Molecular Evidence of the Inhibitory Potential of Melatonin against NaAsO2-Induced Aging in Male Rats
Abstract
:1. Introduction
2. Results
2.1. Total Tissue Arsenic Using an Inductively Coupled Plasma Mass Spectrometer
2.2. Oxidative Stress Biomarkers
2.3. Reactive Oxygen Species
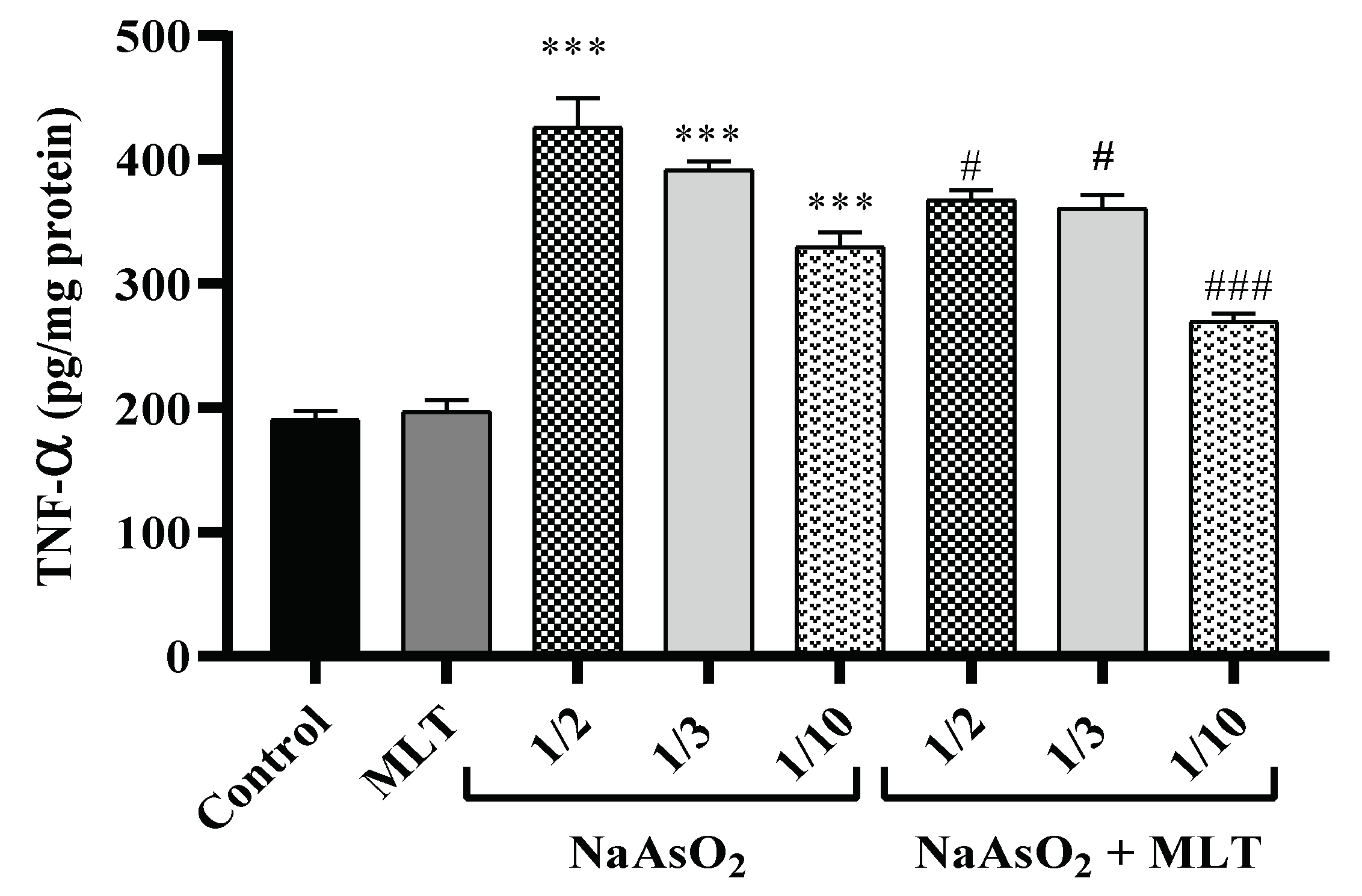
2.4. Pro-Inflammatory TNF-α Cytokine
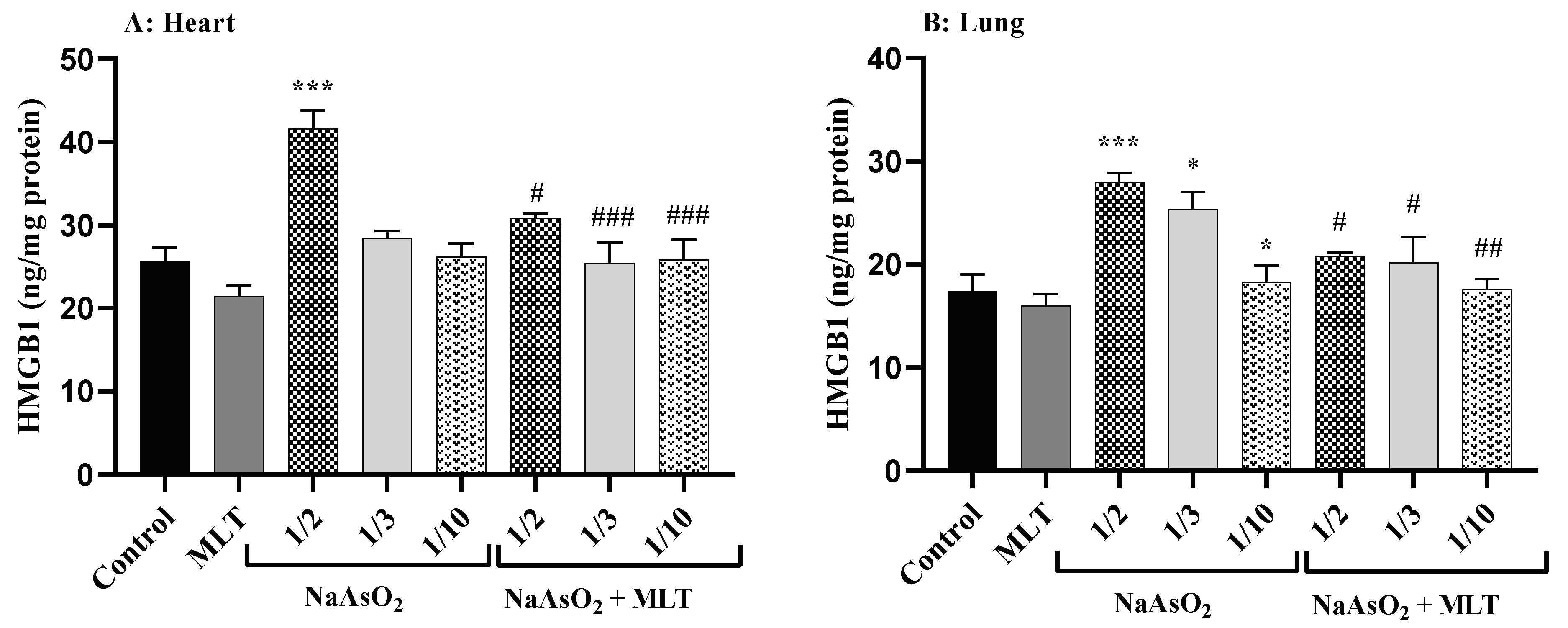
2.5. High Mobility Group Box 1 Levels
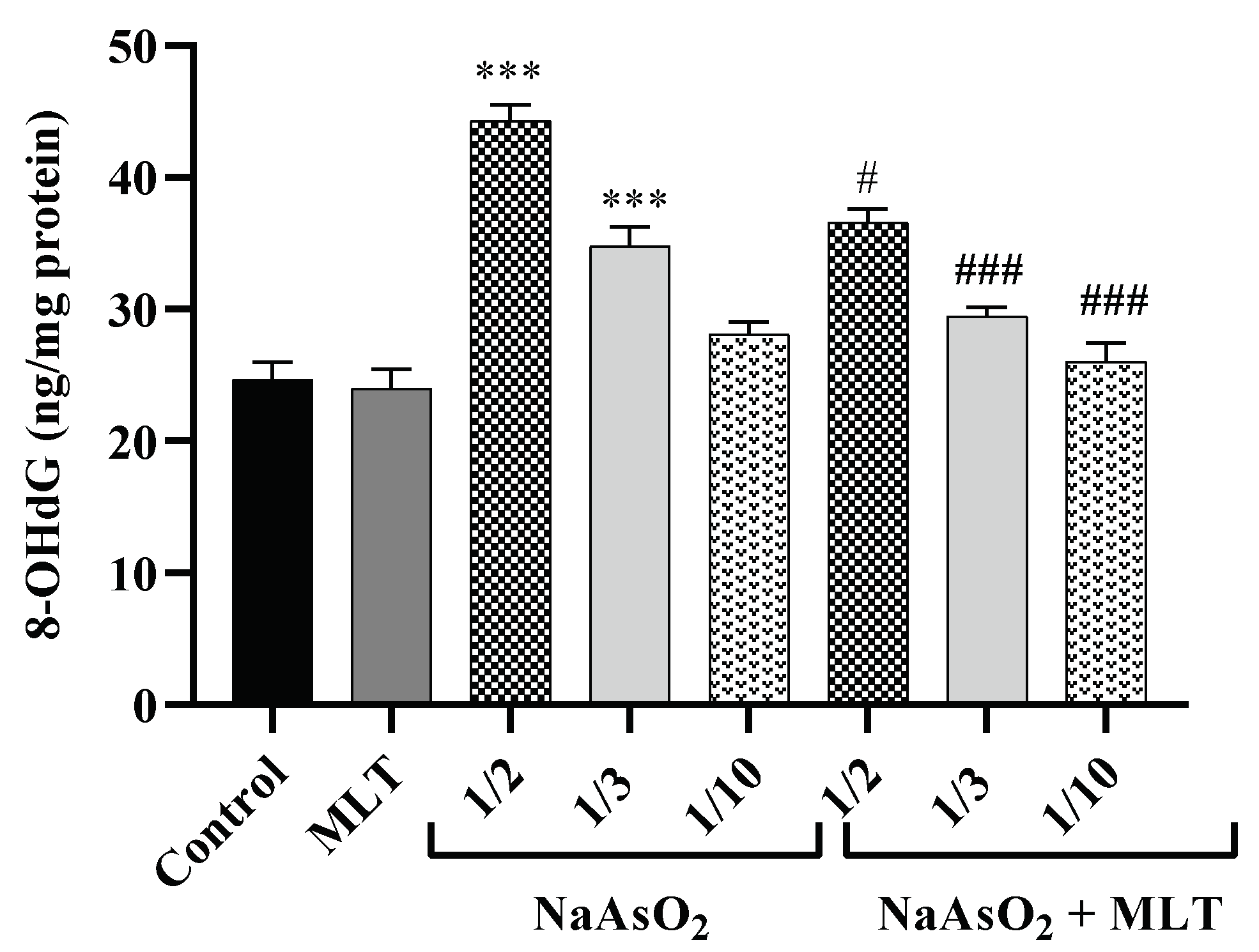
2.6. 8-Hydroxy-2-deoxyguanosine Levels
2.7. Lactate Levels
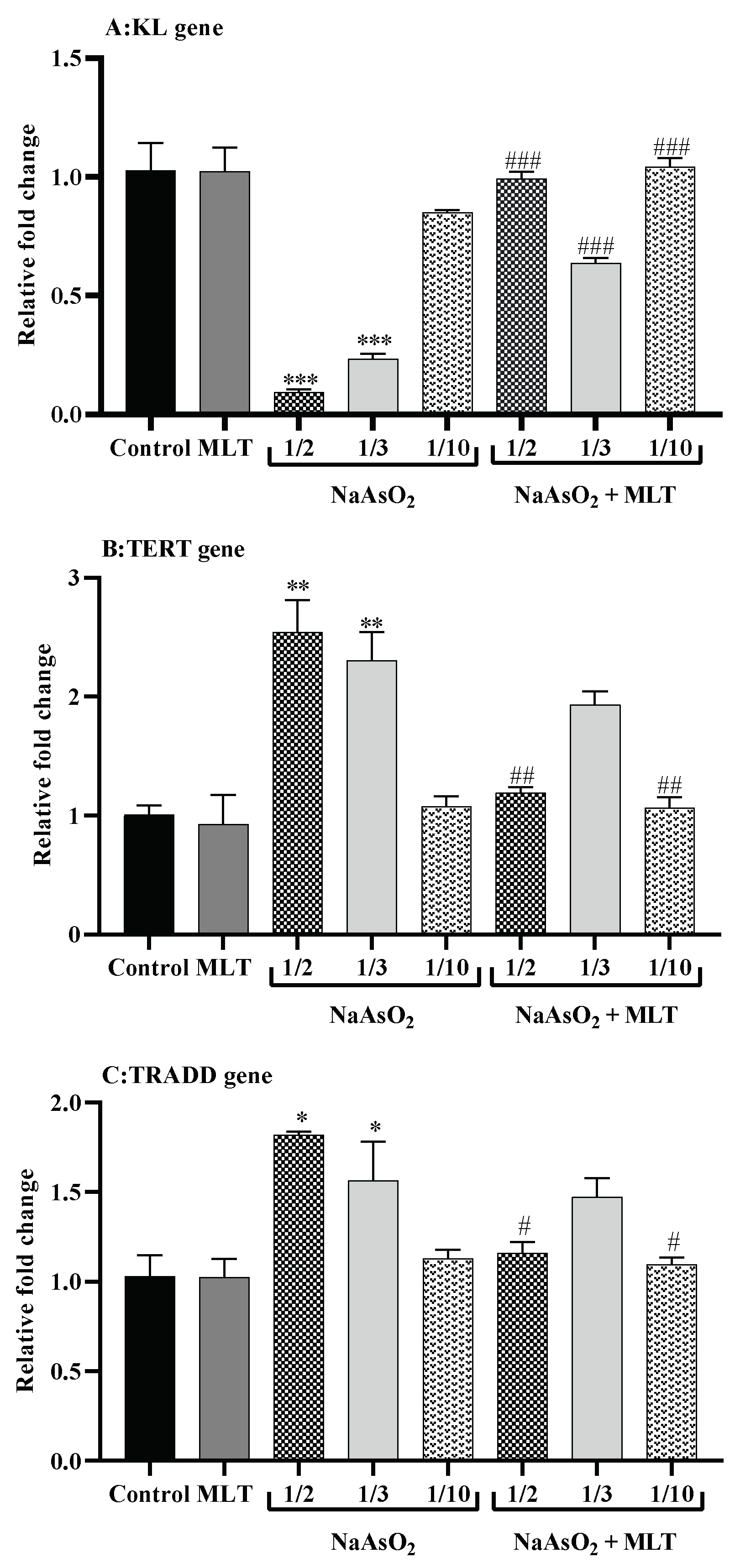
2.8. Real-Time Reverse Transcription PCR for Gene Expression
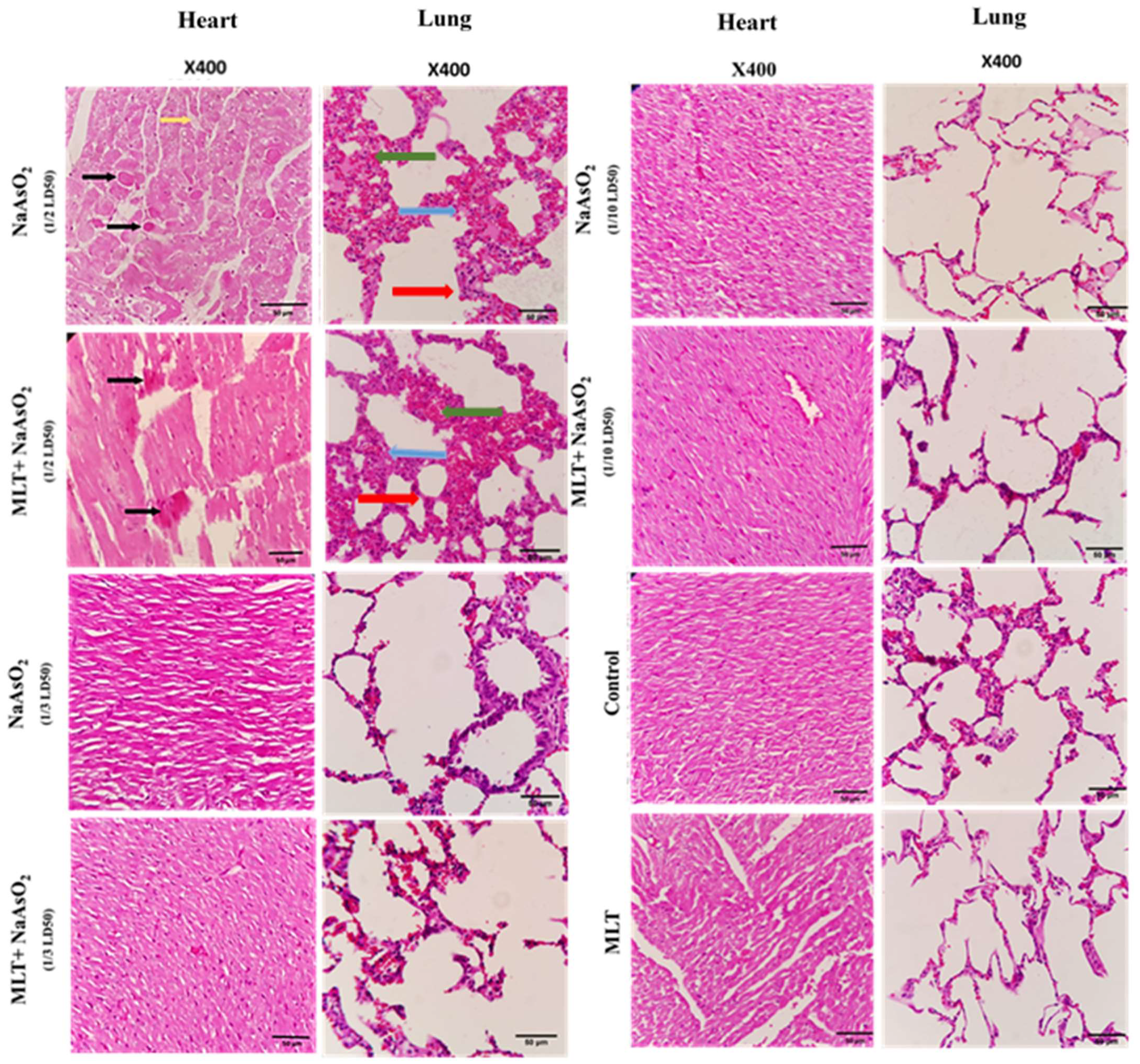
2.9. Histological Evaluations of Heart and Lung Tissues
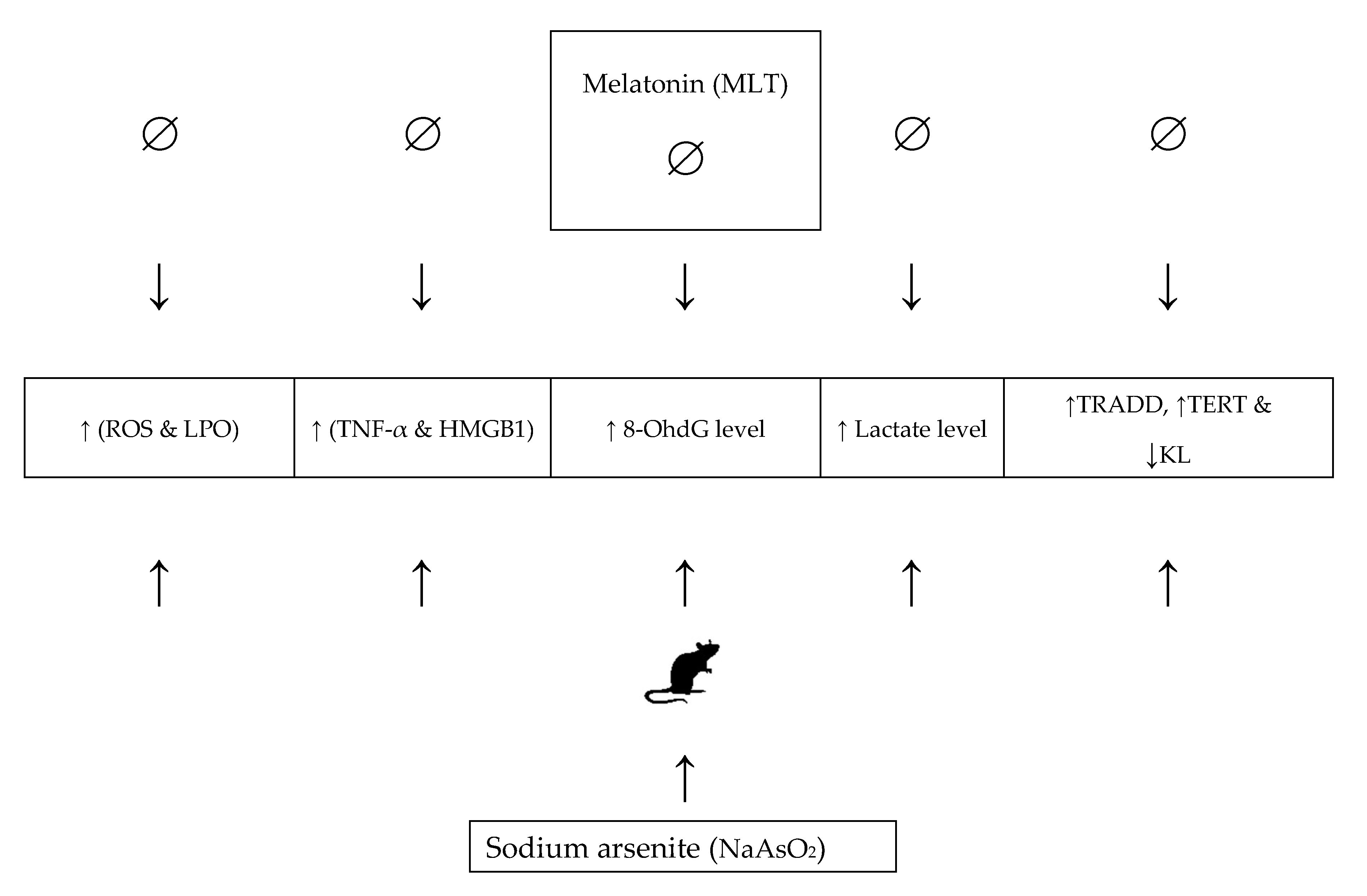
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Ethical Approval
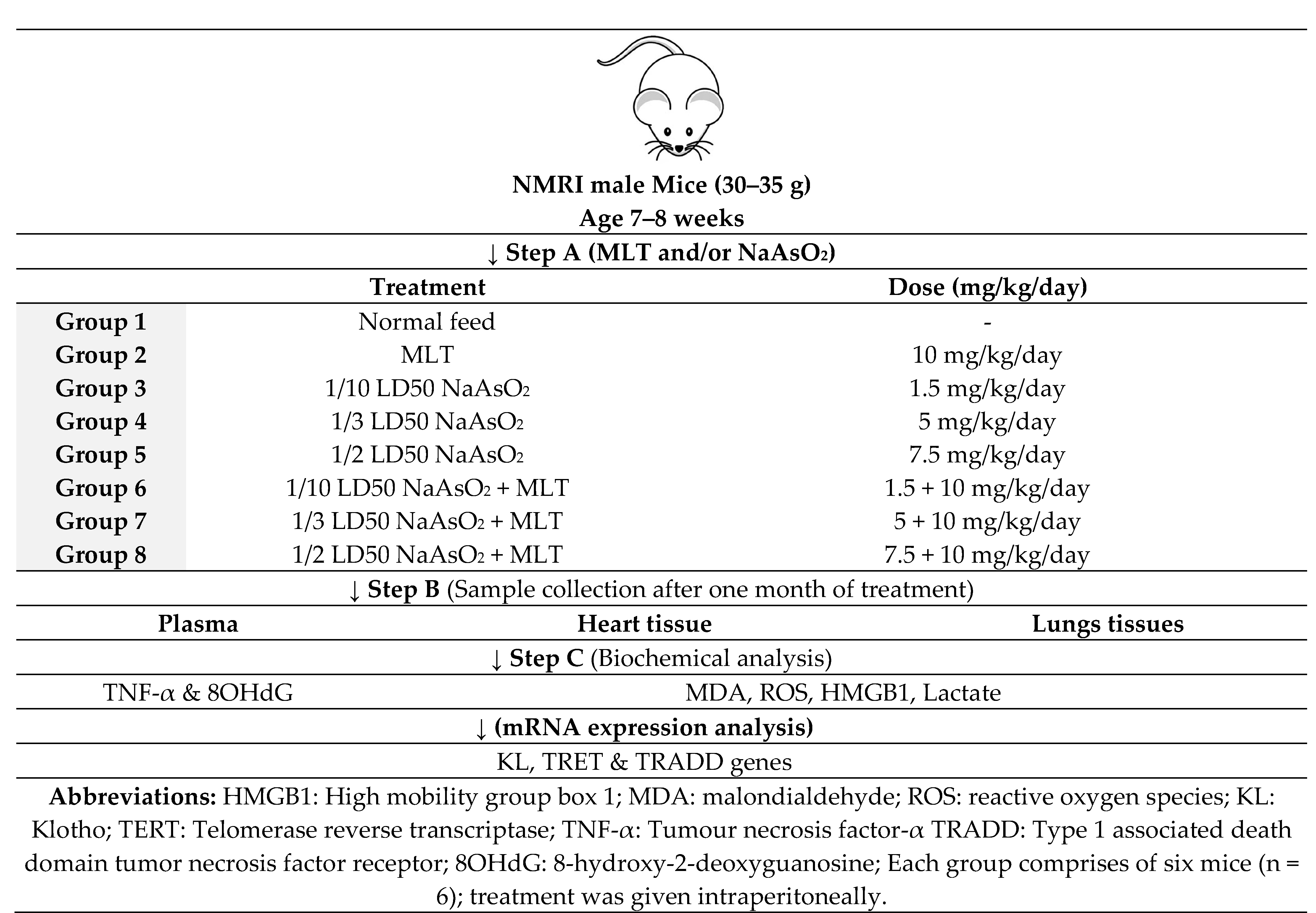
4.3. Study Design
4.4. Assessment of Total Tissue Arsenic Using an Inductively Coupled Plasma Mass Spectrometer
4.5. Assessment of Oxidative Stress Biomarkers
4.6. Assessment of Reactive Oxygen Species
4.7. Assessment of Pro-Inflammatory Cytokines
4.8. Assessment of DNA Damage
4.9. Assessment of Lactate Level
4.10. Quantitative Real-Time Reverse Transcription PCR for Gene Expression
4.11. Histological Ex Vivo Evaluations
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
Sample Availability
References
- Ng, J.C.; Wang, J.; Shraim, A. A Global Health Problem Caused by Arsenic from Natural Sources. Chemosphere 2003, 52, 1353–1359. [Google Scholar] [CrossRef]
- Wu, J. Challenges for Safe and Healthy Drinking Water in China. Curr. Environ. Health Rep. 2020, 7, 292–302. [Google Scholar] [CrossRef] [PubMed]
- Borowska, S.; Brzóska, M.M. Metals in Cosmetics: Implications for Human Health. J. Appl. Toxicol. 2015, 35, 551–572. [Google Scholar] [CrossRef]
- Ratnaike, R.N. Acute and Chronic Arsenic Toxicity. Postgrad. Med. J. 2003, 79, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Kessel, M.; Liu, S.X.; Xu, A.; Santella, R.; Hei, T.K. Arsenic Induces Oxidative DNA Damage in Mammalian Cells. Mol. Cell Biochem. 2002, 234–235, 301–308. [Google Scholar] [CrossRef]
- Yuan, X.; Larsson, C.; Xu, D. Mechanisms Underlying the Activation of TERT Transcription and Telomerase Activity in Human Cancer: Old Actors and New Players. Oncogene 2019, 38, 6172–6183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akincilar, S.C.; Unal, B.; Tergaonkar, V. Reactivation of Telomerase in Cancer. Cell. Mol. Life Sci. CMLS 2016, 73, 1659–1670. [Google Scholar] [CrossRef] [Green Version]
- Ferrario, D.; Collotta, A.; Carfi, M.; Bowe, G.; Vahter, M.; Hartung, T.; Gribaldo, L. Arsenic Induces Telomerase Expression and Maintains Telomere Length in Human Cord Blood Cells. Toxicology 2009, 260, 132–141. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Patel, K.K.; Dehari, D.; Agrawal, A.K.; Singh, S. Melatonin and Its Ubiquitous Anticancer Effects. Mol. Cell. Biochem. 2019, 462, 133–155. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Tan, D.X.; Rosales-Corral, S.; Galano, A.; Zhou, X.J.; Xu, B. Mitochondria: Central Organelles for Melatonin’s Anti-oxidant and Anti-Aging Actions. Molecules 2018, 23, 509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortega-Gutiérrez, S.; López-Vicente, M.; Lostalé, F.; Fuentes-Broto, L.; Martínez-Ballarín, E.; García, J.J. Protective Effect of Melatonin Against Mitomycin C-Induced Genotoxic Damage in Peripheral Blood of Rats. J. Biomed. Biotechnol. 2009, 2009, 791432. [Google Scholar] [CrossRef]
- Nooshinfar, E.; Safaroghli-Azar, A.; Bashash, D.; Akbari, M.E. Melatonin, an Inhibitory Agent in Breast Cancer. Breast Cancer 2017, 24, 42–51. [Google Scholar] [CrossRef]
- Leon-Blanco, M.M.; Guerrero, J.M.; Reiter, R.J.; Calvo, J.R.; Pozo, D. Melatonin Inhibits Telomerase Activity in the MCF-7 Tumor Cell Line both In Vivo and In Vitro. J. Pineal. Res. 2003, 35, 204–211. [Google Scholar] [CrossRef]
- Martínez-Campa, C.M.; Alonso-González, C.; Mediavilla, M.D.; Cos, S.; González, A.; Sanchez-Barcelo, E.J. Melatonin Down-Regulates hTERT Expression Induced by Either Natural Estrogens (17beta-estradiol) or Metalloestrogens (cadmium) in MCF-7 Human Breast Cancer Cells. Cancer Lett. 2008, 268, 272–277. [Google Scholar] [CrossRef]
- Pant, H.H.; Rao, M.V. Evaluation of In Vitro Anti-Genotoxic Potential of Melatonin against Arsenic and Fluoride in Human Blood Cultures. Ecotoxicol. Environ. Saf. 2010, 73, 1333–1337. [Google Scholar] [CrossRef]
- Lin, A.M.; Feng, S.F.; Chao, P.L.; Yang, C.H. Melatonin Inhibits Arsenite-Induced Peripheral Neurotoxicity. J. Pineal. Res. 2009, 46, 64–70. [Google Scholar] [CrossRef]
- Lin, A.M.; Fang, S.F.; Chao, P.L.; Yang, C.H. Melatonin Attenuates Arsenite-Induced Apoptosis in Rat Brain: Involvement of Mitochondrial and Endoplasmic Reticulum Pathways and Aggregation of Alpha-Synuclein. J. Pineal. Res. 2007, 43, 163–171. [Google Scholar] [CrossRef]
- Teng, Y.C.; Tai, Y.I.; Huang, H.J.; Lin, A.M. Melatonin Ameliorates Arsenite-Induced Neurotoxicity: Involvement of Autophagy and Mitochondria. Mol. Neurobiol. 2015, 52, 1015–1022. [Google Scholar] [CrossRef]
- Durappanavar, P.N.; Nadoor, P.; Waghe, P.; Pavithra, B.H.; Jayaramu, G.M. Melatonin Ameliorates Neuropharmacological and Neurobiochemical Alterations Induced by Subchronic Exposure to Arsenic in Wistar Rats. Biol. Trace. Elem. Res. 2019, 190, 124–139. [Google Scholar] [CrossRef]
- Pal, S.; Chatterjee, A.K. Prospective Protective Role of Melatonin against Arsenic-Induced Metabolic Toxicity in Wistar Rats. Toxicology 2005, 208, 25–33. [Google Scholar] [CrossRef]
- Uygur, R.; Aktas, C.; Caglar, V.; Uygur, E.; Erdogan, H.; Ozen, O.A. Protective Effects of Melatonin against Arsenic-Induced Apoptosis and Oxidative Stress in Rat Testes. Toxicol. Ind. Health 2016, 32, 848–859. [Google Scholar] [CrossRef]
- Morales, M.; Munné-Bosch, S. Malondialdehyde: Facts and Artifacts. Plant Physiol. 2019, 180, 1246–1250. [Google Scholar] [CrossRef] [Green Version]
- Jakubczyk, K.; Dec, K.; Kałduńska, J.; Kawczuga, D.; Kochman, J.; Janda, K. Reactive Oxygen Species-Sources, Functions, Oxidative Damage. Pol. Merkur. Lekarski. 2020, 48, 124–127. [Google Scholar]
- Parameswaran, N.; Patial, S. Tumor Necrosis Factor-A Signaling in Macrophages. Crit. Rev.™ Eukaryot. Gene Expr. 2010, 20, 87–103. [Google Scholar] [CrossRef]
- Vijayakumar, E.C.; Bhatt, L.K.; Prabhavalkar, K.S. High Mobility Group Box-1 (HMGB1): A Potential Target in Therapeutics. Curr. Drug Targets 2019, 20, 1474–1485. [Google Scholar] [CrossRef]
- Valavanidis, A.; Vlachogianni, T.; Fiotakis, C. 8-Hydroxy-2′-Deoxyguanosine (8-OHdG): A Critical Biomarker of Oxidative Stress and Carcinogenesis. J. Environ. Sci. Health Part C 2009, 27, 120–139. [Google Scholar] [CrossRef] [Green Version]
- Kraut, J.A.; Madias, N.E. Lactic Acidosis. N. Engl. J. Med. 2014, 371, 2309–2319. [Google Scholar] [CrossRef]
- Punshon, T.; Jackson, B.P.; Meharg, A.A.; Warczack, T.; Scheckel, K.; Guerinot, M.L. Understanding Arsenic Dynamics in Agronomic Systems to Predict and Prevent Uptake by Crop Plants. Sci. Total Environ. 2017, 581–582, 209–220. [Google Scholar] [CrossRef]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free Radicals, Antioxidants and Functional Foods: Impact on Human Health. Pharmacogn. Rev. 2010, 4, 118. [Google Scholar] [CrossRef] [Green Version]
- Tordjman, S.; Chokron, S.; Delorme, R.; Charrier, A.; Bellissant, E.; Jaafari, N.; Fougerou, C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017, 15, 434–443. [Google Scholar] [CrossRef]
- Del Rio, D.; Stewart, A.; Pellegrini, J.N. A Review of Recent Studies on Malondialdehyde as Toxic Molecule and Biological Marker of Oxidative Stress. Nutr. Metab. Cardiovasc. Dis. 2005, 15, 316–328. [Google Scholar] [CrossRef] [PubMed]
- Renu, K.; Saravanan, A.; Elangovan, A.; Ramesh, S.; Annamalai, S.; Namachivayam, A.; Abel, P.; Madhyastha, H.; Madhyastha, R.; Maruyama, M.; et al. An Appraisal on Molecular and Biochemical Signalling Cascades during Arsenic-Induced Hepatotoxicity. Life Sci. 2020, 260, 118438. [Google Scholar]
- Taysi, S.; Koc, M.; Büyükokuroğlu, M.E.; Altinkaynak, K.; Sahin, Y.N. Melatonin Reduces Lipid Peroxidation and Nitric Oxide during Irradiation-Induced Oxidative Injury in the Rat Liver. J. Pineal. Res. 2003, 34, 173–177. [Google Scholar] [CrossRef]
- Galano, A.; Tan, D.X.; Reiter, R.J. Melatonin: A Versatile Protector against Oxidative DNA Damage. Molecules 2018, 23, 530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bakker, J.; Postelnicu, R.; Mukherjee, V. Lactate: Where Are We Now? Crit. Care Clin. 2020, 36, 115–124. [Google Scholar] [CrossRef]
- Buchanan, S.; Combet, E.; Stenvinkel, P.; Shiels, P.G. Klotho, Aging and the Failing Kidney. Front. Endocrinol 2020, 11, 560. [Google Scholar] [CrossRef] [PubMed]
- Sosa, C.; Guillén, N.; Lucea, S.; Sorribas, V. Effects of Oral Exposure to Arsenite on Arsenic Metabolism and Transport in Rat Kidney. Toxicol. Lett. 2020, 333, 4–12. [Google Scholar] [CrossRef]
- Shin, E.J.; Chung, Y.H.; Le, H.L.; Jeong, J.H.; Dang, D.K.; Nam, Y.; Wie, M.B.; Nah, S.Y.; Nabeshima, Y.; Nabeshima, T.; et al. Melatonin Attenuates Memory Impairment Induced by Klotho Gene Deficiency Via Interactive Signaling between MT2 Receptor, ERK, and Nrf2-Related Anti-oxidant Potential. Int. J. Neuropsychopharm. 2014, 18, 1–14. [Google Scholar]
- Srinivas, N.; Rachakonda, S.; Kumar, R. Telomeres and Telomere Length: A General Overview. Cancers 2020, 12, 558. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Yuan, W.; Lin, Z. Functional Roles in Cell Signaling of Adaptor Protein TRADD from a Structural Perspective. Comput. Struct. Biotechnol. J. 2020, 18, 2867–2876. [Google Scholar] [CrossRef]
- Pobezinskaya, Y.L.; Liu, Z. The Role of TRADD in Death Receptor Signaling. Cell Cycle 2012, 11, 871–876. [Google Scholar] [CrossRef] [Green Version]
- Matzneller, P.; Kussmann, M.; Eberl, S.; Maier-Salamon, A.; Jäger, W.; Bauer, M.; Langer, O.; Zeitlinger, M.; Poeppl, W. Pharmacokinetics of the P-Gp Inhibitor Tariquidar in Rats after Intravenous, Oral and Intraperitoneal Administration. Eur. J. Drug Metab. Pharmacokinet. 2018, 43, 599–606. [Google Scholar] [CrossRef] [Green Version]
- Reiter, R.J.; Mayo, J.C.; Tan, D.X.; Sainz, R.M.; Alatorre-Jimenez, M.; Qin, L. Melatonin as an Antioxidant: Under Promises but over Delivers. J. Pineal. Res. 2016, 61, 253–278. [Google Scholar] [CrossRef]
- Baydas, G.; Canatan, H.; Turkoglu, A. Comparative Analysis of the Protective Effects of Melatonin and Vitamin E on Streptozocin-Induced Diabetes Mellitus. J. Pineal. Res. 2002, 32, 225–230. [Google Scholar] [CrossRef]
- Wahab, M.H.A.; Akoul, E.-S.E.; Abdel-Aziz, A.-A.H. Modulatory Effects of Melatonin and Vitamin E on Doxorubicin-Induced Cardiotoxicity in Ehrlich Ascites Carcinoma-Bearing Mice. Tumor. J. 2000, 86, 157–162. [Google Scholar] [CrossRef]
- Montilla, P.; Cruz, A.; Padillo, F.J.; Túnez, I.; Gascon, F.; Muñoz, M.C.; Gómez, M.; Pera, C. Melatonin Versus Vitamin E as Protective Treatment against Oxidative Stress after Extra-Hepatic Bile Duct Ligation in Rats. J. Pineal. Res. 2001, 31, 138–144. [Google Scholar] [CrossRef]
- Hsu, C.-H.; Han, B.; Liu, M.; Yeh, C.; Casida, J.E. Phosphine-Induced Oxidative Damage in Rats: Attenuation by Melatonin. Free Radic. Biol. Med. 2000, 28, 636–642. [Google Scholar] [CrossRef]
- Gultekin, F.; Delibas, N.; Yasar, S.; Kilinc, I. In Vivo Changes in Anti-oxidant Systems and Protective Role of Melatonin and a Combination of Vitamin C and Vitamin E on Oxidative Damage in Erythrocytes Induced by Chlorpyrifos-Ethyl in Rats. Arch. Toxicol. 2001, 75, 88–96. [Google Scholar] [CrossRef]
- Rosales-Corral, S.; Tan, D.X.; Reiter, R.J.; Valdivia-Velázquez, M.; Martínez-Barboza, G.; Acosta-Martínez, J.P.; Ortiz, G.G. Orally Administered Melatonin Reduces Oxidative Stress and Proinflammatory Cytokines Induced by Amyloid-β Peptide in Rat Brain: A Comparative, In Vivo Study Versus Vitamin C and E. J. Pineal. Res. 2003, 35, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Forrest, C.M.; Mackay, G.M.; Stoy, N.; Stone, T.W.; Darlington, L.G. Inflammatory Status and Kynurenine Metabolism in Rheumatoid Arthritis Treated with Melatonin. Br. J. Clin. Pharmacol. 2007, 64, 517–526. [Google Scholar] [CrossRef] [Green Version]
- Kędziora-Kornatowska, K.; Szewczyk-Golec, K.; Czuczejko, J.; Pawluk, H.; van Marke de Lumen, K.; Kozakiewicz, M.; Bartosz, G.; Kedziora, J. Antioxidative Effects of Melatonin Administration in Elderly Primary Essential Hypertension Patients. J. Pineal. Res. 2008, 45, 312–317. [Google Scholar] [CrossRef] [PubMed]
- Tamura, H.; Takasaki, A.; Miwa, I.; Taniguchi, K.; Maekawa, R.; Asada, H.; Taketani, T.; Matsuokam, A.; Yamagata, Y.; Shimamura, K.; et al. Oxidative Stress Impairs Oocyte Quality and Melatonin Protects Oocytes from Free Radical Damage and Improves Fertilization Rate. J. Pineal. Res. 2008, 44, 280–287. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Hodjat, M.; Rahimifard, M.; Nigjeh, M.N.; Azizi, M.; Baeeri, M.; Bayrami, Z.; Gholami, M.; Hassani, S.; Abdollahi, M. Assessment of Arsenic-Induced Modifications in the DNA Methylation of Insulin-Related Genes in Rat Pancreatic Islets. Ecotoxicol. Environ. Saf. 2020, 201, 110802. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Browne, R. The Analysis of Free Radicals, Lipid Peroxides, Anti-oxidant Enzymes and Compounds Related to Oxidative Stress as Applied to the Clinical Chemistry Laboratory. Adv. Exp. Med. Biol. 1994, 366, 43–58. [Google Scholar]
- Baeeri, M.; Rahimifard, M.; Daghighi, S.M.; Khan, F.; Salami, S.A.; Moini-Nodeh, S.; Haghi-Aminjan, H.; Bayrami, Z.; Rezaee, F.; Abdollahi, M. Cannabinoids as Anti-ROS in Aged Pancreatic Islet Cells. Life Sci. 2020, 256, 117969. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Lu, Y.-Q. The Regulatory Role of High-Mobility Group Protein 1 in Sepsis-Related Immunity. Front. Immunol. 2021, 11, 1–7. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A New Mathematical Model for Relative Quantification in Real-Time RT-PCR. Nucleic Acids Res. 2001, 29, 2003–2007. [Google Scholar] [CrossRef] [PubMed]
| Heart Tissue | Lung Tissue | ||
|---|---|---|---|
| Groups | Total As Concentration (mg/g) | Total As Concentration (mg/g) | |
| Control | 50 ± 3 | 25 ± 3 | |
| MLT | 48 ± 2 | 23 ± 2 | |
| NaAsO2 | 1/2 | 475 ± 33 *** | 425 ± 23 *** |
| 1/3 | 325 ± 25 *** | 300 ± 23 *** | |
| 1/10 | 200 ± 20 * | 175 ± 18 * | |
| NaAsO2 + MLT | 1/2 | 375 ± 33 | 300 ± 25 |
| 1/3 | 250 ± 15 # | 200 ± 8 # | |
| 1/10 | 175 ± 13 ## | 100 ± 3 ## | |
| Oxidative Stress Biomarker | |||||
|---|---|---|---|---|---|
| Heart Tissue | Lung Tissue | ||||
| Groups | MDA (µmol/mg protein) | ROS (mol/min/mg protein) | MDA (µmol/mg protein) | ROS (mol/min/mg protein) | |
| Control | 0.71 ± 0.05 | 0.72 ± 0.05 | 0.50 ± 0.05 | 0.40 ± 0.05 | |
| MLT | 0.69 ± 0.05 | 0.69 ± 0.04 | 0.57 ± 0.04 | 0.42 ± 0.03 | |
| NaAsO2 | 1/2 | 1.67 ± 0.11 *** | 1.87 ± 0.07 *** | 1.46 ± 0.11 *** | 1.50 ± 0.09 *** |
| 1/3 | 1.46 ± 0.06 *** | 1.68 ± 0.06 *** | 1.43 ± 0.09 *** | 1.36 ± 0.06 *** | |
| 1/10 | 1.14 ± 0.07 * | 1.33 ± 0.03 *** | 0.93 ± 0.07 * | 1.01± 0.03 *** | |
| NaAsO2 + MLT | 1/2 | 1.30 ± 0.07 # | 1.05 ± 0.06 ### | 1.09 ± 0.07 # | 0.65 ± 0.05 ### |
| 1/3 | 0.97 ± 0.09 ### | 0.89 ± 0.05 ### | 0.84 ± 0.06 ### | 0.64 ± 0.08 ### | |
| 1/10 | 0.88 ± 0.06 ### | 0.74 ± 0.04 ### | 0.62 ± 0.06 ### | 0.43 ± 0.04 ### | |
| Lactate Level | |||
|---|---|---|---|
| Heart Tissue | Lung Tissue | ||
| Groups | Lactate (mmol/mg protein) | Lactate (mmol/mg protein) | |
| Control | 0.73 ± 0.04 | 0.55 ± 0.04 | |
| MLT | 0.71 ± 0.09 | 0.51 ± 0.04 | |
| NaAsO2 | 1/2 | 1.30 ± 0.07 *** | 1.34 ± 0.11 *** |
| 1/3 | 1.25 ± 0.04 *** | 0.95 ± 0.04 * | |
| 1/10 | 1.63 ± 0.06 *** | 0.92 ± 0.05 * | |
| NaAsO2 + MLT | 1/2 | 0.96 ± 0.06 ### | 0.83 ± 0.07 ### |
| 1/3 | 0.93 ± 0.04 ### | 0.71 ± 0.03 ### | |
| 1/10 | 1.07 ± 0.07 ### | 0.65 ± 0.07 ### | |
| Histopathological Changes | |||||
|---|---|---|---|---|---|
| Groups | Edema | Congestion | Muscle Necrosis | Hemorrhage | |
| Control | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| MLT | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | 0.00 ± 0.00 | |
| NaAsO2 | 1/2 | 3.33 ± 0.33 *** | 2.33 ± 0.33 *** | 4.67 ± 0.33 *** | 2.00 ± 0.58 * |
| 1/3 | 0.33 ± 0.58 ### | 0.00 ± 0.00 ### | 0.67 ± 0.33 ### | 0.67 ± 0.33 | |
| 1/10 | 0.00 ± 0.00 ### | 0.00 ± 0.00 ### | 0.00 ± 0.00 ### | 0.00 ± 0.00 # | |
| NaAsO2 + MLT | 1/2 | 2.33 ± 1.52 ** | 1.33 ± 0.33 ***# | 3.00 ± 0.58 ***# | 1.67 ± 0.67 |
| 1/3 | 0 ± 0 ### | 0.00 ± 0.00 ### | 0.33 ± 0.33 ### | 0.33 ± 0.33 | |
| 1/10 | 0 ± 0 ### | 0.00 ± 0.00 ### | 0.00 ± 0.00 ### | 0.00 ± 0.00 # | |
| Gene Name Primer | Gene Symbol | Accession No. | Primer Sequence (5′−3′) |
|---|---|---|---|
| Mus musculus beta actin | beta (Actb) | NM_007393.5 | F: CAGCAAGCAGGAGTACGATGA R: TCAAAGAAAGGGTGTAAAACGCA |
| Mus musculus TNFRSF1A-associated via death domain | TRADD | NM_001033161.2 | F: CGTGATGGGCTATACGAGCA R: CCGTGGGTTTCAAACACTGA |
| Mus musculus klotho | KL | NM_013823.2 | F: GTTCTGCACTTCTACCGCTG R: GTGTTTGGCTCGTTCATGGT |
| Mus musculus telomerase reverse transcriptase | TERT | NM_001362388.1 | F: CTGCAGGACACACCGTCTAT R: GTCACCTGTTGGTTTGCTGT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baeeri, M.; Didari, T.; Khalid, M.; Mohammadi-Nejad, S.; Daghighi, S.M.; Farhadi, R.; Rahimifard, M.; Bayrami, Z.; Haghi-Aminjan, H.; Foroumadi, R.; et al. Molecular Evidence of the Inhibitory Potential of Melatonin against NaAsO2-Induced Aging in Male Rats. Molecules 2021, 26, 6603. https://doi.org/10.3390/molecules26216603
Baeeri M, Didari T, Khalid M, Mohammadi-Nejad S, Daghighi SM, Farhadi R, Rahimifard M, Bayrami Z, Haghi-Aminjan H, Foroumadi R, et al. Molecular Evidence of the Inhibitory Potential of Melatonin against NaAsO2-Induced Aging in Male Rats. Molecules. 2021; 26(21):6603. https://doi.org/10.3390/molecules26216603
Chicago/Turabian StyleBaeeri, Maryam, Tina Didari, Madiha Khalid, Solmaz Mohammadi-Nejad, Seyed Mojtaba Daghighi, Ramtin Farhadi, Mahban Rahimifard, Zahra Bayrami, Hamed Haghi-Aminjan, Roham Foroumadi, and et al. 2021. "Molecular Evidence of the Inhibitory Potential of Melatonin against NaAsO2-Induced Aging in Male Rats" Molecules 26, no. 21: 6603. https://doi.org/10.3390/molecules26216603
APA StyleBaeeri, M., Didari, T., Khalid, M., Mohammadi-Nejad, S., Daghighi, S. M., Farhadi, R., Rahimifard, M., Bayrami, Z., Haghi-Aminjan, H., Foroumadi, R., Gholami, M., & Abdollahi, M. (2021). Molecular Evidence of the Inhibitory Potential of Melatonin against NaAsO2-Induced Aging in Male Rats. Molecules, 26(21), 6603. https://doi.org/10.3390/molecules26216603







