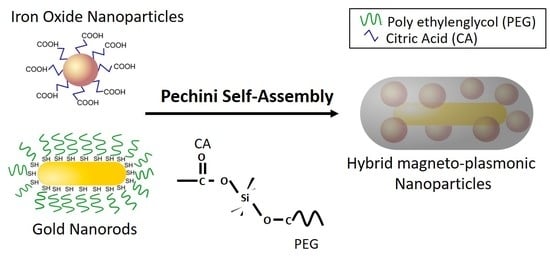
Self-Assembly of Au-Fe3O4 Hybrid Nanoparticles Using a Sol–Gel Pechini Method
Abstract
:1. Introduction
2. Results
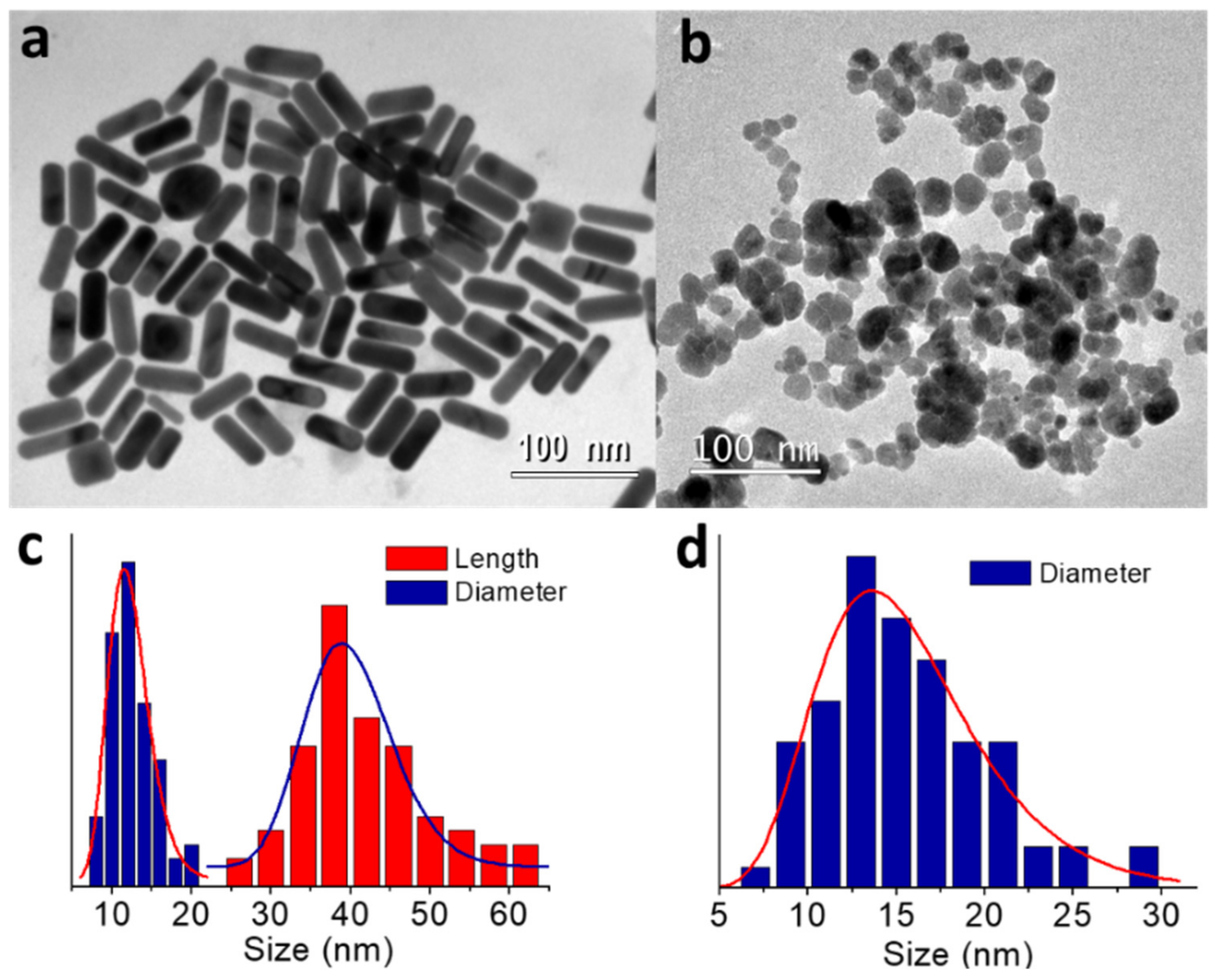
2.1. Synthesis of Primary Nanoparticles and Surface Functionalization with PEG and CA
2.2. Effect of Key Hybridization Parameters on the Nanostructure Geometry
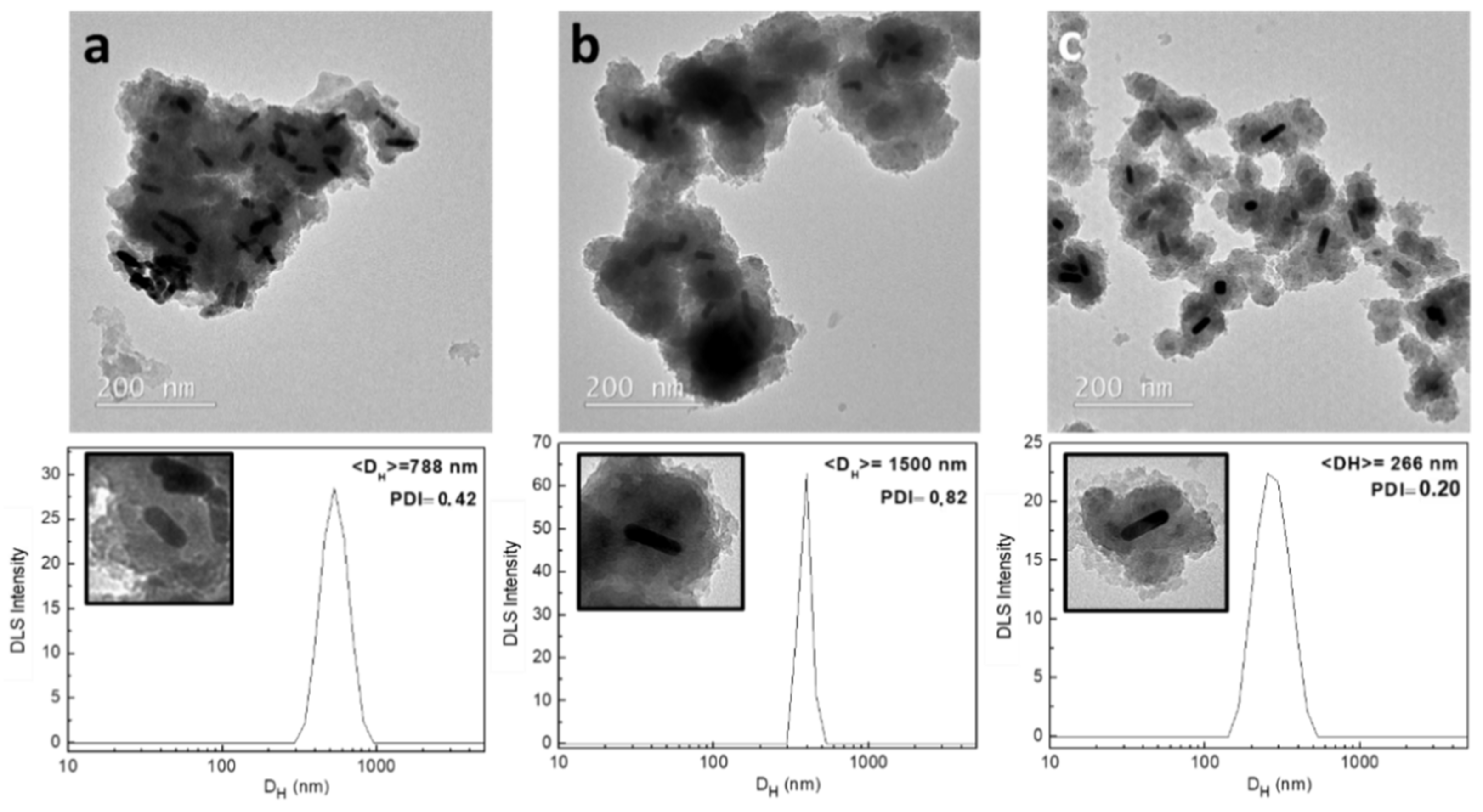
2.2.1. Shaking Conditions
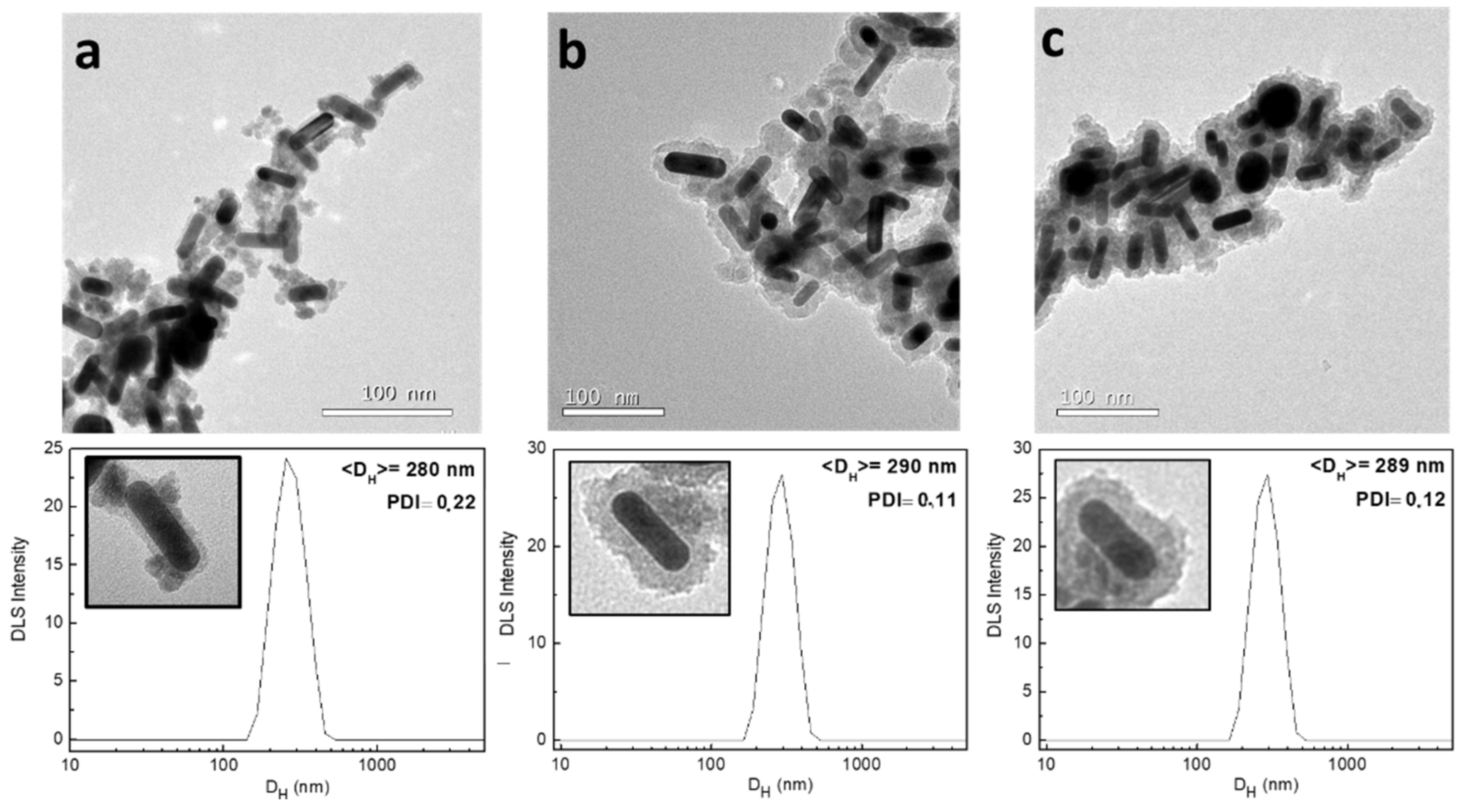
2.2.2. Reaction Time
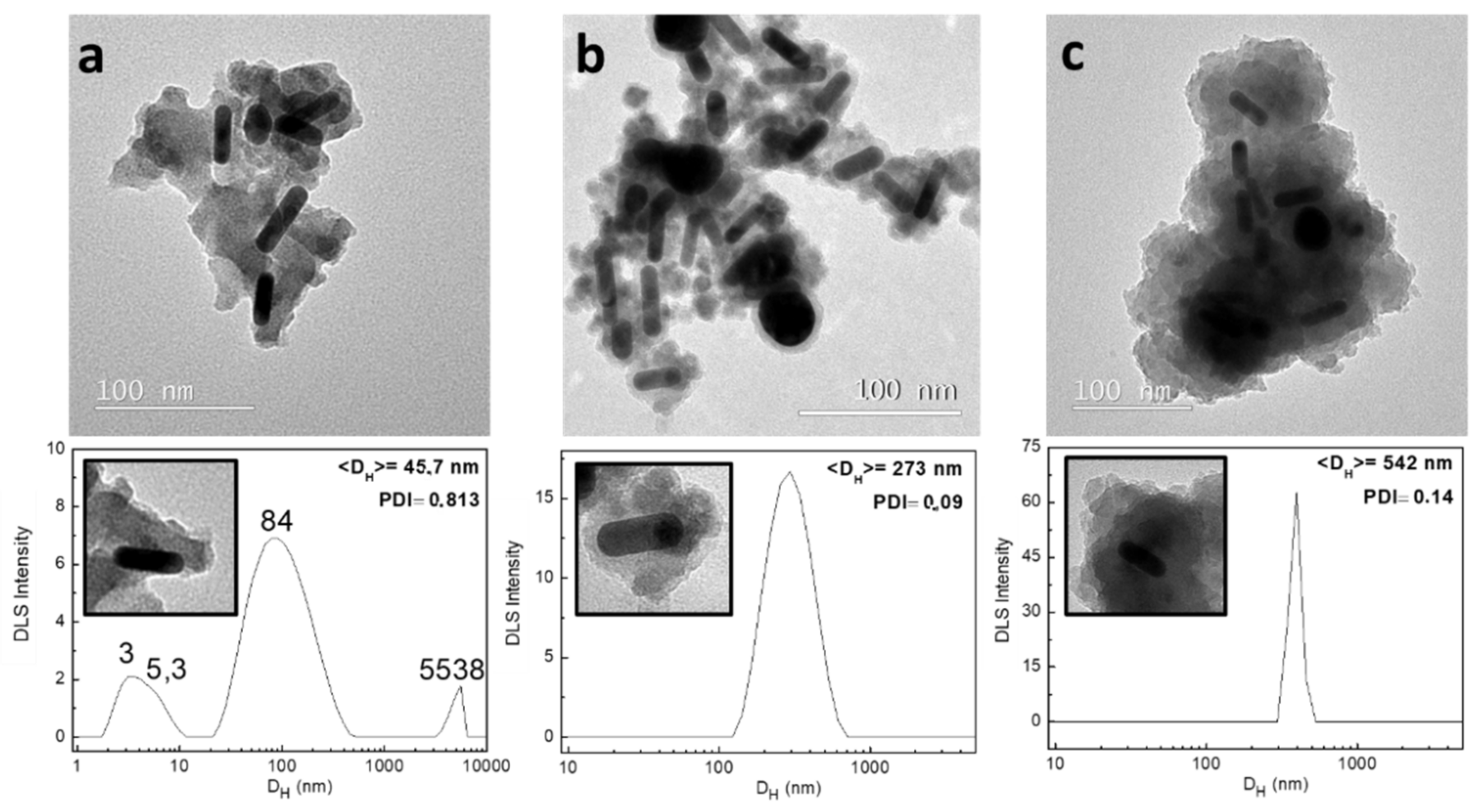
2.2.3. pH Conditions
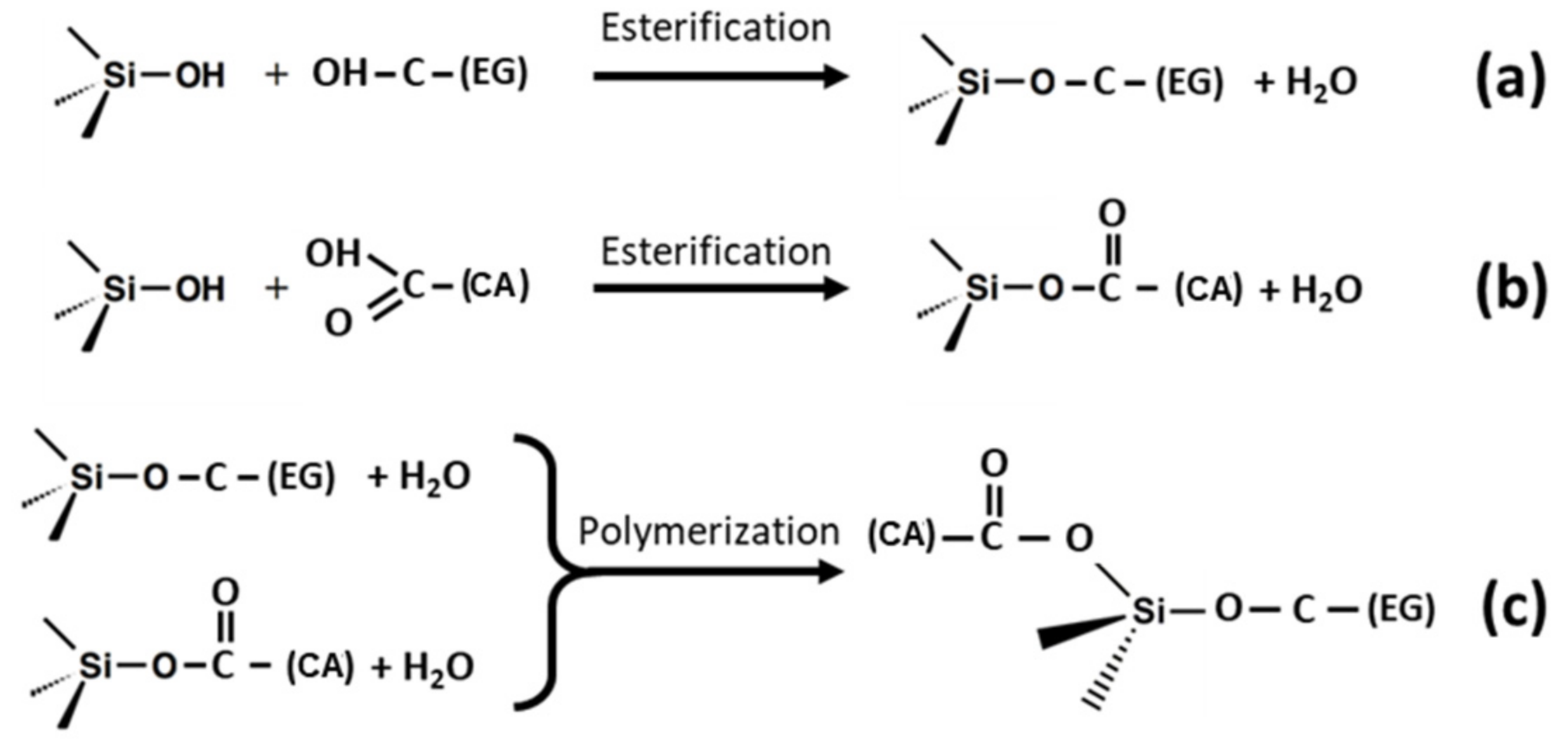
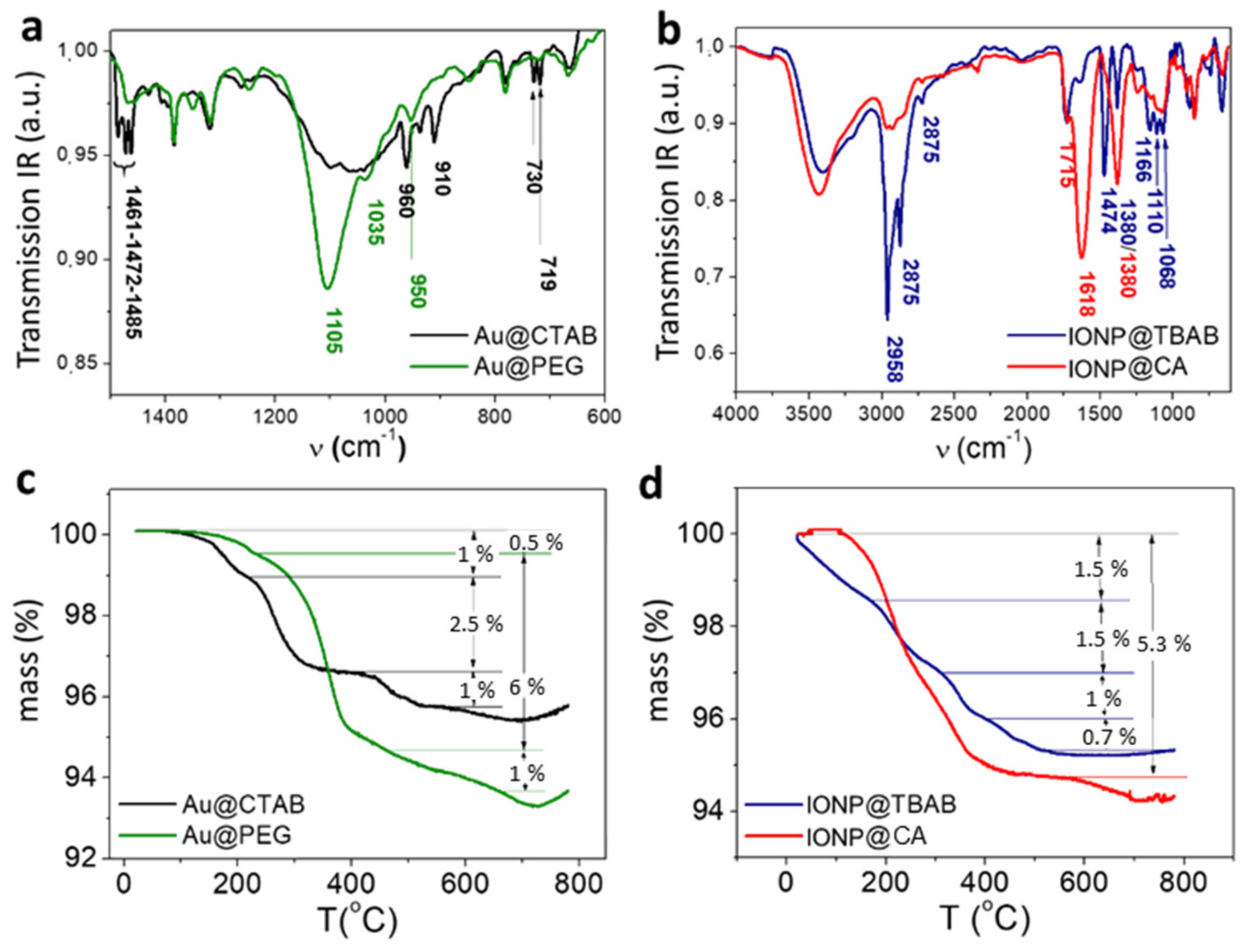
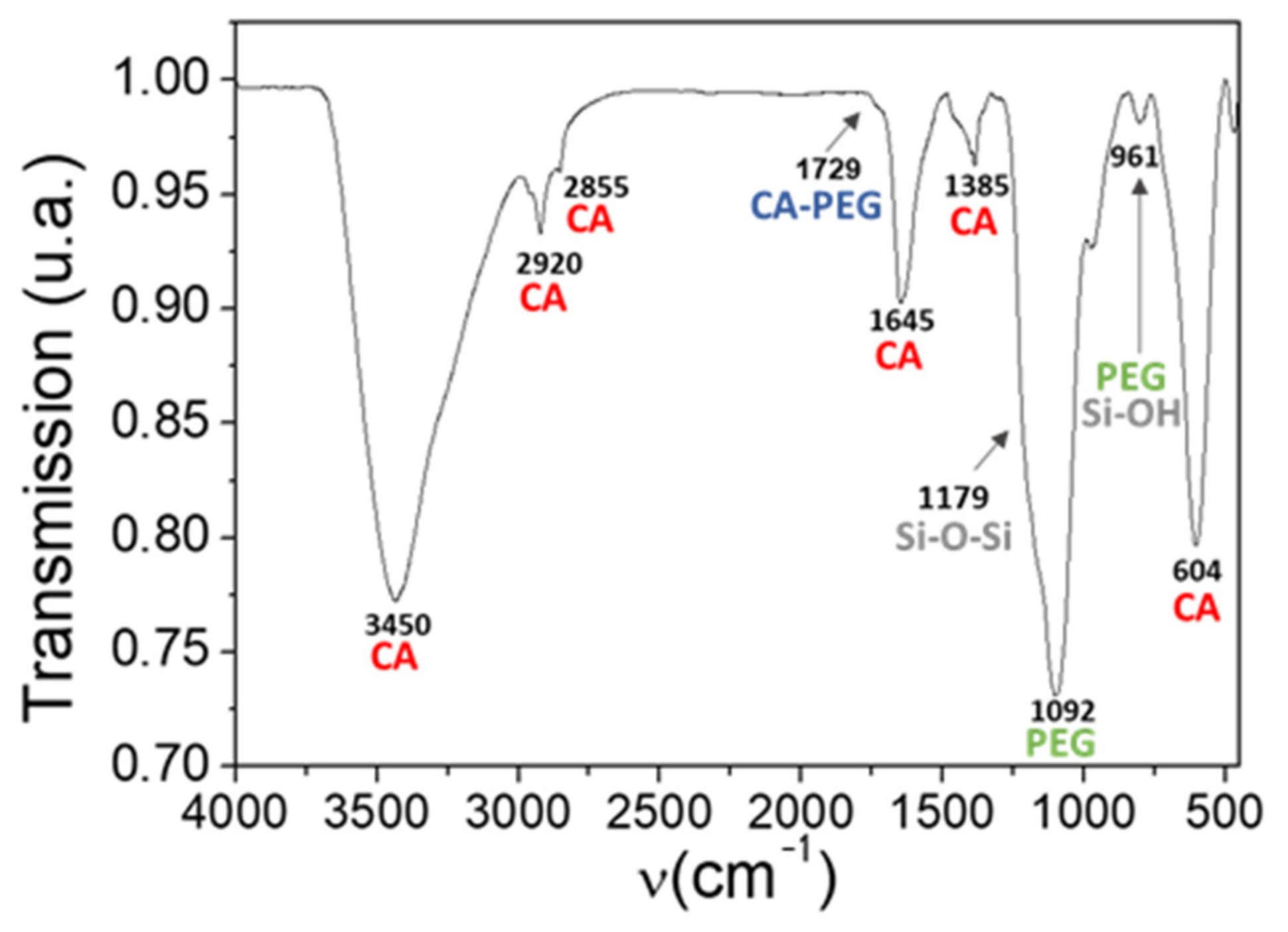
3. Molecular Bonding Mechanism for the Best Hybridization Conditions
- (i)
- The broad band at 3450 cm−1 grows in intensity in respect of the IONP@CA pattern due to the contribution of -OH groups of PEG and hydrolyzed TEOS.
- (ii)
- The contribution of stretching the vibrational mode of Si-OH shifts the small absorption peak observed at 950 cm−1 in the AuNR@PEG pattern to 961 cm−1.
- (iii)
- The low intensity shoulder observed at 1729 cm−1, which is associated to the ester bond between CA and PEG.
- (iv)
- The two differentiated peaks at 1105 cm−1 and 1035 cm−1 observed in the AuNR@PEG pattern become a single broad peak centered at 1105 cm−1, with two shoulders at 1179 cm−1 and 1221 cm−1 (deconvolution in SI).
4. Materials and Methods
4.1. Reactants
4.2. Synthesis of Au NR
4.3. Electrochemical Synthesis of IONP
4.4. Synthesis of Hybrid AuNR-IONP
- The samples analyzed in the study of the shaking conditions were prepared by sitting the plastic tubes, stirring the solution with a magnetic stirrer, and introducing the tube to an ultrasound bath. The rest of parameters were maintained as the standard.
- The samples which analyzed the time of reaction were left in the ultrasound bath for 30, 60 and, 120 min. The rest of the parameters were maintained as the standard.
- The samples analyzed in the study of the pH were prepared using 2.5, 5, and 10 μL of NH3. The rest of parameters were maintained as the standard.
- The resultant hybrid NPs were cleaned by triple centrifugation for 30 min at 3000 rpm. Supernatant was discarded and the hybrid NPs were stored in pure ethanol for the analysis.
4.5. Characterization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- De Julian-Fernandez, C.; Pineider, F. Magneto-plasmonic nanoparticles. In New Trends in Nanoparticle Magnetism; Peddis, D., Laureti, S., Fiorani, D., Eds.; Springer: New York, NY, USA, 2021; pp. 107–136. [Google Scholar]
- Ovejero, J.G.; Herrasti, P. Hybrid magneto-plasmonic nanoparticles in biomedicine: Fundamentals, synthesis and applications. In Nanohybrids: Future Materials for Biomedical Applications; Materials Research Foundations LLC: Millersville, PA, USA, 2021; Volume 87, pp. 1–54. [Google Scholar]
- Hou, X.; Wang, X.; Liu, R.; Zhang, H.; Liu, X.; Zhang, Y. Facile synthesis of multifunctional Fe3O4@SiO2@Au magneto-plasmonic nanoparticles for MR/CT dual imaging and photothermal therapy. RSC Adv. 2017, 7, 18844–18850. [Google Scholar] [CrossRef] [Green Version]
- Di Corato, R.; Béalle, G.; Kolosnjaj-Tabi, J.; Espinosa, A.; Clément, O.; Silva, A.K.A.; Ménager, C.; Wilhelm, C. Combining magnetic hyperthermia and photodynamic therapy for tumor ablation with photoresponsive magnetic liposomes. ACS Nano 2015, 9, 2904–2916. [Google Scholar] [CrossRef] [PubMed]
- Szunerits, S.; Saada, T.N.; Meziane, D.; Boukherroub, R. Magneto-optical nanostructures for viral sensing. Nanomaterials 2020, 10, 1271. [Google Scholar] [CrossRef]
- Espinosa, A.; Reguera, J.; Curcio, A.; Muñoz-Noval, A.; Kuttner, C.; de Van, A.; Liz-Marzán, L.M.; Wilhelm, C. janus magnetic-plasmonic nanoparticles for magnetically guided and thermally activated cancer therapy. Small 2020, 16, 1904960. [Google Scholar] [CrossRef] [PubMed]
- Ovejero, J.G.; Bran, C.; Vilanova, E.; Kosel, J.; Morales, M.P.; Vazquez, M. Electrochemical synthesis of core–shell magnetic nanowires. J. Magn. Magn. Mater. 2015, 389, 144–147. [Google Scholar] [CrossRef] [Green Version]
- Daglar, O.; Çakmakçi, E.; Gunay, U.S.; Hizal, G.; Tunca, U.; Durmaz, H. All in one: The preparation of polyester/silica hybrid nanocomposites via three different metal-free click reactions. Eur. Polym. J. 2021, 154, 110532. [Google Scholar] [CrossRef]
- Mackenzie, J.D.; Bescher, E.P. Chemical routes in the synthesis of nanomaterials using the sol-gel process. Acc. Chem. Res. 2007, 40, 810–818. [Google Scholar] [CrossRef]
- Danks, A.E.; Hall, S.R.; Schnepp, Z. The evolution of ‘sol–gel’ chemistry as a technique for materials synthesis. Mater. Horiz. 2016, 3, 91–112. [Google Scholar] [CrossRef] [Green Version]
- Cannas, C.; Gatteschi, D.; Musinu, A.; Piccaluga, G.; Sangregorio, C. Structural and magnetic properties of Fe2O3 nanoparticles dispersed over a silica matrix. J. Phys. Chem. B 1998, 102, 7721–7726. [Google Scholar] [CrossRef]
- Ennas, G.; Piccaluga, G.; Zedda, D.; Gatteschi, D.; Sangregorio, C.; Stanger, J.L.; Concas, G.; Spano, G. Characterization of iron oxide nanoparticles in an Fe2O3−SiO2 composite prepared by a sol−gel method. Chem. Mater. 1998, 10, 495–502. [Google Scholar] [CrossRef]
- Omri, K.; Najeh, I.; Dhahri, R.; El Ghoul, J.; El Mir, L. Effects of temperature on the optical and electrical properties of ZnO nanoparticles synthesized by sol–gel method. Microelectron. Eng. 2014, 128, 53–58. [Google Scholar] [CrossRef]
- Khan, M.F.; Ansari, A.H.; Hameedullah, M.; Ahmad, E.; Husain, F.M.; Zia, Q.; Baig, U.; Zaheer, M.R.; Alam, M.M.; Khan, A.M.; et al. Sol-gel synthesis of thorn-like ZnO nanoparticles endorsing mechanical stirring effect and their antimicrobial activities: Potential role as nano-antibiotics. Sci. Rep. 2016, 6, 27689. [Google Scholar] [CrossRef] [PubMed]
- Prokopowicz, M. Silica-polyethylene glycol matrix synthesis by sol-gel method and evaluation for diclofenac diethyloammonium release. Drug Deliv. 2007, 14, 129–138. [Google Scholar] [CrossRef]
- Figueira, R.B.; Silva, C.J.R.; Pereira, E.V. Organic–inorganic hybrid sol–gel coatings for metal corrosion protection: A review of recent progress. J. Coat. Technol. Res. 2015, 12, 1–35. [Google Scholar] [CrossRef]
- Pechini, M.P.; Adams, N. Method of Preparing Lead and Alkaline Earth Titanates and Niobates and Coating Method Using the Same to Form a Capacitor. U.S. Patent 3,330,697, 11 July 1967. [Google Scholar]
- Dimesso, L. Pechini processes: An alternate approach of the sol-gel method, preparation, properties, and applications. In Handbook of Sol-Gel Science and Technology: Processing, Characterization and Applications; Klein, L., Aparicio, M., Jitianu, A., Eds.; Springer: New York, NY, USA, 2018; pp. 1067–1087. [Google Scholar]
- Sánchez, C.; Doria, J.; Paucar, C.; Hernandez, M.; Mósquera, A.; Rodríguez, J.E.; Gómez, A.; Baca, E.; Morán, O. Nanocystalline ZnO films prepared via polymeric precursor method (Pechini). Phys. B Condens. Matter 2010, 405, 3679–3684. [Google Scholar] [CrossRef]
- Lin, J.; Yu, M.; Lin, C.; Liu, X. Multiform oxide optical materials via the versatile pechini-type sol−gel process: Synthesis and characteristics. J. Phys. Chem. C 2007, 111, 5835–5845. [Google Scholar] [CrossRef]
- Li, G.; Lin, J. Recent progress in low-voltage cathodoluminescent materials: Synthesis, improvement and emission properties. Chem. Soc. Rev. 2014, 43, 7099–7131. [Google Scholar] [CrossRef]
- Kakihana, M.; Yoshimura, M. Synthesis and characteristics of complex multicomponent oxides prepared by polymer complex method. Bull. Chem. Soc. Jpn. 1999, 72, 1427–1443. [Google Scholar] [CrossRef]
- Campero, A.; Cardoso, J.; Pacheco, S. Ethylene glycol-citric acid-silica hybrid organic-inorganic materials obtained by the sol-gel method. J. Sol-Gel Sci. Technol. 1997, 8, 535–539. [Google Scholar] [CrossRef]
- Leite, E.R.; Souza, F.L.; Bueno, P.R.; de Lazaro, S.; Longo, E. Hybrid Organic−inorganic polymer: A new approach for the development of decoupled polymer electrolytes. Chem. Mater. 2005, 17, 4561–4563. [Google Scholar] [CrossRef]
- Ovejero, J.G.; Yoon, S.J.; Li, J.; Mayoral, A.; Gao, X.; O’Donnell, M.; García, M.A.; Herrasti, P.; Hernando, A. Synthesis of hybrid magneto-plasmonic nanoparticles with potential use in photoacoustic detection of circulating tumor cells. Microchim. Acta 2018, 185, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhuravlev, L.T. The surface chemistry of amorphous silica. Zhuravlev model. Colloids Surf. A Physicochem. Eng. Asp. 2000, 173, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Lu, J.; Liong, M.; Li, Z.; Zink, J.I.; Tamanoi, F. Biocompatibility, biodistribution, and drug-delivery efficiency of mesoporous silica nanoparticles for cancer therapy in animals. Small 2010, 6, 1794–1805. [Google Scholar] [CrossRef] [Green Version]
- Bjugstad, K.B.; Lampe, K.; Kern, D.S.; Mahoney, M. Biocompatibility of poly(ethylene glycol)-based hydrogels in the brain: An analysis of the glial response across space and time. J. Biomed. Mater. Res. Part A 2010, 95, 79–91. [Google Scholar] [CrossRef]
- De Sousa, S.M.G.; Bramante, C.M.; Taga, E.M. Biocompatibility of EDTA, EGTA and citric acid. Braz. Dent. J. 2005, 16, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sotiriou, G.A.; Hirt, A.M.; Lozach, P.-Y.; Teleki, A.; Krumeich, F.; Pratsinis, S.E. Hybrid, silica-coated, Janus-like plasmonic-magnetic nanoparticles. Chem. Mater. 2011, 23, 1985–1992. [Google Scholar] [CrossRef] [Green Version]
- Ovejero, J.G.; Mayoral, A.; Cañete, M.; García, M.; Hernando, A.; Herrasti, P. Electrochemical synthesis and magnetic properties of MFe2O4 (M = Fe, Mn, Co, Ni) nanoparticles for potential biomedical applications. J. Nanosci. Nanotechnol. 2019, 19, 2008–2015. [Google Scholar] [CrossRef]
- Ovejero, J.G.; Morales, I.; de la Presa, P.; Mille, N.; Carrey, J.; Garcia, M.A.; Hernando, A.; Herrasti, P. Hybrid nanoparticles for magnetic and plasmonic hyperthermia. Phys. Chem. Chem. Phys. 2018, 20, 24065–24073. [Google Scholar] [CrossRef] [PubMed]
- Xue, W.; He, H.; Zhu, J.; Yuan, P. FTIR investigation of CTAB-Al-montmorillonite complexes. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2007, 67, 1030–1036. [Google Scholar] [CrossRef]
- Rusen, L.; Mustaciosu, C.; Mitu, B.; Filipescu, M.; Dinescu, M.; Dinca, V. Protein-resistant polymer coatings obtained by matrix assisted pulsed laser evaporation. Appl. Surf. Sci. 2013, 278, 198–202. [Google Scholar] [CrossRef]
- Rahme, K.; Chen, L.; Hobbs, R.G.; Morris, M.A.; O’Driscolle, C.; Holmes, J.D. PEGylated gold nanoparticles: Polymer quantification as a function of PEG lengths and nanoparticle dimensions. RSC Adv. 2013, 3, 6085–6094. [Google Scholar] [CrossRef] [Green Version]
- Hussein-Al-Ali, S.H.; El Zowalaty, M.E.; Hussein, M.Z.; Ismail, M.; Dorniani, D.; Webster, T.J. Novel kojic acid-polymer-based magnetic nanocomposites for medical applications. Int. J. Nanomed. 2014, 9, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Collins, G.; Aureau, D.; Holmes, J.D.; Etcheberry, A.; O’Dwyer, C. Germanium oxide removal by citric acid and thiol passivation from citric acid-terminated Ge(100). Langmuir 2014, 30, 14123–14127. [Google Scholar] [CrossRef]
- Akçay, M. Characterization and adsorption properties of tetrabutylammonium montmorillonite (TBAM) clay: Thermodynamic and kinetic calculations. J. Colloid Interface Sci. 2006, 296, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Nigam, S.; Barick, K.C.; Bahadur, D. Development of citrate-stabilized Fe3O4 nanoparticles: Conjugation and release of doxorubicin for therapeutic applications. J. Magn. Magn. Mater. 2010, 323, 237–243. [Google Scholar] [CrossRef]
- Edrissi, M.; Soleymani, M.; Adinehnia, M. Synthesis of silica nanoparticles by ultrasound-assisted sol-gel method: Optimized by Taguchi robust design. Chem. Eng. Technol. 2011, 34, 1813–1819. [Google Scholar] [CrossRef]
- Dang, F.; Enomoto, N.; Hojo, J.; Enpuku, K. Sonochemical coating of magnetite nanoparticles with silica. Ultrason. Sonochem. 2010, 17, 193–199. [Google Scholar] [CrossRef]
- Montano-Priede, J.L.; Coelho, J.P.; Guerrero-Martínez, A.; Pena-Rodriguez, O.; Pal, U. Fabrication of monodispersed Au@SiO2 nanoparticles with highly stable silica layers by ultrasound-assisted stöber method. J. Phys. Chem. C 2017, 121, 9543–9551. [Google Scholar] [CrossRef]
- Majumder, M.; Rendall, C.S.; Eukel, J.A.; Wang, J.Y.L.; Behabtu, N.; Pint, C.L.; Liu, T.Y.; Orbaek, A.W.; Mirri, F.; Nam, J.; et al. Overcoming the coffee-stain effect by compositional marangoni-flow-assisted drop-drying. J. Phys. Chem. B 2012, 116, 6536–6542. [Google Scholar] [CrossRef]
- Hore, M.J.A.; Ye, X.; Ford, J.; Gao, Y.; Fei, J.; Wu, Q.; Rowan, S.J.; Composto, R.J.; Murray, C.B.; Hammouda, B. Probing the structure, composition, and spatial distribution of ligands on gold nanorods. Nano Lett. 2015, 15, 5730–5738. [Google Scholar] [CrossRef] [PubMed]
- Fernández-López, C.; Mateo-Mateo, C.; Álvarez-Puebla, R.A.; Pérez-Juste, J.; Pastoriza-Santos, I.; Liz-Marzán, L.M. Highly controlled silica coating of PEG-capped metal nanoparticles and preparation of SERS-encoded particles. Langmuir 2009, 25, 13894–13899. [Google Scholar] [CrossRef]
- Rodríguez-Fernández, J.; Pérez-Juste, J.; Liz-Marzán, L.M.; Lang, P.R. Dynamic light scattering of short au rods with low aspect ratios. J. Phys. Chem. C 2007, 111, 5020–5025. [Google Scholar] [CrossRef] [Green Version]
- Tilgner, I.C.; Fischer, P.; Bohnen, F.M.; Rehage, H.; Maier, W.F. Effect of acidic, basic and fluoride-catalyzed sol-gel transitions on the preparation of sub-nanostructured silica. Microporous Mater. 1995, 5, 77–90. [Google Scholar] [CrossRef]
- Coradin, T.; Lopez, P.J. Biogenic silica patterning: Simple chemistry or subtle biology? ChemBioChem 2003, 4, 251–259. [Google Scholar] [CrossRef]
- Prokopowicz, M.; Łukasiak, J. Synthesis and in vitro characterization of freeze-dried doxorubicin-loaded silica/PEG composite. J. Non-Cryst. Solids 2010, 356, 1711–1720. [Google Scholar] [CrossRef]
- Zhou, Y. Organosilicon Chemistry: Special Lectures, Procceedings of the International Symposium on Organosilicon Chemistry; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Ahmadi, A.; Ramezanzadeh, B.; Mahdavian, M. Hybrid silane coating reinforced with silanized graphene oxide nanosheets with improved corrosion protective performance. RSC Adv. 2016, 6, 54102–54112. [Google Scholar] [CrossRef]
- Leite, E.R.; Carreño, N.L.V.; Longo, E.; Pontes, F.M.; Barison, A.; Ferreira, A.G.; Maniette, Y.; Varela, J.A. Development of metal-SiO2 nanocomposites in a single-step process by the polymerizable complex method. Chem. Mater. 2002, 14, 3722–3729. [Google Scholar] [CrossRef]
- Radev, L.; Pavlova, L.; Samuneva, B.; Kashchieva, E.; Mihailova, I.; Zaharescu, M.; Malic, B.; Predoana, L. Sol-gel synthesis and structure of La2O3–CoO–SiO2 powders process. Appl. Ceram. 2008, 2, 103–108. [Google Scholar] [CrossRef]
- Zaharieva, J.; Milanova, M.; Todorovsky, D. SiO2/polyester hybrid for immobilization of Ru(ii) complex as optical gas-phase oxygen sensor. J. Mater. Chem. 2011, 21, 4893–4903. [Google Scholar] [CrossRef]
- Nikoobakht, B.; El-Sayed, M.A. Preparation and growth mechanism of gold nanorods (nrs) using seed-mediated growth method. Chem. Mater. 2003, 15, 1957–1962. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ovejero, J.G.; Garcia, M.A.; Herrasti, P. Self-Assembly of Au-Fe3O4 Hybrid Nanoparticles Using a Sol–Gel Pechini Method. Molecules 2021, 26, 6943. https://doi.org/10.3390/molecules26226943
Ovejero JG, Garcia MA, Herrasti P. Self-Assembly of Au-Fe3O4 Hybrid Nanoparticles Using a Sol–Gel Pechini Method. Molecules. 2021; 26(22):6943. https://doi.org/10.3390/molecules26226943
Chicago/Turabian StyleOvejero, Jesus G., Miguel A. Garcia, and Pilar Herrasti. 2021. "Self-Assembly of Au-Fe3O4 Hybrid Nanoparticles Using a Sol–Gel Pechini Method" Molecules 26, no. 22: 6943. https://doi.org/10.3390/molecules26226943
APA StyleOvejero, J. G., Garcia, M. A., & Herrasti, P. (2021). Self-Assembly of Au-Fe3O4 Hybrid Nanoparticles Using a Sol–Gel Pechini Method. Molecules, 26(22), 6943. https://doi.org/10.3390/molecules26226943