenrich, N. Cannabis Contaminants: Regulating Solvents, Microbes, and Metals in Legal Weed. Environ. Health Perspect. 2019, 127, 82001. [Google Scholar] [CrossRef]
- Montoya, Z.; Conroy, M.; Heuvel, B.D.V.; Pauli, C.S.; Park, S.-H. Cannabis Contaminants Limit Pharmacological Use of Cannabidiol. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- The use of Medicinal Cannabis may Come with Side Effects and Risks. Available online: https://bedrocan.com/cannabis-inside/side-effects-and-risks/ (accessed on 7 December 2020).
- Jamaica Plants and Herbal Remedies. Available online: https://fiwiroots.com/herbal/guineahenweed.html (accessed on 7 December 2020).
- Monera, T.G.; Jani, Z.T.; Maponga, C.C.; Mudzengi, J.; Morse, G.D.; Nhachi, C.F.B. Quality and labeling information of Moringa oleifera products marketed for HIV-infected people in Zimbabwe. J. Public Heal. Afr. 2016, 7, 84–88. [Google Scholar] [CrossRef]
- Moringa Tree and Cancer: Side Effects and Research Studies. Available online: https://www.asbestos.com/blog/2019/11/26/moringa-tree-cancer-research/ (accessed on 7 December 2020).
- Kłębukowska, L.; Zadernowska, A.; Chajęcka-Wierzchowska, W. Microbiological contamination of dried and lyophilized garlic as a potential source of food spoilage. J. Food Sci. Technol. 2013, 52, 1802–1807. [Google Scholar] [CrossRef] [PubMed]
- Garlic Danger Alert: Reasons Why It Can Be Poisonous. Available online: https://timesofindia.indiatimes.com/life-style/health-fitness/photo-stories/garlic-danger-alert-reasons-why-it-can-be-poisonous/photostory/61336724.cms?picid61336731 (accessed on 7 December 2020).
- Garlic Uses, Side Effects & Warnings. Available online: https://www.drugs.com/mtm/garlic.html (accessed on 7 December 2020).
- Badrie, N.; Schauss, A.G. 39—Soursop (Annona muricata L.): Composition, Nutritional Value, Medicinal Uses, and Toxicology. In Bioactive Foods in Promoting Health; 1109039239 838552040 R.R. Watson & 1109039240 838552040 V.R. Preedy (Authors); Elsevier/Academic Press: Amsterdam, The Netherlands, 2010; pp. 621–643. [Google Scholar]
- Graviola: Uses, Side Effects, Interactions, Dosage, and Warning. Available online: https://www.webmd.com/vitamins/ai/ingredientmono-1054/graviola (accessed on 7 December 2020).
- Markets, R. Global Anti-Viral Drug Therapy Market (2020 to 2030)—COVID-19 Implications and Growth. Available online: https://www.globenewswire.com/news-release/2020/04/20/2018628/0/en/Global-Anti-Viral-Drug-Therapy-Market-2020-to-2030-COVID-19-Implications-and-Growth.html (accessed on 20 August 2020).
- Herbal Supplements Market Size & Share: Industry Report, 2018–2025. Available online: https://www.grandviewresearch.com/industry-analysis/herbal-supplements-market (accessed on 20 August 2020).
- Lowe, H.I.; Toyang, N.J. Therapeutic agents containing cannabis flavonoid derivatives targeting kinases, sirtuins and oncogenic agents for the treatment of cancers. U.S. Patent 20180098961A1, 9 March 2019. [Google Scholar]
- Lowe, H.I.; Toyang, N.J.; Bryant, J. Agent Containing Flavonoid Derivatives for Treating Cancer and Inflammation. U.S. Patent 20170360744A1, 8 September 2020. [Google Scholar]
- Lowe, H.I.; Toyang, N.J. Therapeutic Agents Containing Cannabis Flavonoid Derivative for Ocular Disorders. U.S. Patent 10278950B2, 7 May 2019. [Google Scholar]
- Lowe, H.I.; Toyang, N.J. Therapeutic Agents Containing Cannabis Flavonoid Derivatives for the Prevention and Treatment of Neurodegenerative Disorders. U.S. Patent 10751320B2, 25 August 2020. [Google Scholar]
- Lowe, H.I.; Toyang, N.J. Pi 4-Kinase Iinhibitor as a Therapeutic for Viral Hepatitis, Cancer, Malaria. Autoimmune Disorders and Inflammation, and a Radiosensitizer and Immunosuppressant. U.S. Patent WO2018022868A1, 1 February 2018. [Google Scholar]
- Lowe, H.I.; Toyang, N.J. Therapeutic Antiviral Agents Containing Cannabis Cannabinoid Derivatives. U.S. Patent 20180214389A1, 2 August 2018. [Google Scholar]
- Lowe, H.I.; Toyang, N.J.; Bryant, J. Methods for Inhibiting HIV-1 Activity by Inhibitory Mechanisms of Extracts of Guaiacum officinale L. (Zygophyllaceae). U.S. Patent 9814747B2, 14 November 2017. [Google Scholar]
- UTech Researcher, Partner Obtain Patent for Guinea Hen Anti-Cancer Formulation. Available online: http://jamaica-gleaner.com/article/business/20170802/utech-researcher-partner-obtain-patent-guinea-hen-anti-cancer-formulation (accessed on 6 December 2020).
- Verpoorte, R. Pharmacognosy in the New Millennium: Leadfinding and Biotechnology. J. Pharm. Pharmacol. 2000, 52, 253–262. [Google Scholar] [CrossRef] [PubMed]
- Glenz, K.; Warzecha, H. New medicinal plants for the production of vaccines. J. Consum. Prot. Food Saf. 2006, 1, 126–130. [Google Scholar] [CrossRef]
- Mitchell, S.; Burke, A.; McKenzie, C.; Stirling, S.; Ryan, J.; Simpson, W.; McGlashan, D. JAMAICA: Country Report to the FAO International Conference on Plant Genetic Resources for Food and Agriculture; Kingston: Frontenac, ON, Canada, 2008; pp. 2–59. [Google Scholar]
- Palhares, R.M.; Drummond, M.G.; Brasil, B.D.S.A.F.; Cosenza, G.P.; Brandão, M.D.G.L.; Oliveira, G. Medicinal Plants Recommended by the World Health Organization: DNA Barcode Identification Associated with Chemical Analyses Guarantees Their Quality. PLoS ONE 2015, 10, e0127866. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).